Abstract
A five-gene cluster, tolCSm-pcm-smeRo-smeO-smeP, of Stenotrophomonas maltophilia was characterized. The presence of smeOP and smeRo-pcm-tolCSm operons was verified by reverse transcription (RT)-PCR. Both operons were negatively regulated by the TetR-type transcriptional regulator SmeRo, as demonstrated by quantitative RT-PCR and a promoter-fusion assay. SmeO and SmeP were associated with TolCSm (the TolC protein of S. maltophilia) for the assembly of a resistance-nodulation-cell-division (RND)-type pump. The compounds extruded by SmeOP-TolCSm mainly included nalidixic acid, doxycycline, amikacin, gentamicin, erythromycin, leucomycin, carbonyl cyanide 3-chlorophenylhydrazone, crystal violet, sodium dodecyl sulfate, and tetrachlorosalicylanilide.
TEXT
Multidrug efflux transporters capable of active extrusion of noxious compounds are classified into five families, including the resistance-nodulation-cell-division (RND) family, the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, the ATP binding cassette (ABC) family, and the multidrug and toxic compound extrusion (MATE) family (1). The RND systems generally form tripartite components composed of a periplasmic membrane fusion protein (MFP), an inner membrane RND transporter, and an outer membrane protein (OMP) (2).
Stenotrophomonas maltophilia is a nonfermentative Gram-negative bacillus. Eight RND-type efflux systems, SmeABC, SmeDEF, SmeGH, SmeIJK, SmeMN, SmeOP, SmeVWX, and SmeYZ, are postulated to be in the S. maltophilia genome (3). Among them, SmeABC, SmeDEF, SmeIJK, SmeVWX, and SmeYZ have been characterized (4–7). A possible promiscuous OMP, TolCSm, involved in multidrug resistance, has been proposed (8). Referencing the genome sequence of S. maltophilia, we noted that the tolC gene of S. maltophilia (tolCSm) and the smeOP system are located nearby (Fig. 1), implying that smeOP and tolCSm may be involved in a common mechanism for antibiotic extrusion. smeO and smeP were predicted to encode an MFP and an RND-type inner membrane transporter. A TetR-type transcription regulator (annotated as SmeRo here) was located immediately upstream of smeOP and divergently transcribed. The genes downstream of smeRo were the pcm-tolCSm operon, which has been known to contribute to multidrug resistance (8).
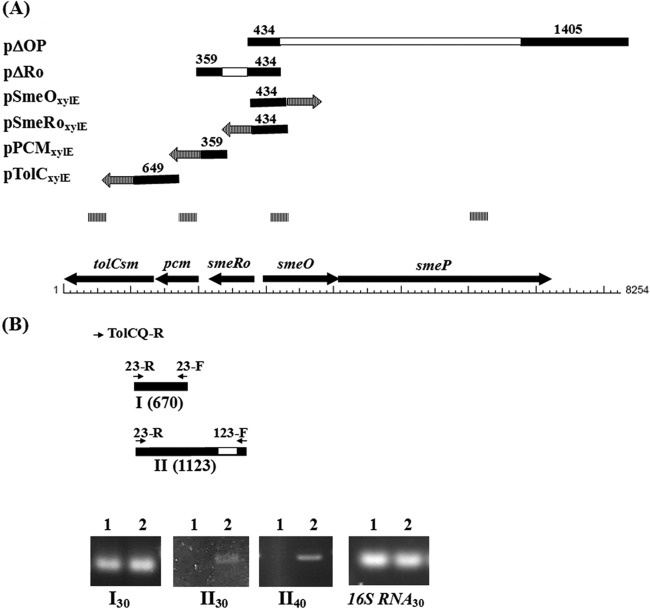
FIG 1.
