Abstract
Although conventional amphotericin B was for many years the drug of choice and remains an important agent against invasive aspergillosis, reliable susceptibility breakpoints are lacking. Three clinical Aspergillus isolates (Aspergillus fumigatus, Aspergillus flavus, and Aspergillus terreus) were tested in an in vitro pharmacokinetic-pharmacodynamic model simulating the biphasic 24-h time-concentration profile of free amphotericin B concentrations in human serum with free peak concentrations (fCmax) of 0.1, 0.3, 0.6, 1.2, and 2.4 mg/liter administered once daily. Drug concentrations were measured with a bioassay, and fungal growth was monitored for 72 h with galactomannan production. The fCmax/MIC corresponding to half-maximal activity (P50) was determined for each species, and the percentage of target attainment was calculated for different MICs for the standard (1 mg/kg of body weight) and a lower (0.6-mg/kg) dose of amphotericin B with Monte Carlo simulation analysis. The fCmax/MICs (95% confidence intervals) corresponding to P50 were 0.145 (0.133 to 0.158), 0.371 (0.283 to 0.486), and 0.41 (0.292 to 0.522) for A. fumigatus, A. flavus, and A. terreus, respectively. The median percentages of P50 attainment were ≥88%, 47%, and 0% for A. fumigatus isolates with MICs of ≤0.5, 1, and ≥2 mg/liter, respectively, and ≥81%, 24%, and 0% and ≥75%, 15%, and 0% for A. flavus and A. terreus isolates with MICs of ≤0.25, 0.5, and ≥1 mg/liter, respectively. The lower dose of 0.6 mg/kg would retain efficacy for A. fumigatus, A. flavus, and A. terreus isolates with MICs of ≤0.25, ≤0.125, and ≤0.125 mg/liter, respectively. The susceptibility, intermediate susceptibility, and resistance breakpoints of ≤0.5, 1, and ≥2 mg/liter for A. fumigatus and ≤0.25, 0.5, and ≥1 mg/liter for A. flavus and A. terreus were determined for conventional amphotericin B with a pharmacokinetic-pharmacodynamic model simulating free-drug serum concentrations.
INTRODUCTION
Invasive aspergillosis is a life-threatening disease among patients with leukemia and bone marrow and solid-organ transplantation (1). Aspergillus fumigatus is the most common pathogen causing invasive aspergillosis, accounting for >70% of the cases, followed by Aspergillus flavus, Aspergillus terreus, and Aspergillus niger (2). Conventional amphotericin B was for many years the drug of choice and is still used against invasive aspergillosis. Despite its in vitro potent antifungal activity against Aspergillus spp., clinical trials have shown that conventional amphotericin B therapy is associated with <60% survival in patients with invasive aspergillosis (3–6). Although underlying disease, immunosuppression, toxicity, and timing of antifungal therapy affect the mortality of these infections, pathogen susceptibility and drug serum concentrations also represent important contributing factors. In vivo studies have shown that the best efficacy was achieved when the maximum concentration of amphotericin B in serum exceeded 2.4 times the MIC of the A. fumigatus isolate (7). However, in vitro antifungal susceptibility testing of amphotericin B is challenged by the lack of reliable susceptibility breakpoints for each Aspergillus species.
The epidemiological cutoff values 2, 2, and 4 mg/liter for the CLSI method and 1, 4, and 4 mg/liter for the EUCAST method were determined for A. fumigatus, A. flavus, and A. terreus, respectively, in order to detect isolates with extreme MICs (8, 9). The current susceptibility breakpoints for Aspergillus spp. with the CLSI and EUCAST methodologies are ≤1 mg/liter (9, 10). However, the supporting clinical and experimental in vivo data are poor, and no pharmacokinetic-pharmacodynamic (PK-PD) studies have validated this breakpoint (9). In a retrospective case-control study of 29 patients, 22/23 patients with isolate MICs of ≥2 mg/liter died, whereas 6/6 patients with isolate MICs of ≤1 mg/liter survived (11). Of note, 5/6 survivors with isolate MICs of ≤1 mg/liter underwent surgery or resolved their neutropenia. In two other large retrospective studies of 160 and 40 patients, no correlation was found between an Aspergillus MIC of ≥2 mg/liter and clinical outcome in patients with invasive aspergillosis treated with conventional amphotericin B (12, 13). Notably, more than 95% of A. fumigatus isolates have MICs of ≤1 mg/liter (8). Therefore, the majority of the isolates would be considered wild-type susceptible based on the 1-mg/liter cutoff, while conventional amphotericin B therapy has been associated with <60% of survival in all clinical trials (3, 4, 14). Furthermore, treatment failure was observed in patients infected with A. fumigatus and A. flavus isolates with CLSI MICs of 0.25 to 0.5 mg/liter and 1 mg/liter, respectively, when treated with the standard dose of 1 mg/kg of conventional amphotericin B (15, 16). Thus, a clinically relevant and useful susceptibility breakpoint for amphotericin B and Aspergillus spp. is warranted.
An in vitro pharmacokinetic-pharmacodynamic model was recently developed for Aspergillus spp. simulating pharmacokinetics of antifungal drugs (17). This model revealed considerable pharmacodynamic differences among Aspergillus isolates reflecting differences in time- and concentration-dependent inhibitory and fungicidal activity that were not captured by the MIC (18). Using the same model and simulating multidose human pharmacokinetics of voriconazole, in vitro data were predictive of experimental animal data and clinical outcome for A. fumigatus, enabling the determination of susceptibility breakpoints and target values for therapeutic drug monitoring (19).
