Abstract
Nephrotoxicity is the main complication of gentamicin (GM) treatment. GM induces renal damage by overproduction of reactive oxygen species and inflammation in proximal tubular cells. Phenolic compounds from ginger, called gingerols, have been demonstrated to have antioxidant and anti-inflammatory effects. We investigated if oral treatment with an enriched solution of gingerols (GF) would promote a nephroprotective effect in an animal nephropathy model. The following six groups of male Wistar rats were studied: (i) control group (CT group); (ii) gingerol solution control group (GF group); (iii) gentamicin treatment group (GM group), receiving 100 mg/kg of body weight intraperitoneally (i.p.); and (iv to vi) gentamicin groups also receiving GF, at doses of 6.25, 12.5, and 25 mg/kg, respectively (GM+GF groups). Animals from the GM group had a significant decrease in creatinine clearance and higher levels of urinary protein excretion. This was associated with markers of oxidative stress and nitric oxide production. Also, there were increases of the mRNA levels for proinflammatory cytokines (tumor necrosis factor alpha [TNF-α], interleukin-1β [IL-1β], IL-2, and gamma interferon [IFN-γ]). Histopathological findings of tubular degeneration and inflammatory cell infiltration reinforced GM-induced nephrotoxicity. All these alterations were attenuated by previous oral treatment with GF. Animals from the GM+GF groups showed amelioration in renal function parameters and reduced lipid peroxidation and nitrosative stress, in addition to an increment in the levels of glutathione (GSH) and superoxide dismutase (SOD) activity. Gingerols also promoted significant reductions in mRNA transcription for TNF-α, IL-2, and IFN-γ. These effects were dose dependent. These results demonstrate that GF promotes a nephroprotective effect on GM-mediated nephropathy by oxidative stress, inflammatory processes, and renal dysfunction.
INTRODUCTION
Gentamicin (GM) is a typical aminoglycoside antibiotic agent that is widely used in clinical practice for the treatment of Gram-negative infections (1, 2). However, its use is complicated by a great risk of nephrotoxicity, which affects 10 to 30% of patients, especially after long-term use (3–5).
In its elimination route, GM accumulates in the renal proximal tubular cells through the megalin/cubilin complex receptor, which is responsible for GM transportation inside the cell. Aminoglycoside-induced nephrotoxicity is characterized by tubular cell apoptosis and/or necrosis, predominantly in the proximal tubules (3, 6). Clinically, it results in acute kidney injury (AKI) with elevations of serum creatinine (SCr) and urea, in addition to proteinuria (5, 7). Pathological findings can be seen as proximal tubular edema, tubular necrosis, and desquamation, as well as inflammation and diffuse interstitial edema.
Several studies have shown the involvement of reactive oxygen species (ROS) in gentamicin-induced AKI. Gentamicin has been shown to enhance the generation of superoxide anion (O2−), peroxynitrite anion (ONOO−), hydrogen peroxide (H2O2), and hydroxyl radical (OH) production from renal cortical mitochondria, which is associated with an increase in lipid peroxidation and decrease in antioxidant enzymes (8, 9). These ROS act directly on cells, affecting their structure and functionality (2, 5).
Several phytotherapeutic agents have been used to prevent or ameliorate GM-induced AKI (5, 10–12), including ginger extract. Ginger (Zingiber officinale) is one of the world's best-known spices and is cultivated in several countries. It has been used since antiquity for its health benefits (13). Extracts obtained from its roots usually contain polyphenol compounds, such as [6]-gingerol, [8]-gingerol, and [10]-gingerol, which have been cited as the main components responsible for its pharmacological effects (14, 15). Among other potential mechanisms, ginger has antioxidant (15–17) and anti-inflammatory properties (15, 18, 19).
Ginger has been shown to protect against renal ischemia-reperfusion injury and cisplatin-related nephrotoxicity (20, 21). In the former case, its use has been associated with an antiapoptotic effect. However, ginger's anti-inflammatory effects on acute nephrotoxicity have scarcely been studied. In the present study, we aimed to evaluate the effects of a gingerol-enriched fraction of ginger extract in a model of gentamicin-induced nephrotoxicity, focusing mainly on gentamicin's oxidative and inflammatory effects (15, 18).
MATERIALS AND METHODS
Animals and experimental drug.
Experiments were performed with male Wistar rats weighing 240 to 280 g. The animals were housed under standard laboratory conditions and maintained on a 12-h light-dark cycle, and they had free access to food (Biotec, Agua Fria, Santa Catarina, Brazil) and water. The experimental protocols and all procedures were conducted according to the norms of the National Council for Control of Animal Experimentation (CONCEA) and were approved by the Ethics Committee of Animal Research of the Federal University of Ceara, Fortaleza, Brazil (protocol 68/12).
GM was purchased from Sigma and was administered intraperitoneally (i.p.) for 7 days. The GM solution was prepared so that the maximum injected volume was 0.5 to 0.6 ml/rat.
GF.
Nine grams of ginger extract obtained from fresh ginger rhizomes was fractionated into 14 fractions (F1 to F14) by column chromatography (18.0 cm by 4.2-cm internal diameter [i.d.]) on silica gel (70-230 mesh) with gradients of n-hexane and ethyl acetate. After high-pressure liquid chromatography (HPLC) analysis, the gingerols were identified in F3 (gingerol fraction [GF]). The standard gingerols used in the analysis had already been isolated and identified in previous studies (22). The gingerols present in the GF were quantified on a semipreparative HPLC system through a sample injection (50 mg of GF), collection of the fractions that contained each gingerol, and further weighing (22). The GF contained 41.7% [6]-gingerol (the main ginger compound), 4.8% [8]-gingerol, 2.4% [10]-gingerol, and 51.1% other minor compounds. GF was diluted in a 2% Tween 80 solution and orally administered in a maximum volume of 2.5 to 3.5 ml per rat.