Schematic organization of the smeOP and smeRo-pcm-tolCSm operons from S. maltophilia strain KJ. The smeOP operon contains genes for a membrane fusion protein (SmeO) and an RND transporter (SmeP). A TetR-type transcriptional regulator gene, smeRo, is located immediately upstream of the smeOP operon and is divergently transcribed. The smeRo, pcm, and tolCSm genes form an operon. Solid arrows represent open reading frames (ORFs) and the direction of transcription. (A) Structures of recombinant plasmids. The solid lines represent the PCR amplicons, and each empty bar represents the deleted region for each plasmid construct. The numbers above the solid bars represent the PCR amplicon sizes (in bp). The arrows with vertical lines indicate the xylE gene. The vertical-line bars indicate the products of qRT-PCR. (B) Presence of the smeRo-pcm-tolCSm operon. The solid bars labeled I and II indicate the products of RT-PCR generated by primers 23-F/23-R and 123-F/23-R, respectively. The numbers in parentheses represent the PCR amplicon sizes (in bp). The arrows indicate the positions of primers. The empty bar represents the deleted region in strain KJΔRo. The agarose gels show the RT-PCR products of strains KJ and KJΔRo. Lanes 1, strain KJ; lanes 2, strain KJΔRo. The RT-PCR products, labeled I and II, were generated by primers 23-F/23-R and 123-F/23-R, respectively. The subscript numbers indicate the numbers of PCR cycles.
SmeRo acts as a regulator for the expression of smeOP.
To evaluate the regulatory role of smeRo, a smeRo deletion mutant, KJΔRo, was constructed. A 359-bp DNA fragment (the 27-bp N terminus of pcm, the intergenic region of pcm and smeRo, and the 198-bp C terminus of smeRo) and a 434-bp DNA fragment (the 276-bp N terminus of smeRo, the intergenic region of smeRo and smeO, and the 33-bp N terminus of smeO) were amplified with the primers SmeRo3-F/SmeRo3-R and SmeRo5-F/SmeRo5-R (Fig. 1; see also Table S1 in the supplemental material), respectively, and sequentially cloned into pEX18Tc, yielding plasmid pΔRo for the construction of KJΔRo. The resultant in-frame deletion mutant, KJΔRo, included an internal deletion in the smeRo gene from nucleotide (nt) 273 to nt 494. The transcripts of smeO, smeP, pcm, and tolCSm in KJ and KJΔRo were determined by reverse transcription-quantitative PCR (qRT-PCR). The qRT-PCR was carried out at least in triplicate (6), and the primers we used are listed in Table S1. The transcript levels of smeO, smeP, pcm, and tolCSm in KJΔRo were greater than those in KJ by factors of 4.6 ± 2.1, 3.8 ± 1.9, 1.5 ± 0.6, and 1.4 ± 0.5, respectively (means and standard deviations). The effects of smeRo deletion on the expression levels of pcm and tolCSm were notably minor. Therefore, SmeRo plays a regulatory role, presumably as a repressor, in the expression of smeOP.
smeRo, pcm, and tolCSm form an operon.
Based on the genetic organization, we considered the possible presence of the smeRo-pcm-tolCSm operon. To test this possibility, an RT-PCR analysis was performed on the mRNA extracted from strains KJ and KJΔRo. Primer TolCQ-R (Fig. 1B; see also Table S1 in the supplemental material) was used to obtain cDNA. A 670-bp amplicon I (generated using primers 23-F/23-R) and a 1,123-bp amplicon II (generated with primers 123-F/23-R) were detected in KJΔRo, but only amplicon I was observed in KJ (Fig. 1B). KJΔRo is an in-frame deletion mutant with an internal deletion in the smeRo gene from nt 273 to nt 494. The primers 123-F and 23-R targeted nt 221 to 241 of the smeRo gene and nt 48 to 65 of the tolCSm gene (Fig. 1B). Therefore, a 1,123-bp amplicon II can be amplified only if smeRo, pcm, and tolCSm are cotranscribed. A smeRo-pcm-tolCSm transcript was observed in KJΔRo but not in KJ, even though the number of PCR cycles was increased to 40 (Fig. 1B). These observations support that a smeRo-pcm-tolCSm transcript was slightly expressed in KJΔRo and that a pcm-tolCSm transcript was expressed in KJ and KJΔRo.
Assays of promoter activities of smeRo-pcm-tolCSm and smeOP operons.
