Abstract
NAI-603 is a ramoplanin derivative designed to overcome the tolerability issues of the parent drug as a systemic agent. NAI-603 is highly active against aerobic and anaerobic Gram-positive bacteria, with MICs ranging from 0.008 to 8 μg/ml. MICs were not significantly affected by pH (range, 6 to 8), by inoculum up to 108 CFU/ml, or by addition of 50% human serum. Against staphylococci and enterococci, NAI-603 was rapidly bactericidal, with minimum bactericidal concentration (MBC)/MIC ratios never exceeding 4. The frequency of spontaneous resistance was low at 2× to 4× MIC (≤1 × 10−6 to ≤1 × 10−8) and below the detection limit (about ≤1 × 10−9) at 8× MIC. Serial subcultures at 0.5× MIC yielded at most an 8-fold increase in MICs. In a systemic infection induced by methicillin-resistant Staphylococcus aureus (MRSA), the 50% effective dose (ED50) of intravenous (i.v.) NAI-603 was 0.4 mg/kg, lower than that of oral (p.o.) linezolid (1.4 mg/kg) and subcutaneous (s.c.) teicoplanin (1.4 mg/kg) or vancomycin (0.6 mg/kg). In neutropenic mice infected with vancomycin-resistant enterococci (VRE), the ED50s for NAI-603 were 1.1 to 1.6 mg/kg i.v., compared to 0.5 mg/kg i.v. of ramoplanin. The bactericidal activity was confirmed in vivo in the rat granuloma pouch model induced by MRSA, where NAI-603, at 40 mg/kg i.v., induced about a 2- to 3-log10-reduction in viable bacteria in the exudates, which persisted for more than 72 h. The pharmacokinetic (PK) profiles of NAI-603 and ramoplanin at 20 mg/kg show similar half-lives (3.27 and 3.80 h, respectively) with the maximum concentration (Cmax) markedly higher for NAI-603 (207 μg/ml versus 79 μg/ml). The favorable pharmacological profile of NAI-603, coupled with the absence of local tolerability issues, supports further investigation.
INTRODUCTION
In 2009, the WHO identified antibiotic resistance as one of the greatest threats to human health (1). Indeed, figures are frightening: in Europe about 25,000 patients die every year from infection with multidrug-resistant (MDR) bacteria, and such infections result in health care costs and lost productivity of at least €1.5 billion per year. Methicillin-resistant Staphylococcus aureus (MRSA) alone infects more than 94,000 people and kills nearly 19,000 in the United States every year, more deaths than are caused by HIV/AIDS, Parkinson's disease, emphysema, and homicide combined (1). Despite that, we are witnessing the steady rise of drug-resistant bacteria while the treatment of infection remains an area where there have been few major advances in recent years and while the number of companies in this area of research has dropped off steadily. New, safe, and effective antibiotics are therefore needed to limit the dramatic effects of increasing antibiotic resistance. Ramoplanin is a glycolipodepsipeptide antibiotic obtained from fermentation of Actinoplanes ATCC 33076 (2). The compound, discovered more than 20 years ago, is active against multidrug-resistant Gram-positive aerobic and anaerobic bacteria (3, 4). Its rapid bactericidal activity (5, 6) made ramoplanin a promising candidate for treating serious, life-threatening infections. Ramoplanin is effective in systemic use in animal models of infection although only a few studies have reported efficacy data (7, 8). Such use, however, has been hampered by ramoplanin hemolytic activity when the compound is administered intravenously (i.v.). As a consequence of this limitation, ramoplanin has been under clinical investigations for topical applications, including as an oral (per os, p.o.), nonabsorbable antibiotic for the prevention of infections by vancomycin-resistant enterococci (VRE) and for the treatment of Clostridium difficile-associated diarrhea (9, 10).
Because of ramoplanin's favorable antibacterial properties, efforts have been made to generate ramoplanin analogs by total synthesis (11–14) or by semisynthesis of the natural compound (15). Alanine scanning experiments demonstrated that hydroxyphenylglycine in position 11 and the ornithine in position 10 are essential for the antibiotic activity (11, 13), together with the presence of the fatty acid (11). After semisynthesis, removal of the dimannosyl residue and guanylation of the amino groups of the two ornithines maintained an antimicrobial activity comparable to that of ramoplanin but did not improve tolerability. On the contrary, modifications of the fatty acid chain influenced both antibiotic and hemolytic activities. In particular, the transformation of the α,β-double bond in an aldehyde and its subsequent conversion into an amine moiety allowed selective and smooth removal of the original fatty acid chain and its replacement with a set of different fatty acids. Among these compounds, the most promising molecule is one in which ramoplanin's di-unsaturated fatty acid chain is replaced by a 2-methyl-phenylacetic acid residue (15). This modification led to a significant reduction in hemolytic activity, and testing a limited number of strains suggested that this derivative retained the antibacterial spectrum and potency of the parent compound. We report here the characterization of this promising derivative, designated NAI-603, with respect to its in vitro and in vivo properties and demonstrate that the compound maintains the favorable properties of the parent compound, including a favorable pharmacokinetics (PK).
(This study was presented in part as posters at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 13 to 17 September 2003 [16, 39].)
MATERIALS AND METHODS
Bacterial strains and antimicrobials.
The strains used in this study (154 Gram-positive aerobic strains and 40 Gram-positive anaerobes, all part of the NAICONS strain collection) were laboratory strains or clinical isolates collected from different sources, with over 60% of the isolates resistant to antibacterial agents of common nosocomial use. NAI-603, which corresponds to compound 48 in the study of Ciabatti et al. (15), and ramoplanin were prepared and used as described previously (15). Teicoplanin, metronidazole, vancomycin, daptomycin, and linezolid, all from commercial sources, were dissolved at 10 mg/ml in dimethyl sulfoxide and diluted with the appropriate medium before use.
Media.
Todd Hewitt broth (THB) and agar (THA), Mueller-Hinton broth (MHB), brucella broth (BB), brain heart infusion (BHI) broth, and antibiotic medium agar number 2 (AM2) were from Difco Labs (Detroit, MI, USA). Cation-adjusted Mueller-Hinton broth (CAMHB), prepared by adding 20 mg/liter CaCl2 (50 mg/liter CaCl2 for testing daptomycin) and 10 mg/liter MgCl2 to MHB, was used for all aerobic strains, except for streptococci, which were grown in THB. Anaerobic bacteria were grown in BB supplemented with hemin and vitamin K1 (5 and 1 μg/ml, respectively). Oxyrase (Oxyrase, Inc., Mansfield, OH, USA) was added to BB (1:25, vol/vol) only for cultures of Clostridium spp.
Antibacterial activities.
