Abstract
Carbapenem-hydrolyzing class D β-lactamases (CHDLs) are enzymes of the utmost clinical importance due to their ability to produce resistance to carbapenems, the antibiotics of last resort for the treatment of various life-threatening infections. The vast majority of these enzymes have been identified in Acinetobacter spp., notably in Acinetobacter baumannii. The OXA-2 and OXA-10 enzymes predominantly occur in Pseudomonas aeruginosa and are currently classified as narrow-spectrum class D β-lactamases. Here we demonstrate that when OXA-2 and OXA-10 are expressed in Escherichia coli strain JM83, they produce a narrow-spectrum antibiotic resistance pattern. When the enzymes are expressed in A. baumannii ATCC 17978, however, they behave as extended-spectrum β-lactamases and confer resistance to carbapenem antibiotics. Kinetic studies of OXA-2 and OXA-10 with four carbapenems have demonstrated that their catalytic efficiencies with these antibiotics are in the same range as those of some recognized class D carbapenemases. These results are in disagreement with the classification of the OXA-2 and OXA-10 enzymes as narrow-spectrum β-lactamases, and they suggest that other class D enzymes that are currently regarded as noncarbapenemases may in fact be CHDLs.
INTRODUCTION
The major mechanism of resistance to β-lactams in Gram-negative pathogens is the production of β-lactamases, which are enzymes capable of hydrolyzing the four-membered β-lactam ring of antibiotics, rendering them inactive. More than 1,000 individual β-lactamases have been reported, and their number is steadily growing. Based on the conservation of the active-site amino acid residues and motifs that are critical for substrate recognition and catalysis, β-lactamases are classified into classes A through D. Class D β-lactamases, also known as OXA-type enzymes or oxacillinases, are represented by more than 350 genetically diverse enzymes (see http://www.lahey.org/studies/webt.asp) that are widely disseminated in Gram-negative bacteria. They are broadly classified into narrow- and extended-spectrum enzymes based upon the conferred resistance profile against β-lactam antibiotics (1–3). The OXA-2 and OXA-10 β-lactamases exemplify the narrow-spectrum enzymes capable of producing resistance to penicillins and some early cephalosporins (4). Both enzymes, however, can extend their substrate profile to produce resistance to expanded-spectrum cephalosporins, such as ceftazidime, by accumulating one to several amino acid substitutions (5–12). Class D carbapenemases, also known as carbapenem-hydrolyzing class D β-lactamases (CHDLs) (4), represent a further expansion of the substrate profile of class D enzymes to include carbapenem antibiotics. CHDLs are most problematic clinically, as they produce resistance to the antibiotics of last resort, carbapenems, thus severely limiting therapeutic options. Based on their amino acid sequence identity, CHDLs have been subdivided into several subgroups. Enzymes belonging to the OXA-23, OXA-24/40, OXA-48, OXA-51, OXA-58, and OXA-143 subgroups are of major clinical importance due to their wide dissemination in bacterial pathogens. The majority of these carbapenemases, except for OXA-48, have been identified in various Acinetobacter isolates, predominantly in Acinetobacter baumannii (4, 13, 14). Among them, OXA-51-related enzymes are the most abundant and are found on the chromosomes of all A. baumannii strains tested. Recently, the gene encoding this enzyme has been identified in plasmids, which allows it to spread among various Acinetobacter species (15). Another CHDL, OXA-48, is the notorious class D carbapenemase of Klebsiella pneumoniae and, to a lesser extent, this enzyme has spread among other members of the Enterobacteriaceae family (16–20).
Due to the utmost clinical importance of class D carbapenemases, numerous studies of these enzymes, addressing their genetic diversity, epidemiology, and conferred resistance spectra, have been undertaken. To gain insights into the mechanisms of the carbapenemase activity of class D β-lactamases, the catalytic properties and structural features of some CHDLs have been elucidated and compared with those of class D enzymes that are considered noncarbapenemases. The premise of such comparisons is that knowledge of structural and kinetic differences between carbapenemases and noncarbapenemases would facilitate understanding of the evolution of carbapenemase activity in class D β-lactamases. Here we present evidence that the OXA-2 and OXA-10 β-lactamases, enzymes that are currently regarded as noncarbapenemases, have catalytic efficiencies against carbapenems similar to those of well-recognized CHDLs and are capable of conferring resistance to these last-resort antibiotics when expressed in A. baumannii.
MATERIALS AND METHODS
Strains and plasmids.
The genes encoding the class D β-lactamases OXA-2 (GenBank accession no. X07260.1), OXA-10 (GenBank accession no. U37105.2), OXA-23 (GenBank accession no. AJ132105.1), OXA-24/40 (GenBank accession no. AJ239129.2), OXA-48 (GenBank accession no. AY236073.2), and OXA-58 (GenBank accession no. AY665723.1) were custom synthesized using the nucleotide sequences reported in GenBank and were cloned into the unique NdeI-HindIII sites of the shuttle vector pNT221 (21) (NdeI-HindIII restriction sites were present in some of the sequences and were removed by making a silent mutation using A. baumannii codon usage). This vector can be transformed into both Escherichia coli and A. baumannii and uses ISAba3 (from the insertion sequence of Acinetobacter baumannii) as the promoter sequence. Acquisition of this ISAba element by clinical Acinetobacter isolates provides a strong promoter that results in the observed high level of resistance to carbapenems. The shuttle vector pNT221 with cloned genes for the OXA β-lactamases was subsequently introduced into E. coli strain JM83 by chemical transformation and into A. baumannii ATCC 17978 by electroporation. The presence of the plasmids in the transformants was verified by isolation of plasmid DNA and restriction digestion analysis. For purification of the enzymes, the genes for different β-lactamases were optimized for expression in E. coli and custom synthesized. The 5′ ends of the genes encoding the signal peptides (codons for the first 18, 20, 17, 19, 21, and 20 amino acids for OXA-10, OXA-2, OXA-23, OXA-24/40, OXA-48, and OXA-58, respectively) were removed, and a unique NdeI restriction site was introduced at the 5′ end of the genes. The genes were cloned into the NdeI-HindIII restriction sites of the pET24a(+) vector (Invitrogen) and transformed into chemically competent E. coli BL21(DE3). To evaluate the levels of expression of OXA β-lactamases in an Acinetobacter host, the enzymes were labeled with a 6×His tag at the C termini, cloned into the NdeI-HindIII sites of the shuttle vector pNT221, and introduced into A. baumannii ATCC 17978 by electroporation.
