Abstract
Heat shock protein 90 (Hsp90) is an essential chaperone involved in the fungal stress response that can be harnessed as a novel antifungal target for the treatment of invasive aspergillosis. We previously showed that genetic repression of Hsp90 reduced Aspergillus fumigatus virulence and potentiated the effect of the echinocandin caspofungin. In this study, we sought to identify sites of posttranslational modifications (phosphorylation or acetylation) that are important for Hsp90 function in A. fumigatus. Phosphopeptide enrichment and tandem mass spectrometry revealed phosphorylation of three residues in Hsp90 (S49, S288, and T681), but their mutation did not compromise Hsp90 function. Acetylation of lysine residues of Hsp90 was recovered after treatment with deacetylase inhibitors, and acetylation-mimetic mutations (K27A and K271A) resulted in reduced virulence in a murine model of invasive aspergillosis, supporting their role in Hsp90 function. A single deletion of lysine K27 or an acetylation-mimetic mutation (K27A) resulted in increased susceptibility to voriconazole and caspofungin. This effect was attenuated following a deacetylation-mimetic mutation (K27R), suggesting that this site is crucial and should be deacetylated for proper Hsp90 function in antifungal resistance pathways. In contrast to previous reports in Candida albicans, the lysine deacetylase inhibitor trichostatin A (TSA) was active alone against A. fumigatus and potentiated the effect of caspofungin against both the wild type and an echinocandin-resistant strain. Our results indicate that the Hsp90 K27 residue is required for azole and echinocandin resistance in A. fumigatus and that deacetylase inhibition may represent an adjunctive anti-Aspergillus strategy.
INTRODUCTION
Invasive aspergillosis (IA) is a life-threatening infection in the increasing population of patients with depressed immune systems, such as cancer patients or transplant recipients (1). Three antifungal drug classes are approved for the treatment of IA: the triazoles (e.g., voriconazole), the polyenes (e.g., amphotericin B), and the echinocandins (e.g., caspofungin), all of them targeting the fungal cell membrane or cell wall (2). The emergence in Aspergillus fumigatus of resistance to triazoles, the preferred primary therapy against IA, is a growing concern (3). Moreover, the toxicity of polyenes often limits their use, and the echinocandins possess only fungistatic activity against Aspergillus spp. Combination antifungal therapy with existing classes of agents has shown limited promise (4), suggesting the need for new therapeutic options.
One novel therapeutic approach consists of the development of drugs targeting intracellular signaling proteins involved in compensatory mechanisms of the cell wall. The heat shock protein 90 (Hsp90)-calcineurin axis is a crucial element governing stress adaptation processes in fungi (5). Calcineurin inhibitors (FK506 and cyclosporine) and Hsp90 inhibitors (geldanamycin, 17-allylamino-17-demethoxygeldanamycin [17-AAG], and 17-demethylaminoethylamino-17-demethoxygeldanamycin [17-DMAG]) are active against A. fumigatus and potentiate the effect of caspofungin (6–8), but the lack of fungal specificity of these compounds and cross-reactivity against the human proteins prevent their use for the treatment of fungal diseases.
Hsp90 is an essential molecular chaperone that activates multiple client proteins (9). Fungal Hsp90 promotes resistance to azole and echinocandin drugs, which has been well demonstrated in the pathogenic yeast Candida albicans (6, 10, 11). We have previously shown that genetic repression of A. fumigatus Hsp90 abolishes virulence in a murine model of IA (12). Compromising Hsp90-mediated stress responses by genetic modifications of the hsp90 promoter also potentiated the effect of caspofungin and abolished the paradoxical effect of this drug (7, 12). Because Hsp90 is a highly conserved protein in eukaryotes, identification of specific regions of the protein that are important for fungal virulence or antifungal resistance would be a critical step toward development of novel fungal-specific Hsp90 inhibitors.
Activation of Hsp90 and interactions with its client proteins and cochaperones are mediated by posttranslational modifications, such as phosphorylation, acetylation, oxidation, S-nitrosylation, and ubiquitination (13). Reversible phosphorylation is the addition of a phosphate group to serine, threonine, or tyrosine residues, a process regulated by kinases and phosphatases. Multiple phosphorylation sites have been identified as regulatory elements of Hsp90 function in human and yeast (13–15). Phosphorylation of A. fumigatus Hsp90, however, has not been previously investigated. Reversible internal acetylation, which is the addition of an N-α-acetyl group from acetyl-coenzyme A (CoA) to the side chain of a lysine residue, may also represent an important regulatory mechanism of Hsp90 (16). Hyperacetylation of Hsp90 can be induced by inhibitors of the lysine deacetylases (KDACs) and is associated with altered chaperone activity (17–20). Acetylation of the K294 residue in human Hsp90 and its corresponding residue K270 in Saccharomyces cerevisiae is important for Hsp90 function (16, 21). The K27 residue was also found to be acetylated in yeasts (21).
In the present study, we identified the phosphorylation and acetylation sites of A. fumigatus Hsp90 and determined their role in the key pathogenic processes of virulence and antifungal resistance.
MATERIALS AND METHODS
Nano-flow LC-ESI-MS/MS analysis.
