Abstract
Carbapenemase-producing Klebsiella pneumoniae strains (CP-Kps) are currently among the most important nosocomial pathogens. An observational study was conducted during 2009 to 2010 in two hospitals located in a high-prevalence area (Athens, Greece). The aims were (i) to evaluate the clinical outcome of patients with CP-Kp bloodstream infections (BSIs), (ii) to identify predictors of mortality, and (iii) to evaluate the various antibiotic schemes employed. A total of 205 patients with CP-Kp BSIs were identified: 163 (79.5%) were infected with KPC or KPC and VIM, and 42 were infected with VIM producers. For definitive treatment, 103 patients received combination therapy (two or more active drugs), 72 received monotherapy (one active drug), and 12 received therapy with no active drug. The remaining 18 patients died within 48 h after the onset of bacteremia. The all-cause 28-day mortality was 40%. A significantly higher mortality rate was observed in patients treated with monotherapy than in those treated with combination therapy (44.4% versus 27.2%; P = 0.018). The lowest mortality rate (19.3%) was observed in patients treated with carbapenem-containing combinations. In the Cox proportion hazards model, ultimately fatal disease (hazards ratio [HR], 3.25; 95% confidence interval [CI], 1.51 to 7.03; P = 0.003), the presence of rapidly fatal underlying diseases (HR, 4.20; 95% CI, 2.19 to 8.08; P < 0.001), and septic shock (HR, 2.15; 95% CI, 1.16 to 3.96; P = 0.015) were independent predictors of death. Combination therapy was strongly associated with survival (HR of death for monotherapy versus combination, 2.08; 95% CI, 1.23 to 3.51; P = 0.006), mostly due to the effectiveness of the carbapenem-containing regimens.
INTRODUCTION
Carbapenemase-producing Klebsiella pneumoniae strains (CP-Kps) have become a major public health problem worldwide. The carbapenemases commonly encountered in CP-Kps are the KPC variants and the zinc-dependent metallo-β-lactamases (VIM, IMP, and NDM types) that inactivate most clinically available β-lactams (1, 2). Also, virtually all CP-Kps exhibit resistance to important antibacterials, such as aminoglycosides and fluoroquinolones (3, 4). The most active agents in vitro remain colistin and tigecycline, although a steady increase in frequencies of resistance to these drugs is already evident (3–6).
While CP-Kps can affect any patient with significant health care exposure in a setting where infection is endemic, they mainly cause life-threatening infections, such as bacteremia and pneumonia in critically ill patients. Several factors, including advanced age, severity of underlying disease, poor functional status, and comorbid conditions, have been associated repeatedly with increased mortality in patients with CP-Kp infections (1, 7, 8).
The dearth of therapeutic options, a consequence of the extensive resistance phenotypes of CP-Kps, poses additional difficulties in the patients' management. Indeed, based on in vitro susceptibilities, use of a single antimicrobial drug, such as colistin or tigecycline, was—and in some settings still is—the standard therapy for CP-Kp infections, often with poor results (9–11). According to recent clinical observations, CP-Kp-infected patients may benefit from the use of specific combinations of antibiotics (some of which include a carbapenem), but this issue remains unsettled (10–13). Nevertheless, in the absence of controlled trials on which to base firm conclusions, observational studies including large numbers of CP-Kp patients would likely help improve treatment approaches.
Herein, we have attempted an analysis of the mortality-associated factors in a cohort of 205 patients with bloodstream infections (BSIs) caused by CP-Kps. Emphasis was given to the role of antimicrobial treatment.
MATERIALS AND METHODS
Setting.
The study was conducted between August 2009 and December 2010 in two tertiary care hospitals located in Athens: hospital A with 1,000 beds and 82,000 admissions per year and hospital B with 500 beds and 55,000 admissions per year. CP-Kps were endemic in both institutions.
Study design.
This was a retrospective observational study that included consecutive patients with either primary or secondary K. pneumoniae BSIs. Enrolled subjects had at least one blood culture positive for K. pneumoniae and clinical findings consistent with the systemic inflammatory response syndrome (14). Only the first K. pneumoniae BSI episode was considered. Patients were followed up twice a week until discharge or death. Patients with polymicrobial bacteremia were included in the analysis only if they had received antibiotics active in vitro against the other coinfecting organism(s). Pertinent information, including demographic characteristics, underlying diseases, severity of sepsis, prior hospitalizations, prior exposure to health care facilities, treatment of the bacteremia episode, and outcome, were extracted from medical records in a predesigned form. Charlson's comorbidity index was calculated as described previously (15). The institutional review board of each hospital approved the study with a waiver of informed consent.
Microbiology.
