Abstract
Topical administration of live commensal bacteria to the vaginal tract holds significant potential as a cost-effective strategy for the treatment of sexually transmitted infections and the delivery of mucosal vaccines. Probiotic-releasing intravaginal rings (IVRs) embody significant theoretical advantages over traditional daily-dosage forms, such as sustained and controlled delivery leading to improved adherence to therapy compared to that of frequent dosing. The conventional IVR designs, however, are not amenable to the delivery of live bacteria. We have developed a novel pod-IVR technology where polymer-coated tablets (“pods”) of Lactobacillus gasseri strain ATCC 33323, a commensal microorganism of human origin, are embedded in silicone IVRs. The release rate of bacterial cells is controlled by the diameter of a delivery channel that exposes a portion of the pod to external fluids. In vitro studies demonstrated that the prototype devices released between 1.1 × 107 and 14 × 107 cells per day for up to 21 days in a controlled sustained fashion with stable burst-free release kinetics. The daily release rates were correlated with the cross-sectional area of the delivery channel. Bacteria in the IVR pods remained viable throughout the in vitro studies and formed biofilms on the surfaces of the devices. This proof-of-principle study represents the first demonstration of a prolonged, sustained release of bacteria from an intravaginal device and warrants further investigation of this device as a nonchemotherapeutic agent for the restoration and maintenance of normal urogenital flora.
INTRODUCTION
The burden of sexually transmitted infections (STIs) among women, particularly in resource-poor regions, highlights the urgent need for female-controlled cost-effective approaches to prevention and treatment (1). Strategies involving the administration of commensal bacteria to the vaginal tract are emerging as a promising platform to achieve these important goals (2). Probiotics, “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (3), have been shown to promote and restore healthy vaginal microbiota in clinical trials (2–4). Both oral and intravaginal probiotic regimens for the prevention and treatment of bacterial vaginosis (BV) (5–8) and urinary tract infections (UTIs) (9, 10) have demonstrated clinical efficacy. Because probiotic lactobacilli express a number of characteristics that are antagonistic to pathogens but complementary to host immunity, their use has been proposed to improve reproductive health and pregnancy outcomes (11).
Commensal and attenuated pathogenic bacteria also are being developed as vectors for mucosal vaccines against STIs (2). Listeria monocytogenes is a promising candidate vaccine vector against HIV because it induces a strong cell-mediated immune response and can be readily manipulated to express viral antigens. Proof-of-concept studies have been performed using recombinant L. monocytogenes strains that express HIV Gag in feline (12) and nonhuman primate (13) HIV models. Similarly, human vaginal isolates of Lactobacillus jensenii were genetically modified to secrete functional two-domain CD4 proteins, thereby inhibiting HIV-1 entry into target cells in a dose-dependent manner (14). A recombinant L. jensenii organism expressing the HIV-1 entry inhibitor cyanovirin-N demonstrated a 63% reduction in the transmission of a chimeric simian HIV strain (SHIVSF162P3) following repeated vaginal challenges in macaques (15). L. monocytogenes expressing the H-2K(b) glycoprotein B peptide from herpes simplex virus 1 (HSV-1) triggered a robust CD8 T cell response providing protective immunity against HSV infection (16). Intravaginally administered recombinant Streptococcus gordonii and Salmonella enterica engineered to express antigens of human papillomavirus type 16 were evaluated in mice (17, 18) and cynomolgus macaques (19) in an effort to develop effective topical vaccines for cervical cancer. Recently, attenuated bacterial pathogens have been investigated as delivery vectors for heterologous antigens that may simultaneously vaccinate against two pathogens (20).
The intravaginal administration of probiotics traditionally has been achieved using a variety of dosage forms, including tablets (21), capsules (7, 8), suppositories (9), and tampons (22). Collectively, these approaches have led to detectable, but not optimally sustainable, levels of the delivered bacteria. In women, these levels may not be sufficient to overcome existing biofilms refractory to the administered organism. The topical delivery of commensal bacteria using intravaginal rings (IVRs) holds significant potential for female-controlled STI prevention and treatment. The microorganisms can be administered in a controlled manner in sustained-release formulations, and adherence issues are significantly reduced compared to daily dosing. Intravaginal rings are being explored for the delivery of small-molecule antiviral agents (microbicides) to protect against sexually contracted HIV (23) and HSV acquisition (24), as well as for the treatment of recurrent genital herpes (25). However, microbicidal IVR technologies based on the established matrix and reservoir designs (23, 26), in which the antimicrobial agent is dispersed and diffuses through the ring elastomer, are not amenable to the delivery of live bacteria.
Consequently, we have developed a novel pod-IVR (27) formulated with the commensal organism Lactobacillus gasseri ATCC 33323, a neotype strain of human origin (28, 29). The sustained delivery of L. gasseri was obtained for up to 21 days in vitro with controlled-release kinetics. The bacteria remained viable in the IVRs throughout the study.