Experimental design.
Animals were assigned to 6 different groups containing 7 or 8 animals each. The sham group (CT group) received 0.4-ml i.p. injections of saline solution (0.9%) for 7 consecutive days and oral treatment with 2% Tween 80 solution in the last 5 days. The second group (GF group) also received 0.4-ml i.p. injections of saline for 7 consecutive days, plus 5 days of gingerol-enriched solution (25 mg/kg of body weight) through the oral route. Another group (GM group) received i.p. injections of GM (100 mg/kg) for 7 consecutive days (23) and oral treatment with 2% Tween 80 solution for 5 days. Finally, three other groups (GM+GF groups) received i.p. injections of GM (100 mg/kg) for 7 consecutive days and three different doses of GF (6.25, 12.5, and 25 mg/kg) for 5 days. The oral administration of 2% Tween 80 or GF was always given on the fifth day after the first GM or saline injection.
Evaluation of renal functions.
The rats were kept individually in metabolic cages, and urine was collected for a 24-h period after the last oral administration. The animals were anesthetized, and a blood sample was obtained from the abdominal aorta. Blood plasma was separated for biochemical measurements. The left kidney was removed and immediately stored at −80°C. The right kidney was stored in 10% formalin for the histological studies. Plasma and urine samples were used for the analysis of urea, uric acid, and urinary protein by use of standard diagnostic kits (Labtest, Fortaleza, Brazil). Creatinine was also measured spectrophotometrically in order to calculate its clearance (CLCR) and was used to evaluate renal function.
Oxidative stress measurement.
We investigated oxidative damage through the malondialdehyde (MDA) content, which is an indicative measurement of lipid peroxidation. MDA was assayed by measuring thiobarbituric acid-reactive substances (TBARS). The reaction mixture consisted of a 10% renal tissue homogenate solution with 1% phosphoric acid (H3PO4) plus a 0.6% solution of thiobarbituric acid. The mixture of these reagents was maintained at 95°C for 45 min. The mixture was cooled in running water, and n-butanol was added. The tube was vortexed for 1 min and centrifuged at 294 × g for 15 min. After centrifugation, the organic phase was removed for a spectrophotometry reading (520 to 535 nm). The protein concentration was measured using the modified Bradford method (24).
Nitrite measurement.
The stable nitrite level was measured as an indirect method of detecting nitric oxide by using Griess reagent, based on a colorimetric assay described by Green et al. (25). Renal tissue homogenate (100 μl) was mixed with 100 μl Griess reagent, which consists of 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid. After 10 min, the absorbance was read at 540 nm. A standard curve was obtained using sodium nitrite.
Reduced GSH content.
The reduced glutathione (GSH) content in renal tissue was estimated according to the method described by Sedlak and Lindsay (26), with a few modifications. For the GSH assay, each kidney was homogenized in an ice-cold 0.02 M EDTA solution. Aliquots (400 μl) of tissue homogenate were mixed with 320 μl of distilled water and 80 μl of 50% (wt/vol) trichloroacetic acid in glass tubes and centrifuged at 3,000 × g for 15 min. Supernatants (400 μl) were mixed with 800 μl Tris buffer (0.4 M; pH 8.9), and 20 μl of 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB; 0.01 M) was added. After shaking of the reaction mixture, the absorbance was measured at 412 nm within 5 min of DTNB addition, against a blank with no homogenate. The absorbance values were extrapolated from a glutathione standard curve, and the level of GSH was expressed in grams of GSH per milligram of protein.
SOD activity.
Superoxide dismutase (SOD) activity was measured according to the method of Sun et al. (27). The activity of this enzyme was evaluated by measuring its capacity to inhibit the photochemical reduction of nitroblue tetrazolium (NBT). In this assay, the photochemical reduction of riboflavin generates O2, which reduces NBT to produce formazan salt, which has maximal absorbance at 560 nm. In the presence of SOD, the reduction of NBT is inhibited, as the enzyme converts the superoxide radical to peroxide. The results are expressed as amounts of SOD needed to inhibit the rate of NBT reduction by 50%, in units of enzyme per gram of protein. Homogenates (10% tissue in phosphate buffer) were centrifuged (10 min, 2,608 × g, 4°C), and the supernatant was removed and centrifuged a second time (20 min, 15,294 × g, 4°C). The supernatant was then assayed. In a dark chamber, 1 ml of the reactant (50 mM phosphate buffer, 100 nM EDTA, and 13 mM l-methionine, pH 7.8) was mixed with 30 μl of the sample, 150 μl of NBT (75 μM), and 300 μl of riboflavin (2 μM). The tubes containing the resulting solution were exposed to fluorescent light bulbs (15 W) for 15 min and then read using a spectrophotometer at 560 nm.
Inflammatory status.
Gene expression of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-2, and gamma interferon (IFN-γ) was assayed using a CFX96 Touch detection system (Bio-Rad). Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta polypeptide (YWHAZ) was used as the reference (28). DNA primers for all genes (Table 1) were designed on the basis of mRNA sequences obtained from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov; accessed 4 February 2011).