Therefore, in the present study, we simulated multidose pharmacokinetics of amphotericin B and studied its activity against A. fumigatus, A. flavus, and A. terreus. The in vitro PK-PD relationship was described and bridged with human PKs in order to determine susceptibility breakpoints for each species.
MATERIALS AND METHODS
Isolates.
Three clinical strains of A. fumigatus (AFM 4215; ATCC MYA-1163), A. flavus (AFL 113), and A. terreus (AT 137) isolated from patients with invasive aspergillosis were studied. The MICs of amphotericin B as determined thrice according to the CLSI broth microdilution method were 1 mg/liter for all Aspergillus species. The strains were maintained at −70°C in 10% glycerol and cultured twice in Sabouraud dextrose agar at 30°C for 5 to 7 days. A conidial suspension was prepared in normal saline with 1% Tween 20. Conidia were counted with a Neubauer chamber in order to obtain a final suspension of 1 × 103 CFU/ml, and their concentration was confirmed by quantitative cultures on Sabouraud dextrose agar.
Antifungal drug and medium.
Pure powder of amphotericin B (Sigma-Aldrich, Bioline, Athens, Greece) was reconstituted at 5,000 mg/liter in dimethyl-sulfoxide and was stored at −70°C. The medium contained 10.4 g/liter RPMI 1640 with glutamine without sodium bicarbonate (Sigma-Aldrich, St. Louis, MO) and 0.165 M buffer morpholinepropanesulfonic acid (MOPS) (Invitrogen, Carlsbad, CA), pH 7.0, with 100 mg/liter chloramphenicol (Sigma-Aldrich, St. Louis, MO).
In vitro pharmacokinetic-pharmacodynamic model.
The in vitro pharmacokinetic simulation model consisted of (i) a glass beaker containing 700 ml medium (external compartment [EC]) in which was placed (ii) a dialysis tube of a 10-ml volume (internal compartment [IC]) made of a permeable cellulose membrane, which allowed free diffusion of molecules with a molecular mass of <20 kDa, and (iii) a peristaltic pump (Minipuls Evolution, Gilson, France), which removed the content of EC and added fresh medium within it at a rate equivalent to drug removal from human serum (17). The conidial suspension was inoculated in the IC, where it remained trapped together with the galactomannan (molecular mass of 20 kDa to 60 kDa) produced by the growing mycelia, while nutrients and drug diffused freely between the IC and EC. The concentration of the galactomannan increased with fungal growth, as found previously (20). After inoculation of the IC, the drug was injected into the EC and IC (for rapid equilibration between the two compartments) every 24 h, and its concentration declined over time, with a half-life (t1/2) observed in human serum after intravenous administration of amphotericin B. Because amphotericin B degrades in vitro at 37°C, concentrations were adjusted to account for the higher clearance. The EC was covered with aluminum foil in order to minimize light exposure and placed on a heated magnetic stirrer (37°C). At the beginning of, during, and at the end of each experiment, temperature and flow rate were measured to ensure that they were at the expected values. All experiments were repeated twice.
Bioassay.
The drug levels in the IC were determined by a microbiological agar diffusion method using the strain Paecilomyces variotii ATCC 22319 (18, 21). Specifically, P. variotii conidia at final concentration of 5 × 104 CFU/ml were inoculated into prewarmed (54°C) RPMI 1640 medium and MOPS with 15 g/liter agar and poured onto 10- by 10-cm plastic plates. After solidification of the agar, 1-cm-diameter holes were opened and filled with 100 μl of known drug dilutions (range of 0.1 to 8 mg/liter), as well as 100 μl of IC samples. The plates were incubated at 37°C for 24 h, when diameters of inhibition zones were measured. Unknown drug concentrations in the IC samples were determined using the standard curve constructed from known drug concentrations and corresponding diameters of inhibition zones. The diameters of inhibition zones correlated linearly with amphotericin B concentrations (R2 > 0.9). The lowest limit of detection was 0.1 mg/liter, and the inter- and intraday variations were <10% within the detection range.
Pharmacokinetic analysis.
The time-concentration profile of free amphotericin B observed in human serum after intravenous administration of 0.6 and 1 mg/kg/day (22, 23) was simulated in the in vitro model. Thus, the free peak concentrations (fCmax) of 0.1, 0.3, 0.6, 1.2, and 2.4 mg/liter were introduced in the system once daily for 3 days (for A. flavus and A. terreus, the fCmax of 0.8 and 1 mg/liter were also tested in order to differentiate their pharmacodynamics). Although fCmax of >1 mg/liter are not clinically achievable in human serum because of the drug's solubility limitation, these concentrations were simulated in order to fully describe the in vitro exposure-effect relationship. The biphasic 24-h time-concentration profiles of amphotericin B with an alpha phase with a short half-life of <1 h observed within 2 h after drug administration followed by a beta phase with a longer half-life of 6 to 10 h observed 2 to 24 h after drug administration were simulated. Amphotericin B concentrations were determined at 0 h, 4 h, 6 h, 8 h, 20 h, 24 h, 44 h, 48 h, and 72 h after the introduction of the drug in the IC using the bioassay. The data were analyzed with nonlinear regression based on a two-compartment model described by the equation C = Cαekαt + Cβekβt, where kα and kβ are the rate constants, Cα and Cβ are the y intercepts for alpha and beta, respectively, and C is the concentration at a given time t. The half-lives of alpha and beta phases were calculated using the equations t1/2,α = kα/ln(2) and t1/2,β = kβ/ln(2), respectively.
