Abstract
Transcription factor Nrf2 (NF-E2-related factor 2) coordinately regulates cytoprotective gene expression, but under unstressed conditions, Nrf2 is degraded rapidly through Keap1 (Kelch-like ECH-associated protein 1)-mediated ubiquitination. Nrf2 harbors two Keap1-binding motifs, DLG and ETGE. Interactions between these two motifs and Keap1 constitute a key regulatory nexus for cellular Nrf2 activity through the formation of a two-site binding hinge-and-latch mechanism. In this study, we determined the minimum Keap1-binding sequence of the DLG motif, the low-affinity latch site, and defined a new DLGex motif that covers a sequence much longer than that previously defined. We have successfully clarified the crystal structure of the Keap1-DC-DLGex complex at 1.6 Å. DLGex possesses a complicated helix structure, which interprets well the human-cancer-derived loss-of-function mutations in DLGex. In thermodynamic analyses, Keap1-DLGex binding is characterized as enthalpy and entropy driven, while Keap1-ETGE binding is characterized as purely enthalpy driven. In kinetic analyses, Keap1-DLGex binding follows a fast-association and fast-dissociation model, while Keap1-ETGE binding contains a slow-reaction step that leads to a stable conformation. These results demonstrate that the mode of DLGex binding to Keap1 is distinct from that of ETGE structurally, thermodynamically, and kinetically and support our contention that the DLGex motif serves as a converter transmitting environmental stress to Nrf2 induction as the latch site.
INTRODUCTION
Transcription factor Nrf2 (NF-E2-related factor 2) plays important roles in the cellular defense against various electrophilic and oxidative stresses (1–3). Nrf2 binds ARE/EpRE (antioxidant/electrophile response element) by forming a heterodimer with small Maf proteins and activates the transcription of a battery of cytoprotective genes, including genes encoding phase II detoxifying enzymes and anti-oxidative-stress enzymes/proteins that protect cells from toxic chemicals and reactive oxygen species (2). We previously found that Keap1 (Kelch-like ECH-associated protein 1) negatively controls Nrf2 signaling (4). Keap1 contains several functional domains: from the N terminus, the domains include the BTB (Broad complex, Tramtrack, and Bric-a-Brac) domain, the IVR (intervening region), the DGR (double glycine repeat) domain, and the CTR (C-terminal region) (2). The BTB domain is required for homodimerization, while the IVR contains two reactive cysteine residues, Cys273 and Cys288, that are important for the electrophilic activation of Nrf2. The DGR domain and the CTR form a β-propeller structure in collaboration, and we refer to the DGR domain and the CTR collectively as the DC domain (5). The Keap1 DC domain interacts with the Neh2 domain of Nrf2.
Under unstressed conditions, Nrf2 is rapidly ubiquitinated by Keap1-Cul3 (Cullin 3) E3 ligase. One Nrf2 molecule associates with a Keap1 homodimer or two Keap1 molecules by exploiting two distinct binding motifs within the Neh2 domain, the low-affinity DLG motif and the high-affinity ETGE motif. This two-site binding of Nrf2 to the Keap1 homodimer allows for the efficient ubiquitination of Nrf2, which leads to the rapid degradation of Nrf2 through the proteasome pathway (6). There are seven lysine residues between the DLG and ETGE motifs; thus, the two-site binding of Nrf2 to Keap1 is assumed to be critical for efficient Nrf2 ubiquitination (7–9). This rapid turnover of Nrf2 maintains a constitutively low cellular Nrf2 level. In contrast, upon exposure to toxic electrophiles, the electrophiles modify reactive cysteine residues of Keap1 and disrupt the two-site binding process by altering the conformation of the Keap1 homodimer. Consequently, Nrf2 is no longer degraded and accumulates in the nucleus, where it activates the expression of cytoprotective genes. Indeed, experimental results supporting the notion that the low-affinity DLG site is dissociated from Keap1 upon exposure to electrophiles have been accumulating (9–11). On the basis of these observations, we have proposed a two-site binding hinge-and-latch model in which the dissociation of the DLG-Keap1 interaction is important for the sensing of electrophilic stress (5).