MATERIALS AND METHODS
Preparation of L. gasseri tablets.
L. gasseri (ATCC 33323) cultures were inoculated from frozen stock into de Man, Rogosa, and Sharpe (MRS) medium and incubated for 24 h at 37°C and 130 rpm. The cells were harvested via centrifugation for 30 min at 3,000 × g and 4°C. Pooled cell pellets (ca. 15 g) were dried by lyophilization, and the resulting powder was blended with sodium carboxymethyl cellulose (CMC) (25% [wt/wt]) by gentle tumbling for 24 h. The resulting mixtures were compacted into 3.0-mm (outer diameter) tablets in a manual pellet press (Parr Instrument Company).
Manufacture of silicone intravaginal rings.
Human-sized polydimethylsiloxane (PDMS; silicone) pod-IVRs were prepared in a multistep process that has been described in detail elsewhere (27). Tablets containing ca. 30 mg of lyophilized L. gasseri (ca. 7 × 1014 cells) were coated with 5% (wt/vol) poly(d,l-lactide) in dichloromethane-ethyl acetate (1:1 [vol/vol]) to produce pods that were dried at room temperature for 72 h. The pods were embedded in IVRs (10 per ring) with a single mechanically punched delivery channel for each pod. The delivery channels were 2.0, 1.5, 1.0, or 0.75 mm in diameter, depending on the target release rate. The IVRs were cut into single-pod segments for in vitro evaluation. The viability of the bacteria encapsulated in the IVRs was compared to that of the lyophilized material by culturing and typically exceeded 90%. The viability of the IVR bacteria after 21 days of evaluation in vitro (see below) remained at 90% relative to that of the lyophilized cells stored at 4°C.
Bacterial enumeration.
The concentrations of bacterial cells in 150-μl aliquots of release medium collected at predetermined time points were measured as a function of the optical density at 600 nm (OD600) in a 96-well format using a SpectraMax Plus absorbance microplate reader (Molecular Devices, Inc.). The OD600 reading was converted to the number of viable bacterial cells per ml of medium (N) according to the equation N = 2.35 × 108 × 2.38 × OD600.
The factor 2.35 × 108 represents the number of viable cells ml−1 providing an extinction of 1 absorbance unit (AU) cm−1 at 600 nm. This value was determined experimentally and is well within the normal range for bacterial cells (30). The factor 2.38 corrects the optical path length to the 150-μl volume in a 96-well plate.
In vitro studies.
In vitro release studies were carried out in triplicate using procedures presented elsewhere (27). Briefly, the IVR segments were placed in dissolution medium consisting of 1 ml sterile 1× phosphate-buffered saline (PBS; pH 7.2) and were incubated at room temperature (25 ± 2°C) with shaking. The medium was replaced every 24 h and the segments were thoroughly rinsed with 1× PBS before placing into fresh sterile release buffer.
Bacterial viability.
The viability of the L. gasseri cells in the release medium was measured every 6 days by culture. The aliquots (150 μl) were inoculated into 100 μl MRS medium and incubated for 24 h at 37°C and 130 rpm. The OD600 of the resulting culture was used as a surrogate measurement of growth.
SEM.
IVR segments with 2.0-mm-diameter delivery channels were incubated in release medium for 16 days at 25°C and 100 rpm. The segments were rapidly frozen by immersion in liquid propane and were prepared for scanning electron microscopy (SEM) as described previously (31, 32). Dehydration of the frozen segments was carried out by freeze-substitution in ethanol at −80°C, followed by warming to ambient temperature and critical point drying. During this process, much of the biological material became detached from the IVR surface. The dried ring segments were cut lengthwise, mounted on metal specimen stubs, coated with a 10-nm-thick platinum film, and imaged using an XL30-SFEG 6 SEM (FEI Company, Hillsboro, OR) operating at 5 kV.
Statistical analysis.
The data were analyzed using GraphPad Prism version 6.02 (GraphPad Software, Inc.).
RESULTS
In vitro kinetic studies demonstrated sustained controlled release profiles for up to 21 days.
