Abstract
By global standards, the prevalence of community-onset expanded-spectrum-cephalosporin-resistant (ESC-R) Escherichia coli remains low in Australia and New Zealand. Of concern, our countries are in a unique position, with high extramural resistance pressure from close population and trade links to Asia-Pacific neighbors with high ESC-R E. coli rates. We aimed to characterize the risks and dynamics of community-onset ESC-R E. coli infection in our low-prevalence region. A case-control methodology was used. Patients with ESC-R E. coli or ESC-susceptible E. coli isolated from blood or urine were recruited at six geographically dispersed tertiary care hospitals in Australia and New Zealand. Epidemiological data were prospectively collected, and bacteria were retained for analysis. In total, 182 patients (91 cases and 91 controls) were recruited. Multivariate logistic regression identified risk factors for ESC-R among E. coli strains, including birth on the Indian subcontinent (odds ratio [OR] = 11.13, 95% confidence interval [95% CI] = 2.17 to 56.98, P = 0.003), urinary tract infection in the past year (per-infection OR = 1.430, 95% CI = 1.13 to 1.82, P = 0.003), travel to southeast Asia, China, the Indian subcontinent, Africa, and the Middle East (OR = 3.089, 95% CI = 1.29 to 7.38, P = 0.011), prior exposure to trimethoprim with or without sulfamethoxazole and with or without an expanded-spectrum cephalosporin (OR = 3.665, 95% CI = 1.30 to 10.35, P = 0.014), and health care exposure in the previous 6 months (OR = 3.16, 95% CI = 1.54 to 6.46, P = 0.02). Among our ESC-R E. coli strains, the blaCTX-M ESBLs were dominant (83% of ESC-R E. coli strains), and the worldwide pandemic ST-131 clone was frequent (45% of ESC-R E. coli strains). In our low-prevalence setting, ESC-R among community-onset E. coli strains may be associated with both “export” from health care facilities into the community and direct “import” into the community from high-prevalence regions.
INTRODUCTION
Despite a dramatic global rise in the prevalence of expanded-spectrum-beta-lactamase (ESBL)-producing Escherichia coli, infections by expanded-spectrum-cephalosporin-resistant (ESC-R) E. coli in Australia, New Zealand, North America, and selected European countries remain at relatively low levels. Recent Australian national data show that 3.2% of community isolates carry such resistance. Approximately 80% of these harbor a globally dominant blaCTX-M ESBL gene and 12% a plasmid-borne AmpC-type mechanism (1). European surveillance data show that a significant proportion of countries have ESC resistance rates rates below 10% among invasive E. coli isolates (2). In the United States, a recent large sample of E. coli isolates indicated that 3.9% were ESBL-producing strains (3). Although these low rates offer reassurance in the near term, a year-on-year rise in the incidence of community-onset ESC-R E. coli infections in low-prevalence countries is of concern (2, 4).
Australia and New Zealand are in a globally unique position. We have low rates of use of antimicrobials traditionally identified as a risk factor for ESC-R E. coli. This includes very low fluoroquinolone use among humans and a ban on the use of ESC and fluoroquinolones in food production (5, 6). In contrast, we have considerable extramural pressure on antimicrobial resistance rates. Our countries are located within the Asia-Pacific region, with which we share a mobile population (7) and frequent commerce (although no land borders). A high proportion of our regional neighbors have rates of ESC-R among E. coli strains in excess of 25% (8, 9).
The aim of our study was to define the risk factors for, and dynamics of, ESC-R among community-onset E. coli infections in the low-prevalence settings of Australia and New Zealand by using a case-control methodology. Furthermore, we characterized the resistance genes and membership of the worldwide pandemic clone ST131 in implicated isolates.
MATERIALS AND METHODS
The COOEE Study (COmmunity Onset ESBL and AmpC E. coli Study) was a multisite case-control study, with prospective recruitment of patients and data collection. Six geographically dispersed tertiary centers in Australia (n = 5) and New Zealand (n = 1) participated. The human research ethics committees at The University of Queensland and participating sites approved this study.
Definitions.
E. coli infection was defined as “community onset” where a patient was resident in the community (including nursing homes) or had been hospitalized less than 48 h at the time of onset; “expanded-spectrum cephalosporin resistance” included all “nonsusceptible” isolates and was identified phenotypically. For ceftriaxone, MIC > 1 mg/liter was used. For ceftazidime, laboratories used MIC > 1 mg/liter or MIC > 4 mg/liter, depending on their use of EUCAST or Clinical and Laboratory Standards Institute (CLSI) criteria, respectively (10, 11); “site of infection” was determined by the researcher from available information. Guidance for urinary tract infections (UTI) was given as follows: “asymptomatic” = a positive urine culture, with no attributable symptoms; “lower tract infection” = lower-urinary-tract-only symptoms such as urgency, frequency, and dysuria; and “upper urinary tract infection” = temperature ≥ 38°C, flank pain, or costovertebral angle tenderness and/or any bacteremia from a urinary source. “Immune suppression” referred to use at the time of the sample collection of corticosteroids (>15 mg/day prednisolone or equivalent), calcineurin inhibitors, other nonbiologics (e.g., mycophenylate and methotrexate), cytotoxic agents, biological agents, or radiation therapy; the Charlson comorbidity index (12) was calculated on the basis of data available from the survey, with the exception of neurological impairment (dementia and hemiplegia), which was inadvertently omitted from the survey questioning. A McCabe score was assigned based on the investigator's estimate of participant survival (<1 month, 1 month to 2 years, or >2 years) (13). “International travel” (excluding travel between Australia and New Zealand) was classified into geographical regions as follows: South Pacific islands, southeast Asia, Indian subcontinent, China, Japan, North America, Europe, and Africa/the Middle East. “High-risk travel” (regions of the Indian subcontinent, southeast Asia, Africa, the Middle East, and China) was defined a priori based on Australian data (14). “Health care exposure” was assessed by the Friedman criteria (15) with two modifications: (i) day procedures were recorded, and (ii) the criteria were assessed in three “discrete” time periods (<1 month earlier, 1 to 6 months earlier, and 7 to 12 months earlier). In addition, exact dates and details of any hospital admissions or surgical procedures were recorded and the interval (in days) from the termination of health care contact to the date of the first medical review with the enrolling E. coli infection subject was calculated. Further definitions are provided in the supplemental material.
Clinical methods.
A case-control methodology was used. Case patients with community-onset ESC-R E. coli in a culture of blood or urine were identified in the microbiology laboratory of participating hospitals. Control patients had community-onset ESC-susceptible (ESC-S) E. coli isolated from the same specimen type (urine or blood) as the case. Controls were not matched by any clinical presentation, comorbidity, or demographic factors. They were selected as the next appropriate patient, after an enrolled case patient, within the same laboratory's specimen registration system. If the next appropriate control patient could not be recruited, the process was repeated, at the same time of day and day of week, in a later week of the study. A single control was recruited for each case.