Antimicrobial susceptibility testing.
The MICs of a total of 20 different β-lactams against E. coli strain JM83 and A. baumannii ATCC 17978 harboring the empty shuttle vector or expressing the different β-lactamase enzymes were determined by the broth microdilution method, as described in the Clinical and Laboratory Standards Institute guidelines (22, 23). The MICs were determined in triplicate in Mueller-Hinton II broth (Difco), using a final inoculum of 5 × 105 CFU/ml. Microtiter plates were incubated at 37°C, and the results were interpreted after 16 to 20 hours (E. coli) or 20 to 24 hours (A. baumannii) of incubation.
Enzyme purification.
All OXA enzymes used in this study were purified from 500 ml of LB broth supplemented with 60 μg/ml of kanamycin. After purification to >95% homogeneity (as judged by analysis of up to 30 μg of protein by SDS-PAGE), the enzymes were concentrated using a Centricon Plus 70 concentrator (Millipore) and were dialyzed against 25 mM HEPES (pH 7.5).
Cells harboring the gene encoding OXA-2 were grown at 37°C with shaking until an optical density at 600 nm (OD600) of 0.8 was achieved, expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and cells were grown for 5 h at 37°C with shaking. The bacterial cells were harvested by centrifugation, resuspended in 50 mM Tris (pH 8.0), and disrupted by sonication. The cell extract was then centrifuged for 1 h at 18,000 × g at 4°C. The supernatant was recovered and loaded onto a High Q anion-exchange column (Bio-Rad) equilibrated with the same buffer. The flowthrough fraction contained the purified OXA-2.
The induction of OXA-10 expression was achieved with 0.4 mM IPTG at an OD600 of 0.8, and the cells were grown overnight at 22°C. The soluble protein was isolated in 20 mM morpholineethanesulfonic acid (MES) (pH 6.0), as described above, and was loaded onto a High Q anion-exchange column equilibrated with the same buffer. The flowthrough fraction, containing OXA-10, was recovered and loaded onto a High S cation-exchange column (Bio-Rad) equilibrated with the same buffer. The column was washed with 2 column volumes of 20 mM MES (pH 6.0), and the protein was eluted using a NaCl gradient. The fractions containing the pure enzyme were identified by reaction with the chromogenic cephalosporin nitrocefin and SDS-PAGE.
Cells expressing OXA-24/40 were treated with 0.4 mM IPTG when an OD600 of 0.6 was reached. After the addition of IPTG, the temperature was decreased to 22°C and the cells were grown overnight. The soluble fraction containing the protein was prepared in 20 mM Tris (pH 8.0), as for OXA-2, and loaded onto a High Q anion-exchange column equilibrated with the same buffer. The flowthrough fraction was recovered and dialyzed against 20 mM MES (pH 6.0). After dialysis, the OXA-24/40-containing fraction was loaded onto a CM cation-exchange column (Bio-Rad). The column was washed with 2 column volumes of the same buffer, and the protein was eluted using a NaCl gradient. Fractions containing the pure enzyme were identified by reaction with nitrocefin and SDS-PAGE.
The OXA-48 gene-containing cells were grown until an OD600 of 0.8 was reached, expression was induced with 1 mM IPTG, and the cells were grown overnight at 22°C. The soluble fraction was prepared in 50 mM Tris (pH 8.0), as with OXA-2, and loaded onto a High Q anion-exchange column. The purified OXA-48 was in the flowthrough fraction.
For the purification of OXA-58, cells were treated with 1 mM IPTG at an OD600 of 0.8 and were grown overnight at 22°C. The soluble fraction containing the protein was prepared in 20 mM MES (pH 6.0) and loaded onto a High Q anion-exchange column. The flowthrough fraction, containing OXA-58, was collected and loaded onto a CM cation-exchange column. OXA-58 was eluted with a NaCl gradient, and fractions containing the purified enzyme were identified by their reaction with nitrocefin and SDS-PAGE. Finally, OXA-23 was purified as described previously (21).
Determination of steady-state kinetic parameters.
All kinetic data were collected with a Cary 50 (Varian) or Cary 60 (Agilent) spectrophotometer. The reactions were performed at room temperature in 100 mM sodium phosphate (pH 7.0) supplemented with 50 mM NaHCO3, 0.2 mg/ml bovine serum albumin (BSA), and various concentrations of the β-lactam substrate. All reactions were initiated by the addition of enzyme. The following wavelengths and extinction coefficients were used in the assays: oxacillin, Δε260 = 440 cm−1 M−1; imipenem, Δε297 = −10,930 cm−1 M−1; meropenem, Δε298 = −7,200 cm−1 M−1; ertapenem, Δε295 = −10,940 cm−1 M−1; doripenem, Δε299 = −11,540 cm−1 M−1; nitrocefin, Δε500 = 15,900 cm−1 M−1. The steady-state velocities (v) were obtained from the linear phase of the reaction time course, which allowed determination of the observed rate constant (kobs = v/[E]). The kcat and Km values were calculated by plotting kobs as a function of the concentration of the β-lactam and fitting the results with nonlinear regression using the Michaelis-Menten equation.
Determination of dissociation constants.
The dissociation constant (Ks) values for the tested carbapenems were determined using the Dixon method (24), with nitrocefin as the reporter substrate. The reactions were performed at room temperature in 100 mM sodium phosphate (pH 7.0) supplemented with 50 mM NaHCO3, 0.2 mg/ml BSA, two fixed concentrations of nitrocefin, and various concentrations of the carbapenem. The reactions were started by the addition of enzyme, with final concentrations ranging between 80 and 200 pM.
Quantification of OXA β-lactamases.