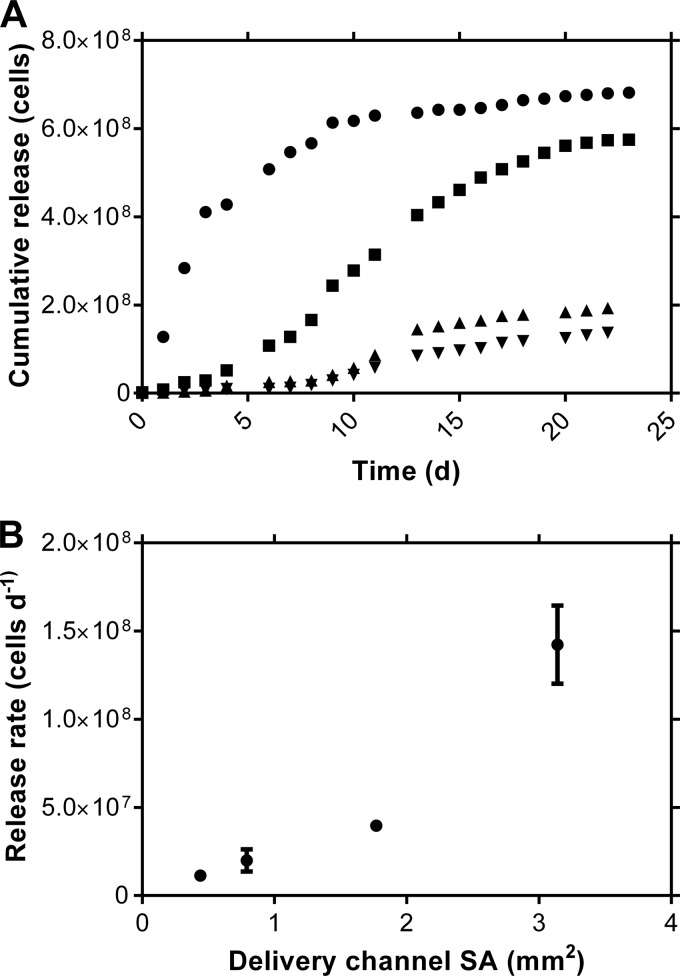
In vitro cumulative and daily release profiles (Fig. 1 and Table 1) from the L. gasseri IVR formulation exhibited burst-free sustained release, as is typical for pod-IVRs that deliver small molecules (27, 33, 34). The daily release rates, calculated from the linear portion of the cumulative release profile, displayed the expected (27) dependence on the delivery channel cross-sectional area (Fig. 1B).
FIG 1.
In vitro release kinetics of live L. gasseri from single-pod-IVR segments as a function of delivery channel size (n = 3). (A) Median cumulative release versus time (●, 2.0-mm diameter; ◾, 1.5-mm diameter; ▲, 1.0-mm diameter; ▼, 0.75-mm diameter). (B) Mean (± standard deviation) release rates as a function of delivery channel cross-sectional area (SA).
TABLE 1.
Daily Lactobacillus gasseri release rates as a function of IVR configuration
| No. of viable cells | Daily bacterial release ratea for IVR delivery channel diam (mm) of: |
|||
|---|---|---|---|---|
| 2.0 | 1.5 | 1.0 | 0.75 | |
| 107 viable cells per day | 14 ± 2.2 | 4.0 ± 0.15 | 2.0 ± 0.63 | 1.1 ± 0.26 |
Mean ± standard deviation (n = 3).
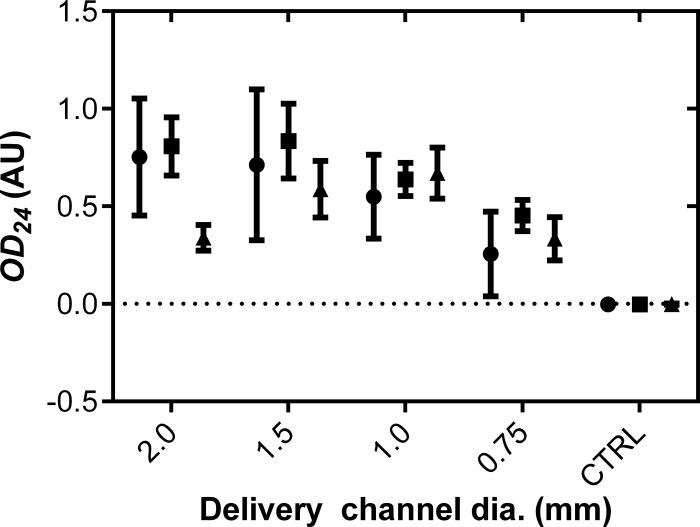
IVR bacteria remained viable, even after 21 days.
The viability of the L. gasseri bacteria in the IVR formulations was maintained (90%, compared to lyophilized cells stored at 4°C), even after 21 days of incubation in release medium (Fig. 2). These results were confirmed by labeling (with the LIVE/DEAD BacLight bacterial viability kit; Life Technologies Corporation) the excised pod core and examining by fluorescence microscopy (data not shown). The concentration of viable cells in the release medium, measured in terms of the OD600 (Fig. 2), was representative of the daily release rate at those time points (Fig. 1A).
FIG 2.
Viability of L. gasseri in IVR release medium at day 10 (●), day 12 (■), and day 21 (▲). The OD24 readings correspond to the absorbance at 600 nm of MRS medium inoculated from the release buffer and incubated for 24 h at 37°C and 130 rpm. CTRL, negative control using MRS medium with no inoculation.