Our Hsp90-EGFP strain, in which Hsp90 is tagged with the enhanced green fluorescent protein (EGFP) (7), was used for determination of the phosphorylation and acetylation status of Hsp90 by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) as previously described (22). Briefly, the strain was grown in liquid glucose minimal medium (GMM) (23) for 24 h at 37°C. For determination of acetylation sites, the lysine deacetylase inhibitor trichostatin A (TSA) was added to the medium at a concentration of 2.5 μg/ml. Protein extraction and quantification were performed as previously described (22). Hsp90 was purified by GFP-trap affinity purification (Chromotek). After proteolytic digestion, peptides were subjected to titanium dioxide (TiO2) phosphopeptide enrichment and chromatographic separation on a Waters NanoAquity ultraperformance liquid chromatograph (UPLC). MS/MS spectra were acquired for the three most abundant precursor ions using a linear trap quadrupole (LTQ)-Orbitrap XL mass spectrometer. Raw data files were processed in Mascot distiller (Matrix Science) and submitted to Mascot database searches (Matrix Science) against a Swiss-Prot fungal taxonomy. The probability of correct phosphorylation site localization was assessed by the Ascore algorithm (24).
Generation of mutant strains and culture conditions.
Strains generated in this study are described in Fig. 1C. Phosphorylated residues of Hsp90 at S49, S288, and T681 were replaced by alanine residues to prevent phosphorylation in the S49A and S288A-T681A strains. The lysine sites K27 and K271 were mutated to alanines individually in the K27A and K271A strains and concomitantly in the K27A-K271A strain. Because alanine is supposed to mimic acetylation status (16, 21, 25), the K27 residue was also mutated to arginine (mimicking deacetylation) in the K27R strain and deleted in the K27Δ strain.
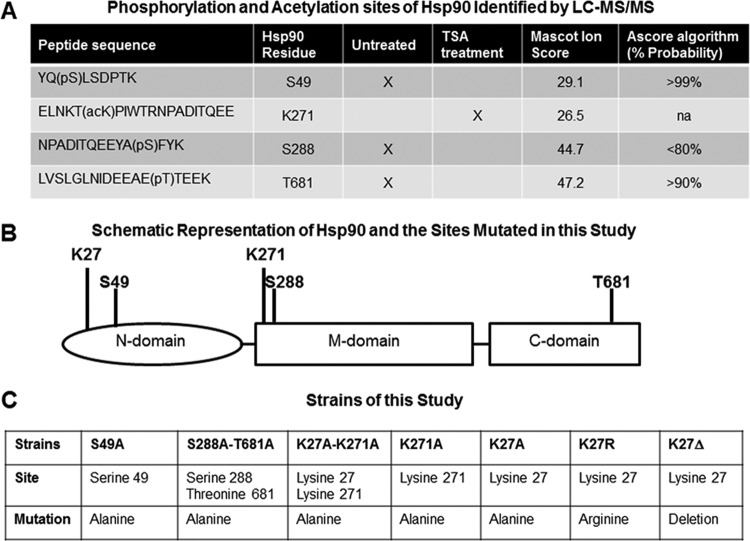
FIG 1.
Mutations of phosphorylation and acetylation sites of Hsp90. (A) Phosphorylation and acetylation sites of Hsp90 identified by TiO2 enrichment and LC-MS/MS analysis. Phosphorylated residues were identified at S49, S288, and T681 in the untreated Hsp90-EGFP strain (standard growth conditions in liquid GMM). Acetylation of K271 was detected after exposure to the lysine deacetylase inhibitor trichostatin A (TSA). Sequences of peptides with phosphorylated serine (pS) and threonine (pT) residues or acetylated lysine (acK) residues are shown in the first column. A Mascot identity score of >41 indicates identity or extensive homology (P < 0.05). The Ascore algorithm indicates the probability of localization of the phosphorylation sites and is not applicable (na) for the acetylation sites. (B) Schematic representation of the A. fumigatus Hsp90 protein with 706 amino acids consisting of the N-terminal, middle (M), and C-terminal domains. Positions of the K27, S49, K271, S288, and T681 amino acid residues that were mutated in this study are shown. (C) Strains generated in this study. Phosphorylation sites of Hsp90 (S49, S288, and T681) identified by LC-MS/MS were mutated to alanines. The K271 site, which was found to be acetylated after treatment with deacetylase inhibitor, was mutated to alanine. The K27 site, which was previously identified as acetylated in yeasts, was further investigated by mutation to either alanine or arginine or deletion.
Point mutations were induced by fusion PCR with complementary primers containing the base pair substitution as previously described (26). Primers used in this study are listed in Table S1 in the supplemental material. Plasmids pUCGH (27) and pBluescript II SK(−) containing the hygromycin resistance cassette (12) were used for cloning (see Fig. S1 in the supplemental material). Transformations were performed in the A. fumigatus akuBKU80 strain as previously described (28). Homologous recombination and integration of the mutations were verified by sequencing.
In vitro growth was assessed for each mutant strain and compared to that of the akuBKU80 (control) strain on GMM agar plates after a 5-day incubation at 37°C under standard and various stress conditions. Conidia were quantified on day 5 for strains exhibiting an apparent conidiation defect.
Western analysis.
Expression of the Hsp90 protein was verified for some of the mutant strains exhibiting a Hsp90 functional defect and compared to that of the akuBKU80 strain. Protein extraction was performed as previously described after 24 h of growth in liquid GMM at 37°C (29). Samples were normalized to a total protein of 50 μg that was separated through a 12% SDS-polyacrylamide gel using a Miniprotean electrophoresis cell (Bio-Rad Laboratories, Inc.). Proteins were electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Inc.) and hybridized with C. albicans anti-Hsp90 antibodies (a gift from Leah Cowen, University of Toronto) (30). Detection was performed by a SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
Murine inhalational model of invasive aspergillosis.