Cells harboring the shuttle vector pNT221 with the His-tag-labeled genes for OXA β-lactamases were incubated overnight at 37°C in LB medium supplemented with 40 μg/ml kanamycin, were diluted 100-fold into fresh LB medium, and were grown to an optical density at 600 nm of 0.6 (Ultrospec 10; Amersham Biosciences). At this optical density, the bacterial suspension contained 2.5 × 108 cells/ml (as estimated by counting colony numbers after serial dilution of the bacterial suspensions). The bacterial suspensions were sonicated, cell debris and nonsoluble proteins were pelleted by centrifugation, and the supernatant containing soluble protein was analyzed after mixing with reducing Laemmli sample buffer. The samples were boiled and resolved on 12% TGX precast gels (Bio-Rad). Following separation, the proteins were transferred to 0.2-μm polyvinylidene difluoride (PVDF) membranes using the Trans-Blot Turbo transfer system (Bio-Rad). The 6×His tag was detected by immunoblot analysis with specific primary antibody (monoclonal mouse anti-6×His antibody, no. MA1-21315; Thermo Scientific) and horseradish peroxidase-conjugated anti-mouse IgG secondary antibody (no. 32430; Thermo Scientific). SuperSignal West Dura (no. 34076; Thermo Scientific) was used as a substrate for horseradish peroxidase in the enhanced chemiluminescence reaction. Dilution of antibodies and visualization of 6×His-tagged proteins were completed according to the manufacturer's recommendations. To quantitate and to compare protein expression levels, the gels were imaged using Image Lab software (Bio-Rad).
RESULTS AND DISCUSSION
Cloning of genes and antibiotic susceptibility testing.
Of the six class D enzymes used in this study, two (OXA-2 and OXA-10) were discovered in Pseudomonas aeruginosa and are presently characterized as narrow-spectrum β-lactamases. The remaining four enzymes are carbapenemases; three of them (OXA-23, OXA-24/40, and OXA-58) are well known CHDLs of A. baumannii, while OXA-48 is a carbapenemase that is widely distributed in Enterobacteriaceae, predominantly K. pneumoniae. In fact, OXA-48 is the only clinically important CHDL of non-Acinetobacter origin. On the other hand, the vast majority of >100 class D β-lactamases that have been identified in Acinetobacter spp. are CHDLs that produce high levels of resistance to carbapenems (4, 14). The specific bacterial host plays an important role in determining the levels of β-lactamase-mediated resistance. Acinetobacter is notorious for the low permeability of its cell walls and the expression of various efflux pumps (25). This raises the question of whether the resistance to carbapenem antibiotics that is widely observed in Acinetobacter spp. results primarily from the efficient hydrolysis of antibiotics by CHDLs and/or whether the bacterial host makes a major contribution to the observed high levels of resistance. To answer this question, the OXA β-lactamases were expressed in both E. coli and A. baumannii from the same plasmid and the promoter conferred by ISAba3, an insertion sequence that often provides efficient promoters for class D enzymes circulating in clinical isolates of Acinetobacter spp.
Next, we determined the MICs of a panel of β-lactam antibiotics against strains producing the class D β-lactamases in E. coli strain JM83 and A. baumannii ATCC 17978. When expressed in E. coli JM83, all enzymes produced high levels of resistance to penicillins, including ampicillin, amoxicillin, benzylpenicillin, oxacillin, ticarcillin, and piperacillin (Table 1). OXA-2 and OXA-10 produced 16-fold increases and OXA-23 and OXA-58 produced 8-fold increases in the MICs of the narrow-spectrum cephalosporin cephalothin, while OXA-10 was the only enzyme that also significantly (32-fold) increased the MIC of another narrow-spectrum cephalosporin, i.e., cefuroxime. OXA-10 was also the only enzyme that produced significant (256-fold) increases in resistance to the monobactam aztreonam and moderate (4-fold) decreases in susceptibility to the cephamycins cefoxitin and cefmetazole. Expression in E. coli JM83 of OXA-10 and OXA-58 also resulted in 32-fold increases in the MIC of the oxacephem moxalactam. OXA-10 produced the greatest decrease in susceptibility to expanded-spectrum cephalosporins (cefotaxime, ceftriaxone, and cefepime), although the resulting MICs were below clinically significant values. Similarly, production of OXA-10 resulted in a 32-fold increase in the MIC of ceftazidime. The only other OXA enzyme in this study that increased the MIC of ceftazidime was OXA-2 (64-fold increase over the baseline value). Antibiotic susceptibility testing with carbapenems produced rather unexpected results. The OXA-2 and OXA-10 β-lactamases, which are regarded as noncarbapenemases, produced 2- to 8-fold increases in the MICs for imipenem, meropenem, and doripenem and 32- to 128-fold increases for ertapenem. On the other hand, while two of the well-known carbapenemases, OXA-23 and OXA-24/40, elevated the MICs of carbapenems to even higher levels, the OXA-48 and OXA-58 carbapenemases conferred levels of resistance to carbapenems similar to those produced by OXA-2 and OXA-10.
TABLE 1.