There are ample lines of evidence that dysfunction of the Keap1-Nrf2 system is linked to various human diseases, including cancers (12–14). Nrf2-deficient mice are prone to both chemical toxicity and tumorigenesis (15–18). Recently, somatic mutations of human KEAP1 and NRF2 have also been identified in various types of cancers. Importantly, all somatic mutations in NRF2 reside near the two degron sites, the DLG and ETGE motifs (3). These mutations impair two-site recognition between the KEAP1 homodimer and the Neh2 domain of NRF2 (19), resulting in the constitutive activation or derepression of NRF2. NRF2 protects cancer cells against stress from the microenvironment or anticancer drugs and confers radiation resistance on cancer cells (20–22). Indeed, patients with somatic mutations in NRF2 or KEAP1 have a poor prognosis (19, 22–25).
We have noted that somatic mutations encompassing the DLG motif are distributed more widely than those of the ETGE motif. This observation is particularly interesting, as the DLG and Keap1 interaction has been suggested to be weak and to serve as a converter transmitting electrophilic insults to Nrf2 induction. Therefore, in this study, we have elucidated the molecular and structural basis of the DLG-Keap1 interaction. We found that the DLG motif is much longer than the previously defined “classical” DLG motif; thus, we refer to the extended DLG motif as the DLGex motif, which contains both the classical DLG motif and the DIDLID element (7, 8). We have successfully clarified the crystal structure of Keap1-DC in complex with the DLGex peptide (Met17 to Gln51) at a 1.6 Å resolution. The crystal structure has revealed that DLGex possesses a complicated helix structure, which interprets well the human-cancer-derived loss-of-function mutations in DLGex. We also have noticed that the modes of DLGex and ETGE binding to Keap1 are quite distinct from each other. Keap1-DLGex binding is characterized thermodynamically as both enthalpy and entropy driven and kinetically as a fast-on and fast-off mode. In contrast, ETGE-Keap1 binding is characterized as purely enthalpy driven and seems to involve a two-state reaction that leads to a stable conformation. These unique features of the DLGex-Keap1 interaction support the molecular and structural basis of the rapid response of Nrf2 to oxidative and electrophilic stress.
MATERIALS AND METHODS
Plasmid constructions.
The expression vectors for mouse Keap1-DC (Ala321 to Thr609) fused to a 6×His tag at the N terminus and for mouse Keap1(Met1-Arg614) fused to a 6×His tag (Keap1-His) at the C terminus were described previously (11, 26). An expression vector, pET15b-Neh2, for 6×His-tagged mouse Nrf2-Neh2(Met1-Gly98) was described previously (9). A pET15b-Neh2ΔETGE construct that expresses 6×His-tagged mouse Neh2ΔETGE (Met1 to Gly98 without Glu79 to Glu82) and an S40E mutant form thereof were constructed by the inverse PCR technique with pET15b-Neh2 as the template. A pET15b-GST-Neh2 construct that expresses 6×His–glutathione S-transferase (GST)–mouse Neh2 was constructed by inserting a DNA fragment encoding GST into pET15b-Neh2(Met1-Gly98) via the NdeI site with an In-Fusion Advantage PCR cloning kit (Clontech). Expression vectors for deletion mutant forms of 6×His-GST-Neh2, including Neh2ΔDLGex (Met1 to Gly98 without Met17 to Gln51), were constructed by the inverse PCR technique with pET15b-GST-Neh2 as the template. Expression vectors for His-GST-Neh2(1-56) and mutant forms thereof were similarly constructed by the inverse PCR technique.
Protein expression and purification.