Inclusions and exclusions.
A laboratory-specific protocol was developed by each site to identify all potentially appropriate patients aged ≥16 years with an isolate of ESC-R E. coli managed at the participating site. Patients cared for by external health care providers such as family doctors and external clinics (utilizing the participating laboratories as an external provider) were not considered for recruitment, due to the complex human-research ethics requirements in our jurisdiction. Initial screening to determine likely community onset and the presence of exclusion criteria was by review of available electronic laboratory data and/or contact with the clinician caring for the patient. Two exclusion criteria were applied: (i) inability of the patient to give informed consent to participate, and (ii) extra-anatomical urinary drainage such as an indwelling urinary catheter (in the community), intermittent catheterization, ileal conduit, or similar. These two groups whose members local clinicians had already identified as a high risk factor for resistant infection appeared to have relatively distinct demographic and health profiles. Hence, they were excluded in order to focus study resources on a more generalized population group.
Data collection.
Hospitalized patients or those attending ambulatory clinics were approached for recruitment and data collection in person, whereas the remainder were contacted by telephone. By telephone, at least three contact attempts on different days were made. After informed consent, including explanation of the aims of this study, a structured interview was conducted using a standardized data collection form completed by an investigator under non-blind conditions. Data were primarily self-reported by participants. Where the participant was uncertain of details (e.g., dates of hospitalization or antimicrobial use) or the details were not clear to the investigators on the basis of the answer(s) provided, the investigators were able to review the patient's medical records held at their institution.
For intermittent exposures (e.g., travel, health care exposure, use of antimicrobials, etc.), participants were asked to recall 12 months before presentation. Exact dates of exposure were recorded. If the exact date was not recalled, it was estimated (“start of month” = the 1st of the month, “middle” or no date specified = 15th, “end of month” = last day).
Data were forwarded to a central coordinator where they were checked and entered into a secure database. Any omissions or discrepancies were clarified with the individual sites.
Laboratory methods.
All phenotypic susceptibility data presented in this study have been assessed by EUCAST criteria (10). All nonsusceptible isolates were considered “resistant” for the purpose of this analysis. E. coli isolates from each patient were forwarded to the research laboratory, with phenotypic identification and antimicrobial susceptibility undertaken by the use of disk diffusion susceptibility testing (DST), an automated system (VITEK 2), or agar dilution, based on the criteria in use by the laboratory at the time. Where data for susceptibility to an ancillary antimicrobial (e.g., nitrofurantoin) were not available, this was assessed by DST in the research laboratory. Where an isolate was originally tested by CLSI, DST using EUCAST criteria was undertaken (in the research laboratory) for agents for which the nonsusceptibility breakpoints of these two criteria differ (ceftazidime, cefepime, amikacin, gentamicin, ciprofloxacin, and nitrofurantoin). Where stated, MICs were performed by Etest (bioMérieux, France). For each isolate, a summative antimicrobial resistance score was calculated from 11 antimicrobials tested (ampicillin, amoxicillin plus clavulanate, ceftriaxone, ceftazidime, cefepime, meropenem, trimethoprim-sulfamethoxazole [SXT], ciprofloxacin, nitrofurantoin, gentamicin, and amikacin).
After overnight culture, bacterial DNA was extracted using an UltraClean microbial DNA isolation kit (Mo Bio Laboratories). ESC resistance genes were investigated by PCR using previously published primers and conditions (16–18). A stepwise approach based on local epidemiology of resistance mechanisms was employed. All isolates were investigated for blaCTX-M-1 group and blaCTX-M-9 group genes. Isolates negative for these were investigated for blaCTX-M (consensus sequence), blaCMY, blaDHA, blaTEM, blaSHV, and blaVEB. All isolates were screened for carbapenemase genes (blaNDM, blaKPC, and blaIMP) using an in-house multiplex PCR (19) and a singleplex PCR for blaOXA-48-like enzymes (20). All PCR amplicons were sequenced in the forward and reverse directions using an ABI3730XL (Life Technologies) capillary sequencer and compared to published sequences in GenBank (www.ncbi.nlm.nih.gov/GenBank).
Presumptive ST131 E. coli isolates were determined by use of semiautomated repetitive sequence-based PCR (rep-PCR) (DiversiLab, bioMérieux, France). Isolates clustering within 95% similarity to multilocus sequence type (MLST)-confirmed ST131 reference clones, using a Pearson correlation coefficient, were considered members of this clone (21). A selection of isolates (n = 4) were confirmed as ST131 by formal MLST analysis (22).
Statistical methods.
Sample sizes with overseas travel as a risk factor for resistant infection were calculated. With an estimated annual rate of overseas travel of 250/1,000 population (7), a sample size of 95 cases with matched controls was required to detect this risk with odds ratio ≥ 2.5 (power of 0.8 and two-sided alpha of 0.05).
Continuous data on health care exposure was right-censored at 365 days. Univariate comparison was undertaken by a χ2 test, Fischer's exact test, Wilcoxon rank sum test, and logistic regression as indicated. Interactions were examined. A multivariate logistic regression model with variables significant in univariate analysis at a P = 0.2 level was constructed. Using backward selection, variables were retained in the final logistic regression model if their significance remained below P = 0.2. Models were assessed by calculation of a receiver operating characteristic (ROC) curve and Hosmer-Lemeshow goodness of fit. All statistical tests were two-tailed, and P < 0.05 was considered significant. STATA version 12.1 (Statacorp) was used.
RESULTS
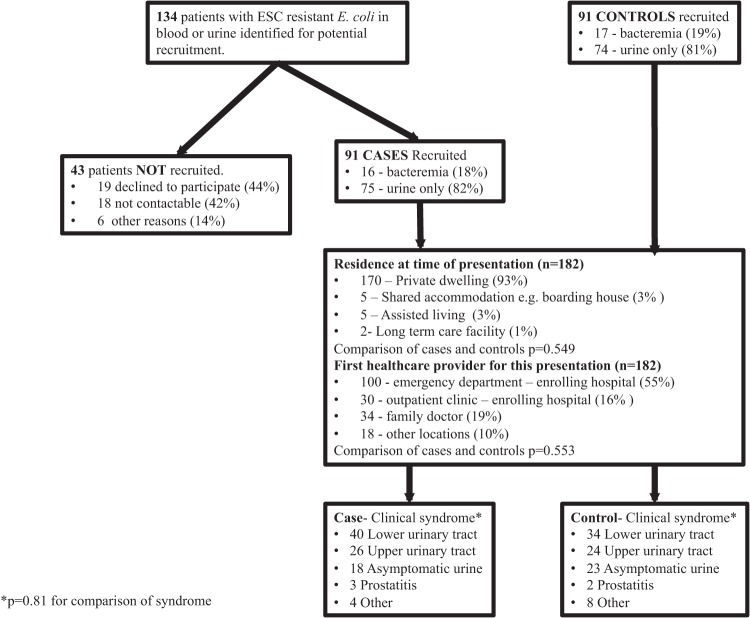
In total, 182 patients (91 cases and 91 controls) were recruited between March 2011 and October 2012 (Fig. 1). Patients were recruited over 12 continuous months at five sites and over 9 months at one site. Sites contributed between 8 and 58 patients.