Virulence of the K27A-K271A strain was assessed in our murine inhalational model of invasive aspergillosis (31). Forty male mice (CD1; Charles River Laboratories, Raleigh, NC) were immunosuppressed with cyclophosphamide (150 mg/kg of body weight intraperitoneally on days −2 and +3) and triamcinolone acetonide (40 mg/kg subcutaneously on days −1 and +6). Mice were exposed to an aerosolized conidial suspension of either the K27A-K271A strain or the akuBKU80 strain as previously described (31). Survival was plotted on a Kaplan-Meier curve using a log rank test for pairwise comparison. Lung sections were obtained for one mouse of each group euthanized on day 7 after infection and examined by staining with Gomori methenamine silver and hematoxylin-eosin. Animal experiments were conducted in accordance with the Animal Care and Use Program of the Duke University Medical Center.
Antifungal susceptibility testing.
The antifungal activity of the lysine deacetylase inhibitor trichostatin A (TSA) was tested against the A. fumigatus AF293 (wild-type) strain, four multiazole-resistant clinical isolates harboring various mutations of the cyp51a gene (strains F16134, F14946, F16216, and F12776; gifts from David Denning) (3), and the pan-echinocandin-resistant laboratory strain EMFR-S678P (a gift from David Perlin) (32). Antifungal susceptibility testing was performed in liquid RPMI 1640 medium according to the Clinical and Laboratory Standards Institute (CLSI) procedure (33). The minimal effective concentration (MEC) and the MIC50 and MIC90 were assessed after 24 h. Checkerboard dilutions were used for drug combination testing, and interactions were described according to the fractional inhibitory concentration index (FICI) as previously described (8). The antifungal activity was also assessed after 5 days of growth at 37°C on GMM agar plates.
RESULTS
A. fumigatus Hsp90 exhibited three phosphorylation sites that were not essential for its function.
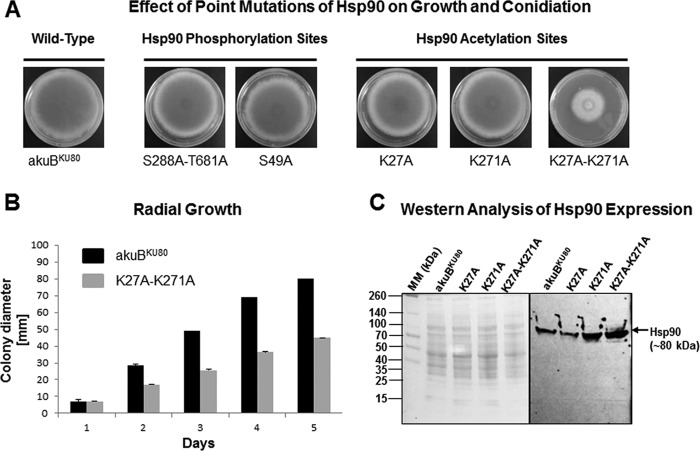
Our LC-MS/MS analysis identified three phosphorylated sites in A. fumigatus Hsp90: S49, S288, and T681 (Fig. 1A and B). While phosphorylation of S49 was previously reported in human Hsp90 (corresponding human site S63) (13), the S288 and T681 residues have never been described as phosphorylated. Thus, we first generated a strain with mutations of both S288 and T681 to alanine (S288A-T681A). This strain did not exhibit any growth defect (Fig. 2A), and its susceptibility to antifungal drugs was similar to that of the wild type (data not shown). We then mutated the S49 residue (S49A) and also did not find any phenotypic alteration (Fig. 2A). We concluded that these phosphorylation sites do not have an essential role for Hsp90 function in A. fumigatus.
FIG 2.
Phenotypic analyses of mutated Hsp90 strains. (A) Mutations of the phosphorylation sites S49, S288, and T681 to alanines (S49A and S288A-T681A strains) did not result in any growth defect compared to growth of the control akuBKU80 strain. While individual mutation of the lysine sites K27 and K271 to alanines (K27A and K271A strains) did not result in any phenotypic alteration under standard growth conditions, the double mutation (K27A-K271A strain) induced a substantial growth and conidiation defect. Pictures were taken after 5 days of growth on GMM agar at 37°C. (B) Radial growth of the K27A-K271A strain was assessed by daily measurement of the colony diameter compared to that of the akuBKU80 strain over 5 days (GMM agar at 37°C). A significant growth defect was apparent from day 2. On day 5, radial growth of the K27A-K271A strain was decreased by 44% (P < 0.0001). (C) Western analysis using anti-Hsp90 antibody showed that expression of the Hsp90 protein was stable in the different lysine mutant strains, K27A, K271A and K27A-K271A, compared to the akuBKU80 strain, as demonstrated by the detection of a band of approximately 80 kDa (corresponding to Hsp90) in all of them (right panel). Fifty micrograms of total protein from each strain was used, as shown by the similar patterns of Ponceau S protein staining of the PVDF membrane (left panel). MM, molecular mass.
The K27 and K271 residues are important for Hsp90 function in A. fumigatus.