Species identification and susceptibility testing were performed in the clinical laboratories by Vitek 2 (bioMérieux) (hospital A) and Wider I (Dade Behring MicroScan) (hospital B). Isolates were delivered to the Infectious Diseases Research Laboratory, First Department of Propaedeutic Medicine of the Medical School of Athens for further study. Susceptibility to imipenem, meropenem, doripenem, colistin, and tigecycline was reevaluated by the Etest (AB Biodisk). The organisms were considered resistant to carbapenems and colistin according to the EUCAST breakpoints (>8 μg/ml for imipenem and meropenem, >4 μg/ml for doripenem, and >2 μg/ml for colistin) (16). For tigecycline, the U.S. Food and Drug Administration interpretive criteria were used (17). bla gene content, carbapenemase production, pulsed-field gel electrophoresis (PFGE) types, and sequence types (STs) of the isolates were also determined (18).
Definitions.
Onset of BSI was defined as the date of collection of the first blood culture that yielded the study organism. BSIs were classified as community acquired, health care associated, and hospital acquired according to standard criteria (19). The suspected source of the BSI was identified using the Centers for Disease Control and Prevention definitions (20). The underlying illnesses were classified as rapidly fatal, ultimately fatal, and nonfatal according to the modified McCabe and Jackson classification (21, 22). Neutropenia was defined as a neutrophil count lower than 1,000/mm3. Severity of BSI was distinguished as sepsis, severe sepsis, and septic shock, as proposed by the International Sepsis Definitions Conference (14). Treatment given before obtaining susceptibility results was defined as empirical. Antimicrobial treatment with agents that had no in vitro activity and/or treatment for less than 48 h was considered inadequate. Antimicrobial therapy given after the susceptibility data had become available was defined as “definitive.” Treatment regimens were classified as monotherapy (treatment with one in vitro active agent) or combination therapy (treatment with two or more in vitro active agents).
Statistical analysis.
Data were processed and analyzed using the STATA statistical software (StataCorp LP). The outcome measured was the all-cause mortality within 28 days after the onset of bacteremia. Patients discharged before day 28 were considered survivors. Mortality was analyzed as a binary outcome (yes/no) as well as survival time data with patients discharged before day 28 or hospitalized and alive at day 28 considered censored observations. Survivors and nonsurvivors were compared to identify factors associated with mortality. Bivariate associations between the binary outcome of 28-day mortality and patients' characteristics were assessed using the χ2 test for categorical variables and the Student t test (for normally distributed variables) or the Mann-Whitney U test (for nonnormally distributed variables) for continuous variables. Kaplan-Meier estimates of the probability of survival were obtained. Survival curves were compared between groups using the log rank test. A Cox proportional hazards model was used to identify factors independently associated with mortality (12 patients who received definitive treatment with no active drug were not included in this model). Sets of variables that were either identified from univariate analysis as statistically significant or reported in the literature as being associated with the outcome were entered into the model, and their contribution was assessed using the likelihood ratio test.
RESULTS
Patients.
Of the 338 patients diagnosed with K. pneumoniae BSIs (240 in hospital A and 98 in hospital B) during the study period, 205 (170 in hospital A and 35 in hospital B) were infected with CP-Kps and included in the analysis (Table 1). The incidence rates of CP-Kp bacteremia were 2.8 and 1.2/10,000 patient days for hospitals A and B, respectively. One hundred eighteen patients (57.6%) were male, and 87 (42.4%) were female. The mean patient age was 62.7 years (standard deviation [SD], 17.5 years) (median, 66 years; range, 17 to 90 years). All episodes of CP-Kp BSIs were either hospital acquired (92.7%) or health care associated (7.3%), while none was community acquired. The majority (56.6%) of infections occurred in intensive care units (ICUs), and 46.8% of the patients had rapidly fatal or ultimately fatal underlying disease. The median duration of hospitalization before the onset of bacteremia was 18 days (interquartile range [IQR], 10 to 34 days). The probable source of bacteremia was the lung in 43 patients, the abdomen in 29, the intravascular catheter in 22, the genitourinary tract in 19, the skin or soft tissue in 6, and the central nervous system (CNS) in 3. In 83 cases, no definite portal of entry could be detected.
TABLE 1.