FIG 1.
Participant identification and recruitment and characteristics of presentation and clinical syndrome.
Bacteremia was detected in 33 patients (18%), and positive cultures were grown from urine samples collected from the remaining 149 (82%). Uneven numbers of bacteremias occurred as one control patient recruited with a positive urine culture subsequently manifested a positive blood culture. The residences of the patients before presentation, clinical syndromes of presentation, and characteristics of hospital presentation did not differ significantly between case and control patients (Fig. 1).
A further 43 patients with presumed community-onset ESC-R E. coli infection and no overt exclusion criteria were not recruited (declined to participate, n = 19; not contactable, n = 18; other, n = 6). On comparison with recruited study participants, the median age (56 years, P = 0.81) and gender (11/43, 26% male, P = 0.39) did not differ significantly from those of the recruited patients and they were not analyzed further.
Close temporal matching of cases and controls was not frequent. Samples from 9 controls originated from the same calendar day as those from the matched case. For the entire cohort, there was a median interval of 22 days between the dates of collection of the case and control samples.
Phenotype, resistance genes, and ST131.
All case patients' E. coli isolates demonstrated phenotypic ESC resistance (ceftriaxone plus ceftazidime = 60 [68%], ceftriaxone only = 28 [32%], ceftazidime only = 3 [3%]). For the three E. coli isolates with ceftazidime resistance, the MICs of ceftazidime in the study laboratory were >256 mg/liter, 2 mg/liter, and 0.25 mg/liter, respectively. All control patient isolates were susceptible to ceftriaxone and ceftazidime. For all antimicrobials studied, with the exception of meropenem (100% susceptible) and amikacin (case = 4 resistant/91 [4%], control = 0 resistant/91 [0%], P = 0.121), resistance was significantly more likely in the ESC-R isolates than in the ESC-susceptible (ESC-S) isolates. For ESC-R E. coli, there was significant resistance to the oral therapeutic options investigated, including amoxicillin plus clavulanate (ESC-R = 59/91 [65%] versus ESC-S = 15/91 [16%], P = <0.001), ciprofloxacin (57/91 [63%] versus 6/91 [7%], P < 0.001) and SXT (64/91 [70%] versus 20/91 [22%], P < 0.01).
E. coli isolates from 89 cases (98%) and 90 (99%) controls were available for further analysis. Carbapenemases were not detected in any isolates. Expanded-spectrum cephalosporinase genes were detected in 87 of 89 (98%) ESC-R E. coli isolates as follows: for ESBLs, the blaCTX-M-1 group (36/89, 40%), blaCTX-M-9 group (35/89, 39%), blaCTX-M-1 and blaCTX-M-9 group (3/89, 3%), and blaSHV-5 group (n = 1; 1%); and for non-ESBLs, the blaCMY-2 group (n = 11; 12%) and blaDHA-1 group (n = 1; 1%). The two remaining samples included two of the three E. coli isolates with ceftazidime resistance (MICs, 2 mg/liter and 0.25 mg/liter) and contained only blaTEM-1, a non-expanded-spectrum beta-lactamase. ESC nonsusceptibility most likely originated from hyperproduction of this enzyme, with loss of this trait during passage and storage in the case of the isolate with the lower drug MIC.
The worldwide pandemic ST131 clone was presumptively identified in 46 patients (24%), who were significantly more likely to be case patients than controls (40/89 [45%] versus 6/90 [7%], P < 0.001). Among ESC-R E. coli isolates, ST131 was not associated with any non-CTX-M enzymes. They constituted 54% of the entire group of CTX-M isolates. In total, 24 (60%) harbored a CTX-M-1 group enzyme and 19 (48%) a CTX-M-9 group enzyme (P = 0.173 for the comparison). This includes three isolates (8%) harboring both enzymes. There was no significant difference in the proportions of ST131 by sample type (blood versus urine, P = 0.514) or hospital site (P = 0.574). With the exception of the smallest site (where 0 of 8 were ST131), the clone constituted 19% to 32% of the isolates from each site.
Demographics, comorbidities, and antimicrobial use.
Age data were compared by visual inspection of histograms. Cases and controls had similar bimodal distributions, with peaks at approximately 25 and 65 years. Median and 25th to 75th percentiles for ages of cases and controls, respectively, were 61 years (21 to 82) and 59 years (19 to 87) (P = 0.769). Results of univariate comparisons of demographic factors and medical comorbidities between cases and controls are shown in Table 1. Male sex was the only variable with a significant difference (odds ratio [OR] = 2.3, 95% confidence interval [CI] = 1.5 to 4.6, P = 0.018).
TABLE 1.