To further study the regulatory circuits, transcriptional xylE fusions to promoters of the smeOP operon (pSmeOxylE), the smeRo-pcm-tolCSm operon (pSmeRoxylE), the pcm-tolCSm operon (pPCMxylE), and tolCSm (pTolCxylE) were constructed (Fig. 1A), and each construct was introduced into strains KJ and KJΔRo. The expressed catechol 2,3-dioxygenase (C23O) activities were monitored as described previously (9). KJΔRo(pSmeOxylE) exhibited a higher C23O activity than KJ(pSmeOxylE) (Table 1), further confirming that SmeRo plays a repressor role in the expression of the smeOP operon. Compared to KJ(pSmeRoxylE), KJΔRo(pSmeRoxylE) had a slightly higher C23O activity (Table 1), which signifies the slightly negative autoregulation of smeRo. KJ(pPCMxylE) and KJΔRo(pPCMxylE) exhibited comparable C23O activities, indicating that the promoter of the pcm-tolCSm operon is constitutively active and not subjected to the regulation of SmeRo. No significant C23O activity was observed in KJ(pTolCxylE) or KJΔRo(pTolCxylE), supporting that there is no promoter activity in the 243-bp upstream region of the tolCSm gene.
TABLE 1.
Analysis of promoter transcription fusion constructs
| Strain | C23O activity (Uc/OD450)a |
|---|---|
| KJ(pSmeOxylE) | 18 ± 1.7 |
| KJΔRo(pSmeOxylE) | 89 ± 9.2 |
| KJ(pSmeRoxylE) | 20 ± 3.0 |
| KJΔRo(pSmeRoxylE) | 54 ± 4.5 |
| KJ(pPCMxylE) | 119 ± 17 |
| KJΔRo(pPCMxylE) | 115 ± 14 |
| KJ(pTolCxylE) | 5 ± 1 |
| KJΔRo(pTolCxylE) | 8 ± 1 |
One unit of catechol 2,3-dioxygenase (Uc) is defined as 1 nmol of catechol hydrolyzed per min. Results are expressed as the means ± standard deviations of three independent determinations. OD450, optical density at 450 nm.
Substrate spectrum of the SmeOP efflux pump.
A 1,405-bp DNA fragment containing the partial C terminus of smeP, amplified with primers SmeP3-F and SmeP3-R, and the aforementioned 434-bp DNA fragment (generated with the primers SmeRo5-F/SmeRo5-R) were sequentially cloned into pEX18Tc, yielding plasmid pΔOP (Fig. 1A; see also Table S1 in the supplemental material). The SmeOP in-frame deletion mutant, KJΔOP, was obtained using the strategy described in reference 6. The 1,103-bp C terminus of the smeO gene and the 2,802-bp N terminus of the smeP gene in the mutant KJΔOP were deleted (Fig. 1A). The substrate spectrum of SmeOP was assessed by comparing the antimicrobial susceptibilities between KJ and KJΔOP and between KJΔRo and KJΔRoΔOP (Table 2). The susceptibility assay was performed by using the agar dilution method, as described previously (6), in at least three replicate experiments. The results indicated that SmeOP was responsible for the extrusion of nalidixic acid, doxycycline, aminoglycosides, macrolides, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), crystal violet, sodium dodecyl sulfate (SDS), and tetrachlorosalicylanilide (TCS). Among these compounds, the aminoglycosides, CCCP, and TCS were the most significant ones.
TABLE 2.