MICs were determined by the broth microdilution method according to CLSI guidelines (17, 18) using inocula of 1 × 105 to 5 × 105 CFU/ml and 24-h incubations at 35°C. Minimal bactericidal concentrations (MBCs) were determined according to CLSI guidelines (19). After a 24-h incubation, cultures containing concentrations higher than the MIC were serially diluted, and duplicate 0.01-ml aliquots were spread on THA plates. Colonies were counted after 48 h at 35°C. The MBC was defined as the concentration that killed ≥99.9% of the initial inoculum.
For determining the pH effect on MIC, CAMHB was adjusted to pH 6, 7, or 8 with appropriate volumes of 0.1 N HCl or 0.5 N NaOH. Inoculum effects were determined in the range of 103 to 108 CFU/ml. The effect of proteins on MICs and MBCs was investigated according to CLSI recommendations (19), starting from an exponentially growing culture prepared as follows. Several colonies from an overnight THA plate were picked and inoculated into MHB supplemented with 50% human serum (Sigma Chemical Co., St. Louis, MO, USA). Cultures were incubated under shaking conditions at 35°C and subsequently diluted to 5 × 105 CFU/ml. MICs and MBCs were determined as previously described.
Resistance selection.
The following strains were used: methicillin-susceptible Staphylococcus aureus (MSSA) 819, methicillin-resistant S. aureus (MRSA) 1400, glycopeptide-intermediate S. aureus (GISA) 3797, and vancomycin-resistant Enterococcus faecalis (VanA) A8. The occurrence of spontaneous resistant mutants was evaluated by plating 25 μl of serial dilutions of late-exponential-phase CAMHB cultures on Mueller-Hinton agar (MHA) plates containing 2×, 4×, or 8× MIC of NAI-603 or ramoplanin. The number of colonies was determined after 2 to 5 days of incubation at 35°C. Randomly selected colonies from each plate were tested to confirm resistance and to determine the frequency of resistant colonies. Experiments were made in duplicate.
The serial passage technique was employed using the same strains. Briefly, 100-μl cultures containing about 1 × 106 CFU/ml and different concentrations of either ramoplanin or NAI-603 were incubated for 24 h at 35°C. For each compound, the culture grown at 0.5× MIC was diluted to about 1 × 107 CFU/ml and supplemented with an equal volume of fresh medium containing serial dilutions of compound for the following passage. The procedure was repeated for up to 22 passages. Samples of each passage were added with 20% glycerol and frozen at −20°C. To control the variability of the MIC values at different days, MIC tests were performed on samples of first and last passage, frozen and thawed, on three different days.
Acute lethal infection model in mice.
Female ICR mice (Harlan Italy), 23 to 25 g, were used. Cultures from frozen vials were incubated for 24 h at 35°C and then appropriately diluted with 5% bacteriological mucin (Difco Labs, Detroit, MI, USA) in BHI broth. Immunocompetent mice were infected intraperitoneally (i.p.) with about 106 CFU of MRSA 3817, while neutropenic mice were infected i.p. with about 106 CFU of E. faecalis VanA A533 or Enterococcus faecium VanA 569, in a final volume of 0.5 ml. In all experiments, these inocula resulted in 100% mortality in untreated controls within 48 to 72 h from infection. Neutropenia was induced as described previously (20), with minor modifications. Cyclophosphamide (Endoxan-Asta; Asta Medica) was administered i.p. on days −4 (200 mg/kg of body weight) and −1 (100 mg/kg) before infection. This schedule was found to be optimal for establishing the desired transient neutropenia (<100 polymorphonuclear leukocytes per mm3 of blood) for at least 4 days after the last dose of cyclophosphamide.
Eight mice per group were treated at each dose level with 0.25 ml of a single-dose regimen of test compound. Treatments started within 10 to 15 min after infection and were intravenous (i.v.) for NAI-603 and ramoplanin, subcutaneous (s.c.) for NAI-603, daptomycin, teicoplanin, and vancomycin, and oral (p.o.) for linezolid. Doses of comparator treatments were selected to mimic therapeutic exposures obtained in patients and were also based on dose regimens used in other animal models of infection (21, 22). Linezolid was dosed orally because of its complete bioavailability following this route of administration (23). Mortality was recorded daily. Mice surviving after 7 days of treatment were euthanized. The 50% effective doses (ED50s) and 95% confidence limits were calculated by the Spearman-Kärber method from the animals surviving to 7 days at each dose. This method was also used to calculate the 50% lethal dose (LD50) of each infecting organism (24).
Rat granuloma pouch model.
Sprague-Dawley rats (Harlan Italy, S. Pietro al Natisone, Italy) of both sexes, weighing 150 to 200 g, were used in the granuloma pouch model as described by Dalhoff (25). Briefly, pouches were produced in five to six rats per group by injecting 30 ml of sterile filtered air followed by injection of 0.5 ml of 0.5% croton oil in sesame seed oil deep into the loose connective dorsal tissue. Two days later, approximately 10 ml of air was withdrawn, and after 2 to 3 more days, 2 ml of 1% peptone and 0.35% agar in saline solution were injected in the pouch. On day 10, rats were infected with 106 CFU of MRSA 1400 suspended in 1.5 ml of THB containing 2.5% gastric mucin and 0.35% agar. Single intravenous (i.v.) treatments with 20 to 40 mg/kg of NAI-603 or intramuscular (i.m.) treatment with 100 mg/kg of vancomycin started 3 h after infection. The selected dose of vancomycin has been shown to achieve plasma peak and trough vancomycin concentrations in rats comparable to those achieved in humans following i.v. administration of 1 g (26). Samples (0.2 ml) of infected exudates were withdrawn before and 24, 48, 72, and 96 h after treatment and plated on THA by inclusion in soft agar to determine bacterial titers, which were expressed as the average log10 CFU/ml of exudates from five or six rats per treatment group. The detection limit of the method was 40 CFU/ml. Infected exudates were collected from pouches using heparinized syringes, followed by centrifugation at 5,000 × g for 5 min. The concentration of NAI-603 in the resulting supernatants was determined by agar diffusion on AM2 using Bacillus subtilis ATCC 6633 as the test organism. The limit of quantification was 0.2 μg/ml, and the calibration curve was in the range of 0.2 to 3.2 μg/ml. When needed, samples were properly diluted to fit within the range of the calibration curve.
Pharmacokinetics.
Sprague-Dawley rats (Harlan Italy, S. Pietro al Natisone, Italy) of both sexes, weighing about 300 g, were used. NAI-603 at 5 and 20 mg/kg i.v. and 20 mg/kg s.c. and ramoplanin at 20 mg/kg i.v., both dissolved in saline, were administered to six rats/dose. Blood samples (0.2 to 0.4 ml) were collected, under light halothane anesthesia, from the retro-orbital sinus into heparinized tubes. Blood samples were centrifuged at 2,000 × g for 10 min at room temperature, and the resulting plasma samples were stored at −20°C until analysis. Compound concentrations were determined by agar diffusion as described above. Pharmacokinetic parameters were determined by noncompartmental analysis (WinNonlin, version 3.1pro; Pharsight).