TABLE 1.
Oligonucleotide sequences of primers used for qPCR
| Gene | Primer direction | Primer sequence (5′-3′) |
|---|---|---|
| TNF-α | Sense | GTACCCACTCGTAGCAAAC |
| Antisense | AGTTGGTTGTCTTTGAGATCCATG | |
| IL-1β | Sense | GACCTGTTCTTTGAGGCTGACA |
| Antisense | CTCATCTGGACAGCCCAAGTC | |
| IL-2 | Sense | CAAGCAGGCCACAGAATTGA |
| Antisense | CCGAGTTCATTTTCCAGGCA | |
| IFN-γ | Sense | ACAGTAAAGCAAAAAAGGATGCA |
| Antisense | GCTGGATCTGTGGGTTGTTC | |
| YWHAZ | Sense | GCTACTTGGCTGAGGTTGCT |
| Antisense | TGCTGTGACTGGTCCACAAT |
Real-time PCR assays were performed in a final volume of 25 μl containing 12.5 μl iQ SYBR green supermix (Bio-Rad), 200 nM (each) primers, and 1 μl cDNA from the sample. Negative samples were also tested, with the cDNA being replaced with autoclaved Milli-Q water. The PCR conditions were as follows: an initial denaturation period of 3 min at 95°C followed by 40 cycles of gene amplification. Each cycle consisted of an initial denaturation step of 20 s at 95°C, followed by an annealing step of 20 s at 60°C and an extension step of 45 s at 72°C. The samples were then subjected to an extension step of 3 min at 72°C.
To measure the specificity of the applied amplifications (i.e., to determine whether the formed products were specific for the tested genes), we performed a melting curve analysis in which the reaction temperature was increased 0.5°C every 15 s, beginning at the annealing temperature of the tested set of primers and ending at 95°C. Throughout the curve construction process, the changes in fluorescence were measured, and the data obtained, using CFX Manager software (version 3.0; Bio-Rad), were based on the values for the threshold cycle, i.e., the cycle where the observed fluorescence was 10-fold higher than the basal fluorescence for each quantitative PCR (qPCR) assay. Gene expression was obtained by applying the mathematical 2−ΔΔCT method (29).
Histological and morphological analyses.
The right kidneys were fixed with paraformaldehyde. Next, they were dehydrated with 70% ethanol and processed in paraffin. The resulting blocks were sliced into 5-μm-thick sections, stained with hematoxylin and eosin (H&E), and observed under a light microscope (×400).
Statistical analysis.
Values were expressed as means ± standard errors of the means (SEM) for statistical analysis. One-way analysis of variance (ANOVA) followed by the Student-Newman-Keul post hoc test was used for parametric data. Gene expression data were evaluated by the Mann-Whitney test. P values of <0.05 were considered significant. Analysis was performed using GraphPad Prism 5.0.
RESULTS
Effect of GF on gentamicin-induced AKI.
The GM group had a significant reduction in CLCR in comparison with both the CT and GF groups. This CLCR reduction was accompanied by increases in uric acid levels and the urine protein excretion rate (Table 2). There was a progressive amelioration of CLCR and a serum creatinine (SCr) reduction in the GM+GF groups, with statistical significance at the 12.5- and 25-mg/kg doses. The 25-mg/kg dose appeared to confer better protection, so it was used in some experiments to explore GF's protective effects.
TABLE 2.
Effects of GF on parameters of overall renal function in GM-induced nephrotoxicity
| Parameter | Value (mean ± SEM) for groupa |
|||||
|---|---|---|---|---|---|---|
| CT | GM | CT-GF (25 mg/kg) | GM+GF (6.25 mg/kg) | GM+GF (12.5 mg/kg) | GM+GF (25 mg/kg) | |
| Serum creatinine (mg/dl) | 0.62 ± 0.03 | 1.05 ± 0.08* | 0.51 ± 0.01# | 0.87 ± 0.06 | 0.78 ± 0.07# | 0.63 ± 0.03# |
| Creatinine clearance (ml/min) | 1.02 ± 0.18 | 0.41 ± 0.08* | 0.97 ± 0.1# | 0.55 ± 0.08 | 0.68 ± 0.09 | 0.88 ± 0.14# |
| Urea (mg/dl) | 47.3 ± 2.61 | 80.6 ± 7.77* | 37.9 ± 2.8# | 62.5 ± 6.7 | 53.8 ± 6.9# | 41.7 ± 1.8# |
| Urinary protein (mg/dl) | 40.5 ± 4.48 | 93.5 ± 9.28* | 44.5 ± 4.82# | 78.3 ± 9.93 | 55.6 ± 7.74# | 51.3 ± 3.24# |
| Uric acid (mg/dl) | 1.33 ± 0.21 | 3.74 ± 0.52* | 1.57 ± 0.29# | 2.5 ± 0.5 | 2.33 ± 0.71 | 1.66 ± 0.33# |
*, statistically significant (P < 0.05) compared to control (CT); #, statistically significant (P < 0.05) compared to GM.
Gentamicin-induced lipid peroxidation is attenuated by gingerols.
Gentamicin treatment increased MDA levels in renal tissue compared to those in the control group. In the GM+GF groups, there was a progressive reduction in the MDA level as the GF dose was increased. At the dose of 25 mg/kg, kidney MDA levels in the GM+GF group were similar to those in the control group (Fig. 1). Without GM-induced renal injury, GF alone had no effect on kidney MDA levels.
FIG 1.