Consistent with previous studies in yeasts (21), no acetylated residues of Hsp90 were identified by LC-MS/MS analysis under standard growth conditions, except for the N-terminal serine residue (S2), which represents a constitutively acetylated site in eukaryotic proteins that has a role in degradation signaling (34). However, in the presence of the lysine deacetylase inhibitor trichostatin A (TSA), acetylation was detected at K271 (Fig. 1A). Acetylation of this site has been previously described in human Hsp90 following TSA treatment (corresponding to human site K294) (16). Because the K27 residue, located in a highly conserved region, was found to be acetylated after genetic knockdown of lysine deacetylases in S. cerevisiae (21), we also considered this site for our mutational analysis. Three strains were created in which the K27 and K271 residues of Hsp90 were mutated to alanines individually (K27A and K271A) and in combination (K27A-K271A). Mutation of lysine to alanine is expected to mimic acetylation (16, 21, 25). Because acetylation was not detected on any internal residue of Hsp90 under standard growth conditions, we hypothesized that mutations mimicking acetylation might destabilize Hsp90 function. While the K27A and K271A strains were able to grow normally, the double mutant strain (K27A-K271A) exhibited a significant growth defect, with a 44% reduction of radial growth compared to the wild type (P < 0.0001) (Fig. 2A and B). Sporulation was also reduced, as shown by the white colony appearance (Fig. 2A), which was confirmed by the conidial count (0.09 ± 0.02 million conidia/mm2 versus 1.32 ± 0.22 million conidia/mm2 in the wild type; P < 0.001). These phenotypic characteristics were similar to those obtained after genetic repression of Hsp90 (7, 12). Western analysis confirmed that the mutated Hsp90 protein was stably expressed in this strain despite these important functional alterations (Fig. 2C).
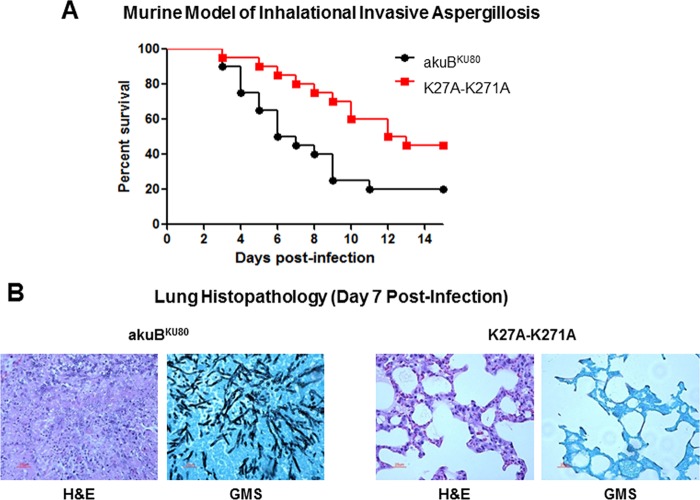
The impact of the K27A-K271A double mutation on A. fumigatus virulence was assessed in a murine inhalational model of invasive aspergillosis. At day 14 postinfection, 45% of the mice infected with the K27A-K271A strain were alive, compared to 20% of those infected with the akuBKU80 strain (P = 0.02) (Fig. 3A). The attenuated virulence of the K27A-K271A strain was also demonstrated by decreased inflammation and hyphal invasion on lung histopathologic sections (Fig. 3B).
FIG 3.
Impact of the mutations of the K27 and K271 residues of Hsp90 on A. fumigatus virulence. (A) Virulence of the K27A-K271A strain was assessed in a murine inhalational model of invasive aspergillosis. At day 14 postinfection, survival was significantly higher among mice infected with the K27A-K271A strain than among those infected with the wild-type akuBKU80 strain (45% versus 20%, respectively; P = 0.02). (B) Histopathological analysis of the lungs at day 7 postinfection shows little inflammatory reaction (hematoxylin-eosin staining [H&E]) and minimal hyphal invasion (Gomori methenamine silver staining [GMS]) in the K27A-K271A strain whereas there was extensive inflammation and hyphal proliferation observed in the akuBKU80 strain.
The K27 residue of Hsp90 is required for both caspofungin and voriconazole resistance.
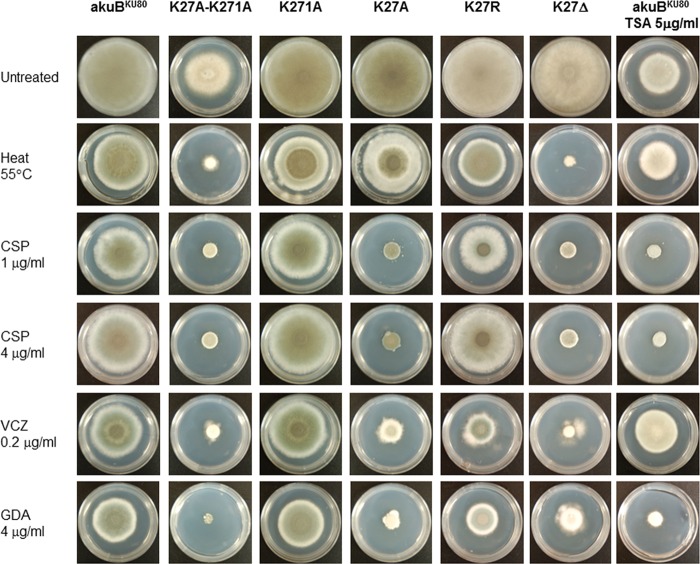
To further characterize the role of these two lysine residues in Hsp90 function, we tested the K27A, K271A, and K27A-K271A strains under various stress conditions. The impact of the double mutation on Hsp90 function was confirmed by the nearly complete growth arrest of the K27A-K271A strain in the presence of the Hsp90 inhibitor geldanamycin and under heat stress (Fig. 4). Hypersensitivity to the antifungal drugs voriconazole and caspofungin, including loss of the caspofungin paradoxical effect (i.e., increase of growth at high concentrations), were also observed (Fig. 4), as previously described following Hsp90 repression (7, 12). Use of an osmotic stabilizer in the growth medium (1.2 M sorbitol) compensated both the growth and conidiation defects (data not shown).