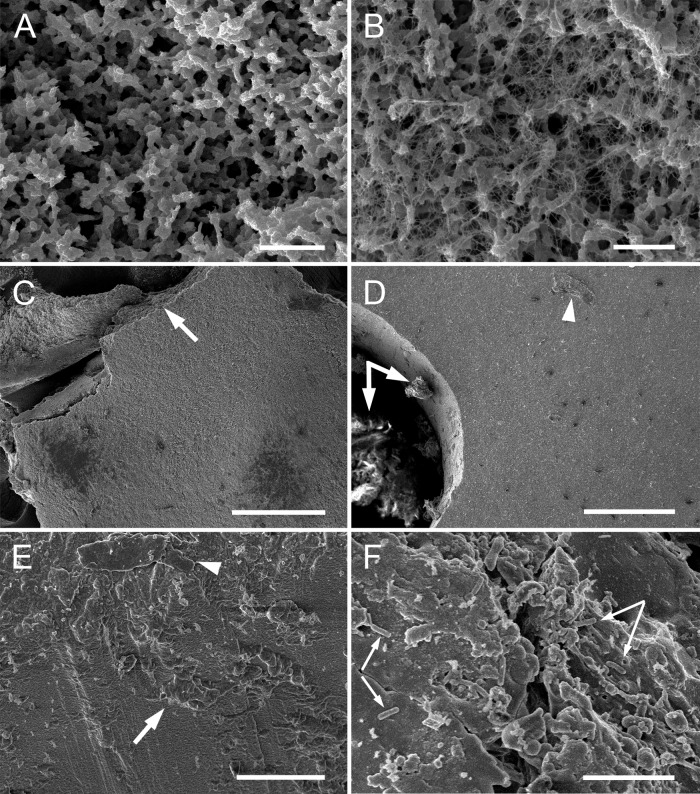
L. gasseri biofilms form on the IVR surface.
Bacterial biofilms were clearly visible on L. gasseri-delivering IVR segments following prolonged incubation in PBS. These structures were delicate and readily detached from the IVR surface during handling. Two principal biofilm morphologies were observed by SEM examination of the cryopreserved specimens (Fig. 3): (i) open channels defined by bacteria embedded in extracellular polymeric substances (EPS) (Fig. 3A), sometimes linked by dense networks of nanofibers (Fig. 3B), and (ii) thick mats of aggregated bacteria (Fig. 3C). Bacterial mats also formed on the inner surface of the delivery channel (Fig. 3D to F).
FIG 3.
SEM images of L. gasseri biofilms that formed on IVR fragments in vitro following 16 days of incubation at 25°C at 100 rpm in PBS. (A and B) Biofilm morphologies displaying typical ultrastructural features of phenotype II (40), including regular open structures and nanowires. (D to F) Biofilms in the IVR drug delivery channel have a different morphology, resembling a dense bacterial mat, similar to phenotype I (40). (A) Bacteria, embedded in EPS, assemble into open architectures, including connective channels. Bar, 2 μm. (B) Portions of the biofilm structures contain dense nanofiber networks attached to the branching bacterial aggregates. Bar, 2 μm. (C) Biofilm fragment at low magnification consists of a thick mat of aggregated bacteria that readily detach from the IVR surface. The broken edge (arrow) represents the fragmentation line that developed during sample processing. Bar, 200 μm. (D) The IVR delivery channel, at low magnification, contains a mass of material attached to the inner surface (arrows). Traces of material on the IVR surface also were present (arrowhead). Bar, 10 μm. (E) The inner surface of the IVR delivery channel is covered with attached biological material. Bar, 20 μm. (F) The attached biofilm contains bacteria (arrows). Bar, 10 μm.
DISCUSSION
The pod-IVR design (27) contains a number of key unique features relevant to this study. The unmedicated structure that holds the bacterial pods can be made of any biocompatible elastomer (e.g., PDMS, ethylene-vinyl acetate copolymer, polyurethane, or latex), providing flexibility in material choice. In conventional IVRs, such as matrix and reservoir designs (23), including segmented (35, 36) and tubular (37, 38) configurations, the device elastomer forms an integral part of the delivery system controlling drug diffusion, an approach that is not amenable to the delivery of live bacterial agents.