Univariate analysis of demographics, comorbidities, antimicrobial use, region of travel and birth, occupational and household exposure
| Subject variable | Frequency in ESC-R cases (%) (n = 91) | Frequency in ESC-S controls (%) (n = 91) | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|
| Demographics + comorbidities | |||||
| Male sex | 30 (33) | 16 (18) | 2.31 | 1.51–4.62 | 0.018b |
| Age < 30 or > 59 yrs | 66 (73) | 60 (66) | 1.36 | 0.72–2.57 | 0.336 |
| Immune suppression | 19 (20) | 10 (11) | 1.99 | 0.87–4.60 | 0.105b |
| Charlson score ≥ 1 | 44 (48) | 34 (37) | 1.57 | 0.87–2.83 | 0.135b |
| Active malignancy | 11 (13) | 9 (8) | 1.43 | 0.55–3.73 | 0.469 |
| Renal failure | 11 (13) | 9 (10) | 1.25 | 0.49–3.19 | 0.636 |
| McCabe score ≥ 2+ | 78 (86) | 76 (84) | 1.18 | 0.53–2.65 | 0.681 |
| Indigenous | 7 (8) | 6 (7) | 1.18 | 0.38–3.66 | 0.774 |
| Heart disease | 7 (8) | 7 (8) | 1 | ||
| Long-term-care-facility resident | 1 (1) | 1 (1) | 1 | ||
| Smoker | 12 (13) | 14 (15) | 0.83 | 0.36–1.92 | 0.672 |
| Liver disease | 3 (3) | 4 (4) | 0.74 | 0.16–3.41 | 0.701 |
| Lung disease | 5 (5) | 7 (8) | 0.70 | 0.21–2.85 | 0.552 |
| Pregnant or postpartum | 3 (3) | 7 (8) | 0.41 | 0.10–1.63 | 0.206 |
| Renal tract background | |||||
| Renal transplant | 8 (9) | 4 (4) | 2.1 | 0.61–7.22 | 0.241 |
| Anatomical or structural abnormality | 23 (25) | 15 (16) | 1.71 | 0.83–3.55 | 0.147b |
| UTIs in past 12 mos (per UTI)c | Median = 1; IQR = 0–3 | Median = 0; IQR = 0–1 | 1.32 | 1.08–1.63 | 0.008b |
| UTIs in lifetime (per UTI)c | Median = 2; IQR = 0–5 | Median = 2; IQR = 0–5 | 1.03 | 0.90–1.18 | 0.657 |
| Antimicrobial use | |||||
| Any antimicrobials in past 12 mos | 69 (76) | 62 (68) | 1.47 | 0.76–2.81 | 0.249 |
| Trimethoprim ± sulfamethoxazole | 16 (17.58) | 6 (6.59) | 3.022 | 1.13–8.12 | 0.028b |
| Expanded-spectrum cephalosporin(s) | 7 (8) | 0 | NA | 0.014b | |
| Fluoroquinolone(s) | 7(8) | 3 (3) | 2.44 | 0.61–9.77 | 0.206 |
| β-Lactam + ß-lactamase inhibitor | 16 (17.58) | 11 (12.09) | 1.552 | 0.68–3.56 | 0.300 |
| Carbapenem(s) | 3 (3.3) | 2 (2.2) | 1.517 | 0.25–9.30 | 0.652 |
| Aminoglycoside(s) | 5 (5) | 4 (4) | 1.26 | 0.33–4.87 | 0.733 |
| Macrolide | 6 (6.59) | 5 (5.49) | 1.214 | 0.36–4.13 | 0.756 |
| “Unknown” antimicrobial(s) | 35 (38) | 33 (36) | 1.1 | 0.60–2.00 | 0.759 |
| Narrow-spectrum cephalosporin(s) | 16 (17.58) | 15 (16.48) | 1.081 | 0.50–2.34 | 0.844 |
| Narrow-spectrum penicillin(s) | 10 (10.99) | 14 (15.38) | 0.679 | 0.28–1.62 | 0.383 |
| Travel by regiona | |||||
| Any overseas travel | 28 (30.8) | 22 (24.18) | 1.39 | 0.72–2.68 | 0.32 |
| High-risk regions | 24 (26) | 14 (15) | 1.97 | 0.94–4.11 | 0.071b |
| Indian subcontinent | 6 (6.59) | 1 (1.1) | 6.928 | 0.75–53.87 | 0.09 |
| North America | 5 (5.49) | 2 (2.20) | 2.199 | 0.49–13.69 | 0.264 |
| Africa + the Middle East | 3 (3.3) | 2 (2.2) | 1.517 | 0.25–9.30 | 0.652 |
| Southeast Asia | 15 (16) | 13 (14) | 1.18 | 0.53–2.65 | 0.681 |
| South Pacific | 3 (3.30) | 3 (3.30) | 1 | ||
| Europe | 3 (3.30) | 5 (5.49) | 0.586 | 0.14–2.53 | 0.474 |
| China | 4 (4.4) | 0 | 0.121 | ||
| Japan | 1 (1.1) | 0 | 0.500 | ||
| Birth by region | |||||
| High-risk region | 18 (20) | 10 (11) | 2.0 | 0.87–4.60 | 0.105 |
| Indian subcontinent | 11 (13) | 2 (2) | 6.12 | 1.32–28.45 | 0.021b |
| Australia + New Zealand | 58 (64) | 59 (65) | 0.95 | 0.52–1.75 | 0.877 |
| Europe | 15 (16) | 18 (20) | 0.80 | 0.38–1.71 | 0.564 |
| Southeast Asia | 3 (3) | 4 (4) | 0.74 | 0.16–3.41 | 0.701 |
| Africa + Middle East | 2 (2) | 4 (4) | 0.49 | 0.09–2.74 | 0.415 |
| China | 2 (2) | 0 | 0.497 | ||
| South Pacific | 0 | 3 (3) | 0.246 | ||
| Latin America | 0 | 1 (1) | 1.0 | ||
| Occupation and household exposure | |||||
| Partner with recent ESC-R E. coli infection | 2 (2) | Not assessed | |||
| Occupational health care exposure | 10 (11) | 7 (8) | 1.48 | 0.54–4.08 | 0.447 |
| Pet cat or dog or both at home | 32 (35) | 33 (36) | 0.95 | 0.52–1.75 | 0.877 |
| Occupational animal exposure | 4 (4) | 5 (5) | 0.79 | 0.21–3.05 | 0.733 |
| Preschoolers at home (<5 yrs of age) | 7 (8) | 9 (10) | 0.76 | 0.27–2.13 | 0.601 |
| Food consumption | |||||
| Any meat in past 12 mos | 89 (98) | 87 (98) | 2.05 | 0.37–11.46 | 0.415 |
| Poultry | 88 (97) | 83 (92) | 2.47 | 0.62–9.89 | 0.206 |
| Processed/preserved meats | 51 (56) | 52 (58) | 0.93 | 0.52–1.68 | 0.814 |
| Pork | 60 (66) | 63 (70) | 0.83 | 0.44–1.55 | 0.558 |
| Red meat | 76 (84) | 78 (88) | 0.72 | 0.31–1.66 | 0.433 |
Destinations of travel by region were as follows: for the Indian subcontinent, India, Pakistan, Nepal, and Bangladesh; for North America, the United States and Canada; for Africa and the Middle East, Zimbabwe, Kenya, Sudan, Liberia, Turkey, and Afghanistan; for Southeast Asia, Malaysia, Singapore, Thailand, Laos, Cambodia, Vietnam, Burma, Indonesia, and The Philippines; for South Pacific, New Caledonia, Papua New Guinea (PNG), Fiji, Samoa, Cook Islands, and boat cruises through the South Pacific; for Europe, the United Kingdom, Italy, Holland, Portugal, and Poland; for China, China, Hong Kong, and Macau; for Japan, Japan. High-risk regions include the Indian subcontinent, Africa, the Middle East, Southeast Asia, and China regions.
Entered into multivariate model.
Infections were recorded numerically on a scale of 0 to 5+, with all 5+ results considered 5 for analysis. Summaries are presented as a median value and an interquartile range (IQR).