Keap1-DC with an N-terminally fused 6×His tag was expressed and purified as previously described (26). The Neh2(1-56)-based cancer mutant forms with N-terminally fused 6×His-GST tags were expressed in Escherichia coli BL21 gold/DE3 (Stratagene). The Neh2ΔETGE-based deletion mutant forms with N-terminally fused 6×His-GST tags, Neh2ΔETGE with an N-terminally fused 6×His tag, and Neh2ΔETGE S40E with an N-terminally fused 6×His tag were expressed in E. coli Rosetta 2/DE3 singles (Novagen). These bacteria were cultured in LB medium at 37°C. The production of recombinant fusion proteins was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM. After an additional 4-h incubation at 37°C, the cells were harvested and mechanically lysed by sonication (Branson Sonifier 450) on ice. The soluble-protein fraction was recovered by centrifugation at 10,000 × g for 30 min at 4°C. The 6×His-GST Neh2(1-56) mutant forms, 6×His-GST-Neh2ΔETGE deletion mutant forms, 6×His-Neh2ΔETGE, and 6×His-Neh2ΔETGE S40E were purified with a His Trap FF crude 1-ml column (GE Healthcare) and desalted with a Bio-Gel P6 desalting cartridge column (Bio-Rad) with the Profinia protein purification system (Bio-Rad). 6×His-Neh2ΔETGE and 6×His-Neh2ΔETGE S40E were further purified with a MonoQ 15/50 column (GE Healthcare). Keap1(Met1-Arg614) with a C-terminally fused 6×His tag was expressed in E. coli Rosetta 2/DE3 singles (Novagen), which were cultured in LB medium at 37°C. The production of Keap1-6×His was induced by adding IPTG to a final concentration of 0.5 mM. After an additional 20-h incubation at 15°C, the cells were harvested and mechanically lysed by sonication (Branson Sonifier 450) on ice. The soluble-protein fraction was recovered by centrifugation at 10,000 × g for 30 min at 4°C. Keap1-6×His was purified with a His Trap FF crude 1-ml column with the Profinia protein purification system and further purified with a Superdex 200 16/60 column (GE Healthcare).
Crystallization and data collection.
Keap1-DC with an N-terminal 6×His tag was purified and concentrated to 15 mg/ml by ultrafiltration in a buffer containing 1 mM TCEP [tris(2-carboxyethyl)phosphine] and 20 mM Tris-HCl (pH 7.7). A 1.0-μl aliquot of a mixture containing a 1:1.2 molar ratio of Keap1-DC to the DLGex peptide (17MDLIDILWRQDIDLGVSREVFDFSQRQKDYELEKQ51 from the Toray Research Center) was mixed with 1.0 μl of crystallization solution and equilibrated by sitting-drop vapor diffusion against 100 μl of reservoir solution. Crystals were obtained in a drop containing 100 mM sodium HEPES (pH 7.6), 0.2 M potassium acetate, and 20% (wt/vol) polyethylene glycol 3350 at 4°C. Diffraction data were collected under cryogenic conditions on BL44XU at SPring-8 (Harima, Japan). Data processing and reduction were conducted with HKL-2000 (27).
Structure determination and refinement.
The structure of the complex of Keap1-DC and the DLGex peptide was solved by molecular replacement with the free-form Keap1-DC structure (Protein Data Bank [PDB] accession number 1X2J) as a search model. Molecular replacement was performed with MOLREP (28). Several rounds of manual fitting and refinement were carried out with the programs Coot (29), REFMAC5 (30), and PHENIX (31). In the resultant Fo-Fc omit map, the electron density map clearly showed the presence of the DLGex motif of Nrf2. For a summary of the data reduction and refinement statistics, see Table 2.
TABLE 2.
Data collection and refinement statics
| Parameter | Value(s) |
|---|---|
| Data collection | |
| Wavelength (Å) | 0.9 |
| Unit cell a, b, c (Å) | 66.138, 84.394, 69.818 |
| Unit cell α, β, γ (degrees) | 90.000, 116.892, 90.000 |
| Space group | P21 |
| Resolution range (Å) | 50.00–1.58 (1.61–1.58)a |
| Total no. of reflections | 344,090 |
| No. of unique reflections | 93450 |
| Completeness (%) | 98.8 (88.7) |
| Redundancy | 3.7 (3.4) |
| Rmerge (%)b | 9.7 (25.3) |
| <I/s (I)> | 18.6 (7.8) |
| Refinement statistics | |
| Resolution range (Å) | 34.78–1.57 (1.61–1.57)a |
| No. of reflections used | 88,724 |
| Free R reflections (%) | 5.0 |
| Rwork/Rfreec (%) | 18.7/22.3 |
| RMSD bond length (Å) | 0.025 |
| RMSD bond angle (degrees) | 2.343 |
| No. of protein atoms | 5,021 |
| No. of solvent molecules | 489 |
| B factors (Å2) | |
| Protein (chains A, L) | 16.5, 16.2 |
| Peptide (chains B, M) | 25.3, 26.1 |
| Water | 25.1 |
| Ramachandran analysis | |
| % of residues in most-favored regions | 89.3 |
| % of residues in additional allowed regions | 10.3 |
| % of residues in generously allowed regions | 0.0 |
| % of residues in disallowed regions | 0.4 |
The values for the highest-resolution shell are in parentheses.