Univariate analysis of factors associated with all-cause 28-day mortality of 205 patients with carbapenemase-producing K. pneumoniae bloodstream infections
| Variable | Result for patients who: |
P | |
|---|---|---|---|
| Survived (n = 123) | Died (n = 82) | ||
| Age, mean yr (SD) | 60.2 (18.9) | 66.4 (14.5) | 0.008 |
| Gender, no. (%) | 0.018 | ||
| Male | 79 (66.9) | 39 (33.1) | |
| Female | 44 (50.6) | 43 (49.4) | |
| Acquisition of infection, no. (%) | 0.584 | ||
| Health care associated | 8 (53.3) | 7 (46.7) | |
| Hospital acquired | 115 (60.5) | 75 (39.5) | |
| Hospital, no. (%) | 1.00 | ||
| A | 102 (60.0) | 68 (40.0) | |
| B | 21 (60.0) | 14 (40.0) | |
| Ward at onset of BSI, no. (%) | 0.687 | ||
| Non-ICU | 52 (58.4) | 37 (41.6) | |
| ICU | 71 (61.2) | 45 (38.8) | |
| Charlson comorbidity index, median (IQR) | 1 (0–2) | 2 (1–4) | 0.001 |
| Severity of underlying disease, no. (%)a | <0.001 | ||
| Nonfatal | 87 (79.8) | 22 (20.2) | |
| Ultimately fatal | 21 (39.6) | 32 (60.4) | |
| Rapidly fatal | 15 (34.9) | 28 (65.1) | |
| Neutropenia, no. (%) | 0.030 | ||
| No | 117 (62.2) | 71 (37.8) | |
| Yes | 6 (35.3) | 11 (64.7) | |
| Severity of sepsis, no. (%) | <0.001 | ||
| Sepsis | 96 (69.6) | 42 (30.4) | |
| Severe sepsis | 12 (42.9) | 16 (57.1) | |
| Septic shock | 15 (38.5) | 24 (61.5) | |
| Polymicrobial BSI, no. (%) | 0.049 | ||
| No | 95 (64.2) | 53 (35.8) | |
| Yes | 28 (49.1) | 29 (50.9) | |
| Source of BSI, no. (%) | 0.844 | ||
| Non-urinary tract | 112 (60.2) | 74 (39.8) | |
| Urinary tract | 11 (57.9) | 8 (42.1) | |
| Carbapenemase type(s), no. (%) | 0.090 | ||
| VIM | 30 (71.4) | 12 (28.6) | |
| KPC/KPC + VIM | 93 (57.1) | 70 (42.9) | |
| ST, no. (%) | 0.269 | ||
| ST258 | 48 (55.8) | 38 (44.2) | |
| Other | 52 (64.2) | 29 (35.8) | |
| Time to initiation of at least 1 active drug, no. (%) | |||
| >48 h | 50 (53.2) | 44 (46.8) | 0.086 |
| ≤48 h | 73 (65.8) | 38 (34.2) | |
| Empirical treatment, no. (%) | 0.250 | ||
| No active drug | 50 (55.5) | 40 (44.5) | |
| At least 1 active drug | 73 (63.5) | 42 (36.5) | |
| Definitive treatment, no. (%)b | 0.018 | ||
| Monotherapy | 40 (55.6) | 32 (44.4) | |
| Combination therapy | 75 (72.8) | 28 (27.2) | |
Microorganisms.
Of the 205 CP-Kps, 163 (79.5%) produced KPC-2 (36 of which coproduced VIM-1). The remaining 42 isolates produced VIM-1. All CP-Kps were resistant to penicillin-inhibitor combinations and expanded-spectrum cephalosporins. The vast majority of the isolates were also resistant to ciprofloxacin (97.6%) and co-trimoxazole (90.3%). Resistance frequencies to meropenem, imipenem, and doripenem were 53.7, 52.7, and 57.1%, respectively. The most active drugs were tigecycline and colistin, although considerable numbers of isolates exhibited resistance to these agents (15.1 and 25.4%, respectively). The resistance frequency to gentamicin was 31.2%, and that to amikacin was 68.3%. PFGE typing of CP-Kps indicated 21 different genomic profiles; however, most isolates (n = 167) were grouped into eight types according to standard criteria (18). Multilocus sequence typing of two to four isolates from each of the main eight PFGE types revealed the predominance of the STs 258 (n = 86), 147 (n = 38), 383 (n = 31), and 945 (n = 12).
Treatment.
Antibiotic therapy was selected at the discretion of the attending physician based on the susceptibility results provided by the respective clinical laboratory. Infection source control, including line removal in 22 cases, debridement in 4, and percutaneous or surgical drainage in 6, was performed in a timely manner in all cases. The definitive antimicrobial therapy was initiated 48 to 72 h after the clinical onset of bacteremia. The total daily dose of colistin was 9 million IU given in two or three divided dosages, and for tigecycline the total daily dose was 100 to 200 mg administered in two divided dosages. High doses of carbapenems were used: 1 g for imipenem and doripenem and 2 g for meropenem every 8 h. Aminoglycosides were administered once daily: 5 mg/kg for gentamicin and 15 mg/kg for amikacin. Dosages were adjusted to creatinine clearance when indicated (23).