Antimicrobial susceptibilities of S. maltophilia strain KJ and its derived deletion mutants
| Compound class and/or agenta | MIC (μg/ml) for strain |
|||||||
|---|---|---|---|---|---|---|---|---|
| KJ | KJΔOP | KJΔRo | KJΔRoΔOP | KJΔDEF | KJΔDEFΔOP | KJΔTolC | KJΔTolCΔOP | |
| Chloramphenicol | 8 | 8 | 16 | 8 | 4 | 4 | 4 | 4 |
| Quinolones | ||||||||
| Nalidixic acid | 8 | 4 | 16 | 4 | 4 | 2 | 2 | 2 |
| Norfloxacin | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Tetracyclines | ||||||||
| Doxycycline | 1 | 0.5 | 2 | 1 | 0.5 | 0.25 | 0.5 | 0.5 |
| Tetracycline | 16 | 16 | 16 | 16 | 8 | 8 | 16 | 16 |
| Aminoglycosides | ||||||||
| Amikacin | 1,024 | 256 | 1,024 | 256 | 1,024 | 256 | 16 | 16 |
| Gentamicin | 1,024 | 256 | 1,024 | 256 | 1,024 | 256 | 8 | 8 |
| Kanamycin | 256 | 128 | 256 | 128 | 256 | 64 | 16 | 16 |
| Macrolides | ||||||||
| Erythromycin | 64 | 32 | 64 | 32 | 32 | 32 | 32 | 32 |
| Leucomycin | 256 | 32 | 256 | 64 | 128 | 16 | 16 | 16 |
| Rokitamycin | 512 | 512 | 512 | 512 | 256 | 256 | 128 | 128 |
| Others | ||||||||
| Acriflavine | >1,024 | 1,024 | >1,024 | 1,024 | 256 | 256 | 1,024 | 1,024 |
| CCCP | 16 | 8 | 128 | 8 | 16 | 8 | 8 | 8 |
| CHH | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| Crystal violet | 8 | 4 | 16 | 4 | 4 | 2 | 4 | 4 |
| Fusaric acid | 512 | 512 | 512 | 512 | 512 | 512 | 256 | 256 |
| Menadione | 64 | 64 | 64 | 64 | 64 | 64 | 32 | 32 |
| Paraquat | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 512 | 512 |
| Plumbagin | 32 | 32 | 32 | 32 | 32 | 32 | 8 | 8 |
| Proflavine | 512 | 512 | 512 | 512 | 64 | 64 | 512 | 512 |
| SDS | 0.08 | 0.04 | 0.08 | 0.04 | 0.04 | 0.02 | 0.04 | 0.04 |
| TCS | 8 | 4 | 32 | 4 | 8 | 4 | 2 | 2 |
CCCP, carbonyl cyanide 3-chlorophenylhydrazone; CHH, 2-chlorophenylhydrazine hydrochloride; SDS, sodium dodecyl sulfate; TCS, tetrachlorosalicylanilide.
Complementation test for the smeRo mutant.
To confirm that the phenotype observed for strain KJΔRo was due to inactivation of the smeRo gene, the plasmid pSmeRo (containing the full-length smeRo gene) was introduced into KJΔRo, yielding KJΔRo(pSmeRo). The transcript levels of smeO, smeP, pcm, and tolCSm in KJΔRo(pSmeRo) were lower than those in KJΔRo(pRK415) by factors of 2.6 ± 1.1, 2.3 ± 1.0, 1.1 ± 0.4, and 1.1 ± 0.5, respectively. The susceptibilities of KJ(pRK415), KJΔRo(pRK415), and KJΔRo(pSmeRo) to aminoglycoside, CCCP, and TCS were tested by the agar dilution method and a disk diffusion assay. The complementation of KJΔRo with pSmeRo decreased resistance to aminoglycosides, CCCP, and TCS (see Table S2 in the supplemental material).
SmeOP requires TolCSm for efflux pump function.
Inactivation of the smeOP operon decreased the MICs to some antibiotics (Table 2), indicating that smeOP and its cognate OMP gene should be expressed constitutively in the wild-type KJ strain. Four possible OMP candidates, SmeC, SmeF, SmeX, and TolCSm, for the RND-type efflux pumps have been proposed (8). Of the four OMPs, the transcripts of smeF and tolCSm were observed, and no significant transcripts of smeC and smeX were detected by RT-PCR (see Fig. S1 in the supplemental material). Therefore, SmeC and SmeX are less likely to be the cognate OMP of the SmeOP efflux pump. To assess the possibility of SmeOP-SmeF as the cognate OMP, the smeDEF operon was deleted from the chromosomes of strains KJ and KJΔOP, generating mutants KJΔDEF and KJΔDEFΔOP, respectively. Compared to KJΔDEF, KJΔDEFΔOP obviously had compromised resistance to aminoglycosides and leucomycin (Table 2), indicating that the SmeOP pump is still functional in the case of smeF inactivation. The possibility of SmeOP-TolCSm as the cognate OMP was also evaluated. The susceptibility of KJΔTolC reported in our previous study (8) is also included in Table 2 for comparison. The introduction of ΔsmeOP into KJΔTolC did not further compromise the resistance of KJΔTolC to any of the compounds tested (Table 2, KJΔTolC versus KJΔTolCΔOP), which lends support for TolCSm being the cognate OMP for the SmeOP pump. Moreover, deletion of the tolCSm gene was associated with greater decreases in MICs than those caused by deletion of smeOP (Table 2), signifying the promiscuous role of TolCSm. TolCSm may participate not only in the function of the SmeOP pump but also in the function of other hitherto-uncharacterized efflux systems.