All in vivo procedures were approved by the Animal Care and Use Committee at Vicuron Pharmaceuticals.
RESULTS
MIC and MBC determinations.
In previous work, NAI-603 was found to be active against a few strains of S. aureus, Streptococcus pyogenes, and Enterococcus spp., with MICs generally comparable to those of ramoplanin (15). These results were expanded and confirmed in the present work, as shown in Table 1. Overall, the NAI-603 MICs are comparable to or lower than those of reference compounds against all of the 102 tested aerobic strains. All Staphylococcus spp. were susceptible to NAI-603, irrespective of resistance to methicillin, with MICs ranging between 0.06 and 1 μg/ml. Streptococcus pyogenes and Streptococcus pneumoniae, including strains with intermediate resistance to penicillin, were highly susceptible to NAI-603 and ramoplanin, with both compounds showing MIC values significantly lower than those of the reference compounds. Against the tested enterococci, the MIC range of NAI-603 was 0.06 to 2 μg/ml, irrespective of the species and of the vancomycin resistance phenotype, and was comparable to previously reported ramoplanin activity (27).
TABLE 1.
MICs of NAI-603 and other antimicrobial agents against aerobic bacteria
| Microorganism | Resistance phenotypea | No. of isolates | MIC range (μg/ml) |
|||
|---|---|---|---|---|---|---|
| NAI-603 | Ramoplanin | Vancomycin | Teicoplanin | |||
| Staphylococcus aureus | MRSA | 17 | 0.06–0.25 | 0.03–0.25 | 1–4 | 1–>16 |
| GISA | 5 | 0.25–1 | 0.06–2 | 1–8 | 2–8 | |
| MSSA | 10 | 0.12–1 | 0.03–0.25 | 0.12–1 | 0.12–2 | |
| Staphylococcus epidermidis | MRSE | 10 | 0.06–1 | 0.015–0.25 | 1–2 | 0.25–16 |
| Streptococcus pneumoniae | PS | 17 | 0.004–0.06 | ≤0.0006–0.06 | 0.25–1 | ≤0.008–0.03 |
| PI | 7 | 0.008–0.03 | 0.002–0.008 | ≤0.06–0.50 | 0.015–0.03 | |
| Streptococcus pyogenes wild type | 1 | ≤0.03 | ≤0.03 | 0.5 | 0.12 | |
| Enterococcus faecium | VanA | 10 | 1–1 | 0.03–0.12 | >128 | >128 |
| VanB | 10 | 0.5–2 | 0.015–0.25 | 32–>128 | 0.25–4 | |
| Enterococcus faecalis | VanA | 14 | 0.25–1 | 0.03–0.12 | 64–>128 | 8–>128 |
| VanB | 10 | 0.5–2 | ≤0.008–0.5 | 8–>128 | 0.12–32 | |
| VanS | 10 | 0.12–4 | ≤0.008–0.5 | 0.5–4 | ≤0.06–0.5 | |
| Enterococcus gallinarum | VanC | 10 | 0.06–1 | ≤0.008–0.125 | 4–64 | 0.25–1 |
| Enterococcus durans | VanA | 5 | 0.12–0.5 | 0.015–0.03 | >128 | >128 |
| VanS | 9 | 0.06–8 | ≤0.008–1 | 0.5–2 | 0.25–1 | |
| Enterococcus hirae wild type | 10 | 0.06–8 | ≤0.008–1 | 0.25–8 | ≤0.06–1 | |
PS, penicillin susceptible; PI, penicillin intermediate; MRSE, methicillin-resistant Staphylococcus epidermidis.
Anaerobic Gram-positive bacteria (Propionibacterium acnes, Clostridium difficile, and other clostridia) were also highly susceptible to NAI-603, which displayed against all tested organisms MIC values equal to or lower than those of reference compounds vancomycin, metronidazole, and clindamycin and comparable to those of ramoplanin (Table 2). This result indicates that, also for anaerobic strains, replacement of the ramoplanin acyl chain with 2-methyl-phenylacetic acid does not adversely impact the MICs.
TABLE 2.
MICs of NAI-603 and other antimicrobial agents against anaerobic bacteria
| Microorganism | No. of isolates | MIC range (μg/ml) |
||||
|---|---|---|---|---|---|---|
| NAI-603 | Ramoplanin | Vancomycin | Metronidazole | Clindamycin | ||
| Clostridium spp. | ||||||
| C. perfringens | 3 | 0.015–0.12 | ≤0.008–0.03 | 0.5–1 | 0.5–8 | ≤0.06–0.5 |
| C. beijerinckii | 5 | ≤0.008–0.12 | ≤0.008–0.12 | 1–4 | 0.25–1 | 2–8 |
| C. difficile | 10 | 0.06–0.25 | 0.06–0.25 | 0.5–2 | 0.12–1 | 4–>64 |
| C. ramosum | 4 | 0.03–0.25 | ≤0.008–0.015 | 4–8 | 0.5–2 | 8 |
| C. septicum | 2 | 0.015 | ≤0.008 | 1 | 1 | 1 |
| C. butyricum | 2 | 0.015 | <0.008 | 1 | 0.5–2 | 0.5–>64 |
| C. clostridioforme | 1 | 2 | 1 | 0.5 | 0.12 | 2 |
| Peptostreptococcus prevotii | 1 | ≤0.008 | ≤0.008 | 1 | 0.12 | 0.12 |
| Propionibacterium acnes | 10 | 0.06–0.25 | ≤0.008–0.03 | 0.06–0.5 | 128–>128 | ≤0.06–>16 |
An important feature of ramoplanin is its high bactericidal activity (5, 6). As shown in Table 3, NAI-603 retained this property against different strains of S. aureus and Enterococcus spp., with MBC/MIC ratios never exceeding 4, similarly to ramoplanin. The presence of 50% human serum increased NAI-603 MICs by 2- to 4-fold, while the MBC/MIC ratio was not affected. In comparison, ramoplanin's MICs and MBC/MIC ratios were more influenced by the presence of human serum (Table 3). It should be noted that our results indicate a limited effect of human serum on ramoplanin's MBC against Enterococcus spp., a result that contrasts with a previous report on the lack of bactericidal activity against this genus in the presence of 50% serum from an unspecified source (28).
TABLE 3.