The pod-IVR platform was specifically designed for the sustained delivery of multiple agents, each with independently and precisely controlled delivery rates (27). We have demonstrated in pig-tailed macaques that the drug release rate can be modulated over a wide range (34). A key feature of the pod-IVR design is its versatility in the agents it can deliver, with drug substances spanning the range from hydrophobic and hydrophilic small molecules (24, 25, 33, 34, 39) to high-molecular-weight highly water-soluble biomolecules (M. Gunawardana, M. M. Baum, A. M. Malone, T. J. Smith, and J. A. Moss, submitted for publication). Moss et al. reported (39) the design and 28-day pharmacokinetic evaluation in sheep of a five-drug pod-IVR as a proof-of-concept advanced multipurpose prevention technology (MPT) that combines three antiretroviral drugs from different mechanistic classes with a proven estrogen-progestogen contraceptive for HIV and unintended-pregnancy prevention. No other IVR design has demonstrated the ability to deliver more than two agents. Pod-IVRs delivering antiviral agents have shown preliminary safety in pig-tailed macaques and women (25, 40), including culture-independent characterization of the vaginal microbiota (41). Based on the extensive in vivo track record of the pod-IVR design in rabbits (24), sheep (24, 33, 39), macaques (34, 40), and women (25, 41), no significant challenges are anticipated in translating the current IVR delivering commensal bacteria to in vivo studies.
Human-sized pod-IVRs can accommodate 10 polymer-coated bacterial tablets, containing up to 200 mg of material each, totaling 2 g per IVR. Each pod can theoretically deliver a different agent at an independently controlled release rate determined by the polymer membrane encapsulating the tablet and by the number and cross-sectional diameter of the delivery channels in the ring, as shown in Fig. 1B. We have demonstrated the simultaneous delivery of multiple agents at controlled rates from pod-IVRs in vivo (24, 39). The sustained delivery of multiple probiotics, such as the combination of Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14 pioneered by Reid and colleagues (42), and probiotic bacteria in tandem with complementary drugs, such as estriol (43–45), a metabolic product of estradiol, and vitamin B complex (46), is possible using the pod-IVR platform.
Unlike oral probiotic dosage regimens (42), the intravaginal probiotic dose required to impact the vaginal microbiota has not been determined clinically; thus, the target probiotic delivery rates for sustained release intravaginal products have not been established. Table 2 summarizes the intravaginal probiotic doses used in a range of clinical studies. Orally administered formulations were not included due to the uncertainty of the vaginal dose received. Based on these data, a 28-day pod-IVR needs to deliver between 0.6 × 104 and 20 × 108 viable organisms per day, a range that is well within the capabilities of the pod-IVR platform discussed here (as supported by Fig. 1). A 10-fold increase in the release rate can be achieved simply by using 10 pods per IVR, and it can be increased further by using multiple delivery channels for each pod (27). Increasing the pod size from 30 mg, as described here, to 200 mg would provide sufficient bacterial loading to last 28 days.
TABLE 2.
Summarya of clinical trials involving intravaginal administration of probiotic formulations
| Reference no. | Reason for treatment | Probiotic(s) | Dose(s) | Form | Regimen |
|---|---|---|---|---|---|
| 49 | BV | Lactobacillus acidophilus | 5 × 108 to 20 × 108 CFU/ml | 5 ml fermented milk product | 2× daily, 7 days |
| 50 | BV | L. acidophilus | 1 × 108 to 10 × 108 CFU/ml | Capsule | 2× daily, 6 days |
| 51 | BV | L. acidophilus | >108 CFU/ml | 10–15 ml yogurt | 2× daily, 7 days; repeat after 1 wk |
| 44 | BV | L. acidophilus | ≥107 CFU | Tablet | 1–2× daily, 6 days |
| Estriol | 30 μg | ||||
| 45 | Vaginitis | L. acidophilus | ≥107 CFU | Tablet | 1× daily, 6 days |
| Estriol | 30 μg | ||||
| Lactose | 600 mg | ||||
| 9 | UTI | Lactobacillus crispatus GAI 98322 | 108 CFU | Suppository | Every 2 days, 1 yr |
| 7 | BV | Lactobacillus rhamnosus | ≥4 × 104 | Capsule | 1× wk, 6 mos |
| 8 | BV | L. rhamnosus | 6.8 × 108 CFU | Capsule | 1× daily, 7 days on, 7 days off, 7 days on |
| L. acidophilus | 0.4 × 108 CFU | ||||
| Streptococcus thermophilus | 0.8 × 108 CFU |
See reference 5.
The two L. gasseri biofilm architectures observed here showed morphological similarities with the phenotypes that were developed in vivo on pod-IVRs delivering antiviral agents in pig-tailed macaques (40) and women (25). In both cases, Lactobacillus spp. were well represented in the vaginal microbiota of the hosts (40, 41). The complete genome of L. gasseri ATCC 33323 has been sequenced (29), and interestingly, was found to encode 14 putative mucus-binding proteins, the highest number among the lactobacilli sequenced to date. In addition, the sequence data were suggestive of a putative exopolysaccharide gene cassette contributing to the features of the cell surface structure (29). These molecular findings are in agreement with our experimental observations regarding IVR surface colonization and biofilm formation by L. gasseri ATCC 33323. The open architecture (Fig. 3A), designated phenotype II (40), contained interwoven networks of uniform fibers (Fig. 3B) reminiscent of structures observed in monospecies Pseudomonas laboratory cultures (32). These so-called nanowires have been observed to be a consistent feature of bacterial biofilms (32, 47, 48). In our in vivo studies, the bacterial biofilms developed on epithelial cell monolayers covering the IVR surface (25, 40). Here, the biofilms easily became detached from the IVR surface during handling, possibly explaining why an epithelial cell monolayer was required to support in vivo surface adhesion of the bacterial EPS. We observed no evidence that the biofilms affected the in vitro release rate of L. gasseri.
