Abstract
We characterized the relative fitness of multiple nonnucleoside reverse transcriptase (RT) inhibitor (NNRTI)-resistant HIV-1 variants in the presence of etravirine (ETV), rilpivirine (RPV), and/or the nucleoside RT inhibitor emtricitabine (FTC) by simultaneous competitive culture and 454 deep sequencing. The E138A substitution, typically associated with decreased virologic responses to ETV- and RPV-containing regimens, confers a clear fitness advantage to the virus in the presence of FTC and decreases FTC susceptibility 4.7-fold.
TEXT
Complera is a fixed-dose antiviral drug combination used as a first-line antiretroviral therapy regimen for the treatment of HIV-1 infection. Complera is composed of the nucleoside reverse transcriptase (RT) inhibitors (NRTIs) emtricitabine (FTC) and tenofovir disoproxil fumarate and the nonnucleoside RT inhibitor (NNRTI) rilpivirine (RPV). In the phase III ECHO and THRIVE clinical trials, the most frequent substitution combination that emerged in the RT gene of Complera virologic failures was E138K and M184I (1, 2). In this regard, Hu and Kuritzkes (3) and Xu et al. (4) reported that the E138K substitution compensated for the poor replicative capacity of M184I. However, Kulkarni et al. found that HIV-1 containing E138K and M184I was less fit than viruses containing either E138K or M184I (5). In addition to E138K, the L100I, K101E/P, E138A/G/Q, Y181C/I/V, Y188L, G190A/S/E, and M230L substitutions are associated with RPV resistance.
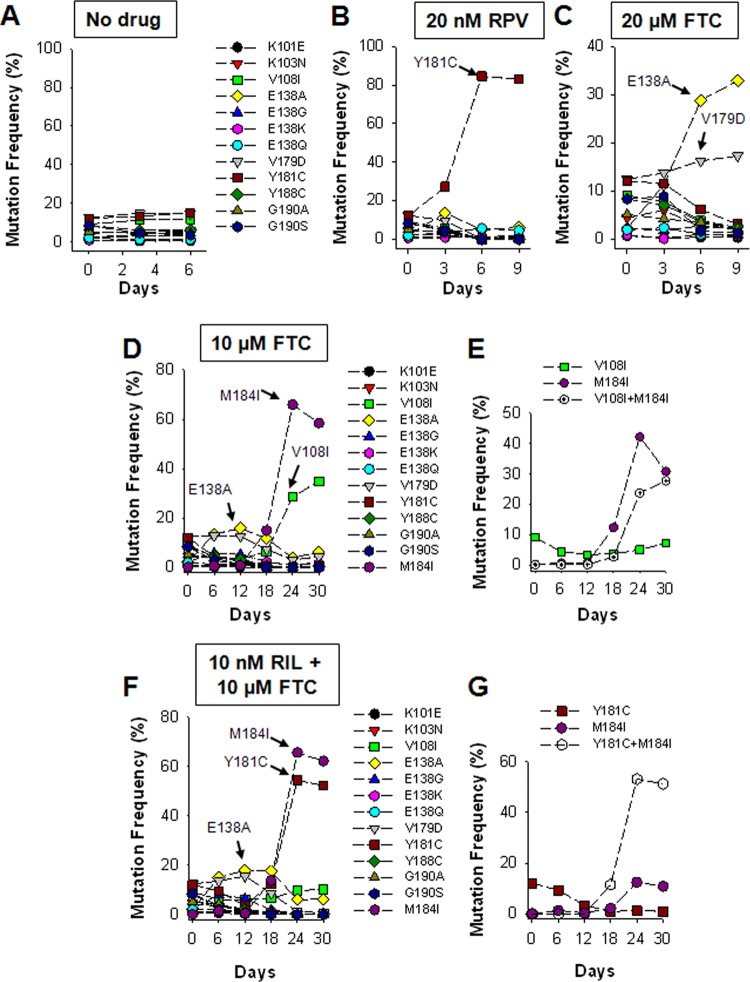
Recently, we developed an efficient method to characterize the relative fitness of multiple HIV-1 mutants simultaneously (6). Briefly, we generated 15 infectious viruses (HIV-1LAI) containing the single NNRTI resistance substitutions V90I, K101P, K103N, V108I, E138A, E138K, V179D, Y181C, Y181I, Y181V, Y188C, G190A, G190S, M230L, and P236L. A wild-type (WT) virus and the 15 mutant viruses were then normalized for relative infectivity (infectious units/ng p24), pooled, and used to infect 1.6 × 106 HUT-78 cells at a multiplicity of infection of 0.004. Culture supernatants were collected at 3-day intervals, viral RNA was extracted, and deep sequencing (454 GS Junior) of an amplicon spanning RT codons 96 to 194 was used to quantify the distribution of mutants at each time point. Using this method, we previously showed that the Y181V substitution in the HIV-1 RT confers a selective advantage to the virus over the 14 other NNRTI resistance substitutions in the presence of the NNRTI etravirine (ETV; Fig. 1B) (6). We next asked whether the fitness landscape was altered if FTC was combined with ETV. In the presence of 200 nM ETV and 10 μM FTC, longitudinal deep sequencing revealed that the Y181V virus again emerged as the most common variant by day 12 (Fig. 1C). Unexpectedly, we found that in experiments carried out in the presence of 10 μM FTC (5× to 10× the EC50), the E138A HIV-1 exhibited a clear fitness advantage (Fig. 1D). The frequency of the E138A variant increased from 9.6% at day 0 to 28% by day 6. The frequencies of HIV-1 containing the V179D or E138K substitutions were also increased, whereas all of the other NNRTI-resistant variants declined over time (Fig. 1D). The K101P, Y181I, and Y181V