Risk from previous urinary tract infection, renal allograft transplant, and anatomical abnormality of the renal tract was investigated (Table 1). The number of urinary tract infections in the previous year was significantly associated with ESC-R E. coli, with an odds ratio of 1.32 (95% CI = 1.08 to 1.63, P = 0.008) per infection.
Results of univariate analysis of antimicrobial use in the previous year are shown in Table 1. Where the patient could not recall the antimicrobial taken, it was recorded as “unknown.” Exposure to trimethoprim or trimethoprim with sulfamethoxazole (SXT) (OR = 3.02, 95% CI = 1.13 to 8.12, P = 0.028) was a significant risk factor for ESC-R E. coli. In addition, 7 of 7 patients who had been exposed to an expanded-spectrum cephalosporin (ceftriaxone, ceftazidime, or cefepime) had ESC-R E. coli isolated.
Health care exposure.
Health care exposure was analyzed using two distinct sets of data. First, health care exposure, classified using Friedman criteria for health care-associated (HA) infection, was analyzed in three time windows, with and without the inclusion of day procedures. Exclusion of day procedures performed marginally better at predicting ESC-R; exposure 0 to 1 month earlier (OR = 3.56, 95% CI = 1.14 to 11.14, P = 0.029) and 2 to 6 months earlier (OR = 2.99, 95% CI = 1.50 to 5.98, P = 0.002) was associated with ESC-R E. coli whereas exposure 7 to 12 months earlier (P = 0.705) was not (full details are provided in the supplemental material).
Second, a continuous model of the temporal risk of ESC-R E. coli infection after health care exposure was generated using the exact time interval since last hospital admission. Day procedures were excluded based on the results of the first analysis. This smoothed curve of the odds ratios shows the lower bound of the 95% CI approaching an odds ratio of 1.0 at approximately 4 to 5 months (Fig. 2).
FIG 2.
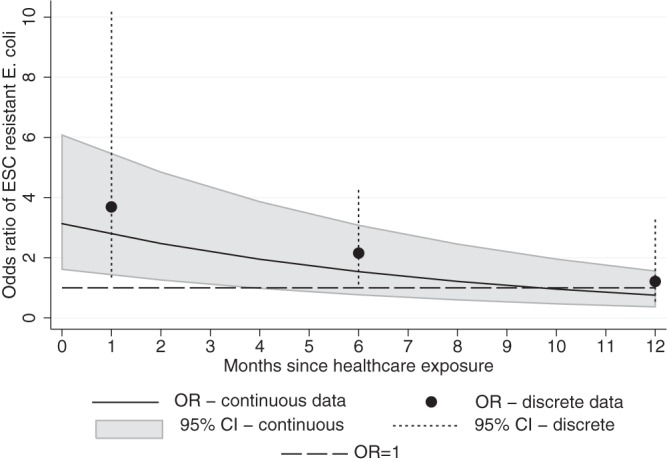
The risk of ESC-R E. coli infection over a 12-month period after the most-recent episode of health care exposure, excluding day procedures, estimated with two data sets. The smoothed curve was calculated using continuous data corresponding to the months since hospital admission (black line; 95% CI in gray). Discrete intervals determined using Friedman criteria are indicated (black dots; 95% CI as vertical dashes). The dashed line represents no increased risk (odds ratio = 1.0).
Travel, community, and occupational exposure.
Travel in the previous year was analyzed by region. Travel to the Indian subcontinent approached but did not achieve significance (P = 0.09). Birth on the Indian subcontinent was a significant risk factor (OR = 6.119, 95% CI = 1.32 to 28.44, P = 0.021) (Table 1).
Occupational exposure to animals, medical patients, and potential household risks was assessed, as was consumption of a variety of meats. No factors were significant (Table 1). Probable household transmission of ESC-R E. coli was suggested in one case where the partner of an enrolled patient had an infection with a highly similar isolate (99% identical by rep-PCR using Diversilab) 3 months prior.
Multivariate analysis.
For the multivariate model, health care exposure in the previous 6 months, excluding day procedures, was selected as a pragmatic option (univariate OR = 2.95, 95% CI = 1.59 to 5.46, P = 0.001). This dichotomous measure was nonsignificantly different from the four-category measurement used earlier (likelihood ratio test P = 0.821). Travel to high-risk regions was selected from the travel group (OR = 1.97, 95% CI = 0.94 to 4.11, P = 0.071). Use of an expanded-spectrum cephalosporin was combined with use of trimethoprim and SXT, in order to enter the former into the model, given its accepted prominence as a risk factor for ESC-R E. coli infection.
Significant variables in multivariate analysis were health care exposure, excluding day procedures in the previous 6 months (P = 0.002), birth on the Indian subcontinent (P = 0.004), travel to high-risk regions (P = 0.011), SXT/ESC use (P = 0.014), and number of UTIs in the previous year (P = 0.003) (Table 2). Assessment of the final model demonstrated an area under the ROC curve of 0.77 and a nonsignificant Hosmer-Lemeshow goodness of fit (P = 0.289).
TABLE 2.
Multivariate logistic regression
| Subject variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Health care exposure in the previous 6 mos | 3.16 | 1.54–6.46 | 0.002 |
| UTIs in previous yr (per UTI) | 1.43 | 1.16–1.82 | 0.003 |
| Birth on the Indian subcontinent | 11.13 | 2.17–56.96 | 0.004 |
| Travel to high-risk region(s) | 3.09 | 1.29–7.38 | 0.011 |
| Trimethoprim ± sulfamethoxazole ± ESC use | 3.67 | 1.30–10.35 | 0.014 |
| Male sex | 2.17 | 0.97–4.84 | 0.060 |
Interactions and alternative models.
A significant correlation between travel to high-risk regions and region of birth occurred. Those born in high-risk regions were more likely to undertake high-risk travel than those born elsewhere (17/28 [61%] versus 21/154 [14%], P < 0.001). This was particularly noted for birth and travel to the Indian subcontinent (7/13 [54%] versus 31/169 [18%], P = 0.002). This correlation, and the use of differing parameters for health care contact and antimicrobial exposure, were explored in alternative multivariate models (see the supplemental material). Specific population subgroups (symptomatic patients only, ESC-R blaCTX-M patients only, and ESC-R ST131 patients only) were also tested in the model. None of the alternative models performed better than the final model, although the levels of significance of health care exposure, male sex, and region of birth/travel differed depending on the model parameters selected.
HA and non-HA ESC-R E. coli.