Conclusion.
The delivery of L. gasseri from pod-IVRs in an in vitro model exhibited a controlled release of viable cells over 21 days. This proof-of-principle study demonstrates that the modular pod-IVR platform holds promise for the sustained release of beneficial bacteria to the vaginal tract and warrants further investigation as a nonchemotherapeutic agent for the restoration and maintenance of normal urogenital flora. Future in vivo evaluations of the devices will be critical to advance them through the development pipeline.
ACKNOWLEDGMENTS
We thank our home organization for continuing institutional support. We also thank R. B. Pyles (University of Texas Medical Branch at Galveston) for insightful discussions.
Footnotes
Published ahead of print 3 February 2014
REFERENCES
- 1.Creese A, Floyd K, Alban A, Guinness L. 2002. Cost-effectiveness of HIV/AIDS interventions in Africa: a systematic review of the evidence. Lancet 359:1635–1642. 10.1016/S0140-6736(02)08595-1 [DOI] [PubMed] [Google Scholar]
- 2.Bolton M, van der Straten A, Cohen CR. 2008. Probiotics: potential to prevent HIV and sexually transmitted infections in women. Sex. Transm. Dis. 35:214–225. 10.1097/OLQ.0b013e31815b017a [DOI] [PubMed] [Google Scholar]
- 3.Reid G, Jass J, Sebulsky MT, McCormick JK. 2003. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 16:658–672. 10.1128/CMR.16.4.658-672.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid G. 2012. Probiotic and prebiotic applications for vaginal health. J. AOAC Int. 95:31–34. 10.5740/jaoacint.SGE_Reid [DOI] [PubMed] [Google Scholar]
- 5.Falagas ME, Betsi GI, Athanasiou S. 2007. Probiotics for the treatment of women with bacterial vaginosis. Clin. Microbiol. Infect. 13:657–664. 10.1111/j.1469-0691.2007.01688.x [DOI] [PubMed] [Google Scholar]
- 6.Hummelen R, Changalucha J, Butamanya NL, Cook A, Habbema JD, Reid G. 2010. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. Int. J. Gynaecol. Obstet. 111:245–248. 10.1016/j.ijgo.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 7.Marcone V, Rocca G, Lichtner M, Calzolari E. 2010. Long-term vaginal administration of Lactobacillus rhamnosus as a complementary approach to management of bacterial vaginosis. Int. J. Gynaecol. Obstet. 110:223–226. 10.1016/j.ijgo.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 8.Ya W, Reifer C, Miller LE. 2010. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 203:120.e1–e6. 10.1016/j.ajog.2010.05.023 [DOI] [PubMed] [Google Scholar]
- 9.Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H. 2006. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int. J. Antimicrob. Agents 28(Suppl 1):S30–S34. 10.1016/j.ijantimicag.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Darouiche RO, Hull RA. 2012. Bacterial interference for prevention of urinary tract infection. Clin. Infect. Dis. 55:1400–1407. 10.1093/cid/cis639 [DOI] [PubMed] [Google Scholar]
- 11.Reid JNS, Bisanz JE, Monachese M, Burton JP, Reid G. 2013. The rationale for probiotics improving reproductive health and pregnancy outcome. Am. J. Reprod. Immunol. 69:558–566. 10.1111/aji.12086 [DOI] [PubMed] [Google Scholar]
- 12.Stevens R, LaVoy A, Nordone S, Burkhard M, Dean GA. 2005. Pre-existing immunity to pathogenic Listeria monocytogenes does not prevent induction of immune responses to feline immunodeficiency virus by a novel recombinant Listeria monocytogenes vaccine. Vaccine 23:1479–1490. 10.1016/j.vaccine.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 13.Jiang SS, Rasmussen RA, Nolan KM, Frankel FR, Lieberman J, McClure HM, Williams KM, Babu US, Raybourne RB, Strobert E, Ruprecht RM. 2007. Live attenuated Listeria monocytogenes expressing HIV Gag: immunogenicity in rhesus monkeys. Vaccine 25:7470–7479. 10.1016/j.vaccine.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, Lewicki JA, Lee PP. 2003. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc. Natl. Acad. Sci. U. S. A. 100:11672–11677. 10.1073/pnas.1934747100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y, Yu R, Venzon D, Lee PP, Hamer DH. 2011. Prevention of vaginal SHIV transmission in macaques by a live recombinant lactobacillus. Mucosal Immunol. 4:648–657. 10.1038/mi.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr MT, Orgun NN, Wilson CB, Way SS. 2007. Cutting edge: recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J. Immunol. 178:4731–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medaglini D, Rush CM, Sestini P, Pozzi G. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330–1337. 10.1016/S0264-410X(97)00026-1 [DOI] [PubMed] [Google Scholar]