For empirical treatment, 111 patients (54.1%) received at least one active drug within 48 h after the onset of bacteremia, while 94 (45.9%) received no active drug during the first 48 h of the infection. For definitive treatment, 175 patients (85.4%) received at least one active drug and 12 (5.9%) received therapy with no active drug. Eight of the latter patients were infected with panresistant CP-Kps. Eighteen patients died within 48 h after the onset of bacteremia, before the susceptibility results were available. Of 175 patients who were treated with at least one active drug, 103 received combination therapy (31 received a carbapenem-containing regimen and 72 a carbapenem-sparing regimen). The remaining 72 patients received monotherapy. The antibiotic regimens used are presented in Table 2. The monotherapy and combination therapy groups of patients were comparable in terms of stay in ICU at the onset of BSI, severity of underlying diseases, comorbidity conditions, presence of neutropenia, severity of sepsis, and source of bacteremia (Table 3). Surprisingly, patients with advanced age were more likely to have received monotherapy. For this observation, no plausible explanation could be provided.
TABLE 2.
Outcome of patients with carbapenemase-producing K. pneumoniae bloodstream infections according to treatment regimen
| Antimicrobial regimen | No. of patients |
Mortality, % | ||
|---|---|---|---|---|
| Total | Survived | Died | ||
| Combination therapy | 103 | 75 | 28 | 27.2 |
| Carbapenem-containing regimen | 31 | 25 | 6 | 19.3 |
| Carbapenem + tigecycline + aminoglycoside or colistin | 11 | 0 | ||
| Carbapenem + tigecycline | 2 | 2 | ||
| Carbapenem + aminoglycoside | 8 | 1 | ||
| Carbapenem + colistin | 4 | 3 | ||
| Carbapenem-sparing regimen | 72 | 50 | 22 | 30.6 |
| Tigecycline + aminoglycoside + colistin | 8 | 3 | ||
| Tigecycline + aminoglycoside | 11 | 9 | ||
| Tigecycline + colistin | 16 | 5 | ||
| Aminoglycoside + colistin | 12 | 5 | ||
| Other | 3 | 0 | ||
| Monotherapy | 72 | 40 | 32 | 44.4 |
| Tigecycline | 16 | 11 | ||
| Colistin | 10 | 12 | ||
| Aminoglycoside | 7 | 2 | ||
| Carbapenem | 5 | 7 | ||
| Other | 2 | 0 | ||
| No active agent | 12a | 8 | 4 | 33.3 |
Eight patients were infected with panresistant Klebsiella pneumoniae.
TABLE 3.
Characteristics of patients with carbapenemase-producing K. pneumoniae bloodstream infections according to treatment regimen
| Characteristic | Result for patients on: |
P | |
|---|---|---|---|
| Monotherapy (n = 72) | Combination therapy (n = 103) | ||
| Age, mean yr (SD) | 66.5 (14.4) | 58.4 (19.5) | 0.002 |
| Gender, no. (%) | 0.846 | ||
| Male | 43 (59.7) | 60 (58.3) | |
| Female | 29 (40.3) | 43 (41.7) | |
| Ward at onset of BSI, no. (%) | 0.427 | ||
| Non-ICU | 33 (45.8) | 41 (39.8) | |
| ICU | 39 (54.2) | 62 (60.2) | |
| Charlson comorbidity index, median (IQR) | 2 (0–3) | 2 (0–3) | 0.474 |
| Severity of underlying disease, no. (%) | 0.376 | ||
| Rapidly fatal | 18 (25.0) | 17 (16.5) | |
| Ultimately fatal | 16 (22.2) | 27 (26.2) | |
| Nonfatal | 38 (52.8) | 59 (57.3) | |
| Neutropenia, no. (%) | 0.483 | ||
| No | 65 (90.3) | 96 (93.2) | |
| Yes | 7 (9.7) | 7 (6.8) | |
| Source of BSI, no. (%) | 0.333 | ||
| Non-urinary tract | 65 (90.3) | 97 (94.2) | |
| Urinary tract | 7 (9.7) | 6 (5.8) | |
| Polymicrobial BSI, no. (%) | 0.418 | ||
| No | 55 (76.4) | 73 (70.9) | |
| Yes | 17 (23.6) | 30 (29.1) | |
| Severity of sepsis, no. (%) | 0.629 | ||
| Sepsis | 49 (68.1) | 73 (70.9) | |
| Severe sepsis | 8 (11.1) | 14 (13.6) | |
| Septic shock | 15 (20.8) | 16 (15.5) | |
Outcome.