FIG 4.
In vitro effect of various genetic and pharmacologic interventions compromising Hsp90 acetylation/deacetylation mechanisms. Compared to the akuBKU80 strain, the K27A-K271A strain exhibited increased susceptibility to heat stress, caspofungin (CSP), including loss of the paradoxical increase of growth at high concentrations (4 μg/ml CSP), voriconazole (VCZ), and the Hsp90 inhibitor geldanamycin (GDA). Mutation of K271 alone was not sufficient to alter Hsp90 function. Mutation of K27 to alanine, mimicking acetylation (K27A), resulted in impaired Hsp90 function (as shown by hypersensitivity to geldanamycin) with a specific impact on Hsp90-mediated responses to the antifungal drugs caspofungin and voriconazole, while the response to heat stress was conserved. The K27 residue was also mutated to arginine (mimicking deacetylation) and deleted in the K27R and K27Δ strains, respectively. While the K27Δ strain showed a phenotypic pattern similar to that of the K27A strain with regard to susceptibility to caspofungin and voriconazole, the K27R mutation partially restored Hsp90 function and antifungal drug resistance. Similar to the genetic acetylation mimicking of Hsp90 K27 and K271, pharmacologic induction of Hsp90 acetylation by the lysine deacetylase inhibitor trichostatin A (TSA) induced a growth and conidiation defect, potentiated the growth-inhibitory effect of caspofungin, and abolished the paradoxical effect of this drug. However, TSA did not potentiate the antifungal activity of voriconazole and did not impact the compensatory response to heat stress. Pictures were taken after 5 days of growth on GMM agar at 37°C.
The single K271A mutation did not result in any impact on the adaptive responses to these stress conditions. However, the K27A mutation was sufficient to impact Hsp90 function, as shown by the hypersensitivity to geldanamycin, and to induce a substantial increase of sensitivity to caspofungin and voriconazole without compromising other Hsp90-mediated stress responses such as to heat stress.
To further assess the role of K27 and the possible effect of acetylation at this site, we mutated it to arginine in the K27R strain. In contrast to alanine, arginine is known to mimic a deacetylation status (16, 21, 25). Despite a slight increase of susceptibility to geldanamycin and voriconazole, the K27R strain was not hypersensitive to caspofungin and was still capable of a paradoxical response (Fig. 4). We then generated another strain in which K27 was deleted (K27Δ), and this strain displayed the same antifungal susceptibility profile as the K27A strain and could not adapt to heat stress. Because Hsp90 function in antifungal resistance was substantially altered after K27 deletion or K27A mutation (mimicking acetylation) and because this defect was partially compensated after K27R mutation (mimicking deacetylation), we conclude that this site should be deacetylated for proper Hsp90-dependent responses to both triazole and echinocandin antifungals.
The lysine deacetylase inhibitor trichostatin A is active against A. fumigatus.
To further investigate the potential of acetylation to inhibit Hsp90 function, we used trichostatin A (TSA), which was shown to induce acetylation of Hsp90 in eukaryotes (17–20). TSA and other related lysine deacetylase (KDAC) inhibitors were previously found to have minimal antifungal activity against C. albicans but to enhance the effect of azole compounds and to reduce resistance of azole-resistant strains (21, 35, 36). This effect on antifungal resistance is supposed to be mediated by Hsp90 (21).
We tested the in vitro activity of TSA against A. fumigatus and found it to be active against the wild-type AF293 with an MEC of 0.5 μg/ml (see Fig. S2 in the supplemental material). Growth inhibition levels of ≥50% (MIC50) and ≥90% (MIC90) were achieved at concentrations of 1 and 4 μg/ml, respectively (see Fig. S2). In checkerboard dilution, the combination of TSA with caspofungin was additive (FICI, 1). No positive interaction was found with voriconazole (FICI, 2; indifference). Azole-resistant strains (F16134, F14946, F16216, and F12776) were also resistant to TSA (MEC of ≥8 μg/ml), and there was no reduction of azole resistance in the presence of TSA. The echinocandin-resistant strain (EMFR-S678P) displayed a higher MEC (4 μg/ml). However, the combination of TSA with caspofungin was synergistic against this strain (FICI of <0.5 for the criterion MIC50) (see Fig. S2 in the supplemental material).
Experiments on solid medium confirmed the substantial growth defect induced by TSA (Fig. 4). A conidiation defect, similar to K27A-K271A mutation, was observed. TSA also potentiated the effect of caspofungin and abolished the paradoxical response. However, there was no positive interaction with voriconazole, whose antifungal activity was even slightly decreased in the presence of TSA. The response to heat stress was conserved. Thus, pharmacologic induction of acetylation by TSA had similar and distinct effects in comparison to acetylation-mimicking mutations of the K27 and K271 residues of Hsp90.