Distinct from the tolC orthologs reported for Enterobacteriaceae and Pseudomonas aeruginosa, tolCSm of S. maltophilia is located in a pcm-tolCSm operon. The protein l-isoaspartate O-methyltransferase (PCM), encoded by pcm, is an enzyme that recognizes and catalyzes the repair of damaged l-isoaspartyl and d-aspartyl groups in proteins. Thus, PCM may be involved in repairing damaged TolCSm and keeping TolCSm in a functional state. Recently, we verified that the pcm gene is less related to the TolCSm function regarding antimicrobial extrusion (8). However, in addition to the antimicrobial efflux function, TolC-associated pumps are also known to play physiological roles for adaptation to stress, such as envelope stress or oxidative stress (10). Therefore, it cannot be immediately ruled out that PCM plays an important role in the physiological function of TolCSm.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the National Science Council (grant NSC 101-2320-B-010-053-MY3).
Footnotes
Published ahead of print 6 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01974-13.
REFERENCES
- 1.Putman M, van Veen HW, Konings WN. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672–693. 10.1128/MMBR.64.4.672-693.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Femandez-Recio J, Walas F, Federici L, Venkatesh Pratap J, Bavro VN, Miguel RN, Mizuguchi K, Luisi B. 2004. A model of a transmembrane drug-efflux pump from Gram-negative bacteria. FEBS Lett. 578:5–9. 10.1016/j.febslet.2004.10.097 [DOI] [PubMed] [Google Scholar]
- 3.Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, Sebaihia M, Saunders D, Arrowsmith C, Carver T, Peters N, Adlem E, Kerhornou A, Lord A, Murphy L, Seeger K, Squares R, Rutter S, Quail MA, Rajandream MA, Harris D, Churcher C, Bentley SD, Parkhill J, Thomson NR, Avison MB. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Gen. Biol. 9:R74. 10.1186/gb-2008-9-4-r74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li XZ, Li Z, Poole K. 2002. SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 46:333–343. 10.1128/AAC.46.2.333-343.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso A, Martinez JL. 2000. Cloning and characterization of SmeDEF, a novel multidrug efflux pump from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 44:3079–3086. 10.1128/AAC.44.11.3079-3086.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CH, Huang CC, Chung TC, Hu RM, Huang YW, Yang TC. 2011. Contribution of resistance-nodulation-division efflux pump operon smeU1-V-W-U2-X to multidrug resistance of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 55:5826–5833. 10.1128/AAC.00317-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould VC, Okazaki A, Avison MB. 2013. Coordinate hyperproduction of SmeZ and SmeJK efflux pumps extends drug resistance in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 57:655–657. 10.1128/AAC.01020-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YW, Hu RM, Yang TC. 2013. Role of the pcm-tolCSm operon in multidrug resistance of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 68:1987–1993. 10.1093/jac/dkt148 [DOI] [PubMed] [Google Scholar]
- 9.Huang YW, Lin CW, Hu RM, Lin YT, Chung TC, Yang TC. 2010. AmpN-AmpG operon is essential for expression of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 54:2583–2589. 10.1128/AAC.01283-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zgurskaya HI, Krishnamoorthy G, Ntreh A, Lu S. 2011. Mechanism and function of the outer membrane channel TolC in multidrug resistance and physiology of Enterobacteria. Front. Microbiol. 2:189. 10.3389/fmicb.2011.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.