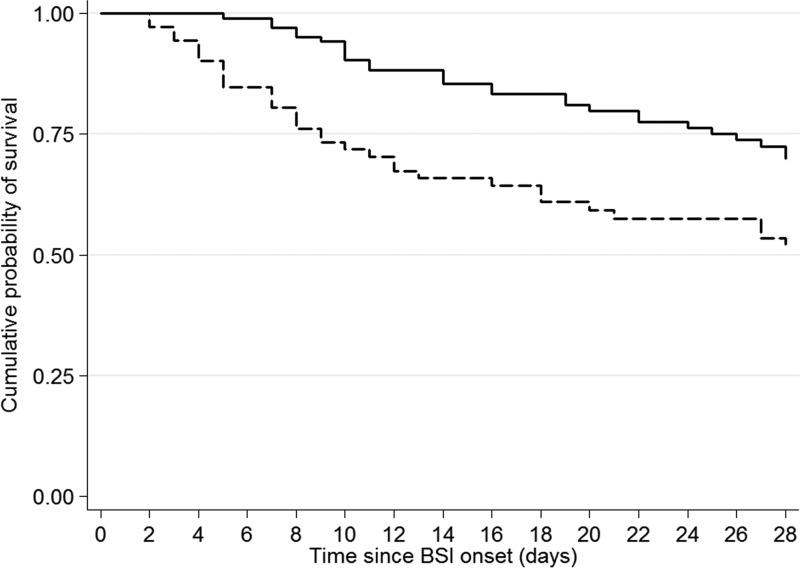
The all-cause 28-day mortality was 40% (82 of 205 patients died). The effects of host-, infection-, and treatment-related factors on 28-day mortality were assessed in a univariate analysis (Table 1). Adverse outcome appeared to be more likely among females and patients with advanced age, a higher Charlson comorbidity index, neutropenia, ultimately fatal or rapidly fatal underlying disease, polymicrobial bacteremia, and severe sepsis or septic shock. Strain-related characteristics (type of carbapenemase, PFGE types, and STs) as well as the timing of administration of effective antimicrobial therapy did not have any apparent predictive value in the outcome. Mortality was higher in patients who received monotherapy than in those treated with combination schemes (44.4% versus 27.2%; P = 0.018). The Kaplan-Meier survival estimates over time were also significantly better for patients treated with combination therapy versus monotherapy (P = 0.003) (Fig. 1). Within the various treatment groups, the lowest mortality rate (19.3%) was observed in patients treated with carbapenem-containing combinations, mainly due to the high efficacy of the triple drug schemes and the carbapenem-aminoglycoside regimens (Table 2). Of note, among the patients who received a carbapenem in combination with other active agents, the mortality rate increased from 19.3% for a carbapenem MIC of ≤8 μg/ml to 35.5% for a MIC of >8 μg/ml. Moreover, the therapeutic benefit provided by carbapenems was also reduced when this class of agents were combined with inactive drugs (Table 4).
FIG 1.
Kaplan-Meier survival estimates of patients with carbapenemase-producing K. pneumoniae bloodstream infections according to treatment regimen: combination therapy (continuous line) versus monotherapy (dotted line). P = 0.003 (log rank test).
TABLE 4.
Outcomes of 79 patients with CP-Kp bloodstream infections treated with carbapenem combinations stratified by carbapenem MIC
| Carbapenem MIC (μg/ml) | Result for carbapenem combination with: |
|||
|---|---|---|---|---|
|
In vitro active agent(s) |
In vitro inactive agent(s) |
|||
| No. of patients who survived/died | Mortality, % | No. of patients who survived/died | Mortality, % | |
| ≤8 | 25/6 | 19.3 | 5/7 | 58.3 |
| >8 | 20/11 | 35.5 | 4/2 | 33.3 |
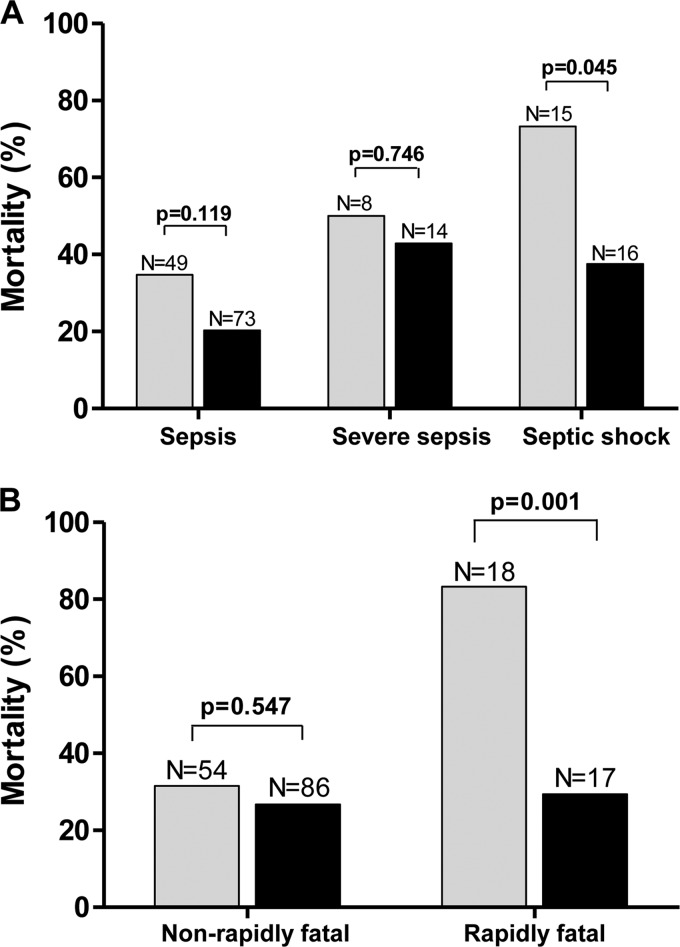
The effect of treatment (combination therapy versus monotherapy) was further assessed in different subsets of patients (Fig. 2). Monotherapy had a declining therapeutic effect at different stages of sepsis, resulting in increasing mortality in patients with sepsis (34%), severe sepsis (49%), and septic shock (73%) as well as in patients with rapidly fatal disease (83.3% mortality) compared to those with nonfatal or ultimately fatal disease (31.5% mortality). In contrast, combination therapy equalized the effect on different patient categories and provided a significant therapeutic advantage over monotherapy in high-risk groups—patients with rapidly fatal underlying disease (odds ratio [OR] of surviving for those receiving combination versus monotherapy, 0.08; 95% confidence interval [CI], 0.01 to 0.52; P = 0.001) (Fig. 2A) and patients with septic shock (OR of surviving for those receiving combination versus monotherapy: 0.22; 95% CI, 0.05 to 1.0; P = 0.045) (Fig. 2B).