DISCUSSION
As a chaperone, Hsp90 interacts with a large network of client proteins, and posttranslational modifications, such as phosphorylation and acetylation, are known to play important roles in its activation and in the regulation of these interactions (13). While Hsp90 is considered a major mammalian phosphoprotein, our analysis revealed only three phosphorylated residues in A. fumigatus Hsp90. Moreover, mutating these three phosphorylated residues did not have any discernible impact on Hsp90 function. A similar mass spectrometric analysis in the yeast S. cerevisiae identified 10 phosphorylation sites in Hsp90 (15). Four serine residues were found to play some role in Hsp90 function (S379, S485, S602, and S604). Our analysis under the same nonstress conditions did not detect phosphorylation at these sites, which suggests that important differences may exist in the phosphorylation profile and mechanisms of activation of Hsp90 between yeasts and molds. However, because phosphorylation is a substoichiometric and dynamic process (i.e., a variable ratio of a given protein being phosphorylated at a given time point), it is possible that some sites may be undetected or become phosphorylated only under specific stress conditions. Thus, despite the high confidence of localization and sensitivity of such advanced phospho-enrichment MS techniques, a complete determination of the phosphorylation profile of a given protein still remains a challenge (37).
Internal acetylation of lysine residues has recently emerged as an important posttranslational modification of Hsp90 (13, 16, 21). Mutation of K270 to arginine or glutamine in S. cerevisiae Hsp90 resulted in increased susceptibility to geldanamycin, indicative of altered Hsp90 function, which was enhanced by additional K27 mutation (21). The K27 residue was found to be acetylated in a lysine deacetylase knockdown strain of S. cerevisiae, but its single mutation to either arginine or glutamine did not result in any impact on geldanamycin or azole susceptibility (21).
Here, we could demonstrate acetylation of K271 in A. fumigatus Hsp90 (corresponding to K270 in S. cerevisiae and K294 in human) after treatment with the deacetylase inhibitor TSA. Mutation mimicking acetylation of this site, which was sufficient to destabilize Hsp90 function in human and yeast (16, 21), had no major impact in A. fumigatus. While acetylation of K27 could not be induced by TSA in our study, mimicking acetylation of this residue (K27A), similar to its deletion, affected Hsp90 function, and the double K27A-K271A mutation significantly reduced A. fumigatus growth and virulence. Most importantly, the single K27A mutation had a specific impact on resistance to two major classes of antifungal drugs, triazoles and echinocandins, while the response to heat stress was conserved. This effect was attenuated by a constitutively unacetylated status (K27R). The key role of K27 in antifungal resistance is unique to A. fumigatus as mutation of K27 and/or K271 in S. cerevisiae to either acetylation- or deacetylation-mimicking residues did not have any impact on azole resistance although this effect was achieved following knockdown of certain lysine deacetylases (KDACs) (21). The acetylation status and role of K27 are unknown in humans. Whether the K27 site could be targeted for a fungal-specific inhibition of Hsp90 remains to be investigated.
Our results seem to indicate that acetylation/deacetylation of Hsp90 is a dynamic process which differentially governs the susceptibility to various stress conditions. For instance, we noted that either K27 or K271 should be unacetylated to counteract heat stress. However, for tolerance to caspofungin or voriconazole, deacetylation of K27 is required. We speculate that there may be a coordinated balance between K27 and K271: the double K27A-K271A mutation potentiated the effect of the single K27A mutation on Hsp90 function, while K271A alone had no impact. It can also be inferred that active deacetylation of K27 is required for Hsp90 activation in antifungal resistance pathways as the K27R mutation only partially improved the response to voriconazole and caspofungin stress. Furthermore, K27 deletion was not equivalent to either K27A or K27R mutations, suggesting that the dynamic process of acetylation/deacetylation is required for proper Hsp90 function. While we do not know under which circumstances acetylation of these sites may occur in vivo, this analysis shows that the critical functions of Hsp90 in virulence and antifungal resistance can be crippled by acetylation. Further investigating the Hsp90 acetylation profile under various stress conditions may provide new insights into the complexity of this dynamic process and its role in various stress responses.
Treatment with the lysine deacetylase inhibitor trichostatin A (TSA) further highlighted the potential of acetylation as a promising antifungal strategy against A. fumigatus. Histone deacetylases (HDACs), also referred to as lysine deacetylases (KDACs), were initially described as enzymes involved in controlling the acetylation status of core histones, which determines the methylation pattern of DNA and modulates gene expression (38). KDACs are divided into three groups: class 1, RPD3 type; class 2, HDA1 type; and a third group that is absent in fungi. The ability of some of them to deacetylate other proteins including Hsp90 was demonstrated previously (17, 18). Here, we showed that TSA, which is a broad-spectrum inhibitor of KDAC classes 1 and 2 and is known to induce Hsp90 acetylation in eukaryotes (16, 18), was active against A. fumigatus within an acceptable range of concentrations and potentiated the effect of caspofungin, but not voriconazole. Notably, TSA decreased resistance of an echinocandin-resistant strain. In yeasts, TSA and other KDAC inhibitors demonstrated very modest antifungal activity, despite their potentiating effect on azoles (21, 35, 36). Recent work showed that this effect on azole resistance is mediated via Hsp90 (21). Our data suggest a similar Hsp90-mediated pathway but also mechanisms of action of TSA that may be independent of Hsp90.