substitutions in HIV-1 RT are seen relatively infrequently in clinical isolates (they all require 2 nucleotide changes) and confer high-level ETV and RPV resistance (6, 7). As such, we generated a new pool of virus that included the K101E, E138G, and E138Q substitutions associated with ETV and RPV resistance (and only require 1 nucleotide change) in lieu of K101P, Y181I, and Y181V. Importantly, the effective concentration of RPV required to inhibit 50% of the pooled virus (i.e., EC50) in TZM-bl cells was found to be 2.6 ± 0.7 nM, which was identical to the concentration required to inhibit the WT virus (EC50 = 2.5 ± 0.9 nM). Interestingly, the pooled virus was ∼4-fold hypersusceptible to FTC (EC50s for FTC for the WT and pooled virus were 1.3 ± 0.3 μM and 0.3 ± 0. μM, respectively). This new pool of virus was then used to infect HUT-78 cells in the presence of RPV, FTC, or a combination of both drugs (Fig. 2). In cultures grown in the presence of 20 nM RPV, the Y181C virus emerged as the most common variant by days 3, 6, and 9 (Fig. 2B). In the presence of 20 μM FTC, the E138A variant again increased in frequency from 2.2% at day 0 to 32.9% at day 9 (Fig. 2C). The frequency of the V179D also marginally increased over time (12.4% at day 0 to 17.3% at day 9). Initially, we tried to culture the pooled virus in the presence of 20 nM RPV and 20 μM FTC. However, we observed no virus outbreak after 24 days (data not shown). Therefore, we reduced the RPV and FTC concentrations to 10 nM and 10 μM, respectively. In cultures grown in the presence of 10 nM RPV, the Y181C virus again emerged as the most common variant (data not shown). In cultures grown in the presence of 10 μM FTC, the E138A variant increased in frequency from 2.2% at day 0 to 15.8% at day 12 (Fig. 2D). However, its frequency began to decline by day 18 and was replaced with virus containing V108I and/or M184I. The M184I substitution in HIV-1 was selected by FTC during the fitness experiment, and pairwise analysis of the sequencing data revealed that it was selected on both WT and V108I backbones (Fig. 2E). In cultures grown in the presence of 10 nM RPV and 10 μM FTC, the E138A variant again increased in frequency from 2.2% at day 0 to 18% by day 18, before it was replaced with a virus population that contained Y181C and M184I (Fig. 2F). Pairwise analysis of the sequencing data showed that the M184I substitution was selected on the Y181C backbone (Fig. 2G). In light of the finding that HIV-1 containing E138A exhibited a clear fitness advantage in the presence of FTC, we performed drug susceptibility assays (Table 1). We found that E138A decreased FTC and lamivudine (3TC) susceptibility 4.7- and 6.0-fold, respectively (P < 0.01), but had no effect on tenofovir susceptibility. Pre-steady-state kinetic experiments revealed that the E138A substitution allows RT to effectively discriminate between the natural nucleotide (dCTP) 3TC-triphosphate (see Table S1 in the supplemental material). 3TC-triphosphate discrimination was driven primarily by a decrease in binding affinity (Kd) and not by a decreased rate of incorporation (kpol). Interestingly, we found that the E138G, E138K, E138Q, and E138R substitutions did not impact FTC susceptibility (Table 1).
FIG 1.