- 18.Echchannaoui H, Bianchi M, Baud D, Bobst M, Stehle JC, Nardelli-Haefliger D. 2008. Intravaginal immunization of mice with recombinant Salmonella enterica serovar Typhimurium expressing human papillomavirus type 16 antigens as a potential route of vaccination against cervical cancer. Infect. Immun. 76:1940–1951. 10.1128/IAI.01484-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Fabio S, Medaglini D, Rush CM, Corrias F, Panzini GL, Pace M, Verani P, Pozzi G, Titti F. 1998. Vaginal immunization of cynomolgus monkeys with Streptococcus gordonii expressing HIV-1 and HPV 16 antigens. Vaccine 16:485–492. 10.1016/S0264-410X(97)80002-3 [DOI] [PubMed] [Google Scholar]
- 20.Saxena M, Van TT, Baird FJ, Coloe PJ, Smooker PM. 2013. Pre-existing immunity against vaccine vectors–friend or foe? Microbiology 159:1–11. 10.1099/mic.0.049601-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggi L, Mastromarino P, Macchia S, Brigidi P, Pirovano F, Matteuzzi D, Conte U. 2000. Technological and biological evaluation of tablets containing different strains of lactobacilli for vaginal administration. Eur. J. Pharm. Biopharm. 50:389–395. 10.1016/S0939-6411(00)00121-1 [DOI] [PubMed] [Google Scholar]
- 22.Eriksson K, Carlsson B, Forsum U, Larsson PG. 2005. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Derm. Venereol. 85:42–46. 10.1080/00015550410022249 [DOI] [PubMed] [Google Scholar]
- 23.Malcolm RK, Edwards KL, Kiser P, Romano J, Smith TJ. 2010. Advances in microbicide vaginal rings. Antiviral Res. 88:S30–S39. 10.1016/j.antiviral.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 24.Moss JA, Malone AM, Smith TJ, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Vincent KL, Motamedi M, Friend DR, Clark MR, Baum MM. 2012. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob. Agents Chemother. 56:875–882. 10.1128/AAC.05662-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller MJ, Malone AM, Carpenter CA, Lo Y, Huang M, Corey L, Willis R, Nguyen C, Kennedy S, Gunawardana M, Guerrero D, Moss JA, Baum MM, Smith TJ, Herold BC. 2012. Safety and pharmacokinetics of acyclovir in women following release from a silicone elastomer vaginal ring. J. Antimicrob. Chemother. 67:2005–2012. 10.1093/jac/dks151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiser PF, Johnson TJ, Clark JT. 2012. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 14:62–77 [PubMed] [Google Scholar]
- 27.Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, Nguyen C, Smith TJ, Friend DR, Clark MR, Moss JA. 2012. An intravaginal ring for the simultaneous delivery of multiple drugs. J. Pharm. Sci. 101:2833–2843. 10.1002/jps.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauer E, Kandler O. 1980. Lactobacillus gasseri sp. nov., a new species of the subgenus Thermobacterium. Zentralbl. Bakteriol. Orig. A 1:75–78 (Article in German) [Google Scholar]
- 29.Azcarate-Peril MA, Altermann E, Goh YJ, Tallon R, Sanozky-Dawes RB, Pfeiler EA, O'Flaherty S, Buck BL, Dobson A, Duong T, Miller MJ, Barrangou R, Klaenhammer TR. 2008. Analysis of the genome sequence of Lactobacillus gasseri ATCC 33323 reveals the molecular basis of an autochthonous intestinal organism. Appl. Environ. Microbiol. 74:4610–4625. 10.1128/AEM.00054-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers JA, Curtis BS, Curtis WR. 2013. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophysics 6:4. 10.1186/2046-1682-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster P, Wu S, Webster S, Rich KA, McDonald K. 2004. Ultrastructural preservation of biofilms formed by non-typeable Haemophilus influenzae. Biofilms 1:165–182. 10.1017/S1479050504001425 [DOI] [Google Scholar]