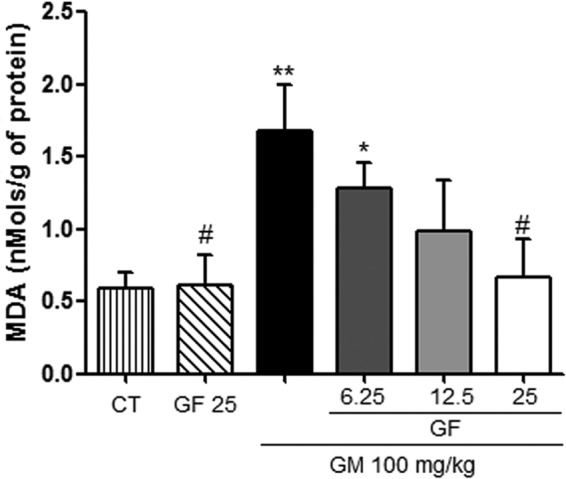
Effects of GF on lipid peroxidation (MDA) in gentamicin-induced nephrotoxicity. The results are expressed as means ± SEM (n = 7). Statistical analysis was performed by ANOVA followed by the Newman-Keuls post hoc test. *, significant difference compared to saline (CT); #, significant difference compared to gentamicin (GM) (P < 0.05); **, significant difference compared to saline (P < 0.01).
Effect of GF on gentamicin-induced nitrosative stress.
The serum nitrite levels in kidney tissue were significantly elevated by GM administration. Animals in the GM+GF group receiving the GF dose of 25 mg/kg (GM+GF25 group) had serum nitrite levels that returned to control (CT) values (Fig. 2).
FIG 2.

Effect of GF on serum nitrite levels in gentamicin-induced nephrotoxicity. The results are expressed as means ± SEM (n = 6). Statistical analysis was performed by ANOVA followed by the Newman-Keuls post hoc test. **, significant difference compared to saline (CT); #, significant difference compared to gentamicin (GM) (P < 0.05); ##, significant difference compared to gentamicin (P < 0.01).
The protective effect of gingerols is associated with increased antioxidant enzyme activity.
The seven consecutive days of gentamicin treatment significantly reduced the levels of reduced GSH (Fig. 3) and SOD activity (Fig. 4). This reduction was significantly attenuated by oral treatment with GF at a dose of 25 mg/kg. GF alone at a dose of 25 mg/kg did not alter these parameters.
FIG 3.

Effect of GF on the amount of glutathione (GSH) in gentamicin-induced nephrotoxicity. The results are expressed as means ± SEM (n = 7). Statistical analysis was performed by ANOVA followed by the Newman-Keuls post hoc test. **, significant difference compared to saline (CT); #, significant difference compared to gentamicin (GM) (P < 0.05).
FIG 4.
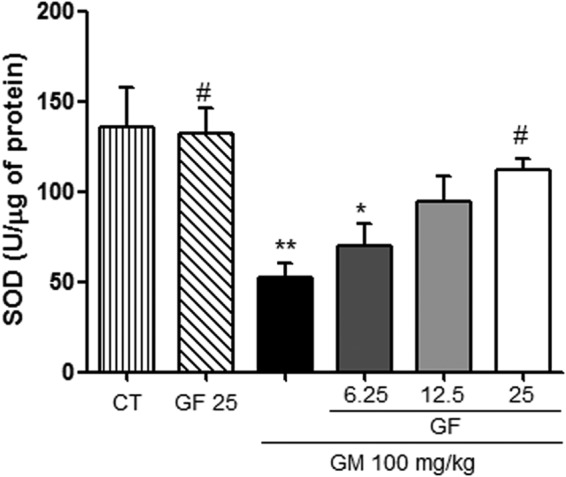
Effect of GF on superoxide dismutase (SOD) activity in gentamicin-induced nephrotoxicity. The results are expressed as means ± SEM (n = 7). Statistical analysis was performed by ANOVA followed by the Newman-Keuls post hoc test. *, significant difference compared to saline (CT); #, significant difference compared to gentamicin (GM) (P < 0.05); **, significant difference compared to saline (P < 0.01).
Gingerol's protective effects are associated with reduced inflammatory gene expression.
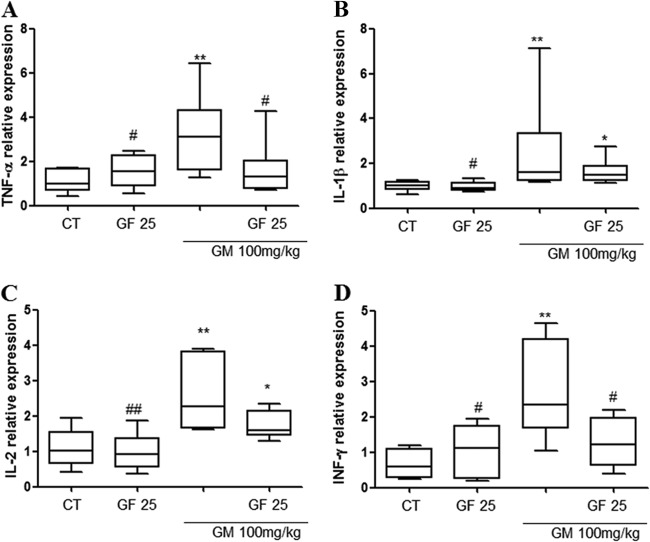
To further explore GF's protection-related mechanisms, we studied the expression of four mRNAs belonging to the innate and adaptive immune responses in kidney tissue samples (mRNAs for TNF-α, IL-1β, IL-2, and IFN-γ). As stated previously, only one group taking GM and GF was evaluated (GM+GF25 group). We observed that TNF-α, IL-2, IFN-γ, and IL-1β expression increased after GM administration (Fig. 5) compared to that in controls (CT) (P < 0.05). Animals in the GM+GF25 group had significant reductions in the expression of these genes only for TNF-α and IFN-γ. There was a tendency for mRNA expression of IL-1β and IL-2 to be blocked after GF treatment, but the difference was not statistically significant. Again, GF did not modify gene expression in animals not treated with GM.