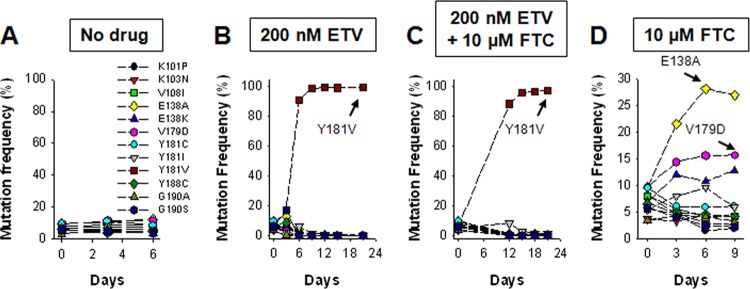
Replication fitness of multiple NNRTI-resistant HIV-1 variants in the presence of ETV and/or FTC. The relative prevalence of 12 viruses, each containing a single resistance substitution, as determined by longitudinal deep sequencing of viral RNA from culture supernatant from cells infected with pooled virus and exposed to no drug (A), 200 nM ETV (B), 200 nM ETV and 10 μM FTC (C), or 10 μM FTC (D). Data are from a single determination.
FIG 2.
Replication fitness of multiple NNRTI-resistant HIV-1 variants in the presence of RPV and/or FTC. The relative prevalence of 12 viruses, each containing a single resistance substitution, as determined by longitudinal deep sequencing of viral RNA from culture supernatant from cells infected with pooled virus and exposed to no drug (A), 20 nM RPV (B), 20 μM FTC (C), 10 μM FTC (D), and 10 nM RPV and 10 μM FTC (F). Pairwise analyses of the sequencing data show that the M184I substitution was selected on a WT and V108I backbone in experiments carried out in the presence of 10 μM FTC (E) and on a Y181C backbone in experiments carried out in the presence of 10 nM RPV and 10 μM FTC (G). Data are from a single determination.
TABLE 1.
Susceptibility to FTC, 3TC, tenofovir, RPV, and ETV of HIV-1 viruses containing substitutions at codon E138a
| Virus | FTC |
3TC |
Tenofovir |
RPV |
ETV |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | Fold R (P value) | EC50 (μM) | Fold R (P value) | EC50 (μM) | Fold R (P value) | EC50 (nM) | Fold R (P value) | EC50 (nM) | Fold R (P value) | |
| WT | 1.2 ± 0.4 | 5.8 ± 2.5 | 16.1 ± 6.3 | 0.3 ± 0.1 | 1.3 ± 0.2 | |||||
| E138A mutant | 6.0 ± 2.3 | 4.7 (0.006) | 35.1 ± 10.0 | 6.0 (0.01) | 8.7 ± 2.4 | 0.5 (>0.05) | 1.5 ± 0.2 | 5.6 (0.03) | 2.9 ± 0.5 | 2.2 (0.05) |
| E138G mutant | 0.5 ± 0.1 | 0.4 (>0.05) | 0.7 ± 0.4 | 2.5 (0.05) | 3.2 ± 0.2 | 2.5 (0.05) | ||||
| E138K mutant | 2.5 ± 1.7 | 1.9 (>0.05) | 10.7 ± 6.7 | 1.8 (>0.05) | 6.6 ± 3.8 | 0.4 (0.05) | 0.8 ± 0.2 | 3.0 (0.04) | 2.8 ± 0.3 | 2.2 (0.05) |
| E138Q mutant | 0.4 ± 0.1 | 0.3 (>0.05) | 5.5 ± 2.4 | 0.3 (0.05) | 1.3 ± 0.1 | 4.8 (0.04) | 4.0 ± 0.1 | 3.1 (0.01) | ||
| E138R mutant | 1.3 ± 0.3 | 1.0 (>0.05) | 1.6 ± 0.4 | 6.0 (0.05) | 5.3 ± 1.6 | 4.1 (0.04) | ||||
EC50s are the concentrations of drug required to inhibit viral replication by 50% from 3 independent experiments that were log10 transformed and compared for statistically significant differences (P < 0.05) by using the two-sample Student paired t test. Data are reported as means ± standard deviations from 3 independent experiments. Fold R is the mean fold change in EC50 of mutant versus WT virus.