Rmerge = ∑hkl∑i|Ii(hkl) − 〈I(hkl)〉|/∑hkl∑iIi(hkl).
Rwork = ∑‖Fo| − |Fc‖/∑ |Fo|, where Fo and Fc are the observed and calculated structure factors, respectively. Rfree is the R value calculated for a subset of 5% of the randomly selected reflections not used in refinement.
GST pulldown assay.
The 6×His-GST-Neh2ΔETGE deletion mutant forms or 6×His-GST-Neh2(1-56) mutant forms were mixed with purified Keap1(Met1-Arg614)–6×His in washing buffer (125 mM Tris-HCl [pH 7.5], 150 mM NaCl). After a 1-h incubation at 4°C, the mixture was incubated with glutathione magnetic beads (Thermo) for 1 h at 4°C, washed three times with washing buffer, and eluted with elution buffer (125 mM Tris-HCl [pH 8.8], 150 mM NaCl, 50 mM reduced glutathione). Bound proteins were analyzed by SDS-PAGE, followed by staining with Oriole fluorescent gel stain (Bio-Rad). The band intensity of the Neh2(1-56) mutant forms and Keap1 was quantified with the ChemiDoc XRS+ system (Bio-Rad).
DSF.
Differential scanning fluorimetry (DSF) was performed with a Stratagene MX3005P machine with an excitation wavelength of 492 nm and an emission wavelength of 610 nm. Each sample was prepared in a total volume of 20 μl containing 2.2 μM Keap1-DC, 3.75× SYPRO orange, 100 mM sodium cacodylate trihydrate (pH 6.0), and each peptide at final concentrations ranging from 0 (reference) to 44 μM (20 times the Keap1-DC concentration). The samples were heated from 25 to 95°C at a rate of 1.0°C/min, and the melting temperature (Tm) was determined with EXCEL-based custom calculation software available at ftp://ftp.sgc.ox.ac.uk/pub/biophysics. The data were fitted to the Boltzmann equation with GraphPad Prism. ΔTm was calculated by subtracting the Tm of the reference cell from that of each peptide.
ITC.
Isothermal titration calorimetry (ITC) was performed at 25°C with the ITC200 system (MicroCal). All protein samples were purified as described above and then dialyzed by ultrafiltration with Amicon Ultra (Millipore) against 60 mM sodium phosphate buffer containing 1 mM Tris(2-carboxyethyl)phosphine hydrochloride (pH 8.1). Then, 1.5-μl aliquots of 1.0 mM DLGex peptide were injected 26 times at 2.5-min intervals from a stirring syringe (1,000 rpm) into the sample cell containing 200 μl of 0.067 mM Keap1-DC; 1.5-μl aliquots of 0.92 mM Neh2ΔETGE were injected 26 times at 2.5-min intervals from a stirring syringe into the sample cell containing 200 μl of 0.061 mM Keap1-DC; 1.5-μl aliquots of 0.98 mM Neh2ΔETGE S40E were injected 26 times at 2.5-min intervals from a stirring syringe into the sample cell containing 200 μl of 0.066 mM Keap1-DC; 1.0-μl aliquots of 0.40 mM ETGE peptide were injected 40 times at 2.0-min intervals from a stirring syringe into the sample cell containing 200 μl of 0.040 mM Keap1-DC. Binding data were analyzed with the computer program Origin, version 7.0, supplied by MicroCal.
SPR measurements.
Surface plasmon resonance (SPR) studies were performed with a Biacore-X100 (GE Healthcare). 6×His-GST-Neh2(1-56) or 6×His-GST-Neh2ΔDLG was diluted into 10 mM sodium acetate (pH 5.5) and immobilized on a CM5 chip to a level ranging from 1,200 to 1,500 resonance units by a standard amine coupling procedure. The binding of Neh2(1-56) to Keap1-DC was measured in multicycle mode without regeneration. The concentrations of the analyte (Keap1-DC) ranged from 0.25 to 8 μM. The binding of Neh2ΔDLG to Keap1-DC was measured in multicycle mode with regeneration with 10 mM glycine-HCl (pH 2.0). The concentrations of the analyte (Keap1-DC) ranged from 1.25 to 80 nM. Both experiments were performed in triplicate at 25°C in HBS-EP+ buffer containing 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% surfactant. Kinetic parameters were calculated with Biacore Evaluation software, version 2.0.
Protein structure accession number.