FIG 5.
Effects of GF on relative expression of the TNF-α, IL-1β, IL-2, and IFN-γ genes in gentamicin-induced nephrotoxicity. Statistical analysis was performed by the Mann-Whitney test (n = 6). *, significant difference compared to saline (CT); #, significant difference compared to gentamicin (GM) (P < 0.05); **, significant difference compared to saline (P < 0.01); ##, significant difference compared to gentamicin (P < 0.01).
Morphological analyses.
In accordance with clearance experiments, morphological analyses demonstrated that the GM+GF groups had less severe hydropic degeneration of the proximal tubular epithelium than the GM group. Also, inflammatory infiltrates were found more often in GM-treated animals than in the GM+GF groups (Fig. 6).
FIG 6.
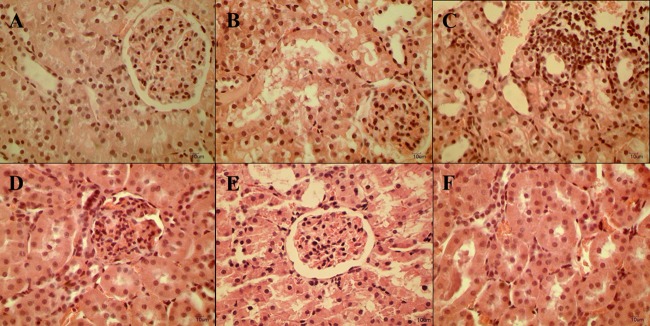
Photomicrographs depicting H&E-stained sections of kidneys from rats receiving saline (control) (A), GM (100 mg/kg) (B and C), saline plus oral treatment with GF at a dose of 25 mg/kg (D), or gentamicin plus GF at 25 mg/kg (E and F). There was vacuolar degeneration in proximal tubule cells (B) and an inflammatory infiltrate (C) associated with GM administration. These features were attenuated by concomitant GF treatment (E and F). Magnification, ×400.
DISCUSSION
In the present study, we demonstrated the protective effects of gingerols in a gentamicin-induced nephrotoxicity model. Attenuation of the GM-induced CLCR reduction by gingerols was associated with reduced oxidative stress, reduced nitric oxide metabolites, and reduced gene expression of important inflammatory cytokines. Previously, ginger extract has been evaluated only fairly in the context of GM-induced nephrotoxicity, with incomplete results (i.e., no data about the glomerular filtration rate—CLCR—or the mechanism associated with this protection) (30).
In a recent report, about 30% of patients treated with GM for more than 7 days showed some sign of nephrotoxicity (4, 31). The severe complications resulting from GM-induced nephropathy are limiting factors for its clinical use (32, 33). Gentamicin-induced kidney injury is characterized by renal tubular epithelial cell necrosis, apoptosis, inflammatory responses, oxidative stress, and vascular contraction (34, 35).
Gingerols are the most abundant pungent compounds in fresh ginger roots. These phenolic compounds have been cited in several studies as the main compounds responsible for the pharmacological effects of ginger, with the most abundant being [6]-gingerol (36). These compounds have, among others, anti-inflammatory and antioxidant properties. These effects have been demonstrated in various organs obtained from diverse injury models. In the context of renal tissue, these compounds have been studied in relation to ischemia-reperfusion injury, cisplatin nephrotoxicity, renal damage caused by carbon tetrachloride, and diabetic nephropathy (37–40).
In our model of GM-induced nephrotoxicity, the beneficial effects of gingerols were remarkable, with almost complete restoration of CLCR by use of a GF dose of 25 mg/kg. This prevention of the CLCR decrease was accompanied by less cell damage, as seen in the histological analysis, and by a reduction in the protein excretion rate. In proximal tubular damage, the renal tubule capacity to reabsorb normally filtered low-weight proteins is impaired. The near normalization of urine protein excretion seen in animals treated with GF is a marker of functional preservation of this tubular segment.
In the present study, we aimed to study several mechanisms associated with gingerol-induced nephroprotection. According to previous reports on the antioxidant effects of ginger extract, rats receiving GM and gingerols show a reduction in GM-induced oxidative stress. Lipid peroxidation is an initial event in the GM-induced nephrotoxicity injury cascade. This view was supported by an increase in MDA level (an index of lipid peroxidation), depletion of the kidney GSH content, and a decrease in SOD activity. In a recent study (41), aminoglycoside and other bactericidal antibiotics induced oxidative damage to DNA, proteins, and membrane lipids. The antioxidant N-acetyl-l-cysteine ameliorates the oxidative stress-related effects. Oxidative substances have been implicated in GM-associated mitochondrial lesions in renal proximal tubular cells as the possible mechanism through which gingerol and other antioxidant substances ameliorate GM-induced nephrotoxicity. Other substances with antioxidant properties have been tested in aminoglycoside-induced nephrotoxicity (42–44). In the present study, we used a model that approximates clinical practice: an in vivo study, protective substance administration initiated after the use of the toxic agent, and evaluation of functional parameters with clinical relevance (e.g., CLCR), in addition to histopathology, inflammatory gene expression, and oxidative status.