In vitro activity of NAI-603 and ramoplanin in the absence or presence of 50% human serum
| Microorganism(resistance phenotype)a | Strain no. | Compound | Bactericidal activity (μg/ml) |
|||
|---|---|---|---|---|---|---|
| No HSb |
+50% HS |
|||||
| MIC | MBC | MIC | MBC | |||
| S. aureus (GISA) | 4061 | NAI-603 | 0.25 | 0.25 | 1 | 1 |
| Ramoplanin | 0.12 | 0.12 | 1 | 1 | ||
| S. aureus (GISA MRSA) | 3797 | NAI-603 | 1 | 2 | 2 | 2 |
| Ramoplanin | 1 | 1 | 4 | 4 | ||
| S. aureus (MSSA) | 3906 | NAI-603 | 0.06 | 0.13 | 0.06 | 0.5 |
| Ramoplanin | 0.03 | 0.03 | 0.06 | 1 | ||
| 819 | NAI-603 | 0.12 | 0.12 | 0.5 | 0.5 | |
| Ramoplanin | 0.12 | 0.12 | 0.5 | 1 | ||
| S. aureus (MRSA) | 1400 | NAI-603 | 0.25 | 0.25 | 1 | 1 |
| Ramoplanin | 0.12 | 0.12 | 1 | 1 | ||
| S. haemolyticus (MRSA) | 3902 | NAI-603 | 0.12 | 0.5 | 0.03 | 0.25 |
| Ramoplanin | 0.03 | 0.03 | 0.03 | 0.03 | ||
| E. faecium (VanA) | B518 | NAI-603 | 0.5 | 1 | 1 | 4 |
| Ramoplanin | 0.03 | 0.03 | 0.5 | 2 | ||
| F524 | NAI-603 | 0.25 | 0.25 | 1 | 1 | |
| Ramoplanin | 0.03 | 0.03 | 0.5 | 0.5 | ||
| D561 | NAI-603 | 0.5 | 0.5 | 1 | 1 | |
| Ramoplanin | 0.03 | 0.06 | 4 | 4 | ||
| 560 | NAI-603 | 0.5 | 0.5 | 1 | 1 | |
| Ramoplanin | 0.03 | 0.06 | 1 | 1 | ||
| E. faecalis (VanA) | 569 | NAI-603 | 0.5 | 0.5 | 2 | 2 |
| Ramoplanin | 0.03 | 0.06 | 4 | 4 | ||
| J1 | NAI-603 | 0.25 | 1 | 0.5 | 0.5 | |
| Ramoplanin | 0.03 | 0.06 | 0.5 | 0.5 | ||
| A8 | NAI-603 | 0.25 | 0.5 | 0.5 | 4 | |
| Ramoplanin | 0.03 | 0.06 | 0.5 | 0.5 | ||
S. haemolyticus, Staphylococcus haemolyticus.
HS, human serum.
The MICs of NAI-603 against Staphylococcus or Enterococcus spp. were only marginally affected by pH in the range of 6 to 8 by or inoculum size between 103 to 108 CFU/ml, a behavior similar to that observed with ramoplanin (Table 4).
TABLE 4.
Effect of pH and inoculum on the MICs of NAI-603 and ramoplanin against Staphylococcus spp. and Enterococcus spp.
| Microorganism (resistance phenotype) | Strain no. | Compound | MIC (μg/ml) under the indicated conditionb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH |
Starting inoculum (CFU/ml) |
||||||||||
| 6 | 7 | 8 | 103 | 104 | 105 | 106 | 107 | 108 | |||
| S. aureus (GISA) | 4061 | NAI-603 | 0.25 | 0.25 | 0.5 | 0.12 | 0.25 | 0.25 | 0.25 | 0.5 | 1 |
| Ramoplanin | 0.12 | 0.03 | 0.12 | ≤0.01 | ≤0.01 | 0.03 | 0.03 | 0.03 | 0.06 | ||
| S. aureus (GISA MRSA) | 3797 | NAI-603 | 1 | 2 | 2 | NT | NT | NT | NT | NT | NT |
| Ramoplanin | 2 | 2 | 2 | NT | NT | NT | NT | NT | NT | ||
| S. aureus (GISA MRSA) | 3798 | NAI-603 | 2 | 2 | 2 | 0.5 | 0.5 | 1 | 1 | 2 | 2 |
| Ramoplanin | 1 | 0.25 | 0.25 | 0.12 | 0.12 | 0.12 | 0.25 | 0.25 | 0.5 | ||
| S. aureus (MSSA) | 4064 | NAI-603 | 0.5 | 1 | 2 | 0.5 | 0.5 | 0.5 | 1 | 1 | 4 |
| Ramoplanin | 0.5 | 0.25 | 0.5 | 0.06 | 0.06 | 0.06 | 0.12 | 0.25 | 2 | ||
| S. aureus (MRSA) | 1400 | NAI-603 | 0.06 | 0.12 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 | 1 |
| Ramoplanin | 0.008 | 0.03 | 0.03 | 0.015 | 0.03 | 0.03 | 0.06 | 0.06 | 0.12 | ||
| E. faecium (VanA) | B518 | NAI-603 | NG | 1 | 2 | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 |
| Ramoplanin | NG | 0.015 | 0.015 | ≤0.01 | ≤0.01 | ≤0.01 | 0.03 | 0.03 | 0.06 | ||
| E. faecium (VanA Lnzr)a | A6349 | NAI-603 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 |
| Ramoplanin | 0.06 | 0.03 | 0.0125 | ≤0.01 | 0.03 | 0.03 | 0.03 | 0.03 | 0.06 | ||
| E. faecalis (VanA) | A6345 | NAI-603 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Ramoplanin | ≤0.03 | 0.008 | 0.008 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | 0.03 | 0.06 | ||
| E. faecalis (VanA) | A533 | NAI-603 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 |
| Ramoplanin | 0.03 | 0.015 | 0.015 | ≤0.01 | ≤0.01 | ≤0.01 | 0.03 | 0.06 | 0.06 | ||
| E. faecalis (wild type) | A6350 | NAI-603 | 0.25 | 0.25 | 0.5 | 0.12 | 0.12 | 0.12 | 0.25 | 0.5 | 1 |
| Ramoplanin | 0.008 | 0.008 | 0.008 | ≤0.01 | ≤0.01 | ≤0.01 | 0.03 | 0.12 | 0.12 | ||
Lnzr, linezolid resistant.
NT, not tested; NG, not grown.
Selection or induction of resistant strains.
Using one MSSA strain, one MRSA strain, and one E. faecalis VanA strain with different susceptibilities to NAI-603 (MIC range, 0.25 to 4 μg/ml), the efficiency of plating decreased rapidly with increasing multiples of the MIC and was below the detection limit (about ≤1 × 10−9) at 8× MIC (Table 5). When colonies grown at 2× or 4× MIC were retested for NAI-603 susceptibility, their MICs were unaltered with respect to the parent strain, suggesting a population effect at small multiples of the MIC (data not shown). These results indicate that the frequency of resistant mutants for all tested strains is below 10−9. Identical results were observed with ramoplanin, consistent with previous data (7).
TABLE 5.