In conclusion, we report that the E138A substitution in HIV-1 RT confers an initial clear selective fitness advantage to HIV-1 in the presence of FTC, before being outcompeted by HIV-1 containing M184I. Of note, we previously reported that an I132M substitution in the β7-β8 loop (that contains residue E138) of the p51 subunit of HIV-1 RT conferred nevirapine resistance but 3TC hypersusceptibility (8). Taken together, these studies suggest that residues in the β7-β8 loop of HIV-1 RT can influence nucleotide selectivity. At present, the clinical significance of this finding is unknown. However, the biological cutoffs for FTC and 3TC in the vircoTYPE HIV-1 phenotype are 3.1- and 2.1-fold changes in the calculated EC50, respectively (Janssen Diagnostics), which suggests that E138A might impact the clinical response to these nucleoside analogs. Of note, E138A is a relatively rare substitution in HIV-1 subtype B (∼2% prevalence in both therapy-naive and NNRTI-experienced HIV-infected individuals) but is quite common in both therapy-naive and NNRTI-experienced individuals, with a prevalence of 5 to 8% (9, 10). Finally, the limitations of this work include the fact that our studies were carried out in a T cell line using a laboratory-adapted strain of HIV-1 with a defined genetic backbone.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants GM068406 and AI081571 (to N.S.-C). P.R.H. is supported by a CIHR/GSK Chair in Clinical Virology and C.J.B by a CIHR Vanier Canada Graduate Scholarship.
Footnotes
Published ahead of print 13 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02114-13.
REFERENCES
- 1.Rimsky L, Vingerhoets J, Van Eygen V, Eron J, Clotet B, Hoogstoel A, Boven K, Picchio G. 2012. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J. Acquir. Immune Defic. Syndr. 59:39–46. 10.1097/QAI.0b013e31823df4da [DOI] [PubMed] [Google Scholar]
- 2.Rimsky L, Van Eygen V, Hoogstoel A, Stevens M, Boven K, Picchio G, Vingerhoets J. 28 May 2013. 96-week resistance analyses of rilpivirine in treatment-naive, HIV-1-infected adults from the ECHO and THRIVE phase III trials. Antivir. Ther. 10.3851/IMP2636 [DOI] [PubMed] [Google Scholar]
- 3.Hu Z, Kuritzkes DR. 2011. Interaction of reverse transcriptase (RT) mutations conferring resistance to lamivudine and etravirine: effects on fitness and RT activity of human immunodeficiency virus type 1. J. Virol. 85:11309–11314. 10.1128/JVI.05578-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HT, Asahchop EL, Oliveira M, Quashie PK, Quan Y, Brenner BG, Wainberg MA. 2011. Compensation by the E138K mutation in HIV-1 reverse transcriptase for deficits in viral replication capacity and enzyme processivity associated with the M184I/V mutations. J. Virol. 85:11300–11308. 10.1128/JVI.05584-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni R, Babaoglu K, Lansdon EB, Rimsky L, Van Eygen V, Picchio G, Svarovskaia E, Miller MD, White KL. 2012. The HIV-1 reverse transcriptase M184I mutation enhances the E138K-associated resistance to rilpivirine and decreases viral fitness. J. Acquir. Immune Defic. Syndr. 59:47–54. 10.1097/QAI.0b013e31823aca74 [DOI] [PubMed] [Google Scholar]
- 6.Brumme CJ, Huber KD, Dong W, Poon AF, Harrigan PR, Sluis-Cremer N. 2013. Replication fitness of multiple NNRTI-resistant HIV-1 variants in the presence of etravirine measured by 454 deep sequencing. J. Virol. 87:8805–8807. 10.1128/JVI.00335-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azijn H, Tirry I, Vingerhoets J, de Béthune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob. Agents Chemother. 54:718–727. 10.1128/AAC.00986-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose Z, Herman BD, Sheen CW, Zelina S, Moore KL, Tachedjian G, Nissley DV, Sluis-Cremer N. 2009. The human immunodeficiency virus type 1 nonnucleoside reverse transcriptase inhibitor resistance mutation I132M confers hypersensitivity to nucleoside analogs. J. Virol. 83:3826–3833. 10.1128/JVI.01968-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan M, Rhee SY, Shafer R, Bertagnolio S, Parkin N. 2012. Elevated prevalence of the E138A mutation in reverse transcriptase of HIV-1 subtypes A and C from recently infected, untreated subjects. Antivir. Ther. 17(Suppl 1):A165 [Google Scholar]
- 10.Sluis-Cremer N, Huber K, Brumme C, Wallis C, Mellors J, Harrigan R. 2013. The E138A mutation in HIV-1 reverse transcriptase is more common in subtype C than B and decreases susceptibility to NNRTIs, abstr 102 Abstr. 20th Conf. Retroviruses Opportunistic Infect. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.