Abstract
We studied the in vitro and in vivo efficacies of the investigational drug isavuconazole against mucormycosis due to Rhizopus delemar. Isavuconazole was effective, with MIC and minimal fungicidal concentration (MFC) values ranging between 0.125 and 1.00 μg/ml. A high dose of isavuconazole prolonged the survival time and lowered the tissue fungal burden of cyclophosphamide/cortisone acetate-treated mice infected with R. delemar and was as effective as a high-dose liposomal amphotericin B treatment. These results support the further development of this azole against mucormycosis.
TEXT
Mucormycoses are among the most common infections afflicting immunocompromised hosts and are occurring at an increasing frequency (1–3). The risk factors for mucormycosis include compromised immune status due to hematologic malignancies, neutropenia, steroid treatment, hyperglycemia, ketoacidosis and other forms of acidosis, deferoxamine therapy, and trauma (4–6). Despite disfiguring surgical debridement and adjunctive antifungal therapy, the overall mortality rate of mucormycosis remains approximately 50% and can approach 100% in hematogenously disseminated and central nervous system disease and in patients with prolonged neutropenia (2, 7–11). Clearly, new strategies for preventing and treating mucormycosis are urgently needed.
The new investigational drug isavuconazole (BAL4815) (ISA) is the active compound of the water-soluble prodrug isavuconazonium sulfate (BAL8557). Following administration, the prodrug isavuconazonium sulfate is rapidly converted by esterases in plasma to isavuconazole (isavuconazole is the term used here to describe the medicinal product). ISA shows broad-spectrum activity against fungi and is currently in late-stage clinical development for invasive fungal diseases, including invasive mucormycosis and invasive aspergillosis. For example, ISA has activity against Aspergillus spp. (12–15) (including those resistant to itraconazole, caspofungin, and amphotericin B [13]), Candida spp. (16, 17) (including isolates resistant to fluconazole [18]), and Cryptococcus spp. (19, 20). Recent studies demonstrated promising in vitro activity of ISA against Mucorales (12, 21, 22) fungi that cause mucormycosis. Therefore, the activity of ISA was compared to that of liposomal amphotericin B (LAmB) in a murine model of mucormycosis. Since Rhizopus species are the most common Mucorales isolates obtained from patients with mucormycosis (8, 23, 24), these studies focused on Rhizopus delemar.
The MIC100 (defined as the lowest concentration that causes 100% growth inhibition relative to the drug-free growth control) values of ISA (Astellas Pharma Global Development, Inc., Northbrook, IL) were determined against four clinical isolates of R. delemar (fumaric-malic acid producers) or four clinical isolates of Rhizopus oryzae (lactic acid producers) (25) using the Clinical Laboratory and Standards Institute (CLSI) M38-A2 method (26). The minimal fungicidal concentrations (MFCs) were also determined by spotting samples from all of the 96-well plates on potato dextrose agar (PDA) plates supplemented with 0.1% Triton X-100 and incubating them at 37°C for 2 days. The MFC was defined as the lowest concentration of the drug at which the organism failed to grow on the PDA plate. Against R. delemar isolates, ISA had median MIC100 and MFC values of 0.188 μg/ml (25th and 75th quartiles, 0.0625 and 0.0625 μg/ml). All tested R. oryzae isolates had ISA MIC100 and MFC values of 0.125 μg/ml. These studies showed that isavuconazole is fungicidal, since the MFC values were equivalent to the MIC values.
Next, the efficacy of the prodrug isavuconazonium sulfate was evaluated in a neutropenic mouse model of intratracheal infection (27) caused by R. delemar 99-880 (a brain isolate with ISA MIC100 and MFC values of 0.25 μg/ml). Male ICR mice (23 to 25 g; Taconic Farms, Germantown, NY) were used in this study. They were given irradiated feed and sterile water containing 50 μg/ml Baytril (enrofloxacin; Bayer) ad libitum (to control for bacterial infection). Neutropenia was induced by cyclophosphamide (200 mg/kg of body weight intraperitoneally [i.p.]) and cortisone acetate (500 mg/kg subcutaneously) on days −2 and 3 relative to infection. This treatment regimen results in ∼10 days of leukopenia with a total white blood cell count dropping from ∼13,0000/cm3 to almost no detectable leukocytes as determined by the Unopette system (Becton-Dickinson and Co., Franklin Lakes, NJ). After sedation with ketamine and xylazine, the mice were intratracheally infected with 2.5 × 105 spores of R. delemar 99-880 (27). Treatment with the prodrug isavuconazonium sulfate (80, 110, and 215 mg/kg, prepared fresh daily in irrigation water and given orally three times daily [t.i.d.]) started 8 h postinfection and continued through day 4. The higher dose of 215 mg/kg t.i.d. of isavuconazonium sulfate demonstrated enhanced efficacy over that of placebo treatment of mice (70% survival in the isavuconazonium sulfate-treated mice versus 10% survival for placebo mice [treated orally with irrigation water] after 21 days) (Fig. 1).