MICs of β-lactam antibiotics against E. coli strain JM83 producing class D β-lactamases
| Antimicrobial | MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| Controla | OXA-2 | OXA-10 | OXA-23 | OXA-24/40 | OXA-48 | OXA-58 | |
| Benzylpenicillin | 16 | 1,024 | 4,096 | 4,096 | 2,048 | 512 | 2,048 |
| Ampicillin | 2 | 1,024 | 16,384 | 4,096 | 2,048 | 256 | 2,048 |
| Amoxicillin | 4 | 1,024 | >2,048 | >2,048 | 2,048 | 512 | 2,048 |
| Oxacillin | 256 | 2,048 | 8,192 | 8,192 | 1,024 | 1,024 | 4,096 |
| Ticarcillin | 4 | 4,096 | 32,768 | 16,384 | 16,384 | 8,192 | 16,384 |
| Piperacillin | 1 | 128 | 512 | 256 | 128 | 16 | 512 |
| Cephalothin | 4 | 64 | 64 | 32 | 8 | 8 | 32 |
| Cefuroxime | 4 | 8 | 128 | 8 | 4 | 4 | 4 |
| Cefoxitin | 2 | 4 | 8 | 2 | 2 | 2 | 2 |
| Cefmetazole | 1 | 2 | 8 | 1 | 1 | 1 | 1 |
| Moxalactam | 0.5 | 1 | 16 | 4 | 2 | 2 | 16 |
| Ceftazidime | 0.125 | 8 | 4 | 0.125 | 0.125 | 0.125 | 0.125 |
| Cefotaxime | 0.03 | 0.125 | 2 | 0.25 | 0.03 | 0.125 | 0.06 |
| Ceftriaxone | 0.03 | 0.06 | 4 | 0.125 | 0.03 | 0.06 | 0.06 |
| Cefepime | 0.016 | 0.06 | 2 | 0.5 | 0.06 | 0.03 | 0.03 |
| Aztreonam | 0.06 | 0.125 | 16 | 0.125 | 0.125 | 0.06 | 0.06 |
| Imipenem | 0.125 | 0.25 | 0.25 | 0.5 | 1 | 0.5 | 0.5 |
| Meropenem | 0.016 | 0.06 | 0.125 | 0.25 | 0.5 | 0.06 | 0.06 |
| Ertapenem | 0.004 | 0.125 | 0.5 | 0.5 | 0.5 | 0.125 | 0.06 |
| Doripenem | 0.03 | 0.125 | 0.125 | 0.25 | 0.5 | 0.125 | 0.06 |
Parental E. coli JM83 strain with the plasmid pNT221 without a β-lactamase gene.
We next evaluated the MICs of various β-lactam antibiotics against A. baumannii ATCC 17978 expressing the same OXA-type enzymes (Table 2). In comparison with E. coli strain JM83, the parental A. baumannii ATCC 17978 strain had decreased susceptibility to penicillins, most noticeably to ampicillin (32-fold) and piperacillin (16-fold). Despite these differences, the MICs of various penicillins against A. baumannii ATCC 17978 expressing one of the six OXA β-lactamases were largely similar to the corresponding MICs with E. coli JM83 producing the same enzyme. The most prominent exceptions were the MICs for piperacillin, which increased significantly (16- to 64-fold) when the OXA-10, OXA-23, and OXA-24/40 enzymes were expressed in the Acinetobacter background. The parental A. baumannii ATCC 17978 strain was even less susceptible to cephalosporins than E. coli JM83, with differences as great as 128- to 256-fold for moxalactam, cefepime, cefotaxime, and ceftriaxone. These differences in MICs for cephalosporins were observed, to various extents, in both E. coli and A. baumannii strains producing the same OXA enzyme. A similar increase was observed with the monobactam aztreonam. More relevant to this study, the MICs of various carbapenem antibiotics were also significantly elevated in the Acinetobacter background, reaching values of up to 512 μg/ml. Of interest, the dramatic increases in the MIC values for carbapenems were observed not only for bona fide CHDLs but also for the two “noncarbapenemases,” OXA-2 and OXA-10. As a result, OXA-10 produced the same MICs for meropenem, ertapenem, and doripenem as the CHDL OXA-48 and higher MICs than another CHDL, OXA-58.
TABLE 2.
MICs of β-lactam antibiotics against A. baumannii ATCC 17978 producing class D β-lactamases
| Antimicrobial | MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| Controla | OXA-2 | OXA-10 | OXA-23 | OXA-24/40 | OXA-48 | OXA-58 | |
| Benzylpenicillin | 32 | 2,048 | 8,192 | 16,384 | 8,192 | 1,024 | 1,024 |
| Ampicillin | 64 | 1,024 | 8,192 | 8,192 | 8,192 | 512 | 1,024 |
| Amoxicillin | 32 | 2,048 | 2,048 | >2,048 | >2,048 | 1,024 | 2,048 |
| Oxacillin | 512 | 1,024 | 8,192 | 8,192 | 1,024 | 512 | 1,024 |
| Ticarcillin | 8 | 2,048 | 16,384 | 16,384 | 8,192 | 4,096 | 4,096 |
| Piperacillin | 16 | 512 | 8,192 | 16,384 | 2,048 | 128 | 1,024 |
| Cephalothin | 256 | 1,024 | 2,048 | 1,024 | 256 | 256 | 256 |
| Cefuroxime | 32 | 32 | 256 | 64 | 32 | 32 | 32 |
| Cefoxitin | 32 | 64 | 64 | 64 | 64 | 64 | 32 |
| Cefmetazole | 64 | 128 | 256 | 128 | 64 | 64 | 64 |
| Moxalactam | 64 | 64 | 256 | 512 | 256 | 128 | 128 |
| Ceftazidime | 2 | 32 | 16 | 4 | 2 | 2 | 2 |
| Cefotaxime | 8 | 8 | 128 | 16 | 8 | 8 | 8 |
| Ceftriaxone | 8 | 8 | 256 | 16 | 8 | 8 | 8 |
| Cefepime | 2 | 2 | 256 | 64 | 8 | 2 | 2 |
| Imipenem | 0.125 | 2 | 4 | 32 | 64 | 16 | 8 |
| Meropenem | 0.25 | 8 | 16 | 64 | 128 | 16 | 4 |
| Ertapenem | 2 | 32 | 128 | 256 | >256 | 128 | 32 |
| Doripenem | 0.125 | 8 | 16 | 32 | 128 | 16 | 4 |
Parental A. baumannii ATCC 17978 strain with the plasmid pNT221 without a β-lactamase gene.
Enzyme kinetics.
While steady-state kinetics have been reported for some class D β-lactamases, the results presented by various groups often contradict each other (20, 26–30). There could be several reasons for such discrepancies. It is well documented that, in class D enzymes, the catalytic lysine is N-carboxylated in vivo (31). Analysis of the published kinetic data indicated that kinetic experiments were often performed without supplementing the reaction mixture with a CO2 source, which is needed for lysine N-carboxylation. The absence of a source of CO2 may result in only partial N-carboxylation of the active-site lysine during in vitro experiments; hence, a portion of the enzyme could be inactive during the analysis. Kinetic studies performed with partially purified enzymes or enzymes that may have lost part of their activity upon purification could also produce questionable results. In this study, we evaluated the steady-state kinetic parameters for turnover of oxacillin, imipenem, meropenem, ertapenem, and doripenem with six class D β-lactamases. To ensure uniformity, all kinetic measurements were performed with a saturating concentration of CO2 and freshly prepared homogeneous enzymes. The results of our studies are presented in Table 3.