Frequency of naturally resistant mutants to ramoplanin or NAI-603 determined by efficiency of plating
| Microorganism (resistance phenotype) | Strain no. | Frequency of resistant mutants with: |
|||||
|---|---|---|---|---|---|---|---|
| Ramoplanin |
NAI-603 |
||||||
| 8× MIC | 4× MIC | 2× MIC | 8× MIC | 4× MIC | 2× MIC | ||
| S. aureus (MSSA) | 819 | <4 × 10−9 | <4 × 10−9 | 3.4 × 10−8 | <2.2 × 10−9 | <2.2 × 10−8 | 2.2 × 10−7 |
| S. aureus (MRSA) | 1400 | 2.86 × 10−9 | 4.7 × 10−8 | 2.9 × 10−7 | <1 × 10−8 | 3.7 × 10−8 | 1 × 10−7 |
| E. faecalis (VanA) | A8 | <1.5 × 10−9 | 1.52 × 10−9 | 9.8 × 10−9 | <2.6 × 10−9 | <2.6 × 10−9 | <2.6 × 10−9 |
The same three strains and two additional strains (one S. aureus GISA and one E. faecium VanA), were also tested for emergence of resistance by exposure to subinhibitory concentrations of NAI-603. The results, illustrated in Fig. 1, indicate that up to 22 serial subcultures at 0.5× MIC yielded at most an 8-fold increase in the MICs against one MRSA and one VRE strain. Similar results were obtained in a parallel experiment with ramoplanin (data not shown).
FIG 1.
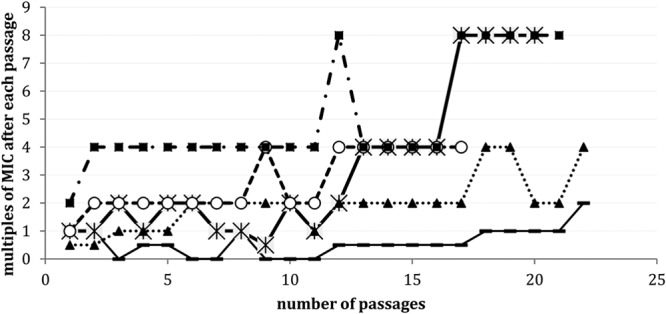
MIC changes of NAI-603 against various pathogens after serial passages at subinhibitory concentrations. —, S. aureus MSSA; *, S. aureus MRSA; ▲, S. aureus GISA; ○, Enterococcus faecium VanA; ■, E. faecalis VanA.
These results confirm that NAI-603 retains the low propensity of ramoplanin to select for resistance, a result expected from a compound that acts by binding to the peptidoglycan intermediate lipid II (2, 29).
In vivo efficacy.
The efficacy of NAI-603, ramoplanin, and comparators in acute lethal infections in mice is reported in Table 6. In immunocompetent mice infected with MRSA 3817, NAI-603 was highly effective, with ED50s of 0.4 and 0.5 mg/kg when administered i.v. and s.c., respectively. Such efficacy is comparable to that of teicoplanin and higher than that of linezolid and vancomycin. In infections induced by vancomycin-resistant E. faecalis A533 and E. faecium 569 in neutropenic mice, NAI-603 administered by i.v. route was 2 to 3 times less effective than ramoplanin, consistent with the respective in vitro activities, and both drugs were always comparable or superior to reference compounds. The ED50 values observed with NAI-603 when it was administered i.v. or s.c. suggest a good exposure of the compound after subcutaneous administration (Table 6).
TABLE 6.
Efficacy of NAI-603 and comparators in murine systemic infections
| Mouse group and organism (resistance phenotype) | Compound | MIC (μg/ml) | Route of administration | ED50 (mg/kg) | 95% Confidence limits (mg/kg) |
|---|---|---|---|---|---|
| Immunocompetent mice | |||||
| S. aureus 3817 (MRSA) | NAI-603 | 0.12 | i.v. | 0.4 | 0.4–0.5 |
| s.c. | 0.5 | 0.4–0.6 | |||
| Linezolid | 2 | p.o. | 1.4 | 1.2–1.7 | |
| Vancomycin | 0.5 | s.c. | 1.4 | 1.2–1.7 | |
| Teicoplanin | ≤0.125 | s.c. | 0.6 | 0.5–0.8 | |
| Neutropenic mice | |||||
| E. faecium 569 (VanA) | NAI-603 | 1 | i.v. | 1.6 | 1.3–1.9 |
| s.c. | 4.0 | 3.2–5.0 | |||
| Daptomycin | 4 | s.c. | 1.4 | 1.1–1.9 | |
| Ramoplanin | 0.06 | i.v. | 0.5 | 0.4–0.6 | |
| Linezolid | 2 | p.o. | 11.3 | 9.5–13.4 | |
| Vancomycin | >128 | s.c. | >90 | ||
| E. faecalis A533 (VanA) | NAI-603 | 0.5 | i.v. | 1.1 | 1.0–1.4 |
| s.c. | 1.6 | 1.3–2.0 | |||
| Daptomycin | 2 | s.c. | 1.4 | 1.2–1.7 | |
| Ramoplanin | ≤0.03 | i.v. | 0.5 | 0.4–0.5 | |
| Linezolid | 2 | p.o. | 12.7 | 10.9–14.7 | |
| Vancomycin | >128 | s.c. | >90 |
Next, we determined the in vivo bactericidal effect of single 20 or 40 mg/kg i.v. NAI-603 doses in an MRSA 1400 infection in rat granuloma pouches in comparison with single i.m. 100 mg/kg vancomycin. In this model, 40 mg/kg NAI-603 reduced the viable cell counts in pouch exudates soon after administration, and this effect lasted for 72 h (Fig. 2). Vancomycin or 20 mg/kg NAI-603 reduced bacterial counts up to 48 h although subsequent growth was observed, resulting at 96 h in a bacterial load similar to that present at time zero (Fig. 2). Importantly, the MRSA colonies (about 6 to 8 colonies/rat) isolated after 96 h from rats treated with 20 mg/kg NAI-603 had the same susceptibility to NAI-603 as the original challenge strain (data not shown), suggesting that bacterial growth at longer times occurred because of washout of the antibiotic and not because of the emergence of resistant mutants in vivo.
FIG 2.
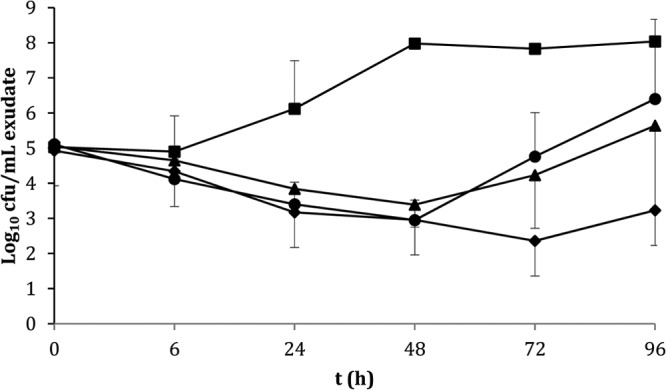
Efficacy of NAI-603 at 20 (▲) and 40 (◆) mg/kg i.v. in comparison with 100 mg/kg s.c. vancomycin (●) and untreated control (■) in the rat granuloma pouch model induced by MRSA 1400.