Determination of fungal growth.
Fungal growth in the IC was assessed in samples of 100 μl at regular time intervals by determining galactomannan production using an enzyme-linked immunosorbent assay (ELISA) (Platellia; Bio-Rad, Athens, Greece). Samples were diluted with 200 μl saline in order to reach the final volume of 300 μl before processing. Results were expressed as a galactomannan index (GI) according to the manufacturer's instructions. Galactomannan levels were also determined in the EC in order to ensure that no galactomannan escaped from the IC.
Pharmacodynamic analysis.
In vitro pharmacodynamics of each amphotericin B dose and Aspergillus species were determined based on the GI-time relationship analyzed with the Emax model: E = Emin + Emax × Tγ/(Tγ + T50γ), where E is the GI (dependent variable), Emax and Emin are the maximum and minimum GI, respectively, T is the incubation time (independent variable), T50 is the time corresponding to 50% of the Emax, and γ is the slope of the curve. As shown previously, the parameters Emax, γ, and T50 describe the extent, rate, and time of galactomannan production, respectively, whereas the area under the galactomannan index-time curve (AUCGI) is a surrogate marker of fungal growth capturing any differences in extent, rate, and time of antifungal activity. The higher the AUCGI, the greater is the fungal growth over time. The area under the galactomannan index-time curve (AUCGI) was calculated for each amphotericin B dose and drug-free control. The percentage of fungal growth at each dose was calculated based on the AUCGI of each dose divided by the AUCGI of the drug-free growth control. The percent growth-fCmax/MIC relationship was analyzed with the Emax model: E = Emin + Emax × Pγ/(Pγ + P50γ), where E is the percent growth (dependent variable), Emax and Emin are the maximum and minimum percent growth, respectively, P is the PK-PD index fCmax/MIC (independent variable), P50 is the fCmax/MIC corresponding to 50% of Emax, and γ is the slope of the curve. In addition, the fCmax/MIC corresponding to near-maximum antifungal activity (i.e., 80% inhibition or 20% growth; P80) was calculated for each Aspergillus species. The 95% confidence interval (CI) of the parameters P50 and P80 obtained with the regression analysis was used as an estimate of both biological and experimental variation as previously described (19).
Monte Carlo simulation.
In order to bridge the in vitro data with human PKs, the steady-state fCmax of 1,000 patients receiving the standard dose of amphotericin B, 1 mg/kg intravenously, were simulated with Monte Carlo analysis. This dosage resulted in steady-state total maximum concentrations in human serum (tCmax) of 2.83 ± 1.17 mg/liter, respectively (23), which corresponds to free maximum concentrations (fCmax) of 0.14 ± 0.06 mg/liter based on the 95% protein binding rate of amphotericin B previously found at these concentrations (24). The percentage of patients with fCmax/MIC exceeding the median, upper, and lower 95% CI limits of the PK-PD targets P50 and P80 were calculated for different MICs and for each Aspergillus species.
Because the dose of 1 mg/kg is associated with significant toxicity, Monte Carlo analysis was also performed for a lower dose of conventional amphotericin B, 0.6 mg/kg, used in clinical practice. This dose resulted in a serum tCmax of 1.43 ± 0.2 mg/liter, corresponding to an fCmax of 0.0715 ± 0.01 mg/liter (22, 24). The median, upper, and lower 95% CI limits of percentage of P50 target attainment were calculated for each species and for different MICs. Finally, because trough levels (Cmin) are clinically easier obtained than Cmax levels, the trough levels were associated using linear regression analysis with Cmax concentrations in the in vitro PK-PD model.
Statistical analysis.
All analysis was performed with the Prism 5.01 software (GraphPad Inc., La Jolla, CA). All Emax models were globally fitted to the data, with Emax and Emin shared among data sets. Comparisons between Emax model parameters among Aspergillus species were assessed using the extra sum-of-squares F test. Monte Carlo analysis was performed with the random function of an Excel spreadsheet (MS Office 97). A P value of <0.05 was considered statistically significant.
RESULTS
Pharmacokinetic analysis.
The time-concentration profiles of amphotericin B doses are shown in Fig. 1. The calculated fCmax in the IC were within ±20% of the target fCmax of 0.1, 0.3, 0.6, 0.8, 1, 1.2, and 2.4 mg/liter. The half-lives of alpha and beta phases were 0.2 to 2 h and 10 to 17 h, respectively.
FIG 1.
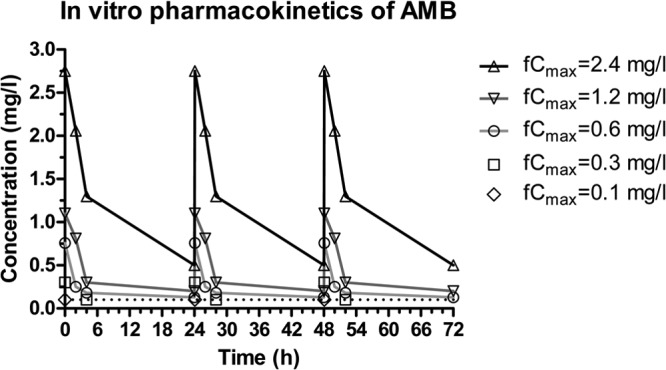
Multidose pharmacokinetics of amphotericin B in the in vitro model with different fCmax and t1/2 of 0.2 to 2 h and 10 to 17 h for alpha and beta phases, respectively. Coefficient of variations was ±15% between two replicates.
Pharmacodynamic analysis.