TABLE 3.
Steady-state kinetic parameters for the hydrolysis of carbapenems by selected OXA enzymes
| Enzyme | Parameter | Oxacillin | Imipenem | Meropenem | Ertapenem | Doripenem |
|---|---|---|---|---|---|---|
| OXA-2 | kcat (s−1) | 1,100 ± 30 | 0.18 ± 0.01 | 0.11 ± 0.01 | 0.085 ± 0.001 | 0.20 ± 0.01 |
| Km (μM) | 210 ± 20 | ≤2.0 | ≤2.0 | ≤2.0 | ≤2.0 | |
| kcat/Km (M−1 s−1) | (5.4 ± 0.5) × 106 | ≥9.0 × 104 | ≥5.5 × 104 | ≥4.3 × 104 | ≥1.0 × 105 | |
| Ks (μM) | NDa | 0.026 ± 0.002 | 0.014 ± 0.001 | 0.0073 ± 0.0004 | 0.034 ± 0.002 | |
| OXA-10 | kcat (s−1) | 530 ± 10 | 0.041 ± 0.001 | 0.039 ± 0.001 | 0.022 ± 0.001 | 0.037 ± 0.001 |
| Km (μM) | 87 ± 5 | 2.0 ± 0.1 | 5.6 ± 0.8 | 4.1 ± 0.5 | 4.8 ± 0.8 | |
| kcat/Km (M−1 s−1) | (6.1 ± 0.3) × 106 | (2.1 ± 0.3) × 104 | (7.0 ± 0.2) × 103 | (5.4 ± 0.7) × 103 | (8 ± 1) × 103 | |
| Ks (μM) | ND | 0.025 ± 0.001 | 0.043 ± 0.005 | 0.0050 ± 0.0009 | 0.035 ± 0.004 | |
| OXA-23 | kcat (s−1) | 320 ± 10 | 0.35 ± 0.01 | 0.068 ± 0.001 | 0.021 ± 0.001 | 0.036 ± 0.001 |
| Km (μM) | 110 ± 10 | ≤2.0 | ≤2.0 | ≤2.0 | ≤2.0 | |
| kcat/Km (M−1 s−1) | (3.1 ± 0.2) × 106 | ≥1.8 × 105 | ≥3.4 × 104 | ≥1.1 × 104 | ≥1.8 × 104 | |
| Ks (μM) | ND | 0.19 ± 0.02 | 0.06 ± 0.01 | 0.011 ± 0.001 | 0.025 ± 0.003 | |
| OXA-24/40 | kcat (s−1) | 170 ± 10 | 1.7 ± 0.1 | 0.11 ± 0.01 | 0.064 ± 0.001 | 0.084 ± 0.001 |
| Km (μM) | 650 ± 50 | ≤2.0 | ≤2.0 | ≤2.0 | ≤2.0 | |
| kcat/Km (M−1 s−1) | (2.6 ± 0.2) × 105 | ≥8.5 × 105 | ≥5.5 × 104 | ≥3.2 × 104 | ≥4.2 × 104 | |
| Ks (μM) | ND | 0.57 ± 0.04 | 0.037 ± 0.005 | 0.012 ± 0.002 | 0.020 ± 0.001 | |
| OXA-48 | kcat (s−1) | 160 ± 10 | 6.7 ± 0.2 | 0.16 ± 0.01 | 0.067 ± 0.001 | 0.14 ± 0.01 |
| Km (μM) | ≤30 | 5.3 ± 0.6 | ≤2.0 | ≤2.0 | ≤2.0 | |
| kcat/Km (M−1 s−1) | ≥6 × 106 | (1.3 ± 0.2) × 106 | ≥8.0 × 104 | ≥3.4 × 104 | ≥7.0 × 104 | |
| Ks (μM) | ND | 2.8 ± 0.3 | 0.075 ± 0.003 | 0.016 ± 0.001 | 0.068 ± 0.003 | |
| OXA-58 | kcat (s−1) | 160 ± 10 | 1.8 ± 0.1 | 0.019 ± 0.001 | 0.011 ± 0.001 | 0.014 ± 0.001 |
| Km (μM) | 53 ± 6 | 6.0 ± 0.5 | ≤2.0 | ≤2.0 | ≤2.0 | |
| kcat/Km (M−1 s−1) | (2.9 ± 0.3) × 106 | (2.9 ± 0.2) × 105 | ≥9.5 × 103 | ≥5.5 × 103 | ≥7.0 × 103 | |
| Ks (μM) | ND | 2.5 ± 0.2 | 0.016 ± 0.002 | 0.0060 ± 0.0007 | 0.014 ± 0.002 |
ND, not determined.
The enzymes had catalytic efficiency (kcat/Km) values with oxacillin in the range of 2.6 × 105 to 6.1 × 106 M−1 s−1. These kinetic parameters are similar to those reported previously for OXA-10, OXA-24/40, OXA-48, and OXA-58 (to our knowledge, kinetic parameters for OXA-2 and OXA-23 with oxacillin have not been published) (20, 30, 32–34). The catalytic efficiency (kcat/Km) of the enzymes with individual carbapenem substrates varied by as much as 3 orders of magnitude (∼103 to 106 M−1 s−1), with OXA-10 being the least efficient carbapenemase. In general, turnover of carbapenems with the enzymes in this study was poor (kcat values of <0.5 s−1), with the exception of OXA-24/40, OXA-48, and OXA-58 with imipenem. For the latter three enzymes and OXA-23, the turnover of imipenem was significantly (5- to 164-fold) higher than that of the other carbapenems tested. In contrast, for OXA-2 and OXA-10, the turnover number was independent of the carbapenem tested.