The mean NAI-603 concentrations observed in infected pouch exudates are shown in Fig. 3. Highest concentrations were 3.88 and 4.98 μg/ml following 20- and 40-mg/kg doses, respectively, with elimination phases in the range of 20 to 27 h. The exudate levels of NAI-603 were above the MBC for MRSA 1400 (Table 3) for up to 24 h following single 20 and 40 mg/kg i.v. doses. Thus, the in vivo bactericidal activity is consistent with the in vitro data.
FIG 3.
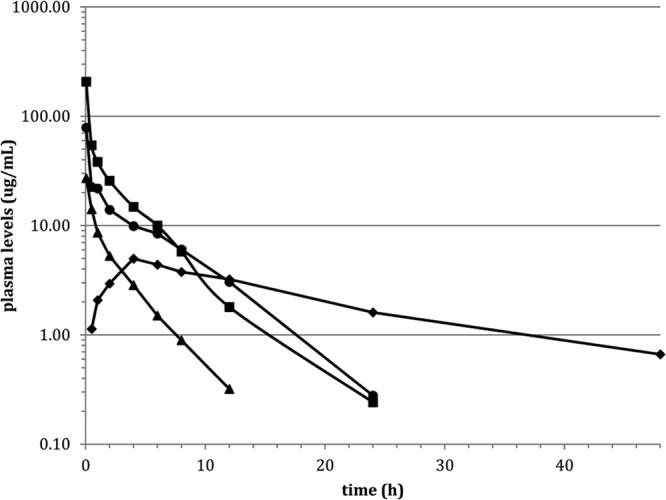
NAI-603 levels in pouch exudates after single i.v. administration of 5 (■), 20 (□), and 40 (▲) mg/kg.
Pharmacokinetics.
Following 5 and 20 mg/kg i.v. doses, plasma concentrations of NAI-603 were determined (Fig. 4). Peak concentrations measured 3 min from dosing were 27 ± 4 and 207 ± 42 μg/ml at 5 and 20 mg/kg, respectively. In both cases, a bi-exponential elimination phase was observed, with compound still detectable in plasma after 24 h at 20 mg/kg. Noncompartmental analysis showed a substantially linear profile in the analyzed range. Comparison of NAI-603 parameters with those achieved with ramoplanin at 20 mg/kg showed that, while the half-lives remained similar at 3.27 and 3.80 h, respectively, the Cmax was markedly higher for NAI-603 than for ramoplanin (207 μg/ml and 79 μg/ml, respectively). The volume of distribution at steady state was lower for NAI-603 (0.3 versus 0.77 liters/kg), suggesting a different early distribution phase.
FIG 4.
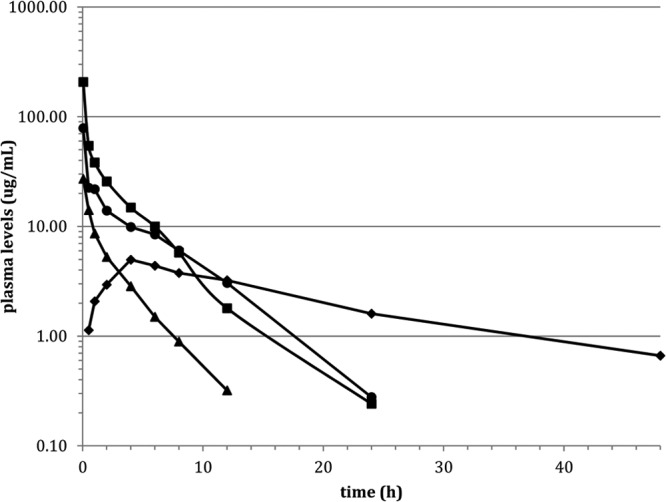
Plasma levels (μg/ml) in rats treated with 20 mg/kg i.v. ramoplanin (●), 20 mg/kg i.v. NAI-603 (■), 5 mg/kg i.v. NAI-603 (▲), and 20 mg/kg s.c. NAI-603 (◆).
Following a single 20 mg/kg s.c. administration of NAI-603, a Cmax of 5.0 ± 1.2 μg/ml was reached 4 h after dosing, while integrating the observed concentrations from time zero to infinity yielded 114 mg · h liter−1, corresponding to a bioavailability of about 52%, consistent with the efficacy observed in murine infections after s.c. administration (Fig. 4 and Table 6).
DISCUSSION
Although treatment of multidrug-resistant systemic infections has received substantial attention, of the six antimicrobials approved over the past 12 years (quinupristin-dalfopristin, linezolid, daptomycin, tigecycline, telavancin, and ceftaroline), only two represent new chemical classes endowed with new mechanisms of action. Resistance to some of these new antibiotics is already showing up in the clinical setting (30–33), making imperative the search of new chemical classes not affected by existing and emerging resistance mechanisms (34, 35).
Ramoplanin disrupts bacterial cell wall synthesis by binding the peptidoglycan intermediate lipid II at a site different from glycopeptides (2). As a result, it shows no cross-resistance with vancomycin or with β-lactams. The rapid bactericidal activity against all Gram-positive aerobic and anaerobic pathogens makes this antibiotic an ideal candidate for prevention and treatment of infections caused by these important pathogens. However, the systemic use of ramoplanin has been limited by the absence of bioavailability following oral administration and by poor local tolerability after i.v. injection. Attempts at overcoming these limitations by protecting formulations have appeared in the patent literature (36, 37); however, injectable formulations of ramoplanin have yet to enter clinical development. Ramoplanin has been under investigation for a decade for the treatment of Clostridium difficile-associated diarrhea (10).
A well-tolerated ramoplanin derivative might represent an innovative antibacterial agent for the systemic treatment of serious nosocomial infections caused by multidrug-resistant Gram-positive bacteria, including VRE and MRSA. Previous studies carried out on the isolated three main components of ramoplanin complex showed that the hemolytic effect was modulated by the length of the fatty acid chain, suggesting that tolerability could also be modulated by the length and nature of the fatty acid residue. Based on this assumption, a program targeting the synthesis of a ramoplanin derivative with the same antibacterial properties of the parent compound but coupled with an improved tolerability profile was initiated (15). Many of the resulting ramoplanin derivatives showed antimicrobial activity similar to that of the natural compound coupled with an improved local tolerability. Among them, the derivative in which the 2-methylphenylacetic acid has replaced the di-unsaturated fatty acid side chain (NAI-603 herein and compound 48 in reference 15) was selected as the most interesting compound: when administered intravenously into rats, no hematuria was observed at a dose of 20 mg/kg, either after single or repeated injections, and no macroscopic lesions, such as dark tail at injection sites, were observed (15).