FIG 1.
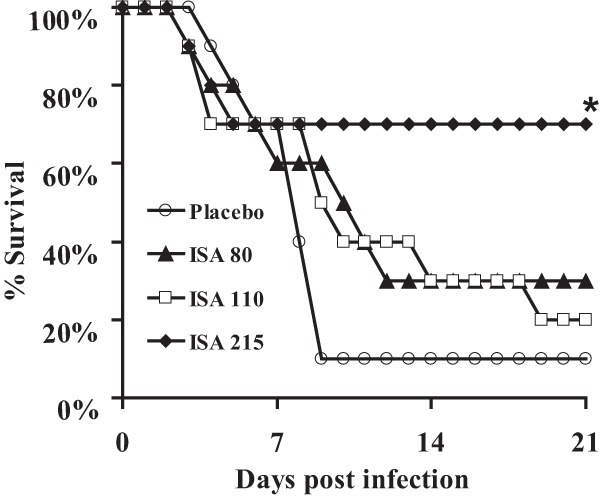
Isavuconazole enhanced survival of neutropenic mice with mucormycosis pneumonia. Mice (n = 10 per arm) were infected intratracheally with 2.5 × 105 spores of R. delemar 99-880 (inhaled inoculum was 4.1 × 103 spores). Isavuconazonium sulfate treatment started 8 h postinfection and continued three times daily at 80, 110, or 215 mg/kg by oral gavage through day 4 postinfection. Placebo mice were infected and treated with sterile irrigation water. *, P < 0.05 compared to placebo-treated mice by log rank test.
Since a high dose of ISA (215 mg/kg t.i.d.) demonstrated efficacy against R. delemar infection, the efficacy of this dose was compared against that of a high dose of LAmB (AmBisome; Gilead Sciences Inc., Forest City, CA) in treating mucormycosis, which is considered the standard therapy for mucormycosis in this model (28). LAmB was dissolved initially in sterile irrigation water and diluted in 5% dextrose water (D5W) according to the manufacturer's instructions. Neutropenic mice were infected intratracheally as described above. Eight hours later, treatment with isavuconazonium sulfate (215 mg/kg t.i.d., given orally) or LAmB (15 mg/kg, given once daily through tail vein injection) started and continued through day 4. Neutropenic mice infected intratracheally and administered a comparable volume of vehicle (i.e., D5W) served as placebo controls. The primary endpoint for efficacy was the time to moribundity of infected mice. ISA was as effective as LAmB in treating neutropenic mice for mucormycosis. The twenty-one-day survival rates for the prodrug isavuconazonium sulfate-, LAmB-, and placebo-treated mice were 65%, 40%, and 15%, respectively (Fig. 2A).
FIG 2.
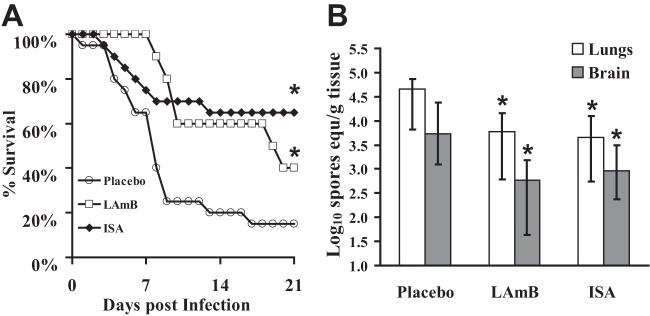
Isavuconazole is as effective as high-dose LAmB in improving survival (A) and reducing fungal burden (B) of neutropenic mice from mucormycosis. Mice (n = 10 per arms for both A and B) were infected intratracheally with 2.5 × 105 spores of R. delemar 99-880 (average inhaled inoculum was 1.3 × 103 cells). Isavuconazonium sulfate (215 mg/kg t.i.d., by oral gavage) or LAmB (15 mg/kg every day by i.v. injection) treatment started 8 h postinfection and continued through day 4 postinfection (A) and day 3 postinfection (B). Placebo mice were infected and treated with 5% dextrose water. (A) Survival of mice through day 21. *, P = 0.025 or 0.004 for LAmB or ISA compared to placebo by log rank test, respectively. (B) Fungal burden was measured by qPCR with a conidial standard curve (29, 34). All qPCR results are expressed as log10 spore equivalents per gram of tissue. *, P < 0.05 compared to placebo by Wilcoxon rank sum test.