Vasodilator effects of NO appear to play a role in GM-induced nephrotoxicity (45). Moreover, NO seems to increase renal injury through its reaction with superoxide radical (O2−), generating the very cytotoxic peroxynitrite species (46). However, the detrimental effect of NO in GM-induced nephrotoxicity appears to be mediated only by inducible nitric oxide synthase (iNOS), not by its constitutive form (eNOS). While treatment with a specific inhibitor of iNOS, aminoguanidine, reduces GM-induced oxidative damage, eNOS inhibition can indeed aggravate it (47, 48). Unfortunately, we were not able to determine which NOS form was attenuated by gingerol administration. We suggest that its inhibitory effect is similar to that attributed to [6]-gingerol, as it inhibited NO production in lipopolysaccharide (LPS)-activated J774.1 macrophages and reduced iNOS protein levels in these cells (49). Reduction of NO synthesis can ameliorate GM-induced nephrotoxicity by itself, but it can also act through the reduction of oxidative stress caused by gingerols.
An increased or unbalanced ROS production and oxidative stress mediate the inflammatory response unleashed by GM. Superoxide anion and hydrogen peroxide activate NF-κB (6, 50), a key mediator of several inflammatory pathways, which induces the expression of proinflammatory cytokines and iNOS (35, 51, 52).
Gentamicin was associated with an increase in inflammatory gene expression of TNF-α, IL-2, IL-1β, and IFN-γ. This upregulation was attenuated by gingerols, except for that of IL-1β and IL-2, for which there was no statistical significance. It has been demonstrated that gingerols can inhibit TNF-α expression in the liver, nervous system, and other tissues (16, 53). Also, it is known that gingerols reduce the expression of IL-2 and IFN-γ by T cells (54, 55). These GM-induced inflammatory molecules participate in the pathogenesis of tubulointerstitial impairment through the promotion of leukocyte attraction and adhesion to inflamed renal tubular cells. In our study, the reduction in inflammatory gene expression is in accordance with the reduced inflammatory infiltrate seen in GM+GF25 animals.
Our data demonstrate a dose-dependent protective effect for all evaluated parameters. Although we were unable to determine the low or high threshold of the dose-effect curve, this relationship is relevant to future clinical studies and to understanding the effects of these substances on oxidative stress. We used a gingerol extract fraction with a predominance of [6]-gingerol, just as reported by others. Although it comprises more than 85% of all gingerols administered, we cannot attribute all beneficial effects to [6]-gingerol. Recently, it was demonstrated that the less abundant compound [10]-gingerol, not [6]-gingerol, was responsible for the anti-inflammatory effects on neuronal cells (53).
In conclusion, a gingerol-enriched fraction reduced GM-induced nephrotoxicity, and this protection was associated with reductions in oxidative stress and NO production and with inhibition of inflammatory gene expression.
ACKNOWLEDGMENTS
We thank the Department of Chemistry at the Federal University of Ceara, which provided the GF.
We are thankful for the financial support of CAPES and INCT-IBISAB.
Footnotes
Published ahead of print 6 January 2014
REFERENCES
- 1.Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, Hernández-Pando R, Pedraza-Chaverrí J. 2003. Antioxidant S-allylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic. Biol. Med. 35:317–324. 10.1016/S0891-5849(03)00312-5 [DOI] [PubMed] [Google Scholar]
- 2.Nitha B, Janardhanan KK. 2008. Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food Chem. Toxicol. 46:3193–3199. 10.1016/j.fct.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 3.Safa J, Argani H, Bastani B, Nezami N, Ardebili BR, Ghorbanihaghjo A, Kalagheichi H, Amirfirouzi A, Mesgari M, Rad JS. 2010. Protective effect of grape seed extract on gentamicin-induced acute kidney injury. Iran. J. Kidney Dis. 4:285–291 [PubMed] [Google Scholar]
- 4.Khan MR, Badar I, Siddiquah A. 2011. Prevention of hepatorenal toxicity with Sonchus asper in gentamicin treated rats. BMC Complement. Altern. Med. 11:1–9. 10.1186/1472-6882-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stojiljkovic N, Stoiljkovicb M, Randjelovica P, Veljkovica S, Mihailovic D. 2012. Cytoprotective effect of vitamin C against gentamicin-induced acute kidney injury in rats. Exp. Toxicol. Pathol. 64:69–74. 10.1016/j.etp.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 6.Quiros Y, Vicente-Vicente L, Morales AL, Lopéz-Novoa JM, Hernandes FJL. 2011. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol. Sci. 119:245–256. 10.1093/toxsci/kfq267 [DOI] [PubMed] [Google Scholar]
- 7.Balakumar P, Rohilla A, Thangathirupathi A. 2010. Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol. Res. 62:179–186. 10.1016/j.phrs.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 8.Said MM. 2011. The protective effect of eugenol against gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Fundam. Clin. Pharmacol. 25:708–716. 10.1111/j.1472-8206.2010.00900.x [DOI] [PubMed] [Google Scholar]