A difference in the levels of risk of health care-associated (HA) ESC-R E. coli and non-HA ESC-R E. coli was separately investigated by analysis of risks within the HA (n = 73) and non-HA (n = 109) cohorts (full details are provided in the supplemental material). Several of the identified risks for ESC-R E. coli appeared to be most concentrated in one cohort. Data corresponding to travel to high-risk regions (P = 0.001), birth on the Indian subcontinent (P = 0.006), and male sex (P = 0.018) were statistically significant only among the members of the non-HA group. Conversely, data corresponding to a risk from SXT or ESC use were statistically significant only among members of the HA group (P = 0.026). The numbers of UTIs in the previous year were nonsignificantly different among the members of either group assessed separately.
Correlates of the classes of ESC resistance enzymes.
Correlates of ESC resistance enzyme classes were investigated by a comparison of patients harboring E. coli with CTX-M group enzyme to those harboring other enzymes (“non-CTX-M” = CMY, DHA, SHV, TEM). Full details are provided in the supplemental material.
There was no statistically significant difference with respect to the site of infection between CTX-M-harboring and non-CTX-M-harboring participants (P = 0.473), and although bacteremia was more frequent in the CTX-M group, this did not reach statistical significance (15/73 [21%] versus 0/15 [0%], P = 0.063). A significantly higher median resistance score was present in CTX-M isolates than in non-CTX-M isolates (median = 6 [interquartile range = 5 to 7] versus median = 4 [interquartile range = 4 to 5], P = 0.001). Notable differences included higher rates of resistance to the non-beta-lactam oral agents ciprofloxacin (56/74 [76%] versus 1/15 [7%], P < 0.001) and SXT (60/74 [81%] versus 4/15 [27%], P < 0.001) among the members of CTX-M group.
In regard to potential risk factors, the members of the CTX-M group were significantly more likely to have had health care exposure in the previous 6 months than the members of the non-CTX-M group (45/74 [61%] versus 3/15 [20%], P = 0.005) although the same was not true with respect to health care exposure in the previous 12 months (P = 0.72). Other factors used in the multivariate model that trended toward significance among the members of the CTX-M group included more high-risk travel (P = 0.052) and fewer reported UTIs in the previous 12 months (P = 0.054). Comparisons of factors not included in the multivariate model showed that “any overseas travel” was more likely in the CTX-M group (27/74 [36%] versus 1/15 [7%], P = 0.033).
DISCUSSION
This multicenter prospective case-control study of community-onset ESC-R E. coli infection has several key findings that have implications for risk-based empirical antibiotic prescription and infection control practices and for control of ESC-R E. coli infections within communities.
First, we established that 6 months is a practical, evidence-based definition for the duration of increased risk of a community-onset E. coli isolate harboring ESC-R after health care exposure. The time-dependent relationship of health care exposure to resistance seems intuitive in nature; however, previously there has been little supporting data. Hence, authors have used a variety of definitions from 1 to 6 months (23–25).
Overall, the significant contribution of health care exposure (OR = 3.15) as an ongoing “exporter” of resistant infection in a low-prevalence setting highlights the importance of controlling ESC resistance in the health care system. Supporting this hypothesis, United Kingdom data have recently demonstrated a broad-based decrease in the rate of ESC resistance among invasive Enterobacteriaceae strains following a reduction in the use of ESC and fluoroquinolones within the hospital system (26).
The “importation” of ESC-R E. coli after travel to countries with a high community incidence of ESBLs is starting to be defined (27), although fewer studies have identified infection rather than carriage (25, 28, 29). While the pathophysiology seems clear, the temporality of this remains to be confirmed. In our study, analysis of temporality, as presented for health care exposure, was precluded by the imprecision of data from the composite “high-risk” group and the low numbers involved. However, in absolute terms, 21 of 24 (87.5%) case participants with travel to high-risk regions departed those regions within the 6 months before presentation of infection. This fits with our previous research demonstrating mostly short-lived carriage of ESBL E. coli following travel overseas and with other studies demonstrating a decrease in the risk of resistant infection beyond 6 weeks after return from travel (29, 30).
Investigation of risks for community acquisition in the low-prevalence countries of Australia and New Zealand showed that one-quarter (n = 23) of ESC-R E. coli patients reported neither health care exposure nor high-risk travel, suggesting there are as-yet-undefined risk factors for transmission within the community (25, 31).
While there was some correlation between birth and travel region data, the identification of birth on the Indian subcontinent (OR = 11.12) as a risk factor for ESC-R E. coli infection in our cohort appears genuine. The etiology of this risk could stem from prolonged carriage of ESC-R E. coli after travel more than 1 year previous, leading to delayed community-onset ESC-R E. coli infection. Alternatively, our observation of a mostly short interval between travel and infection supports the possibility of domestic (within Australia and New Zealand) transmission of this resistance. Transmission of ESC-R E. coli from others within the household or community who have had recent travel to the Indian subcontinent may occur. Although the true magnitude of risk and the broader applicability require further study, this observation is consistent with a previously published study from one of our participating sites and with other descriptions of household transmission (32, 33). Recently, “birth outside Europe” was identified as a risk factor for infection by CTX-M-producing E. coli in another study, although comparison with our data is complicated, as the European study did not fully account for recent travel (31).
Our molecular epidemiology data serve to confirm a number of key observations made in other regions. The first is a distinct difference between the epidemiology of CTX-M ESBLs and that of other expanded-spectrum cephalosporinase enzymes, which may be mediated by the differing modes of acquisition, phenotypes, and characteristics of the E. coli strains harboring them (16, 34). Second, the high proportion of the ST131 clone among ESBLs is no surprise given its global prevalence (35). More surprising is its predominance without significant fluoroquinolone use (<6% of all participants in this study), one of the likely drivers in other regions (36). Exposure to this class of antimicrobials within Australia and New Zealand is very low (5).
Male sex has been defined by other researchers as a risk factor for community-onset ESC-R E. coli infection (23, 37–40) and became significant in some of our alternative models. The patient population of studies with this finding gives a clue to the etiology of this risk. On the whole, they are of older age with frequent health care exposure. This contrasts with studies conducted with a more traditional UTI population of young females that did not identify male sex as a risk factor (28, 29). In addition to males experiencing an age-dependent rise in the overall rates of E. coli infection (41), a limitation associated with case-control studies may also contribute to this finding. Aging patients certainly experience changes in the nuances and dynamics of health care exposure and other potential risk factors for ESC-R E. coli infection not identified with the data corresponding to dichotomous measures such as hospitalization and antimicrobial use that are most often collected.
The strengths of our study include its prospective collaborative nature, a geographically broad sample range, and the case-control methodology used. The low background rates of ESC-R E. coli infection in Australia and New Zealand have likely led to more discrete exposures and easier delineation of temporal risks than in communities where participants are frequently exposed to this form of resistance.