In this study, we show that NAI-603 possesses a similar antibacterial spectrum as the parent compound, covering all MDR Gram-positive aerobic and anaerobic pathogens, with somewhat lower activity against vancomycin-susceptible and -resistant enterococci (MIC range, 0.06 to 2 mg/liter). The two compounds share the same properties, including rapid bactericidal activity and no significant pH or inoculum effect or reduction in potency in the presence of serum. Moreover, the lack of cross-resistance with other cell wall-inhibiting antibiotics and the low propensity of developing resistance represent added values for a drug that may find application in severely ill and hospitalized patients. Indeed, the frequency of natural resistance for NAI-603, determined on clinically relevant pathogens like MRSA, GISA, and VRE, was very low (about ≤1 × 10−9) at concentrations easily attainable in vivo at therapeutic doses (Table 5).
At the time of the discovery of ramoplanin, its in vitro properties led to the suggestion of this antibiotic as the drug of choice for treating systemic infections by Gram-positive bacteria (38). However, we were able to find only two reports of in vivo efficacy: one in the patent literature, in which ED50 values ranging from 1 to 5 mg/kg for mouse septicemia induced by S. aureus or E. faecium were reported (36), and the other in a peer-reviewed journal, where efficacy in rabbit endocarditis was observed only in combination with penicillin (8). Several publications commented on instability of ramoplanin in animal serum (1, 12) although we could not find any data substantiating such a claim. The data reported in Table 6 indicate that, with a half-life of 3 to 4 h, ramoplanin is sufficiently stable, at least in rat plasma. Similar values are observed also for NAI-603, suggesting that this compound is equally stable.
NAI-603 possesses an interesting antibacterial profile, covering all MDR Gram-positive pathogens, as well as a rapid and potent bactericidal activity. These data have been substantiated by different models of experimental infections caused by these difficult pathogens, as documented by the present work. In an acute murine lethal infection induced by MRSA or VRE, the efficacy of intravenously administered NAI-603 was comparable to the efficacies of reference compounds administered in dosages and by routes that reflect the exposures attained in humans at therapeutic doses. NAI-603 was more effective when administered i.v. than s.c., suggesting limited bioavailability by the latter route (Table 6). The rapid bactericidal effect observed for NAI-603 in the rat granuloma pouch model induced by MRSA is consistent with its high potency and suggests good tissue penetration. Interestingly, 20 mg/kg NAI-603 was sufficient to produce the same reduction of bacterial counts achieved by 100 mg/kg i.m. vancomycin. However, while in the vancomycin and 20 mg/kg NAI-603 groups bacterial regrowth was observed starting 72 h after treatment, a low bacterial titer was maintained for up to 96 h in the 40 mg/kg NAI-603 group (Fig. 3).
Comparison of NAI-603 parameters with those achieved with ramoplanin at 20 mg/kg shows that, while half-lives remained similar at 3.27 and 3.80 h, respectively, the Cmax was markedly higher for NAI-603 than for ramoplanin (207 μg/ml and 79 μg/ml, respectively). The volume of distribution at steady state was lower for NAI-603 (0.3 versus 0.77 liters/kg), suggesting a different early distribution phase. Investigation of the PK/pharmacodynamic (PD) properties of NAI-603 are ongoing.
In conclusion, the data reported here indicate that NAI-603, with its high activity against MDR Gram-positive pathogens, rapid bactericidal activity, efficacy in multiple models of infection, and favorable pharmacokinetic profile, together with the good tolerability previously reported (15), is a compound worthy of further investigation.
Footnotes
Published ahead of print 13 January 2014
REFERENCES
- 1.Anonymous. 2009. Urgently needed: new antibiotics. Lancet 374:1868. 10.1016/S0140-6736(09)62076-6 [DOI] [PubMed] [Google Scholar]
- 2.Walker S, Chen L, Hu Y, Rew Y, Shin D, Boger DL. 2005. Chemistry and biology of ramoplanin: a lipoglycodepsipeptide with potent antibiotic activity. Chem. Rev. 105:449–476. 10.1021/cr030106n [DOI] [PubMed] [Google Scholar]
- 3.Rolston KV, Dholakia N, Ho DH, LeBlanc B, Dvorak T, Streeter H. 1996. In-vitro activity of ramoplanin (a novel lipoglycopeptide), vancomycin, and teicoplanin against gram-positive clinical isolates from cancer patients. J. Antimicrob. Chemother. 38:265–269. 10.1093/jac/38.2.265 [DOI] [PubMed] [Google Scholar]
- 4.Citron DM, Merriam CV, Tyrrell KL, Warren YA, Fernandez H, Goldstein EJ. 2003. In vitro activities of ramoplanin, teicoplanin, vancomycin, linezolid, bacitracin, and four other antimicrobials against intestinal anaerobic bacteria. Antimicrob. Agents Chemother. 47:2334–2348. 10.1128/AAC.47.7.2334-2338.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Hare MD, Felmingham D, Grüneberg RN. 1989. In vitro bactericidal activity of the glycopeptide compounds vancomycin, teicoplanin and ramoplanin (A-16686/MDL 62,198). J. Chemother. 1(Suppl 4):210–211 [PubMed] [Google Scholar]
- 6.Johnson CC, Taylor S, Pitsakis P, May P, Levison ME. 1992. Bactericidal activity of ramoplanin against antibiotic-resistant enterococci. Antimicrob. Agents Chemother. 36:2342–2345. 10.1128/AAC.36.10.2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pallanza R, Berti M, Scotti R, Randisi E, Arioli V. 1984. A-16686, a new antibiotic from Actinoplanes. II. Biological properties. J. Antibiot. (Tokyo) 37:318–324. 10.7164/antibiotics.37.318 [DOI] [PubMed] [Google Scholar]
- 8.Landman D, Quale JM, Burney S, Kreiswirth B, Willey BM. 1996. Treatment of experimental endocarditis caused by multidrug resistant Enterococcus faecium with ramoplanin and penicillin. J. Antimicrob. Chemother. 37:323–329. 10.1093/jac/37.2.323 [DOI] [PubMed] [Google Scholar]
- 9.Montecalvo MA. 2003. Ramoplanin: a novel antimicrobial agent with the potential to prevent vancomycin-resistant enterococcal infection in high-risk patients. J. Antimicrob. Chemother. 51(Suppl 3):iii31–iii35. 10.1093/jac/dkg274 [DOI] [PubMed] [Google Scholar]