The relative affinity (Km) values of the enzymes for carbapenem antibiotics were in the nanomolar range, with the exception of OXA-10 (all carbapenems), OXA-48 (imipenem), and OXA-58 (imipenem), for which values were in the low micromolar range. As can be seen from Table 3, the Km values for selected carbapenems could not be evaluated with all class D enzymes studied, with the exception of OXA-10. In order to measure Km values accurately, velocity data must be obtained in the range of Km, ideally from 10-fold below to 10-fold above its value. In reality, this is rarely achieved and Km values are evaluated using a much narrower range of data for which reliable signals can be obtained. Carbapenems, with extinction coefficients ranging from ∼7,000 to 12,000 cm−1 M−1, allow determination of Km values in the low micromolar range; determinations below that range (<0.5 to 1.0 μM) are unreliable due to the low signal changes.
When Km is low and cannot be determined, it is often possible to evaluate the parameter Ks, the dissociation constant for dissociation of the substrate from the enzyme. The Ks values for meropenem, ertapenem, and doripenem with all OXA enzymes studied were in the low nanomolar range (5 to 75 nM). Similar to the trend observed for kcat values, Ks values were independent of the carbapenem with OXA-2 and OXA-10 and higher for imipenem with the remaining class D enzymes. While Ks does set the lower limit for Km, it should not be used as a substitute for Km, as it has been shown that these parameters may vary by 1 order of magnitude or more (35). In fact, we observed this trend with some of our enzymes, most notably OXA-10, for which Ks values were significantly lower than Km values.
Evaluation of enzyme expression levels.
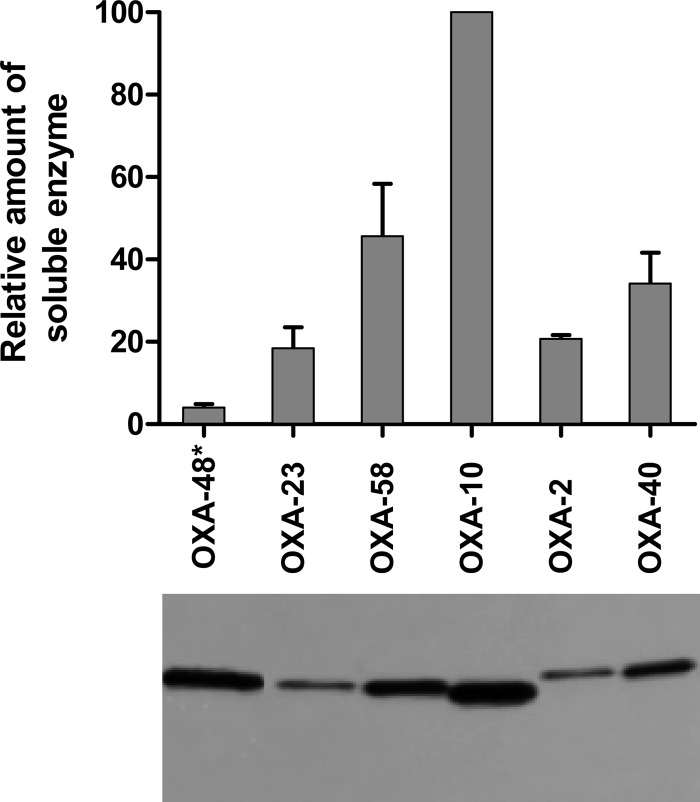
Results of the antibiotic susceptibility tests and enzyme kinetic studies have clearly demonstrated that all class D enzymes relevant to this study, including the OXA-2 and OXA-10 β-lactamases, are CHDLs. For some enzymes, however, no clear correlation between the catalytic efficiencies and conferred MIC values was observed. This discrepancy was most profound when comparing the OXA-10 and OXA-48 β-lactamases. While the enzymes produced identical MICs for meropenem, ertapenem, and doripenem in A. baumannii ATCC 17978 (Table 2), OXA-48 demonstrated significantly higher catalytic efficiencies with these substrates than did OXA-10 (Table 3). Apart from the catalytic efficiency of the β-lactamase, the amount of soluble enzyme in the bacterial cells is a major contributor to the levels of antibiotic resistance produced. To minimize the number of factors that can influence the amounts of β-lactamase produced by the bacteria, we cloned all enzymes of interest for this study into the same vector under the same promoter. The enzymes were subsequently expressed in the same bacterial host, to ensure that host-dependent factors such as the permeability of the outer membrane or the function of efflux pumps would not contribute to the observed levels of antibiotic resistance. Still, additional factors such as the enzyme translation efficiency and solubility can have significant effects on the amounts of soluble β-lactamase in the bacterial cells. To evaluate the relative amounts of various class D β-lactamases produced in A. baumannii ATCC 17978, a hexahistidine tag was introduced at their C termini. Introduction of the hexahistidine tag had no influence on the MIC values for the enzymes (data not shown). Next, we evaluated the amounts of soluble class D β-lactamases produced in A. baumannii ATCC 17978. The immunoblot presented in Fig. 1 shows that the amounts of the OXA-2, OXA-23, OXA-24/40, and OXA-58 enzymes in the cells were relatively similar (maximum of 2.5-fold difference), while the amount of OXA-48 was significantly smaller. Quantitative analysis of the soluble enzyme fraction from the immunoblot showed 25-fold differences in the amounts of the OXA-10 and OXA-48 β-lactamases per bacterial cell. This large difference in the amounts of soluble enzyme produced in A. baumannii ATCC 17978 explains why OXA-10 and OXA-48 produce similar levels of resistance to carbapenem antibiotics while differing significantly in their catalytic efficiencies with these substrates. With respect to other class D β-lactamases used in this study, there were relatively good correlations between the amounts of soluble enzyme produced, the catalytic efficiencies, and the antibiotic resistance levels conferred.
FIG 1.
Expression levels of OXA enzymes in A. baumannii. (Bottom) Representative immunoblot of the amounts of soluble OXA β-lactamases produced by A. baumannii ATCC 17978 from the plasmid pNT221. All lanes contain the soluble protein from equal numbers of cells, with the exception of lane OXA-48*, which contains protein from 25 times more cells. (Top) Relative amounts of enzymes produced, normalized to OXA-10 expression.