- 9.Tavafi M, Ahmadvand H. 2011. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell 43:392–397. 10.1016/j.tice.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 10.Karahan I, Ates S, Ahin A, Yilmaz S, Eribas AOC, Sakin F. 2005. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology 215:198–204. 10.1016/j.tox.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Sayed-Ahmed MM, Nagi MN. 2007. Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin. Exp. Pharmacol. Physiol. 34:399–405. 10.1111/j.1440-1681.2007.04560.x [DOI] [PubMed] [Google Scholar]
- 12.Karadeniz A, Yildirim A, Simsek N, Kalkan Y, Celebi F. 2008. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother. Res. 22:1506–1510. 10.1002/ptr.2522 [DOI] [PubMed] [Google Scholar]
- 13.Shanmugam KR, Ramakrishna CH, Mallikarjunaa K, Reddy KS. 2010. Protective effect of ginger against alcohol-induced renal damage and antioxidant enzymes in male albino rats. Indian J. Exp. Biol. 48:143–149 [PubMed] [Google Scholar]
- 14.Wang W. 2009. Simultaneous determination of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol in rat plasma by liquid chromatography-mass spectrometry: application to pharmacokinetics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:671–679. 10.1016/j.jchromb.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 15.Dugasani S, Pichikac MR, Nadarajahc VD, Balijepallic MK, Tandraa S, Korlakunta JN. 2010. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 127:515–520. 10.1016/j.jep.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda-Bukhsh AR. 2012. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol. Lett. 210:34–43. 10.1016/j.toxlet.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Chari NKL, Manasa D, Srinivas P, Sowbhagyaet HB. 2013. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 139:509–514. 10.1016/j.foodchem.2013.01.099 [DOI] [PubMed] [Google Scholar]
- 18.Lantz RC, Chena GJ, Sarihana M, Solyomb M, Joladb SD, Timmermannb BN. 2007. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 14:123–128. 10.1016/j.phymed.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Funk JL, Frye JB, Oyarzo JN, Timmermann B, Marthritis R. 2009. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J. Nat. Prod. 72:403–407. 10.1021/np8006183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maghsoudi S, Gol A, Dabiri S, Javadi A. 2011. Preventive effect of ginger (Zingiber officinale) pretreatment on renal ischemia-reperfusion in rats. Eur. Surg. Res. 10:45–51. 10.1159/000321704 [DOI] [PubMed] [Google Scholar]
- 21.Ali DA, Abdeen AM, Ismail MF, Mostafa MA. 2013. Histological, ultrastructural and immunohistochemical studies on the protective effect of ginger extract against cisplatin-induced nephrotoxicity in male rats. Toxicol. Ind. Health 29:1–12. 10.1177/0748233713483198 [DOI] [PubMed] [Google Scholar]
- 22.Silva JA, Beccenerib AB, Muttib HS, Martin ACBM, Silva MFGF, Fernandes JB, Vieira PC, Cominetti MR. 2012. Purification and differential biological effects of ginger-derived substances on normal and tumor cell lines. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 903:157–162. 10.1016/j.jchromb.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 23.Bae WK, Lee JU, Park JW, Bae EU, Ma SK, Kim SH, Kim SW. 2008. Decreased expression of Na+/K+-ATPase, NHE3, NBC1, AQP1 and OAT in gentamicin-induced nephropathy. Korean J. Physiol. Pharmacol. 12:331–336. 10.4196/kjpp.2008.12.6.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 25.Green LC, Tannenbaun SR, Goldman P. 2000. Nitrate synthesis in Parkinson's disease using the model of the 6-hydroxydopamine and MPTP. Ann. N. Y. Acad. Sci. 899:262–273 [DOI] [PubMed] [Google Scholar]
- 26.Sedlak J, Lindsay RH. 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25:192–205. 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Oberley LW, Li Y. 1988. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 34:497–500 [PubMed] [Google Scholar]
- 28.Lisowski P, Pierzchała M, Goœcik J, Pareek CS, Zwierzchowski L. 2008. Evaluation of reference genes for studies of gene expression in the bovine liver, kidney, pituitary, and thyroid. J. Appl. Genet. 49:367–372. 10.1007/BF03195635 [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 30.Nasri H, Nematbakhsh M. 2013. Preventive and curative effects of ginger extract against histopathologic changes of gentamicin-induced tubular toxicity in rats. Int. J. Prev. Med. 4:315–321 [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. 2009. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol. Pharm. Bull. 32:61–67. 10.1248/bpb.32.61 [DOI] [PubMed] [Google Scholar]
- 32.Julien N, Karzazi M, Labrecque G, Beauchamp D, Thibault L. 2000. Temporal modulation of nephrotoxicity, feeding, and drinking in gentamicin-treated rats. Physiol. Behav. 68:533–541. 10.1016/S0031-9384(99)00217-6 [DOI] [PubMed] [Google Scholar]
- 33.Oliveira JFP, Silva CA, Barbieri CD, Oliveira GM, Zanetta DMT, Burdmann EA. 2009. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob. Agents Chemother. 53:2887–2891. 10.1128/AAC.01430-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sue YM, Cheng CF, Chang CC, Chou Y, Chen CH, Juan SH. 2009. Antioxidation and anti-inflammation by haem oxygenase-1 contribute to protection by tetramethylpyrazine against gentamicin-induced apoptosis in murine renal tubular cells. Nephrol. Dial. Transplant. 24:769–777. 10.1093/ndt/gfn545 [DOI] [PubMed] [Google Scholar]