FIG 2.
Graphic presentation of the effect of treatment (monotherapy [gray bars] versus combination therapy [black bars]) by severity of underlying disease (A) and by severity of sepsis (B). Numbers above columns indicate the number of patients.
By entering the host-, infection-, and treatment-related variables with potential effect on mortality in the Cox proportional hazards model, the factors ultimately fatal disease (hazards ratio [HR] of death compared to nonfatal, 3.25; 95% CI, 1.51 to 7.03; P = 0.003), rapidly fatal underlying disease (HR of death compared to nonfatal, 4.20; 95% CI, 2.19 to 8.08; P < 0.001), and septic shock (HR of death compared to sepsis, 2.15; 95% CI, 1.16 to 3.96; P = 0.015) were identified as independent predictors of adverse outcome, whereas combination therapy remained an independent predictor of survival (HR of death for monotherapy versus combination therapy, 2.08; 95% CI, 1.23 to 3.51; P = 0.006) (Table 5).
TABLE 5.
Cox proportional hazards model of factors associated with all-cause 28-day mortality in 175 patients with carbapenemase-producing K. pneumoniae bloodstream infections
| Variable | HR (95% CI) | P |
|---|---|---|
| Age (per 1-yr increase) | 1.01 (0.99–1.03) | 0.198 |
| Gender (female/male) | 1.45 (0.82–2.55) | 0.198 |
| Severity of underlying disease | ||
| Ultimately fatal/nonfatal | 3.25 (1.51–7.03) | 0.003 |
| Rapidly fatal/nonfatal | 4.20 (2.19–8.08) | <0.001 |
| Charlson comorbidity index | 1.01 (0.85–1.20) | 0.879 |
| Severity of sepsis | ||
| Severe sepsis/sepsis | 1.63 (0.74–3.59) | 0.227 |
| Septic shock/sepsis | 2.15 (1.16–3.96) | 0.015 |
| Polymicrobial bacteremia | ||
| Yes/no | 1.29 (0.74–2.23) | 0.371 |
| Ward at onset of bacteremia | ||
| ICU/non-ICU | 1.36 (0.72–2.57) | 0.342 |
| Monotherapy/combination therapy | 2.08 (1.23–3.51) | 0.006 |
DISCUSSION
The findings presented herein demonstrate that severe underlying disease, septic shock, and treatment with a single active agent are independent predictors for death in patients with CP-Kp BSIs, while combination therapy provides significant survival benefit that appears to be more pronounced when a carbapenem is included in the regimen.
During the study period, the majority of K. pneumoniae BSIs (60.6%) were caused by carbapenemase-positive organisms, 79.5% of which produced KPC or KPC and VIM and the remainder of which produced VIM. The patient population affected by CP-Kps had several distinct characteristics; all episodes of BSIs were either hospital or health care associated. Also, approximately half of the infections occurred in critically ill patients, many of whom had severe underlying disease. The all-cause 28-day mortality of CP-Kp-infected patients was higher than that observed in the patients infected with non-CP-Kps (40% versus 23%) (data not shown) and in accordance with those reported by others (10, 11).
In view of the high mortality associated with CP-Kp infections, as shown herein and in other studies (10, 11, 24, 25), it could be hypothesized that at least some CP-Kp strains possess an increased pathogenic potential. Yet, this notion has been challenged by our analysis, in which no particular strain types were associated with adverse outcomes. Moreover, recent experimental findings revealed that the predominant KPC ST258 clone—responsible for a significant proportion (51.5%) of BSIs in the present study—should be considered a low-virulence organism (26). Based on these observations, it appears that other factors, not related to virulent traits of the organisms, should account for the apparent high mortality associated with CP-Kp infections.