Because ISA increased the survival rate of neutropenic mice infected with R. delemar, the effect of drug treatment on the tissue fungal burden in target organs was determined. The mice were infected as described above and treated until day 3 relative to infection when the mice were sacrificed and their lungs and brains harvested and tested for tissue fungal burden (representing primary and secondary target organs [27]) by quantitative PCR (qPCR) (29). Treatment of the mice with the prodrug resulted in an approximately 1-log decrease in lung and brain fungal burdens compared to those of placebo-treated controls. This reduction in tissue fungal burden was comparable to that elicited by LAmB treatment (Fig. 2B).
A recent study demonstrated that oral-gastric doses of the prodrug isavuconazonium sulfate at 10, 40, 160, and 640 mg/kg produced serum peak levels of 0.51 to 25.4 μg/ml ISA and an elimination half-life of 1 to 5 h (30). The short half-life of this drug is in contrast to the long half-life of >50 h seen in humans following a single-dose administration (31). Therefore, the prodrug was administered three times daily over a series of doses that would provide a range of exposures, some of which should result in serum peak levels of ISA above the registered MIC and MFC values of 0.125 to 1.0 μg/ml. Indeed, the higher dose of 215 mg/kg (prodrug isavuconazonium sulfate) given three times daily, which would have resulted in serum peak levels of >12.5 μg/ml ISA and a half-life of >3.1 h (30), demonstrated enhanced protection of mice from mucormycosis. This protection was equivalent to the efficacy demonstrated by a high dose of LAmB.
In addition to the activity in the cyclophosphamide/cortisone acetate-treated mice, ISA activity has been demonstrated in animal models of other fungal infections, including invasive aspergillosis and disseminated candidiasis (14, 32, 33). Finally, the availability of ISA in oral and intravenous formulations provides a clear advantage for this azole for use in different medical scenarios. However, given the frequency of this infection in patients with diabetes, future studies of ISA efficacy should also be carried out in a diabetic murine mucormycosis model (8, 9). In summary, given the in vitro and in vivo evidence of the activity of ISA against Rhizopus, these results warrant further development of this azole for the treatment of mucormycosis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R01 AI063503 and a research and educational grant from Astellas Pharma US to A.S.I.
The research described in this article was conducted at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles Medical Center.
Footnotes
Published ahead of print 3 February 2014
REFERENCES
- 1.Chakrabarti A, Chatterjee SS, Das A, Panda N, Shivaprakash MR, Kaur A, Varma SC, Singhi S, Bhansali A, Sakhuja V. 2009. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad. Med. J. 85:573–581. 10.1136/pgmj.2008.076463 [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Carter RA, Crippa F, Wald A, Corey L. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909–917. 10.1086/339202 [DOI] [PubMed] [Google Scholar]
- 3.Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, Lortholary O. 2012. Healthcare-associated mucormycosis. Clin. Infect. Dis. 54(Suppl 1):S44–S54. 10.1093/cid/cir867 [DOI] [PubMed] [Google Scholar]
- 4.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. 2012. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 54(Suppl 1):S23–S34. 10.1093/cid/cir866 [DOI] [PubMed] [Google Scholar]
- 5.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 367:2214–2225. 10.1056/NEJMoa1204781 [DOI] [PubMed] [Google Scholar]
- 6.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne JR, Ganesan A, Li P, Bradley W, Gaskins LJ, Seillier-Moiseiwitsch F, Murray CK, Millar EV, Keenan B, Paolino K, Fleming M, Hospenthal DR, Wortmann GW, Landrum ML, Kortepeter MG, Tribble DR. 2012. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 55:1441–1449. 10.1093/cid/cis749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. 2004. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma 45:1351–1360. 10.1080/10428190310001653691 [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim AS, Edwards JE, Jr, Filler SG, Spellberg B. 2011. Mucormycosis and entomophthoromycosis (zygomycosis), p 265–280 In Kauffman CA, Pappas PG, Sobel JD, Dismukes WE. (ed), Essentials of clinical mycology, 2nd ed. Springer, New York, NY [Google Scholar]
- 9.Kauffman CA. 2004. Zygomycosis: reemergence of an old pathogen. Clin. Infect. Dis. 39:588–590. 10.1086/422729 [DOI] [PubMed] [Google Scholar]
- 10.Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. 2000. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 30:851–856. 10.1086/313803 [DOI] [PubMed] [Google Scholar]
- 11.Husain S, Alexander BD, Munoz P, Avery RK, Houston S, Pruett T, Jacobs R, Dominguez EA, Tollemar JG, Baumgarten K, Yu CM, Wagener MM, Linden P, Kusne S, Singh N. 2003. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin. Infect. Dis. 37:221–229. 10.1086/375822 [DOI] [PubMed] [Google Scholar]