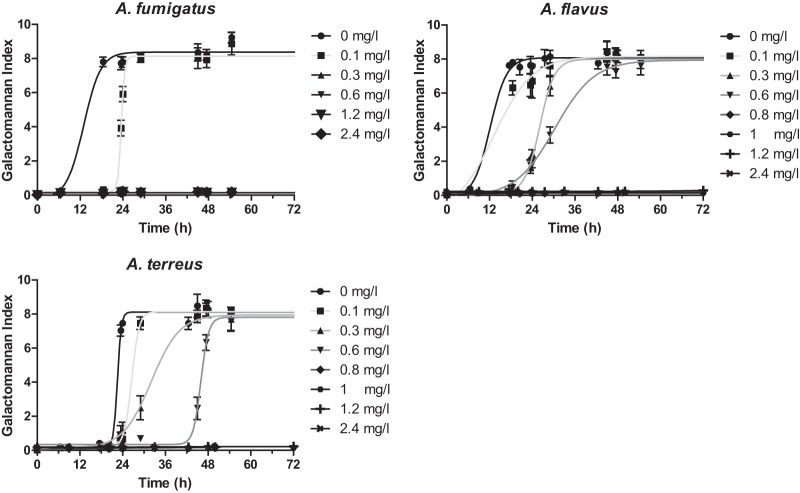
Fungal growth was progressively inhibited at higher concentrations of amphotericin B. The GI-time curves followed a sigmoid pattern described well by the Emax model (R2 > 0.82), and they were characterized by the same Emax but different slopes and T50s for the different doses of amphotericin B and Aspergillus species tested (Fig. 2). Complete inhibition of fungal growth was found for A. fumigatus with an amphotericin B fCmax of ≥0.3 mg/liter, while for A. flavus and A. terreus, complete inhibition was obtained with an amphotericin B fCmax of ≥0.8 mg/liter. In particular, the percentages of fungal growth at fCmax of 0.1, 0.3, and 0.6 mg/liter were 97%, 78%, and 74% for A. flavus and 96%, 82%, and 67% for A. terreus, respectively.
FIG 2.
Multidose pharmacodynamic analysis of simulated amphotericin B doses with fCmax of 0.1, 0.3, 0.6, 0.8, 1, 1.2, and 2.4 mg/liter against A. fumigatus, A. flavus, and A. terreus isolates with a modal CLSI MIC of 1 mg/liter as determined by the galactomannan index in the in vitro PK-PD model.
The fCmax/MIC relationship followed a sigmoid pattern (global R2 = 0.96) for all three species (Fig. 3). The fCmax/MIC (95% CI) associated with 50% growth for A. fumigatus was 0.145 (0.133 to 0.158), which was statistically significantly lower than the corresponding fCmax/MIC of A. flavus (0.371; 0.283 to 0.486) and A. terreus (0.41; 0.292 to 0.522) (P < 0.001). The fCmax/MICs corresponding to near maximal activity (20% growth) were 0.188 (0.166 to 0.213), 0.474 (0.362 to 0.655), and 0.545 (0.376 to 0.714) for A. fumigatus, A. flavus, and A. terreus, respectively.
FIG 3.
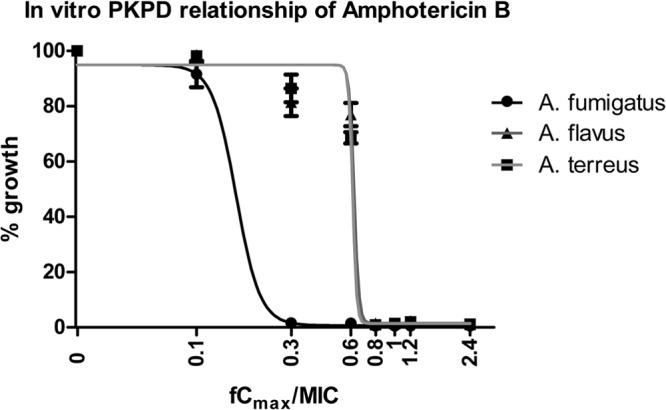
Multidose exposure-efficacy relationship of amphotericin B against each Aspergillus species with modal CLSI MICs of 1 mg/liter for A. fumigatus, A. flavus, and A. terreus in the in vitro PK-PD model simulating amphotericin B human serum levels based on the increasing amphotericin B fCmax (maximum concentration) and the galactomannan index as a marker of fungal growth.
Monte Carlo simulation analysis.
Monte Carlo simulation analysis of 1,000 patients receiving the standard amphotericin B dose of 1 mg/kg resulted in mean tCmax ± standard deviation (SD) values of 2.8 ± 1.2 mg/liter (fCmax ± SD of 0.14 ± 0.06 mg/liter). These values are close to the target values previously found in clinical studies (23). The percentage of patients attaining the PK-PD target (fCmax/MIC) associated with half-maximal (P50) activity is shown in Fig. 4. Higher-than-75% and lower-than-15% P50 target attainment was found for A. fumigatus isolates with MICs of ≤0.5 and ≥2 mg/liter and for A. flavus and A. terreus isolates with MICs of ≤0.25 and ≥1 mg/liter. In particular, the median (range) percentages of target attainment for A. fumigatus, A. flavus, and A. terreus isolates with a MIC of 1 mg/liter were 47% (39 to 57%), 0% (0 to 0%), and 0% (0 to 0%) and with a MIC of 0.5 mg/liter were 88% (87 to 90%), 24% (5 to 50%), and 15% (2 to 46%), respectively. The percentages of P80 target attainment for A. fumigatus, A. flavus, and A. terreus isolates with a MIC of 1 mg/liter were 22%, 0%, and 0%, respectively (data not shown).