Indeed, the data presented here suggest that the nature and severity of underlying diseases as well as the presence of septic shock were of paramount importance in determining survival or death. Also, antibiotic therapy per se had a significant impact on outcome; treatment with a single antimicrobial agent was independent predictor for death, while combination therapy was associated with lower risk of mortality. The latter effect was more apparent among the patients in septic shock as well as in those with severe underlying diseases.
To further assess the efficacy of treatment schemes, we divided the patients who received combination therapy into two groups on the basis of inclusion of a carbapenem. The respective analysis showed that the carbapenem-containing regimens resulted in the lowest mortality rate (19.3%), potentially indicating additive or even synergistic effects: however, neither effect was further explored. Analogous findings have been presented in two studies conducted in the United States and Italy that included cohorts of patients infected with KPC-producing K. pneumoniae (10, 11). Also, a recent systemic review of the literature, including 432 patients with CP-Kp BSIs, came to the conclusion that treatment with combination schemes, especially those containing a carbapenem, were far superior to either colistin or tigecycline monotherapy (27). It must be pointed out, however, that the positive “carbapenem effect” is rather unlikely to occur against CP-Kps either when these drugs are used in monotherapy or when their MICs are ≥16 μg/ml, a limit that is in line with the EUCAST breakpoint and the respective pharmacokinetic/pharmacodynamic (PK/PD) features of this group of β-lactams (1, 11, 12, 28).
Based on the imipenem and meropenem Etest MIC distributions, approximately half of the patients (n = 105) for whom the infecting organisms had MICs of ≤8 μg/ml, could have received the potential therapeutic benefit of a carbapenem-containing combination. Nevertheless, in the present series, only 31 patients received such treatment. Apparently, this happened since the treatment decision was based on the susceptibility results reported by automated systems, which on several occasions had classified the infecting organisms as carbapenem resistant (40% of the isolates) (data not shown), while they were tested as “not resistant” by Etest (29–31). The problem may be partly due to the fact that at the time of the study, carbapenem susceptibility data were subjected to expert rules interpretation. Yet, a systematic comparison of the performance of broth microdilution, Etest, and the main automated systems using CP-Kps from this setting is warranted.
Another treatment-related factor that could have affected outcome is the time to initiation of effective antibiotic treatment. Although it has been widely accepted that prompt initiation of treatment during the initial critical hours of the infection process has an important impact on survival (11, 32), in the present study only a vague association was found between the timing of effective treatment and outcome. We cannot provide a plausible explanation for this observation; nevertheless, other investigators have reported similar findings for this type of infection (9, 10, 33).
The present study has several potential caveats. First, it was an observational study without matching for confounders between the treatment groups. Thus, several variables with potential effects on mortality might have influenced the results. However, with the exception of age, these variables were balanced in the two treatment groups (monotherapy versus combination); additionally, multivariate analysis was performed to control for such confounders. Second, at least during the first year of the study, it was not common practice to administer a loading dose of colistin. Thus, in an undefined number of patients, the PK/PD targets for the latter agent might have been attained with some delay. Notwithstanding its limitations, this study, which to our knowledge included the so-far largest cohort of CP-Kp BSIs, provides information that may assist physicians to adopt more effective approaches for treatment of CP-Kp infections.
ACKNOWLEDGMENTS
This study was supported by the National and Kapodistrian University of Athens Special Account for Research Grants.
We thank Maria Grammatikou for technical assistance with the phenotypic tests.