Are all class D β-lactamases carbapenemases?
Currently, CHDLs are defined as class D enzymes that produce clinical levels of resistance to carbapenem antibiotics. The OXA-2 and OXA-10 β-lactamases are currently regarded as narrow-spectrum enzymes, based on the MICs produced by these enzymes in E. coli or P. aeruginosa backgrounds. In this study, we demonstrate that, when expressed in the A. baumannii ATCC 17978 background, these enzymes produce high levels of resistance to carbapenems, similar to those conferred by classic CHDLs (Table 2). In contrast, when well-recognized CHDLs are expressed in the E. coli background, the MICs produced are similar to those of OXA-2 and OXA-10 and are well below clinically significant levels (Table 1), which would not classify them as carbapenemases. These data clearly demonstrate the importance of the bacterial host in determining whether an enzyme can be classified as a CHDL. Similar results have been reported by other investigators who observed that the expression of CHDLs in the E. coli background produces only low levels of resistance to carbapenems (36, 37). Acinetobacter is unique due to the low permeability of its outer membrane and the expression of efflux pumps, which reduce the concentrations of antibiotics in the periplasm and significantly contribute to increased levels of resistance (36, 37). These properties of the bacterial host can explain why the vast majority of clinically important CHDLs are found in Acinetobacter spp. Thus, to answer the question of whether all or the majority of class D enzymes are carbapenemases, we have to evaluate the ability of each individual β-lactamase to produce clinical levels of resistance to carbapenems when expressed in Acinetobacter. Another important issue that has yet to be addressed is the evaluation of the relative contributions of other parameters (the β-lactamase turnover rate, the affinity of the enzyme for the substrate, and the amount of enzyme produced by the cells) to the produced levels of resistance to carbapenems. Our kinetic data demonstrate that all enzymes studied have very low turnover rates (kcat) and very high affinities (as defined by Ks) for carbapenems. It is thus tempting to suggest that, in the Acinetobacter background (characterized by low concentrations of antibiotics in the periplasm), the affinities of enzymes for the carbapenems play more important roles in resistance than do their turnover rates, due to drug sequestration by these tight-binding enzymes. Finally, the amounts of enzymes produced by the bacteria significantly influence the antibiotic resistance levels produced, as exemplified here by comparison of the levels of expression of the OXA enzymes used in this study. When the levels of β-lactamase expression are very high, it may be expected that a tight-binding enzyme with relatively low turnover rates would still be able to confer resistance to carbapenems in the Acinetobacter background, mainly as a result of antibiotic sequestration. In contrast, low levels of enzyme expression would require higher turnover rates to reach similar levels of resistance.
Lastly, the demonstration that OXA-2 and OXA-10 have kinetic properties similar to those of CHDLs has to be taken into consideration when elucidating the mechanisms of carbapenemase activity for class D β-lactamases. Their structures have been used in comparative studies with bona fide CHDLs. The rationale behind these studies was that structural differences between OXA-2 and OXA-10, which are regarded as narrow-spectrum enzymes, and CHDLs would permit the identification of structural features responsible for the extension of the substrate profile of class D β-lactamases. Our data suggest that conclusions regarding the structural requirements for the carbapenemase activity of CHDLs that are based primarily on such comparisons need to be critically reevaluated. The kinetics of other class D enzymes that are currently classified as narrow- or extended-spectrum β-lactamases also may need to be carefully reexamined prior to use of the enzymes for comparative studies with CHDLs.
Conclusions.
The most troubling development in the field of class D β-lactamases is the spread of enzymes conferring resistance to carbapenem antibiotics. Except for OXA-48, the enzyme that circulates in Enterobacteriaceae (predominantly K. pneumoniae), the majority of clinically important class D carbapenemases (CHDLs) are present in Acinetobacter spp. CHDLs confer high levels of resistance to the last-resort antibiotics carbapenems, severely compromising available therapeutic options, which results in very high mortality rates. Our studies have demonstrated that the OXA-2 and OXA-10 β-lactamases, which are currently classified as narrow-spectrum enzymes, are in fact CHDLs. Their abilities to produce high levels of resistance to carbapenem antibiotics in A. baumannii may have serious clinical implications. Both enzymes have recently been identified in A. baumannii isolates (14, 38), raising the possibility that many, if not all, class D β-lactamases could potentially be exploited by this deadly bacterial pathogen for self-protection against the deleterious effects of carbapenem antibiotics.