Analyses of the KDAC activity pattern in fungi revealed important differences among yeasts and molds (39, 40), which could explain these discrepancies. In Aspergillus nidulans, RpdA (class 1) and HdaA (class 2) were shown to be the major contributors to the total KDAC activity (40). Deletion of hdaA led to a major defect of KDAC activity and hypersensitivity to oxidative stress (39). Interestingly, deletion of rpdA (a class 1 KDAC), whose contribution to KDAC activity is less important, was lethal in A. nidulans, and its repression resulted in growth and conidiation defects that were comparable to the phenotypic consequences of Hsp90 repression in our previous works (7, 12, 41). Yeasts with deletion of the ortholog gene (rpd3) were still viable, and deletion of both rpd3 and hda1 resulted in decreased azole resistance similar to the effect of pharmacologic inhibition by TSA (21). Differences regarding susceptibility to azoles were also observed following Hsp90 repression in A. fumigatus and C. albicans (6, 7, 10, 12). Taken together, these data may explain the discrepancies in TSA activity against A. fumigatus and C. albicans and further support the existence of distinct activity patterns of KDACs in governing acetylation/deacetylation processes and antifungal resistance between yeasts and molds.
Further investigating the relationship between Hsp90 and KDACs and their pathways in fungal virulence and resistance may open perspectives for drug development of new antifungal classes targeting acetylation mechanisms. The short half-life of TSA does not make it a good candidate for in vivo use (42), but more stable and more specific KDAC inhibitors have been developed and are contemplated for application in anticancer therapy (43, 44). The antifungal properties of these compounds deserve further consideration.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Christopher Gehrke for technical assistance.
F.L. is supported by the Swiss Foundation for Grants in Biology and Medicine and the Swiss National Science Foundation (PASMP3-142746). W.J.S. is supported by NIH/NIAID (1R21AI097541-01A1).
Footnotes
Published ahead of print 6 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02286-13.
REFERENCES
- 1.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J. Infect. 65:453–464. 10.1016/j.jinf.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360. 10.1086/525258 [DOI] [PubMed] [Google Scholar]
- 3.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076. 10.3201/eid1507.090043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marr K, Schlamm H, Rottinghaus ST, Jagannatha S, Bow EJ, Wingard JR, Pappas P, Hebrecht R, Walsh TJ, Maertens J. 2012. A randomised, double-bind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis, abstr LB2812. Absourcestr. 22nd Eur. Congr. Clin. Microbiol. Infect. Dis., London, United Kingdom, 31 March to 3 April 2012 [Google Scholar]
- 5.Cowen LE. 2008. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 6:187–198. 10.1038/nrmicro1835 [DOI] [PubMed] [Google Scholar]
- 6.Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, Lindquist S. 2009. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. U. S. A. 106:2818–2823. 10.1073/pnas.0813394106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamoth F, Juvvadi PR, Fortwendel JR, Steinbach WJ. 2012. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot. Cell 11:1324–1332. 10.1128/EC.00032-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamoth F, Juvvadi PR, Gehrke C, Steinbach WJ. 2013. In vitro activity of calcineurin and heat shock protein 90 Inhibitors against Aspergillus fumigatus azole- and echinocandin-resistant strains. Antimicrob. Agents Chemother. 57:1035–1039. 10.1128/AAC.01857-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitesell L, Lindquist SL. 2005. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 5:761–772. 10.1038/nrc1716 [DOI] [PubMed] [Google Scholar]
- 10.Cowen LE, Lindquist S. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189. 10.1126/science.1118370 [DOI] [PubMed] [Google Scholar]
- 11.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 5:e1000532. 10.1371/journal.ppat.1000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamoth F, Juvvadi PR, Gehrke C, Asfaw YG, Steinbach WJ. 4 October 2013. Transcriptional activation of heat shock protein 90 (Hsp90) mediated via a proximal promoter region as trigger of caspofungin resistance in Aspergillus fumigatus. J. Infect. Dis. 10.1093/infdis/jit530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollapour M, Neckers L. 2012. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim. Biophys. Acta 1823:648–655. 10.1016/j.bbamcr.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollapour M, Tsutsumi S, Kim YS, Trepel J, Neckers L. 2011. Casein kinase 2 phosphorylation of Hsp90 threonine 22 modulates chaperone function and drug sensitivity. Oncotarget 2:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soroka J, Wandinger SK, Mausbacher N, Schreiber T, Richter K, Daub H, Buchner J. 2012. Conformational switching of the molecular chaperone Hsp90 via regulated phosphorylation. Mol. Cell 45:517–528. 10.1016/j.molcel.2011.12.031 [DOI] [PubMed] [Google Scholar]
- 16.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. 2007. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol. Cell 25:151–159. 10.1016/j.molcel.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. 2005. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 280:26729–26734. 10.1074/jbc.C500186200 [DOI] [PubMed] [Google Scholar]
- 18.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. 2005. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell 18:601–607. 10.1016/j.molcel.2005.04.021 [DOI] [PubMed] [Google Scholar]