FIG 4.
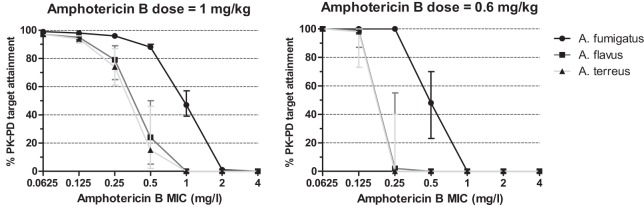
The percentage of patients attaining the PK-PD target associated with half-maximal activity (P50) for A. fumigatus, A. flavus, and A. terreus isolates with different MICs based on the Monte Carlo analysis simulating 1,000 patients treated with 1 or 0.6 mg/kg of conventional amphotericin B. Error bars represent the range of percentage of target attainment for the upper and lower 95% CI limit of the P50 target.
For the lower amphotericin B dose of 0.6 mg/kg, a >98% P50 target attainment was found for MICs of ≤0.25, ≤0.125, and ≤0.125 mg/liter for A. fumigatus, A. flavus, and A. terreus, respectively (Fig. 4). A linear correlation (r2 = 0.98) was found between the Cmin and the Cmax levels in the in vitro model with a slope of 0.20 ± 0.03, indicating that Cmin levels correspond to 1/5 of the Cmax concentrations, as previously found in humans (22, 23).
DISCUSSION
The multidose pharmacodynamic study of amphotericin B using an in vitro pharmacokinetic-pharmacodynamic model that simulated free serum amphotericin B concentrations revealed differences among the three Aspergillus species. Complete growth inhibition was observed at an fCmax of ≥0.3 mg/liter for A. fumigatus and ≥0.8 mg/liter for A. flavus and A. terreus despite their identical MIC values. The fCmax/MICs (95% CI) corresponding to near-maximal activity were 0.188 (0.166 to 0.213), 0.474 (0.362 to 0.655), and 0.545 (0.376 to 0.714) for A. fumigatus, A. flavus, and A. terreus, respectively. These differences are in agreement with our previous study where single-dose pharmacokinetics and a higher initial inoculum were used, providing better discrimination of the in vitro activity of amphotericin B against the three species (18). The observed differences among the three species were attributed to the fast inhibitory action and increased killing rate against A. fumigatus, a slower inhibitory action and reduced killing efficiency against A. flavus, and the slowest inhibitory action and no killing against A. terreus (18).
The PK-PD target of near maximal antifungal activity found in the present study against A. fumigatus is in agreement with that previously obtained in a murine neutropenic model of experimental pulmonary aspergillosis demonstrating that the tCmax/MIC of 2.4 was associated with near-maximal survival (7). Considering that the free-drug percentage of conventional amphotericin B in mouse serum corresponded to 7.4% (25), the in vivo fCmax/MIC corresponds to 0.178, which is very close to the in vitro fCmax/MIC of 0.188 found in the present study. In addition, in neutropenic murine models of experimental aspergillosis, the dose of 5 mg/kg of amphotericin B was associated with >80% survival against A. fumigatus isolates with MICs of 0.5 and 1 mg/liter and <40% against A. terreus and A. flavus isolates with MICs of 1 to 2 mg/liter (26 to 29). The dose of 5 mg/kg, which corresponds to a tCmax around 2.5 mg/liter (i.e., fCmax of 0.185 mg/liter) in mouse serum (7, 30), would attain the PK-PD targets determined in the present study for A. fumigatus but not for A. terreus and A. flavus isolates. However, there are many confounding factors, like immunosuppression, type and severity of infection, dosing regimens, time of initiation of antifungal therapy, pharmacokinetic variation, and in vitro susceptibility testing, that prohibited direct in vitro-in vivo correlation (31 to 33). These factors can be simulated in the present model in order to study the impact of host defense cells, delayed drug administration, and disease progression on the magnitude of the PK-PD parameter.
The present in vitro model indicated that the standard dose of 1 mg/kg of amphotericin B is associated with 47% target attainment using a half-maximum activity PK-PD target (P50) and 22% using a near-maximum activity PK-PD target (P80) for Aspergillus fumigatus isolates with an amphotericin B MIC of 1 mg/liter. This is in agreement with a previous retrospective study where the MICs of 274 clinical Aspergillus isolates from transplant recipients with proven or probable invasive aspergillosis were analyzed together with survival after 6 and 12 weeks of treatment (34). In that study, isolates with amphotericin B MICs of 1 mg/liter belonging mainly to Aspergillus fumigatus species were associated with 45% 6-week and 25% 12-week survival, in agreement with the percentages of P50 and P80 target attainment found in the present study, respectively.
The choice of the in vitro PK-PD target (P50 or P80) for correlating in vitro data with clinical outcome remains controversial. The percentage of P80 target attainment was comparable to 12-week survival, whereas the percentage of P50 target attainment was comparable to 6-week survival. High serum drug exposure ( P80) may result in high concentrations at the site of infection and/or fungicidal actions that will likely produce a long-lasting effect (12-week survival) compared to the lower drug exposure (P50) that will produce a short-lasting effect (6-week survival). However, since most deaths during the second 6-week period of 12-week therapy were attributed to causes other than invasive aspergillosis, albeit with some uncertainty, the 6-week survival endpoint was suggested to be the preferable endpoint to judge the effectiveness of antifungal therapy (35). Because the percentage of P50 target attainment found by Monte Carlo analysis of the present study for A. fumigatus isolates with an amphotericin B MIC of 1 mg/liter was correlated with the 6-week survival found previously in a retrospective study of the MIC-clinical outcome correlation for the same isolates (34), we used the P50 endpoint to bridge the in vitro PK-PD data with human PK in Monte Carlo analysis and to determine susceptibility breakpoints.