- 10.Gerding DN, Muto CA, Owens RC., Jr 2008. Treatment of Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl 1):S32–S42. 10.1086/521860 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Yuan Y, Helm JS, Hu Y, Rew Y, Shin D, Boger DL, Walker S. 2004. Dissecting ramoplanin: mechanistic analysis of synthetic ramoplanin analogues as a guide to the design of improved antibiotics. J. Am. Chem. Soc. 126:7462–7463. 10.1021/ja047879t [DOI] [PubMed] [Google Scholar]
- 12.Shin D, Rew Y, Boger DL. 2004. Total synthesis and structure of the ramoplanin A1 and A3 aglycons: two minor components of the ramoplanin complex. Proc. Natl. Acad. Sci. U. S. A. 101:11977–11979. 10.1073/pnas.0401419101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam J, Shin D, Rew Y, Boger DL. 2007. Alanine scan of [l-Dap(2)]ramoplanin A2 aglycon: assessment of the importance of each residue. J. Am. Chem. Soc. 129:8747–8755. 10.1021/ja068573k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, Nam J, Shin D, Rew Y, Boger DL, Walker S. 2009. Functional and biochemical analysis of a key series of ramoplanin analogues. Bioorg. Med. Chem. Lett. 19:6189–6191. 10.1016/j.bmcl.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciabatti R, Maffioli SI, Panzone G, Canavesi A, Michelucci E, Tiseni PS, Marzorati E, Checchia A, Giannone M, Jabes D, Romanò G, Brunati C, Candiani G, Castiglione F. 2007. Synthesis and preliminary biological characterization of new semisynthetic derivatives of ramoplanin. J. Med. Chem. 50:3077–3085. 10.1021/jm070042z [DOI] [PubMed] [Google Scholar]
- 16.Jabes D, Romano G, Brunati C, Rossi R, Maffioli S, Ciabatti R. 2003. In vitro characteristics of VIC-200603; a new anti-Gram-positive antibiotic, poster F-2112 Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Approved standard M100-S16 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 19.National Committee for Clinical and Laboratory Standards. 1999. Method for determining bactericidal activity of antimicrobial agents; approved guideline, vol. 19 NCCLS document M26-A National Committee for Clinical and Laboratory Standards, Wayne, PA [Google Scholar]
- 20.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandekar PK, Tessier PR, Williams P, Nightingale CH, Nicolau DP. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405–411. 10.1093/jac/dkg337 [DOI] [PubMed] [Google Scholar]
- 22.Knudsen JF, Fuursted K, Raber S, Espersen F, Frimodt-Møller N. 2000. Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob. Agents Chemother. 44:1247–1254. 10.1128/AAC.44.5.1247-1254.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slatter JG, Adams LA, Bush EC, Chiba K, Daley-Yates PT, Feenstra KL, Koike S, Ozawa N, Peng GW, Sams JP, Schuette MR, Yamazaki S. 2002. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 32:907–924. 10.1080/00498250210158249 [DOI] [PubMed] [Google Scholar]
- 24.Finney DJ. 1952. The Spearman-Kärber method, p 524–530 In Finney DJ. (ed), Statistical method in biological assay. Charles Griffin & Co., Ltd., London, United Kingdom [Google Scholar]
- 25.Dalhoff A. 1986. The granuloma pouch, p 123–137 In Zak O, Sande MA. (ed), Experimental models in antimicrobial chemotherapy. Academic Press, London, United Kingdom [Google Scholar]
- 26.Voorn GP, Kuyvenhoven J, Goessens WH, Schmal-Bauer WC, Broeders PH, Thompson J, Michel MF. 1994. Role of tolerance in treatment and prophylaxis of experimental Staphylococcus aureus endocarditis with vancomycin, teicoplanin, and daptomycin. Antimicrob. Agents Chemother. 38:487–493. 10.1128/AAC.38.3.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goossens H, Jabes D, Rossi R, Lammens C, Privitera G, Courvalin P. 2003. European survey of vancomycin-resistant enterococci in at-risk hospital wards and in vitro susceptibility testing of ramoplanin against these isolates. J. Antimicrob. Chemother. 51(Suppl 3):iii5–iii12. 10.1093/jac/dkg271 [DOI] [PubMed] [Google Scholar]
- 28.Mobarakai N, Quale JM, Landman D. 1994. Bactericidal activities of peptide antibiotics against multidrug-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 38:385–387. 10.1128/AAC.38.2.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider T, Sahl HG. 2010. An oldie but a goodie—cell wall biosynthesis as antibiotic target pathway. Int. J. Med. Microbiol. 300:161–169. 10.1016/j.ijmm.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 30.Rahim S, Pillai SK, Gold HS, Venkataraman L, Inglima K, Press RA. 2003. Linezolid-resistant, vancomycin-resistant Enterococcus faecium infection in patients without prior exposure to linezolid. Clin. Infect. Dis. 36:E146–E148. 10.1086/374929 [DOI] [PubMed] [Google Scholar]
- 31.Kelly S, Collins J, Maguire M, Gowing C, Flanagan M, Donnelly M, Murphy PG. 2008. An outbreak of colonization with linezolid-resistant Staphylococcus epidermidis in an intensive therapy unit. J. Antimicrob. Chemother. 61:901–907. 10.1093/jac/dkn043 [DOI] [PubMed] [Google Scholar]
- 32.Julian K, Kosowska-Shick K, Whitener C, Roos M, Labischinski M, Rubio A, Parent L, Ednie L, Koeth L, Bogdanovich T, Appelbaum PC. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445–3448. 10.1128/AAC.00559-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershberger E, Donabedian S, Konstantinou K, Zervos MJ, Eliopoulos GM. 2004. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin. Infect. Dis. 38:92–98. 10.1086/380125 [DOI] [PubMed] [Google Scholar]
- 34.Donadio S, Maffioli S, Monciardini P, Sosio M, Jabes D. 2010. Antibiotic discovery in the twenty-first century: current trends and future perspectives. J. Antibiot. 63:423–430. 10.1038/ja.2010.62 [DOI] [PubMed] [Google Scholar]
- 35.Moir DT, Opperman TJ, Butler MM, Bowlin TL. 2012. New classes of antibiotics. Curr. Opin. Pharmacol. 12:535–544. 10.1016/j.coph.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 36.Candiani G, Cavaleri M, Ciabatti R, Parenti F. July 1999. New injectable formulations containing ramoplanin. European patent EP1100463B1
- 37.Pelzer S, Vente A. May 2007. New lipoglycodepsipeptide compositions. European patent EP1994938A1
- 38.Dixson S, Brumfitt W, Hamilton-Miller JM. 1988. In vitro evaluation of ramoplanin (MDL 62198, A 16686). Eur. J. Clin. Microbiol. Infect. Dis. 7:819–821. 10.1007/BF01975062 [DOI] [PubMed] [Google Scholar]
- 39.Candiani G, Romanó G, Riva S, Maffioli S, Ciabatti R, Jabes D. 2003. Efficacy of VIC-200603, a new anti Gram-positive antibiotic, in experimental infections, poster F-2113 Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]