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25:682–707. 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13:785–796. 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37:415–419. 10.1016/j.ijantimicag.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 4.Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, Wang JT, Wang LS, Siu LK, Yeh KM. 2013. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 8:e69428. 10.1371/journal.pone.0069428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, Saporito MA, Verde MS, Tetamo R, Palma DM. 2012. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011 Euro Surveill. 17:20248. [PubMed] [Google Scholar]
- 6.Kontopidou F, Giamarellou H, Katerelos P, Maragos A, Kioumis I, Trikka-Graphakos E, Valakis C, Maltezou HC, Group for the Study of KPC-producing Klebsiella pneumoniae Infections in Intensive Care Units 18 July 2013. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin. Microbiol. Infect. 10.1111/1469-0691.12341 [DOI] [PubMed] [Google Scholar]
- 7.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028–1033. 10.1128/AAC.01020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. 2012. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin. Microbiol. Infect. 18:439–448. 10.1111/j.1469-0691.2012.03823.x [DOI] [PubMed] [Google Scholar]
- 9.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17:1798–1803. 10.1111/j.1469-0691.2011.03514.x [DOI] [PubMed] [Google Scholar]
- 10.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 56:2108-2113. 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin. Infect. Dis. 55:943–950. 10.1093/cid/cis588 [DOI] [PubMed] [Google Scholar]
- 12.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin. Microbiol. Infect. 17:1135–1141. 10.1111/j.1469-0691.2011.03553.x [DOI] [PubMed] [Google Scholar]
- 13.Petrosillo N, Giannella M, Lewis R, Viale P. 2013. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev. Anti Infect. Ther. 11:159–177. 10.1586/eri.12.162 [DOI] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2003. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31:1250–1256. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:379–383 [DOI] [PubMed] [Google Scholar]
- 16.European Committee on Antimicrobial Susceptibility Testing. 2013. Breakpoint tables for interpretation of MICs and zone diameters, version 3.1, 2013. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf
- 17.Food and Drug Administration. Highlights of prescribing information Tygacil. Food and Drug Administration, Silver Spring, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf [Google Scholar]
- 18.Giakkoupi P, Papagiannitsis CC, Miriagou V, Pappa O, Polemis M, Tryfinopoulou K, Tzouvelekis LS, Vatopoulos AC. 2011. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009–10). J. Antimicrob. Chemother. 66:1510–1513. 10.1093/jac/dkr166 [DOI] [PubMed] [Google Scholar]
- 19.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 137:791–797. 10.7326/0003-4819-137-10-200211190-00007 [DOI] [PubMed] [Google Scholar]
- 20.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting Am. J. Infect. Control 36:309–332. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 21.McCabe MR, Jackson GG. 1962. Gram-negative bacteremia. II. Clinical, laboratory and therapeutic observations. Arch. Intern. Med. 110:856–864 [Google Scholar]
- 22.ECDC 2011. Technical report. Point prevalence survey of health-care associated infections and antimicrobial use in European acute care hospitals (May). European Centre for Disease Prevention and Control, Stockholm, Sweden: http://www.ecdc.europa.eu/en/publications/publications/0512-ted-pps-hai-antimicrobial-use-protocol.pdf [Google Scholar]
- 23.Gilbert ND, Moellering RC, Eliopoulos GM, Chambers HF, Saag SA. (ed). 2010. The Sanford guide to antimicrobial therapy. Antimicrobial Therapy, Inc, Sperryville, VA [Google Scholar]
- 24.Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, Tsioka A, Roilides E, Sofianou D, Gritsi-Gerogianni N. 2010. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect. Control Hosp. Epidemiol. 31:1250–1256. 10.1086/657135 [DOI] [PubMed] [Google Scholar]
- 25.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N, SEERBIO-GRAB Network 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 19:E23–E30. 10.1111/1469-0691.12070 [DOI] [PubMed] [Google Scholar]
- 26.Tzouvelekis LS, Miriagou V, Kotsakis SD, Spyridopoulou K, Athanasiou E, Karagouni E, Tzelepi E, Daikos GL. 2013. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob. Agents Chemother. 57:5144–5146. 10.1128/AAC.01052-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daikos GL, Markogiannakis A, Souli M, Tzouvelekis LS. 2012. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae: a clinical perspective. Expert Rev. Anti Infect. Ther. 10:1393–1404. 10.1586/eri.12.138 [DOI] [PubMed] [Google Scholar]
- 28.Kuti JL, Dandekar P, Nightgale CH, Nicolau DP. 2003. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J. Clin. Pharmacol. 43:1116–1123. 10.1177/0091270003257225 [DOI] [PubMed] [Google Scholar]
- 29.Giakkoupi P, Tzouvelekis LS, Daikos GL, Miriagou V, Petrikkos G, Legakis NJ, Vatopoulos AC. 2005. Discrepancies and interpretation problems in susceptibility testing of VIM-1-producing Klebsiella pneumoniae isolates. J. Clin. Microbiol. 43:494–496. 10.1128/JCM.43.1.494-496.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson KS, Cornish NE, Hong SG, Hemrick K, Herdt C, Moland ES. 2007. Comparison of Phoenix and VITEK 2 extended-spectrum-beta-lactamase detection tests for analysis of Escherichia coli and Klebsiella isolates with well-characterized beta-lactamases. J. Clin. Microbiol. 45:2380–2384. 10.1128/JCM.00776-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulik CC, Fauntleroy KA, Jenkins SG, Abuali M, LaBombardi VJ, Nicolau DP, Kuti JL. 2010. Comparison of meropenem MICs and susceptibilities for carbapenemase-producing Klebsiella pneumoniae isolates by various testing methods. J. Clin. Microbiol. 48:2402–2406. 10.1128/JCM.00267-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwaber MJ, Carmeli Y. 2007. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J. Antimicrob. Chemother. 60:913–920. 10.1093/jac/dkm318 [DOI] [PubMed] [Google Scholar]
- 33.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106. 10.1086/592412 [DOI] [PubMed] [Google Scholar]