An important assumption when bridging in vitro concentrations to serum PK is that the serum concentrations correspond to the tissue concentrations at the site of infection, which is the lung in the case of invasive pulmonary aspergillosis. In vivo pharmacokinetic data of conventional amphotericin B in mice showed that although the tCmax in the lung was lower than the tCmax in serum (3.33 and 4.58 mg/liter, respectively), the fCmax values were similar (0.33 and 0.34 mg/liter, respectively) based on the different degree of protein binding in the lung (10%) compared to that in serum (7.4%) (30). In two clinical studies, a wide range of amphotericin B lung concentrations (0.1 to 23.3 μg/g) were detected with high-performance liquid chromatography (HPLC) in patients with mean concentrations of 11.29 and 5.29 μg/g (36, 37). However, only a small (1.5% to 30%) proportion of HPLC-detected amphotericin B was bioactive, and an even smaller proportion of the latter (<10%) corresponded to free amphotericin B (36, 37). Thus, the free concentration of amphotericin B in the lung may be similar to the free drug concentrations in human serum. For other sites of infections where amphotericin B penetrates poorly (e.g., brain), the tissue-to-serum ratio should be taken into account. Microdialysis experiments may provide better estimates of free amphotericin B in tissues (38).
The results obtained by the Monte Carlo analysis point to the following breakpoints for susceptibility, intermediate susceptibility, and resistance: ≤0.5, 1, and ≥2 for A. fumigatus and ≤0.25, 0.5, and ≥1 for A. flavus and A. terreus. Based on these breakpoints and previous epidemiological susceptibility data, 40% of A. fumigatus, 90% of A. flavus, and 98% of A. terreus isolates would be considered nonsusceptible (8). In a randomized prospective clinical trial of invasive aspergillosis mainly by A. fumigatus, conventional amphotericin B was associated with 42% 6-week mortality, which is in agreement with the 40% of A. fumigatus isolates estimated in the present study being nonsusceptible (3). Furthermore, in a review paper of 60 clinical cases of invasive A. terreus infections published from 1966 to 2003, 28 cases were treated with conventional amphotericin B, and the mortality rate was 96% (27/28), which is also in agreement with the 98% of A. terreus isolates estimated in the present study being nonsusceptible (39). Finally, in a cohort study of 11 cases of invasive A. flavus infections treated only with conventional amphotericin B, the mortality rate was 81% (9/11), in agreement with the 90% of A. flavus isolates estimated in the present study being nonsusceptible (16). Interestingly, in the latter study, all patients with A. flavus isolate MICs of ≥1 mg/liter died. These clinical data provide further support of the breakpoints determined in the present PK-PD model.
In order to maximize the efficacy and minimize the toxicity of conventional amphotericin B treatment, one could increase or decrease the dose based on the MIC of isolated pathogens or epidemiological data of each center. Thus, for a small number of A. fumigatus, A. flavus, and A. terreus strains with MICs of ≤0.25, ≤0.125, and ≤0.125 mg/liter, respectively, the lower dose of 0.6 mg/kg would retain efficacy of the 1-mg/kg dosage but reduce its toxicity. The 0.6-mg/kg dose was associated with 30% renal toxicity compared to >50% renal toxicity associated with the 1-mg/kg dose of amphotericin B (5, 40). For isolates with intermediate susceptibility, therapeutic drug monitoring (TDM) with the goal to attain the PK-PD target could increase the efficacy of the standard dose of 1 mg/kg of amphotericin B as previously suggested (41). Since TDM is easier performed using trough (Cmin) levels than Cmax levels, the fCmin/MIC (tCmin/MIC) ratios based on the in vitro model were 0.029 (0.58), 0.074 (1.48), and 0.082 (1.64) for A. fumigatus, A. flavus, and A. terreus, respectively. Since in vivo amphotericin B is bound by tissue and slowly released, accounting for the prolonged (serum) terminal elimination half-life of the drug, the correlation between fCmin and fCmax may be clinically relevant only for the first few days of therapy. However, the unavoidable toxicity of conventional amphotericin B, which often results in drug discontinuation, will be the limiting factor even for susceptible isolates, limiting the use of conventional amphotericin B, in agreement with the ECIL guidelines for the management of invasive aspergillosis (42). Therefore, lipid formulations of amphotericin B could be used efficiently even for isolates with reduced susceptibility to conventional amphotericin B, as previously shown in an animal model infected by an A. fumigatus isolate with a MIC of 1 mg/liter (43). Lipid formulations can safely deliver high concentrations of amphotericin B in the fungal target and at the site of infection (44).
In conclusion, a PK-PD model was used to simulate free amphotericin B serum concentrations and to study the in vitro activity against three Aspergillus species. The PK-PD targets were 0.145, 0.371, and 0.41 fCmax/MIC for A. fumigatus, A. flavus, and A. terreus, respectively, and the susceptibility breakpoints derived were ≤0.5, 1, and ≥2 mg/liter for A. fumigatus and ≤0.25, 0.5, and ≥1 mg/liter for A. flavus and A. terreus. The suggested breakpoints are substantiated with results obtained from previously published clinical studies of invasive aspergillosis.