Limitations of our study include the moderate sample size, rate of nonrecruitment, and risk of bias due to an absence of blind investigator or patient procedures and reliance on patient recall for many exposures. Recruiting a higher ratio of controls (1:2 or 1:3) would have increased our study power and might have delineated further unidentified risks. The use of a third group of uninfected patients (a case-case-control design) would have allowed for delineation of risk factors associated with de novo acquisition of ESC-R E. coli, as opposed to delineation of risk factors for ESC-R within those that have E. coli (42). However, pragmatic limitations precluded these options.
Some unique features of Australia and New Zealand may limit extrapolation of our findings to other regions. The exclusion of day procedures in this study's definition of health care exposure correlated with our local epidemiology and would need to be reconsidered elsewhere. Furthermore, in cases in which blaCTX-M was not the predominant ESC resistance mechanism in a local population, risk data might differ.
The use of only hospital patients for recruitment allowed consistent access to participants and samples, although it might limit the applicability of some risks to the wider community. The exclusion of patients unable to consent meant that we could not define risks for patients in long-term-care facilities, a known reservoir of ESBL E. coli in Australia and overseas (43–45).
In conclusion, we have defined a critical ESC-R risk period after health care exposure among community-onset E. coli infections and demonstrated that ESC-R E. coli infection in a low-prevalence settings may be driven by “export” from health care exposure in the previous 6 months and importation after travel to regions with a high incidence of community ESBLs.
Supplementary Material
ACKNOWLEDGMENTS
We thank the clinical and microbiology laboratory staff at all sites. They greatly contributed to this study. In particular, we thank Tiffany Brown, Anna Sartor, and Wan Keat Yam (UQ), Julian Williams and Narelle George (RBWH), Cheryll McCullough (PathWest, RPH), and James Pollard and Owen Harris (St John of God Pathology, Geelong).
The laboratory component of this work was supported by a grant from The Royal Brisbane and Womens Hospital Foundation. The use of the Diversilab for this study was supported by bioMérieux (Australia), who supplied consumables for the Diversilab at a discounted cost. B.A.R. is funded by an Australian Postgraduate Award Scholarship. D.L.P. has received honoraria from AstraZeneca, Merck, and Pfizer. P.R.I., N.R., M.C.P., J.T.F., E.A., S.M.H., H.E.S., M.J., E.G., M.D.A., and K.S. received no funding.
Footnotes
Published ahead of print 27 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02052-13.
REFERENCES
- 1.Turnidge J, Gottlieb G, Mitchell D, Pearson J, Bell J, on behalf of The Australian Group on Antimicrobial Resistance January 2012, posting date Gram-negative survey, community-onset infections, 2010 antimicrobial susceptibility report. http://www.agargroup.org/files/AGAR%20GNB10%20REAL%20FINAL.pdf
- 2.European Centre for Disease Prevention and Control. 2012. Antimicrobial resistance surveillance in Europe 2011. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). http://www.ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-surveillance-europe-2011.pdf
- 3.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, II, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin. Infect. Dis. 56:641-648. 10.1093/cid/cis942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Looke DF, Gottlieb T, Jones CA, Paterson DL. 2013. Gram-negative resistance: can we combat the coming of a new “Red Plague”? Med. J. Aust. 198:243–244. 10.5694/mja13.10190 [DOI] [PubMed] [Google Scholar]
- 5.Cheng AC, Turnidge J, Collignon P, Looke D, Barton M, Gottlieb T. 2012. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 18:1453–1460. 10.3201/eid1809.111515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnidge J. 1999. The use of antibiotics in food-producing animals: antibiotic-resistant bacteria in animals and humans. Commonwealth of Australia. http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubs-jetacar-cnt.htm/$FILE/jetacar.pdf
- 7.Australian Bureau of Statistics. 8 February 2010. International movements, Australia, December 2009. Document 3401.0: overseas arrivals and departures, Australia Australian Bureau of Statistics, Canberra, Australia [Google Scholar]
- 8.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. 2009. Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob. Agents Chemother. 53:3280–3284. 10.1128/AAC.00426-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu PL, Liu YC, Toh HS, Lee YL, Liu YM, Ho CM, Huang CC, Liu CE, Ko WC, Wang JH, Tang HJ, Yu KW, Chen YS, Chuang YC, Xu Y, Ni Y, Chen YH, Hsueh PR. 2012. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 40(Suppl):S37–S43. 10.1016/S0924-8579(12)70008-0 [DOI] [PubMed] [Google Scholar]
- 10.European Committee on Antimicrobial Susceptibility Testing. 2011. Clinical breakpoints-bacteria (v 1.3). European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing; 20th information supplement. CLSI document M100-S20 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 13.McCabe WR, Jackson GG. 1962. Gram-negative bacteremia. I. etiology and ecology. Arch. Intern. Med. 110:847–855. 10.1001/archinte.1962.03620240029006 [DOI] [Google Scholar]
- 14.Kennedy K, Collignon P. 2010. Colonisation with Escherichia coli resistant to “critically important” antibiotics: a high risk for international travellers. Eur. J. Clin. Microbiol. Infect. Dis. 29:1501–1506. 10.1007/s10096-010-1031-y [DOI] [PubMed] [Google Scholar]
- 15.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 137:791–797. 10.7326/0003-4819-137-10-200211190-00007 [DOI] [PubMed] [Google Scholar]
- 16.Sidjabat HE, Paterson DL, Adams-Haduch JM, Ewan L, Pasculle AW, Muto CA, Tian GB, Doi Y. 2009. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob. Agents Chemother. 53:4733-4739. 10.1128/AAC.00533-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima A, Ishii Y, Ishihara K, Esaki H, Asai T, Oda C, Tamura Y, Takahashi T, Yamaguchi K. 2005. Extended-spectrum-beta-lactamase-producing Escherichia coli strains isolated from farm animals from 1999 to 2002: report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. Antimicrob. Agents Chemother. 49:3533–3537. 10.1128/AAC.49.8.3533-3537.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasterán F, Rapoport M, Petroni A, Faccone D, Corso A, Galas M, Vázquez M, Procopio A, Tokumoto M, Cagnoni V. 2006. Emergence of PER-2 and VEB-1a in Acinetobacter baumannii strains in the Americas. Antimicrob. Agents Chemother. 50:3222–3224. 10.1128/AAC.00284-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidjabat H, Silvey A, Paterson DL, Lim S. 2012. Development of Multiplex PCR: beta-lactamase genes and virulence determinants in E. coli, p 45 Abstr. Antimicrob. 2012, 13th Annu. Sci. Meet. Aust. Soc. Antimicrob., Brisbane, Australia [Google Scholar]