- 12.Perkhofer S, Lechner V, Lass-Florl C. 2009. In vitro activity of isavuconazole against Aspergillus species and zygomycetes according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 53:1645–1647. 10.1128/AAC.01530-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warn PA, Sharp A, Denning DW. 2006. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J. Antimicrob. Chemother. 57:135–138. 10.1093/jac/dki399 [DOI] [PubMed] [Google Scholar]
- 14.Warn PA, Sharp A, Mosquera J, Spickermann J, Schmitt-Hoffmann A, Heep M, Denning DW. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198–11207. 10.1093/jac/dkl396 [DOI] [PubMed] [Google Scholar]
- 15.Rudramurthy SM, Chakrabarti A, Geertsen E, Mouton JW, Meis JF. 2011. In vitro activity of isavuconazole against 208 Aspergillus flavus isolates in comparison with 7 other antifungal agents: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Diagn. Microbiol. Infect. Dis. 71:370–377. 10.1016/j.diagmicrobio.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Seifert H, Aurbach U, Stefanik D, Cornely O. 2007. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob. Agents Chemother. 51:1818–1821. 10.1128/AAC.01217-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 52:1396–1400. 10.1128/AAC.01512-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamazaki T, Inagaki Y, Fujii T, Ohwada J, Tsukazaki M, Umeda I, Kobayashi K, Shimma N, Page MG, Arisawa M. 2010. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int. J. Antimicrob. Agents 36:324–331. 10.1016/j.ijantimicag.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 19.Thompson GR, III, Wiederhold NP, Fothergill AW, Vallor AC, Wickes BL, Patterson TF. 2009. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob. Agents Chemother. 53:309–311. 10.1128/AAC.01216-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illnait-Zaragozi MT, Martinez GF, Curfs-Breuker I, Fernandez CM, Boekhout T, Meis JF. 2008. In vitro activity of the new azole isavuconazole (BAL4815) compared with six other antifungal agents against 162 Cryptococcus neoformans isolates from Cuba. Antimicrob. Agents Chemother. 52:1580–1582. 10.1128/AAC.01384-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarti A, Shivaprakash MR, Curfs-Breuker I, Baghela A, Klaassen CH, Meis JF. 2010. Apophysomyces elegans: epidemiology, amplified fragment length polymorphism typing, and in vitro antifungal susceptibility pattern. J. Clin. Microbiol. 48:4580–4585. 10.1128/JCM.01420-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verweij PE, Gonzalez GM, Wiedrhold NP, Lass-Florl C, Warn P, Heep M, Ghannoum MA, Guinea J. 2009. In vitro antifungal activity of isavuconazole against 345 Mucorales isolates collected at study centers in eight countries. J. Chemother. 21:272–281. 10.1179/joc.2009.21.3.272 [DOI] [PubMed] [Google Scholar]
- 23.Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236–301. 10.1128/CMR.13.2.236-301.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634–653. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 25.Abe A, Oda Y, Asano K, Sone T. 2007. Rhizopus delemar is the proper name for Rhizopus oryzae fumaric-malic acid producers. Mycologia 99:714–722. 10.3852/mycologia.99.5.714 [DOI] [PubMed] [Google Scholar]
- 26.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Document M38-A2. CLSI, Wayne, PA [Google Scholar]
- 27.Luo G, Gebremariam T, Lee H, French SW, Wiederhold NP, Patterson TF, Filler SG, Ibrahim AS. 2013. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob. Agents Chemother. 57:3340–3347. 10.1128/AAC.00313-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim AS, Avanessian V, Spellberg B, Edwards JE., Jr 2003. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 47:3343–3344. 10.1128/AAC.47.10.3343-3344.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim AS, Bowman JC, Avanessian V, Brown K, Spellberg B, Edwards JE, Jr, Douglas CM. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-beta-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721–727. 10.1128/AAC.49.2.721-727.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepak AJ, Marchillo K, Vanhecker J, Diekema D, Andes DR. 2013. Isavuconazole pharmacodynamic target determination for Candida species in an in vivo murine disseminated candidiasis model. Antimicrob. Agents Chemother. 57:5642–5648. 10.1128/AAC.01354-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:279–285. 10.1128/AAC.50.1.279-285.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. 2009. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J. Antimicrob. Chemother. 63:161–166. 10.1093/jac/dkn431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. 2009. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob. Agents Chemother. 53:3453–34561. 10.1128/AAC.01601-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474–3481. 10.1128/AAC.45.12.3474-3481.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]