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, Rinaldi MG, Stevens DA, Graybill JR. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine 79:250–260 [DOI] [PubMed] [Google Scholar]
- 2.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 65:453–464. 10.1016/j.jinf.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415. 10.1056/NEJMoa020191 [DOI] [PubMed] [Google Scholar]
- 4.Popp AI, White MH, Quadri T, Walshe L, Armstrong D. 1999. Amphotericin B with and without itraconazole for invasive aspergillosis: a three-year retrospective study. Int. J. Infect. Dis. 3:157–160. 10.1016/S1201-9712(99)90038-3 [DOI] [PubMed] [Google Scholar]
- 5.Bowden R, Chandrasekar P, White MH, Li X, Pietrelli L, Gurwith M, van Burik JA, Laverdiere M, Safrin S, Wingard JR. 2002. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35:359–366. 10.1086/341401 [DOI] [PubMed] [Google Scholar]
- 6.White MH, Anaissie EJ, Kusne S, Wingard JR, Hiemenz JW, Cantor A, Gurwith M, Du Mond C, Mamelok RD, Bowden RA. 1997. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis. Clin. Infect. Dis. 24:635–642 [PubMed] [Google Scholar]
- 7.Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469–473. 10.1128/AAC.50.2.469-473.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob. Agents Chemother. 55:5150–5154. 10.1128/AAC.00686-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW. 2012. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin. Microbiol. Infect. 18:E248–E250. 10.1111/j.1469-0691.2012.03890.x [DOI] [PubMed] [Google Scholar]
- 10.Canton E, Espinel-Ingroff A, Peman J. 2009. Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev. Anti. Infect. Ther. 7:107–119. 10.1586/14787210.7.1.107 [DOI] [PubMed] [Google Scholar]
- 11.Lass-Florl C, Kofler G, Kropshofer G, Hermans J, Kreczy A, Dierich MP, Niederwieser D. 1998. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497–502. 10.1093/jac/42.4.497 [DOI] [PubMed] [Google Scholar]
- 12.Lionakis MS, Lewis RE, Chamilos G, Kontoyiannis DP. 2005. Aspergillus susceptibility testing in patients with cancer and invasive aspergillosis: difficulties in establishing correlation between in vitro susceptibility data and the outcome of initial amphotericin B therapy. Pharmacotherapy 25:1174–1180. 10.1592/phco.2005.25.9.1174 [DOI] [PubMed] [Google Scholar]
- 13.Torres HA, Rivero GA, Lewis RE, Hachem R, Raad II, Kontoyiannis DP. 2003. Aspergillosis caused by non-fumigatus Aspergillus species: risk factors and in vitro susceptibility compared with Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 46:25–28. 10.1016/S0732-8893(03)00013-0 [DOI] [PubMed] [Google Scholar]
- 14.van't Wout JW, Novakova I, Verhagen CA, Fibbe WE, de Pauw BE, van der Meer JW. 1991. The efficacy of itraconazole against systemic fungal infections in neutropenic patients: a randomised comparative study with amphotericin B. J. Infect. 22:45–52. 10.1016/0163-4453(91)90954-Q [DOI] [PubMed] [Google Scholar]
- 15.Paterson PJ, Seaton S, Prentice HG, Kibbler CC. 2003. Treatment failure in invasive aspergillosis: susceptibility of deep tissue isolates following treatment with amphotericin B. J. Antimicrob. Chemother. 52:873–876. 10.1093/jac/dkg434 [DOI] [PubMed] [Google Scholar]
- 16.Hadrich I, Makni F, Neji S, Cheikhrouhou F, Bellaaj H, Elloumi M, Ayadi A, Ranque S. 2012. Amphotericin B in vitro resistance is associated with fatal Aspergillus flavus infection. Med. Mycol. 50:829–834. 10.3109/13693786.2012.684154 [DOI] [PubMed] [Google Scholar]
- 17.Meletiadis J, Al-Saigh R, Velegraki A, Walsh TJ, Roilides E, Zerva L. 2012. Pharmacodynamic effects of simulated standard doses of antifungal drugs against Aspergillus species in a new in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 56:403–410. 10.1128/AAC.00662-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Saigh R, Siopi M, Siafakas N, Velegraki A, Zerva L, Meletiadis J. 2013. Single-dose pharmacodynamics of amphotericin B against Aspergillus species in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 57:3713–3718. 10.1128/AAC.02484-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siopi M, Mavridou E, Verweij P, Mouton JW, Zerva L, Meletiadis J. 17 February 2014. Susceptibility breakpoints and target values for therapeutic drug monitoring of voriconazole and Aspergillus fumigatus in an in vitro pharmacokinetic-pharmacodynamic model. J. Antimicrob. Chemother. 10.1093/jac/dku023 [DOI] [PubMed] [Google Scholar]
- 20.Al-Saigh R, Elefanti A, Velegraki A, Zerva L, Meletiadis J. 2012. In vitro pharmacokinetic/pharmacodynamic modeling of voriconazole activity against Aspergillus species in a new in vitro dynamic model. Antimicrob. Agents Chemother. 56:5321–5327. 10.1128/AAC.00549-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shadomy S, McCay JA, Schwartz SI. 1969. Bioassay for hamycin and amphotericin B in serum and other biological fluids. Appl. Microbiol. 17:497–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828–833. 10.1128/AAC.46.3.828-833.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayestaran A, Lopez RM, Montoro JB, Estibalez A, Pou L, Julia A, Lopez A, Pascual B. 1996. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob. Agents Chemother. 40:609–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834–840. 10.1128/AAC.46.3.834-840.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbie G, Chiou WL. 1998. Elucidation of human amphotericin B pharmacokinetics: identification of a new potential factor affecting interspecies pharmacokinetic scaling. Pharm. Res. 15:1630–1636. 10.1023/A:1011923704731 [DOI] [PubMed] [Google Scholar]