- 32.Baum MM, Kainovič A, O'Keeffe T, Pandita R, McDonald K, Wu S, Webster P. 2009. Characterization of structures in biofilms formed by a Pseudomonas fluorescens isolated from soil. BMC Microbiol. 9:103. 10.1186/1471-2180-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis R, Vincent KL, Motamedi M, Smith TJ. 2012. Tenofovir and tenofovir disoproxil pharmacokinetics from intravaginal rings. AIDS 26:707–710. 10.1097/QAD.0b013e3283509abb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry RM, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau CP, Srinivasan P, Smith JM, Baum MM. 2012. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob. Agents Chemother. 56:5952–5960. 10.1128/AAC.01198-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Leede LG, Govers CP, de Nijs H. 1986. A multi-compartment vaginal ring system for independently adjustable release of contraceptive steroids. Contraception 34:589–602. 10.1016/S0010-7824(86)80015-4 [DOI] [PubMed] [Google Scholar]
- 36.Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. 2010. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 39:203–212. 10.1016/j.ejps.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Henzl MR, Mishell DR, Jr, Velazquez JC, Leitch WE. 1973. Basic studies for prolonged progestogen administration by vaginal devices. Am. J. Obstet. Gynecol. 117:101–106 [Google Scholar]
- 38.Johnson TJ, Clark MR, Albright TH, Nebeker JS, Tuitupou AL, Clark JT, Fabian J, McCabe RT, Chandra N, Doncel GF, Friend DR, Kiser PF. 2012. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob. Agents Chemother. 56:6272–6283. 10.1128/AAC.01431-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss JA, Malone AM, Smith TJ, Kennedy S, Nguyen C, Vincent KL, Motamedi M, Baum MM. 2013. Pharmacokinetics of a multipurpose pod-intravaginal ring simultaneously delivering five drugs in the ovine model. Antimicrob. Agents Chemother. 57:3994–3997. 10.1128/AAC.00547-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunawardana M, Moss JA, Smith TJ, Kennedy S, Kopin E, Nguyen C, Malone AM, Rabe L, Schaudinn C, Webster P, Srinivasan P, Sweeney ED, Smith JM, Baum MM. 2011. Microbial biofilms on the surface of intravaginal rings worn in non-human primates. J. Med. Microbiol. 60:828–837. 10.1099/jmm.0.028225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ursell LK, Gunawardana M, Chang S, Mullen M, Moss JA, Herold BC, Keller MJ, McDonald D, González A, Knight R, Baum MM. 2014. Comparison of the vaginal microbial communities in women with recurrent genital HSV receiving acyclovir intravaginal rings. Antiviral Res. 102:87–94. 10.1016/j.antiviral.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid G, Beuerman D, Heinemann C, Bruce AW. 2001. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol. Med. Microbiol. 32:37–41. 10.1111/j.1574-695X.2001.tb00531.x [DOI] [PubMed] [Google Scholar]
- 43.Feiks A, Grünberger W. 1991. Therapy of atrophic colpitis–is a reduction of estrogen dosage in local administration possible? Gynakol. Rundsch. 31(Suppl 2):268–271 (In German) [PubMed] [Google Scholar]
- 44.Parent D, Bossens M, Bayot D, Kirkpatrick C, Graf F, Wilkinson FE, Kaiser RR. 1996. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol: a placebo-controlled multicentric clinical trial. Arzneimittelforschung 46:68–73 [PubMed] [Google Scholar]
- 45.Ozkinay E, Terek MC, Yayci M, Kaiser R, Grob P, Tuncay G. 2005. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 112:234–240. 10.1111/j.1471-0528.2004.00329.x [DOI] [PubMed] [Google Scholar]
- 46.Friedlander A, Druker MM, Schachter A. 1986. Lactobacillus acidophillus and vitamin B complex in the treatment of vaginal infection. Panminerva Med. 28:51–53 [PubMed] [Google Scholar]
- 47.Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. U. S. A. 103:11358–11363. 10.1073/pnas.0604517103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaudinn C, Stoodley P, Kainovič A, O'Keeffe T, Costerton JW, Robinson DH, Baum MM, Ehrlich G, Webster PS. 2007. Bacterial biofilms, other structures seen as mainstream concepts. Microbe 2:231–237 [Google Scholar]
- 49.Fredricsson B, Englund K, Weintraub L, Olund A, Nord CE. 1989. Bacterial vaginosis is not a simple ecological disorder. Gynecol. Obstet. Invest. 28:156–160. 10.1159/000293556 [DOI] [PubMed] [Google Scholar]
- 50.Hallén A, Jarstrand C, Påhlson C. 1992. Treatment of bacterial vaginosis with lactobacilli. Sex. Transm. Dis. 19:146–148. 10.1097/00007435-199205000-00007 [DOI] [PubMed] [Google Scholar]
- 51.Neri A, Sabah G, Samra Z. 1993. Bacterial vaginosis in pregnancy treated with yoghurt. Acta Obst. Gynecol. Scand. 72:17–19. 10.3109/00016349309013342 [DOI] [PubMed] [Google Scholar]