- 35.Tugcu V, Ozbek E, Tasci AI, Kemahli E, Somay A, Bas M, Karaca C, Altug T, Cekmen MB, Ozdogan HK. 2006. Selective nuclear factor kappa-B inhibitors, pyrolidium dithiocarbamate and sulfasalazine, prevent the nephrotoxicity induced by gentamicin. BJU Int. 98:680–686. 10.1111/j.1464-410X.2006.06321.x [DOI] [PubMed] [Google Scholar]
- 36.Zick SM, Dujuric Z. 2008. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 17:1930–1936. 10.1158/1055-9965.EPI-07-2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uz E, Karatas OF, Mete E, Bayrak R, Bayrak O, Atmaca AF, Atis O, Yildirim ME, Akcay A. 2009. The effect of dietary ginger (Zingiber officinals Rosc) on renal ischemia/reperfusion injury in rat kidneys. Ren. Fail. 31:251–260. 10.1080/08860220902779921 [DOI] [PubMed] [Google Scholar]
- 38.Ajith TA, Nivitha V, Usha U. 2007. Zingiber officinale Roscoe alone and in combination with a-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem. Toxicol. 45:921–927. 10.1016/j.fct.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 39.Hamed MA, Ali SA, El-Rigal NS. 2012. Therapeutic potential of ginger against renal injury induced by carbon tetrachloride in rats. ScientificWorldJournal 2012:1–12. 10.1100/2012/840421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzeng T, Liou SS, Chang CJ, Liu M. 2013. The ethanol extract of Zingiber zerumbet attenuates streptozotocin-induced diabetic nephropathy in rats. Evid. Based Complement. Alternat. Med. 2013:340645. 10.1155/2013/340645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS, Collins JJ. 2013. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci. Transl. Med. 5:192ra85. 10.1126/scitranslmed.3006055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia P, Teng J, Zou J, Fang Y, Jiang S, Yu X, Kriegel AJ, Liang M, Ding X. 2013. Intermittent exposure to xenon protects against gentamicin-induced nephrotoxicity. PLoS One 8:e64329. 10.1371/journal.pone.0064329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee IC, Kim SH, Lee SM, Baek HS, Moon C, Kim SH, Park SC, Kim HC, Kim JC. 2012. Melatonin attenuates gentamicin-induced nephrotoxicity and oxidative stress in rats. Arch. Toxicol. 86:1527–1536. 10.1007/s00204-012-0849-8 [DOI] [PubMed] [Google Scholar]
- 44.Randjelovic P, Veljkovic S, Stojiljkovic N, Velickovic L, Sokolovic D, Stoiljkovic M, Ilic I. 2012. Protective effect of selenium on gentamicin-induced oxidative stress and nephrotoxicity in rats. Drug Chem. Toxicol. 35:141–148. 10.3109/01480545.2011.589446 [DOI] [PubMed] [Google Scholar]
- 45.Christo JS, Rodrigues AM, Mouro MG, Cenedeze MAA, Simões MJ, Schor N, Higa EMS. 2011. Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide 24:77–83. 10.1016/j.niox.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 46.Koppenol WH, Moreno JJ, Pryor WAT, Ischiropoulos TH, Beckman JS. 1992. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 5:834–842. 10.1021/tx00030a017 [DOI] [PubMed] [Google Scholar]
- 47.Ghaznavi R, Kadkhodaee M. 2007. Comparative effects of selective and non-selective nitric oxide synthase inhibition in gentamicin-induced rat nephrotoxicity. Arch. Toxicol. 81:453–457. 10.1007/s00204-006-0157-2 [DOI] [PubMed] [Google Scholar]
- 48.Polat A, Parlakpinarb H, Tasdemirb S, Colakc C, Vardid N, Ucard M, Emrea MH. 2006. Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem. 108:365–371. 10.1016/j.acthis.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 49.Ippoushi K, Azuma K, Ito H, Horie H, Higashio H. 2003. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci. 73:3427–3437. 10.1016/j.lfs.2003.06.022 [DOI] [PubMed] [Google Scholar]
- 50.Bledsoe G, Shen B, Yao YY, Hagiwara M, Mizell B, Teuton M, Grass D, Chao L, Chao J. 2008. Role of tissue kallikrein in prevention and recovery of gentamicin-induced renal injury. Toxicol. Sci. 102:433–443. 10.1093/toxsci/kfn008 [DOI] [PubMed] [Google Scholar]
- 51.Volpini RA, Costa RS, Coimbra TM. 2006. Increased expression of p38 mitogen-activated protein kinase is related to the acute renal lesions induced by gentamicin. Braz. J. Med. Biol. Res. 39:817–823. 10.1590/S0100-879X2006000600016 [DOI] [PubMed] [Google Scholar]
- 52.Sánchez-López E, Rayego S, Rodrigues-Díez R, Rodriguez JS, Rodrigues-Díez R, Rodríguez-Vita J, Carvajal G, Aroeira LS, Selgas R, Mezzano AS. 2009. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-κB. J. Am. Soc. Nephrol. 20:1513–1526. 10.1681/ASN.2008090999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho S, Chang K, Lin CC. 2013. Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food Chem. 141:3183–3191. 10.1016/j.foodchem.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 54.Ahui MLB, Champy P, Ramadan A, Van LP. 2008. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int. Immunopharmacol. 8:1626–1632. 10.1016/j.intimp.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 55.Tripathi S, Maier KG, Bruch D, Kittur SD. 2007. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J. Surg. Res. 213:209–213. 10.1016/j.jss.2006.07.051 [DOI] [PubMed] [Google Scholar]