- 26.Warn PA, Morrissey G, Morrissey J, Denning DW. 2003. Activity of micafungin (FK463) against an itraconazole-resistant strain of Aspergillus fumigatus and a strain of Aspergillus terreus demonstrating in vivo resistance to amphotericin B. J. Antimicrob. Chemother. 51:913–919. 10.1093/jac/dkg185 [DOI] [PubMed] [Google Scholar]
- 27.Dannaoui E, Borel E, Persat F, Piens MA, Picot S. 2000. Amphotericin B resistance of Aspergillus terreus in a murine model of disseminated aspergillosis. J. Med. Microbiol. 49:601–606 [DOI] [PubMed] [Google Scholar]
- 28.Odds FC, Van Gerven F, Espinel-Ingroff A, Bartlett MS, Ghannoum MA, Lancaster MV, Pfaller MA, Rex JH, Rinaldi MG, Walsh TJ. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najvar LK, Cacciapuoti A, Hernandez S, Halpern J, Bocanegra R, Gurnani M, Menzel F, Loebenberg D, Graybill JR. 2004. Activity of posaconazole combined with amphotericin B against Aspergillus flavus infection in mice: comparative studies in two laboratories. Antimicrob. Agents Chemother. 48:758–764. 10.1128/AAC.48.3.758-764.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andes D, Safdar N, Marchillo K, Conklin R. 2006. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob. Agents Chemother. 50:674–684. 10.1128/AAC.50.2.674-684.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson EM, Oakley KL, Radford SA, Moore CB, Warn P, Warnock DW, Denning DW. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85–93. 10.1093/jac/45.1.85 [DOI] [PubMed] [Google Scholar]
- 32.Verweij PE, Oakley KL, Morrissey J, Morrissey G, Denning DW. 1998. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob. Agents Chemother. 42:873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosquera J, Warn PA, Morrissey J, Moore CB, Gil-Lamaignere C, Denning DW. 2001. Susceptibility testing of Aspergillus flavus: inoculum dependence with itraconazole and lack of correlation between susceptibility to amphotericin B in vitro and outcome in vivo. Antimicrob. Agents Chemother. 45:1456–1462. 10.1128/AAC.45.5.1456-1462.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP, Ito JI, Balajee SA, Pappas PG, Moser SA. 2009. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 47:3271–3275. 10.1128/JCM.00854-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wingard JR, Ribaud P, Schlamm HT, Herbrecht R. 2008. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer 112:2309–2312. 10.1002/cncr.23441 [DOI] [PubMed] [Google Scholar]
- 36.Christiansen KJ, Bernard EM, Gold JW, Armstrong D. 1985. Distribution and activity of amphotericin B in humans. J. Infect. Dis. 152:1037–1043. 10.1093/infdis/152.5.1037 [DOI] [PubMed] [Google Scholar]
- 37.Collette N, van der Auwera P, Lopez AP, Heymans C, Meunier F. 1989. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob. Agents Chemother. 33:362–368. 10.1128/AAC.33.3.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joukhadar C, Thallinger C, Poppl W, Kovar F, Konz KH, Joukhadar SM, Traunmuller F. 2009. Concentrations of voriconazole in healthy and inflamed lung in rats. Antimicrob. Agents Chemother. 53:2684–2686. 10.1128/AAC.00885-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinbach WJ, Perfect JR, Schell WA, Walsh TJ, Benjamin DK., Jr 2004. In vitro analyses, animal models, and 60 clinical cases of invasive Aspergillus terreus infection. Antimicrob. Agents Chemother. 48:3217–3225. 10.1128/AAC.48.9.3217-3225.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subira M, Martino R, Gomez L, Marti JM, Estany C, Sierra J. 2004. Low-dose amphotericin B lipid complex vs. conventional amphotericin B for empirical antifungal therapy of neutropenic fever in patients with hematologic malignancies—a randomized, controlled trial. Eur. J. Haematol. 72:342–347. 10.1111/j.1600-0609.2004.00239.x [DOI] [PubMed] [Google Scholar]
- 41.Emminger W, Lang HR, Emminger-Schmidmeier W, Peters C, Gadner H. 1991. Amphotericin B serum levels in pediatric bone marrow transplant recipients. Bone Marrow Transplant. 7:95–99 [PubMed] [Google Scholar]
- 42.Maertens J, Marchetti O, Herbrecht R, Cornely OA, Fluckiger U, Frere P, Gachot B, Heinz WJ, Lass-Florl C, Ribaud P, Thiebaut A, Cordonnier C. 2011. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3-2009 update. Bone Marrow Transplant. 46:709–718. 10.1038/bmt.2010.175 [DOI] [PubMed] [Google Scholar]
- 43.Mouton JW, te Dorsthorst DT, Meis JF, Verweij PE. 2009. Dose-response relationships of three amphotericin B formulations in a non-neutropenic murine model of invasive aspergillosis. Med. Mycol. 47:802–807. 10.3109/13693780802672644 [DOI] [PubMed] [Google Scholar]
- 44.Adler-Moore JP, Proffitt RT. 2008. Amphotericin B lipid preparations: what are the differences? Clin. Microbiol. Infect. 14(Suppl 4):25–36 [DOI] [PubMed] [Google Scholar]