- 19.Nimmanapalli R, Fuino L, Bali P, Gasparetto M, Glozak M, Tao J, Moscinski L, Smith C, Wu J, Jove R, Atadja P, Bhalla K. 2003. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res. 63:5126–5135 [PubMed] [Google Scholar]
- 20.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. 2002. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J. Natl. Cancer Inst. 94:504–513. 10.1093/jnci/94.7.504 [DOI] [PubMed] [Google Scholar]
- 21.Robbins N, Leach MD, Cowen LE. 2012. Lysine deacetylases Hda1 and Rpd3 regulate Hsp90 function thereby governing fungal drug resistance. Cell Rep. 2:878–888. 10.1016/j.celrep.2012.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juvvadi PR, Belina D, Soderblom EJ, Moseley MA, Steinbach WJ. 2013. Filamentous fungal-specific septin AspE is phosphorylated in vivo and interacts with actin, tubulin and other septins in the human pathogen Aspergillus fumigatus. Biochem. Biophys. Res. Commun. 431:547–553. 10.1016/j.bbrc.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu K, Keller NP. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. 2006. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24:1285–1292. 10.1038/nbt1240 [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Tsay YG, Tan BC, Lo WY, Lee SC. 2003. Identification and characterization of a novel p300-mediated p53 acetylation site, lysine 305. J. Biol. Chem. 278:25568–25576. 10.1074/jbc.M212574200 [DOI] [PubMed] [Google Scholar]
- 26.Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1:3111–3120. 10.1038/nprot.2006.405 [DOI] [PubMed] [Google Scholar]
- 27.Langfelder K, Philippe B, Jahn B, Latge JP, Brakhage AA. 2001. Differential expression of the Aspergillus fumigatus pksP gene detected in vitro and in vivo with green fluorescent protein. Infect. Immun. 69:6411–6418. 10.1128/IAI.69.10.6411-6418.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinchai N, Perfect BZ, Juvvadi PR, Fortwendel JR, Cramer RA, Jr, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. 2009. Aspergillus fumigatus calcipressin CbpA is involved in hyphal growth and calcium homeostasis. Eukaryot. Cell 8:511–519. 10.1128/EC.00336-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juvvadi PR, Fortwendel JR, Rogg LE, Burns KA, Randell SH, Steinbach WJ. 2011. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 82:1235–1259. 10.1111/j.1365-2958.2011.07886.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burt ET, Daly R, Hoganson D, Tsirulnikov Y, Essmann M, Larsen B. 2003. Isolation and partial characterization of Hsp90 from Candida albicans. Ann. Clin. Lab. Sci. 33:86–93 [PubMed] [Google Scholar]
- 31.Steinbach WJ, Benjamin DK, Jr, Trasi SA, Miller JL, Schell WA, Zaas AK, Foster WM, Perfect JR. 2004. Value of an inhalational model of invasive aspergillosis. Med. Mycol. 42:417–425. 10.1080/13693780410001712034 [DOI] [PubMed] [Google Scholar]
- 32.Rocha EM, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:4174–4176. 10.1128/AAC.00917-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed. CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 34.Hwang CS, Shemorry A, Varshavsky A. 2010. N-terminal acetylation of cellular proteins creates specific degradation signals. Science 327:973–977. 10.1126/science.1183147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaller MA, Messer SA, Georgopapadakou N, Martell LA, Besterman JM, Diekema DJ. 2009. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J. Clin. Microbiol. 47:3797–3804. 10.1128/JCM.00618-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith WL, Edlind TD. 2002. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46:3532–3539. 10.1128/AAC.46.11.3532-3539.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyrich B, Sickmann A, Zahedi RP. 2011. Catch me if you can: mass spectrometry-based phosphoproteomics and quantification strategies. Proteomics 11:554–570. 10.1002/pmic.201000489 [DOI] [PubMed] [Google Scholar]
- 38.Cervoni N, Szyf M. 2001. Demethylase activity is directed by histone acetylation. J. Biol. Chem. 276:40778–40787. 10.1074/jbc.M103921200 [DOI] [PubMed] [Google Scholar]
- 39.Tribus M, Galehr J, Trojer P, Brosch G, Loidl P, Marx F, Haas H, Graessle S. 2005. HdaA, a major class 2 histone deacetylase of Aspergillus nidulans, affects growth under conditions of oxidative stress. Eukaryot. Cell 4:1736–1745. 10.1128/EC.4.10.1736-1745.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trojer P, Brandtner EM, Brosch G, Loidl P, Galehr J, Linzmaier R, Haas H, Mair K, Tribus M, Graessle S. 2003. Histone deacetylases in fungi: novel members, new facts. Nucleic Acids Res. 31:3971–3981. 10.1093/nar/gkg473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tribus M, Bauer I, Galehr J, Rieser G, Trojer P, Brosch G, Loidl P, Haas H, Graessle S. 2010. A novel motif in fungal class 1 histone deacetylases is essential for growth and development of Aspergillus. Mol. Biol. Cell 21:345–353. 10.1091/mbc.E09-08-0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanderson L, Taylor GW, Aboagye EO, Alao JP, Latigo JR, Coombes RC, Vigushin DM. 2004. Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice. Drug Metab. Dispos. 32:1132–1138. 10.1124/dmd.104.000638 [DOI] [PubMed] [Google Scholar]
- 43.Elaut G, Laus G, Alexandre E, Richert L, Bachellier P, Tourwe D, Rogiers V, Vanhaecke T. 2007. A metabolic screening study of trichostatin A (TSA) and TSA-like histone deacetylase inhibitors in rat and human primary hepatocyte cultures. J. Pharmacol. Exp. Ther. 321:400–408. 10.1124/jpet.106.116202 [DOI] [PubMed] [Google Scholar]
- 44.Kaliszczak M, Trousil S, Aberg O, Perumal M, Nguyen QD, Aboagye EO. 2013. A novel small molecule hydroxamate preferentially inhibits HDAC6 activity and tumour growth. Br. J. Cancer 108:342–350. 10.1038/bjc.2012.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.