Footnotes
Published ahead of print 27 January 2014
REFERENCES
- 1.Nordmann P, Guibert M. 1998. Extended-spectrum β-lactamases in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 42:128–131. 10.1093/jac/42.2.128 [DOI] [PubMed] [Google Scholar]
- 2.Queenan AM, Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440–458. 10.1128/CMR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walther-Rasmussen J, Hoiby N. 2006. OXA-type carbapenemases. J. Antimicrob. Chemother. 57:373–383. 10.1093/jac/dki482 [DOI] [PubMed] [Google Scholar]
- 4.Poirel L, Naas T, Nordmann P. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 54:24–38. 10.1128/AAC.01512-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert D, Poirel L, Ali AB, Goldstein FW, Nordmann P. 2001. OXA-35 is an OXA-10-related β-lactamase from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 48:717–721. 10.1093/jac/48.5.717 [DOI] [PubMed] [Google Scholar]
- 6.Danel F, Hall LM, Duke B, Gur D, Livermore DM. 1999. OXA-17, a further extended-spectrum variant of OXA-10 β-lactamase, isolated from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1362–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danel F, Hall LM, Gur D, Livermore DM. 1995. OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1881–1884. 10.1128/AAC.39.8.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danel F, Hall LM, Gur D, Livermore DM. 1997. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 41:785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danel F, Hall LM, Gur D, Livermore DM. 1998. OXA-16, a further extended-spectrum variant of OXA-10 β-lactamase, from two Pseudomonas aeruginosa isolates. Antimicrob. Agents Chemother. 42:3117–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall LM, Livermore DM, Gur D, Akova M, Akalin HE. 1993. OXA-11, an extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:1637–1644. 10.1128/AAC.37.8.1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvey MR, Boyd DA, Baker L, Mykytczuk O, Reis EM, Asensi MD, Rodrigues DP, Ng LK. 2004. Characterization of a Salmonella enterica serovar Agona strain harbouring a class 1 integron containing novel OXA-type β-lactamase (blaOXA-53) and 6′-N-aminoglycoside acetyltransferase genes [aac(6′)-I30]. J. Antimicrob. Chemother. 54:354–359. 10.1093/jac/dkh347 [DOI] [PubMed] [Google Scholar]
- 12.Poirel L, Gerome P, De Champs C, Stephanazzi J, Naas T, Nordmann P. 2002. Integron-located oxa-32 gene cassette encoding an extended-spectrum variant of OXA-2 β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:566–569. 10.1128/AAC.46.2.566-569.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans BA, Hamouda A, Amyes SG. 2013. The rise of carbapenem-resistant Acinetobacter baumannii. Curr. Pharm. Des. 19:223–238. 10.2174/138161213804070285 [DOI] [PubMed] [Google Scholar]
- 14.Zhao WH, Hu ZQ. 2012. Acinetobacter: a potential reservoir and dispenser for β-lactamases. Crit. Rev. Microbiol. 38:30–51. 10.3109/1040841X.2011.621064 [DOI] [PubMed] [Google Scholar]
- 15.Lee YT, Turton JF, Chen TL, Wu RC, Chang WC, Fung CP, Chen CP, Cho WL, Huang LY, Siu LK. 2009. First identification of blaOXA-51-like in non-baumannii Acinetobacter spp. J. Chemother. 21:514–520. 10.1179/joc.2009.21.5.514 [DOI] [PubMed] [Google Scholar]
- 16.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. 2011. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob. Agents Chemother. 55:2420–2423. 10.1128/AAC.01452-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giani T, Conte V, Di Pilato V, Aschbacher R, Weber C, Larcher C, Rossolini GM. 2012. Escherichia coli from Italy producing OXA-48 carbapenemase encoded by a novel Tn1999 transposon derivative. Antimicrob. Agents Chemother. 56:2211–2213. 10.1128/AAC.00035-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glupczynski Y, Huang TD, Bouchahrouf W, Rezende de Castro R, Bauraing C, Gerard M, Verbruggen AM, Deplano A, Denis O, Bogaerts P. 2012. Rapid emergence and spread of OXA-48-producing carbapenem-resistant Enterobacteriaceae isolates in Belgian hospitals. Int. J. Antimicrob. Agents 39:168–172. 10.1016/j.ijantimicag.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Kilic A, Aktas Z, Bedir O, Gumral R, Bulut Y, Stratton C, Tang YW, Basustaoglu AC. 2011. Identification and characterization of OXA-48 producing, carbapenem-resistant Enterobacteriaceae isolates in Turkey. Ann. Clin. Lab. Sci. 41:161–166 [PubMed] [Google Scholar]
- 20.Poirel L, Heritier C, Tolun V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22. 10.1128/AAC.48.1.15-22.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CA, Antunes NT, Stewart NK, Toth M, Kumarasiri M, Chang M, Mobashery S, Vakulenko SB. 2013. Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii. Chem. Biol. 20:1107–1115. 10.1016/j.chembiol.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. Document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—7th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 24.Dixon M. 1953. The determination of enzyme inhibitor constants. Biochem. J. 55:170–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471–3484. 10.1128/AAC.01464-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bou G, Oliver A, Martinez-Beltran J. 2000. OXA-24, a novel class D β-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44:1556–1561. 10.1128/AAC.44.6.1556-1561.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Docquier JD, Calderone V, De Luca F, Benvenuti M, Giuliani F, Bellucci L, Tafi A, Nordmann P, Botta M, Rossolini GM, Mangani S. 2009. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem. Biol. 16:540–547. 10.1016/j.chembiol.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 28.Poirel L, Marque S, Heritier C, Segonds C, Chabanon G, Nordmann P. 2005. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:202–208. 10.1128/AAC.49.1.202-208.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santillana E, Beceiro A, Bou G, Romero A. 2007. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 104:5354–5359. 10.1073/pnas.0607557104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma V, Testero SA, Amini K, Wei W, Liu J, Balachandran N, Monoharan T, Stynes S, Kotra LP, Golemi-Kotra D. 2011. Hydrolytic mechanism of OXA-58 enzyme, a carbapenem-hydrolyzing class D β-lactamase from Acinetobacter baumannii. J. Biol. Chem. 286:37292–37303. 10.1074/jbc.M111.280115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golemi D, Maveyraud L, Vakulenko S, Samama JP, Mobashery S. 2001. Critical involvement of a carbamylated lysine in catalytic function of class D β-lactamases. Proc. Natl. Acad. Sci. U. S. A. 98:14280–14285. 10.1073/pnas.241442898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Luca F, Benvenuti M, Carboni F, Pozzi C, Rossolini GM, Mangani S, Docquier JD. 2011. Evolution to carbapenem-hydrolyzing activity in noncarbapenemase class D β-lactamase OXA-10 by rational protein design. Proc. Natl. Acad. Sci. U. S. A. 108:18424–18429. 10.1073/pnas.1110530108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heritier C, Poirel L, Aubert D, Nordmann P. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47:268–273. 10.1128/AAC.47.1.268-273.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poirel L, Castanheira M, Carrer A, Rodriguez CP, Jones RN, Smayevsky J, Nordmann P. 2011. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 55:2546–2551. 10.1128/AAC.00022-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frase H, Shi Q, Testero SA, Mobashery S, Vakulenko SB. 2009. Mechanistic basis for the emergence of catalytic competence against carbapenem antibiotics by the GES family of β-lactamases. J. Biol. Chem. 284:29509–29513. 10.1074/jbc.M109.011262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:2573–2580. 10.1128/AAC.00393-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heritier C, Poirel L, Lambert T, Nordmann P. 2005. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:3198–3202. 10.1128/AAC.49.8.3198-3202.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542–3547. 10.1128/JCM.41.8.3542-3547.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]