- 20.Poirel L, Heritier C, Tolun V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22. 10.1128/AAC.48.1.15-22.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitout JD, Campbell L, Church DL, Wang PW, Guttman DS, Gregson DB. 2009. Using a commercial DiversiLab semiautomated repetitive sequence-based PCR typing technique for identification of Escherichia coli clone ST131 producing CTX-M-15. J. Clin. Microbiol. 47:1212–1215. 10.1128/JCM.02265-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Ami R, Rodriguez-Bano J, Arslan H, Pitout JD, Quentin C, Calbo ES, Azap OK, Arpin C, Pascual A, Livermore DM, Garau J, Carmeli Y. 2009. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin. Infect. Dis. 49:682–690. 10.1086/604713 [DOI] [PubMed] [Google Scholar]
- 24.Azap OK, Arslan H, Serefhanoglu K, Colakoglu S, Erdogan H, Timurkaynak F, Senger SS. 2010. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin. Microbiol. Infect. 16:147–151. 10.1111/j.1469-0691.2009.02941.x [DOI] [PubMed] [Google Scholar]
- 25.Banerjee R, Strahilevitz J, Johnson JR, Nagwekar PP, Schora DM, Shevrin I, Du H, Peterson LR, Robicsek A. 2013. Predictors and molecular epidemiology of community-onset extended-spectrum beta-lactamase-producing Escherichia coli infection in a Midwestern community. Infect. Control Hosp. Epidemiol. 34:947–953. 10.1086/671725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. 2013. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J. Antimicrob. Chemother. 68:2667–2674. 10.1093/jac/dkt212 [DOI] [PubMed] [Google Scholar]
- 27.van der Bij AK, Pitout JD. 2012. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J. Antimicrob. Chemother. 67:2090–2100. 10.1093/jac/dks214 [DOI] [PubMed] [Google Scholar]
- 28.Laupland KB, Church DL, Vidakovich J, Mucenski M, Pitout JD. 2008. Community-onset extended-spectrum beta-lactamase (ESBL) producing Escherichia coli: importance of international travel. J. Infect. 57:441–448. 10.1016/j.jinf.2008.09.034 [DOI] [PubMed] [Google Scholar]
- 29.Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. 2013. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae—a case-control study in a low prevalence country. PLoS One 8:e69581. 10.1371/journal.pone.0069581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers BA, Kennedy KJ, Sidjabat HE, Jones M, Collignon P, Paterson DL. 2012. Prolonged carriage of resistant E. coli by returned travellers: clonality, risk factors and bacterial characteristics. Eur. J. Clin. Microbiol. Infect. Dis. 31:2413–2420. 10.1007/s10096-012-1584-z [DOI] [PubMed] [Google Scholar]
- 31.Nicolas-Chanoine MH, Jarlier V, Robert J, Arlet G, Drieux L, Leflon-Guibout V, Laouenan C, Larroque B, Caro V, Mentre F. 2012. Patient's origin and lifestyle associated with CTX-M-producing Escherichia coli: a case-control-control study. PLoS One 7:e30498. 10.1371/journal.pone.0030498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilty M, Betsch BY, Bogli-Stuber K, Heiniger N, Stadler M, Kuffer M, Kronenberg A, Rohrer C, Aebi S, Endimiani A, Droz S, Muhlemann K. 2012. Transmission dynamics of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin. Infect. Dis. 55:967–975. 10.1093/cid/cis581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman JT, Williamson DA, Heffernan H, Smith M, Bower JE, Roberts SA. 2012. Comparative epidemiology of CTX-M-14 and CTX-M-15 producing Escherichia coli: association with distinct demographic groups in the community in New Zealand. Eur. J. Clin. Microbiol. Infect. Dis. 31:2057–2060. 10.1007/s10096-011-1540-3 [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Baño J, Miró E, Villar M, Coelho A, Gozalo M, Borrell N, Bou G, Conejo MC, Pomar V, Aracil B, Larrosa N, Agüero J, Oliver A, Fernández A, Oteo J, Pascual A, Navarro F. 2012. Colonisation and infection due to Enterobacteriaceae producing plasmid-mediated AmpC beta-lactamases. J. Infect. 64:176–183. 10.1016/j.jinf.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 35.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66:1–14. 10.1093/jac/dkq415 [DOI] [PubMed] [Google Scholar]
- 36.Rogers BA, Doi Y. 2013. Who is leading this dance? Understanding the spread of Escherichia coli sequence type 131. Infect. Control Hosp. Epidemiol. 34:370-372. 10.1086/669874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melzer M, Petersen I. 2007. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J. Infect. 55:254–259. 10.1016/j.jinf.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 38.Courpon-Claudinon A, Lefort A, Panhard X, Clermont O, Dornic Q, Fantin B, Mentre F, Wolff M, Denamur E, Branger C. 2011. Bacteraemia caused by third-generation cephalosporin-resistant Escherichia coli in France: prevalence, molecular epidemiology and clinical features. Clin. Microbiol. Infect. 17:557–565. 10.1111/j.1469-0691.2010.03298.x [DOI] [PubMed] [Google Scholar]
- 39.Pitout JD, Gregson DB, Campbell L, Laupland KB. 2009. Molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007: emergence of clone ST131 as a cause of community-acquired infections. Antimicrob. Agents Chemother. 53:2846–2851. 10.1128/AAC.00247-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodríguez-Baño J, Navarro MD, Romero L, Martínez-Martínez L, Muniain MA, Perea EJ, Pérez-Cano R, Pascual A. 2004. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J. Clin. Microbiol. 42:1089–1094. 10.1128/JCM.42.3.1089-1094.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy KJ, Roberts JL, Collignon PJ. 2008. Escherichia coli bacteraemia in Canberra: incidence and clinical features. Med. J. Aust. 188:209–213 [DOI] [PubMed] [Google Scholar]
- 42.Kaye KS, Harris AD, Samore M, Carmeli Y. 2005. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect. Control Hosp. Epidemiol. 26:346–351. 10.1086/502550 [DOI] [PubMed] [Google Scholar]
- 43.Stuart RL, Kotsanas D, Webb B, Vandergraaf S, Gillespie EE, Hogg GG, Korman TM. 2011. Prevalence of antimicrobial-resistant organisms in residential aged care facilities. Med. J. Aust. 195:530–533. 10.5694/mja11.10724 [DOI] [PubMed] [Google Scholar]
- 44.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect. Control Hosp. Epidemiol. 34:361–369. 10.1086/669865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rooney PJ, O'Leary MC, Loughrey AC, McCalmont M, Smyth B, Donaghy P, Badri M, Woodford N, Karisik E, Livermore DM. 2009. Nursing homes as a reservoir of extended-spectrum beta-lactamase (ESBL)-producing ciprofloxacin-resistant Escherichia coli. J. Antimicrob. Chemother. 64:635–641. 10.1093/jac/dkp220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.