The atomic coordinates have been deposited in the PDB and assigned accession number 3WN7.
RESULTS
Somatic mutations encompassing Nrf2-DLG in human cancers impair binding between Nrf2-DLG and Keap1.
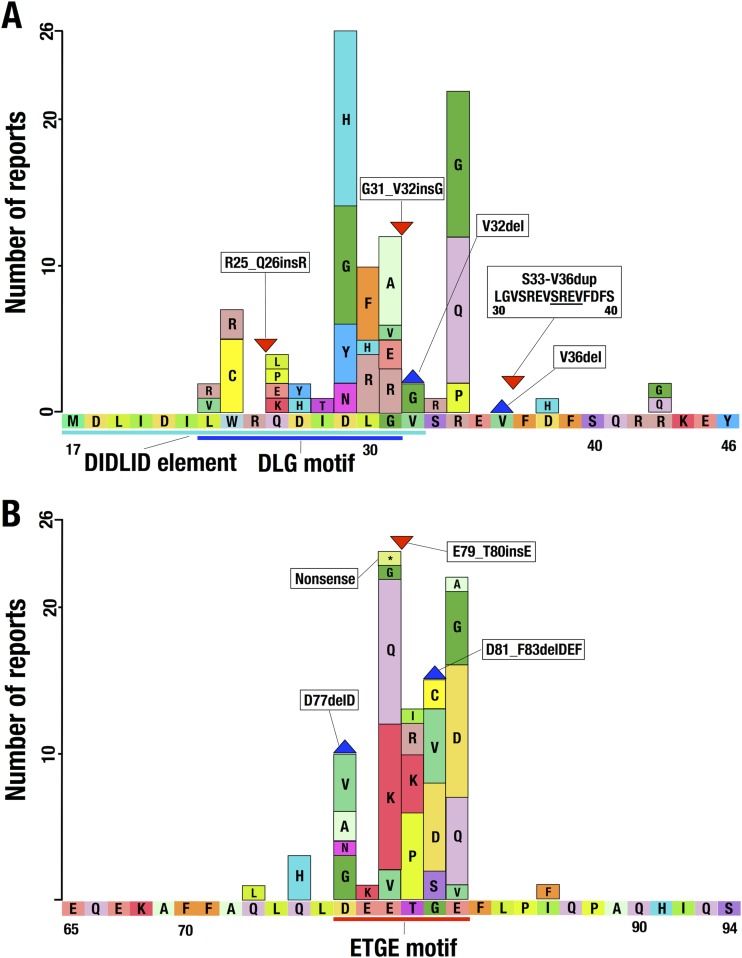
Somatic mutations identified in the NRF2 gene of human cancer patients are located within the 5′ region of the gene encoding the N-terminal Neh2 domain (3), especially in proximity to the DLG and ETGE motifs. We surveyed the current literature (32, 33) and the mutation database of the Catalogue of Somatic Mutations in Cancer at the Sanger Institute (http://www.sanger.ac.uk/cosmic) (34) and compiled the somatic mutations shown in Fig. 1. Through this analysis, we found that the somatic mutations encompassing the Nrf2-DLG motif cover a greater region (21 amino acids; Leu23 to Arg43) (Fig. 1A) than mutations in the ETGE motif (14 amino acids; Gln73 to Ile86) (Fig. 1B). While the mutations encompassing the ETGE motif converge around a region of just 6 amino acid residues (Asp77 to Glu82), mutations in the DLG motif are scattered over 11 amino acid residues (Trp24 to Arg34). Importantly, as shown in Fig. 1A, we have noticed that some of the mutations in the DLG motif are found outside the previously described DLG motif (Leu23 to Gly31) (dark blue line in Fig. 1A) (7) and DIDLID element (Met17 to Val32) (sky blue line in Fig. 1A) (8). These results suggest that the DLG motif binds in a manner distinct from binding by the ETGE motif.
FIG 1.
Distribution of somatic mutations within the NRF2 gene encompassing the Nrf2-DLG motif or the DIDLID element (A) and the Nrf2-ETGE motif (B). The horizontal and longitudinal axes represent the amino acid sequence and number of reports, respectively. The boxes, blue triangle, and red triangle indicate substitution mutations, a deletion mutation, and an insertion mutation, respectively. Sky blue, dark blue, and red lines indicate the DIDLID element, the DLG motif, and the ETGE motif, respectively.
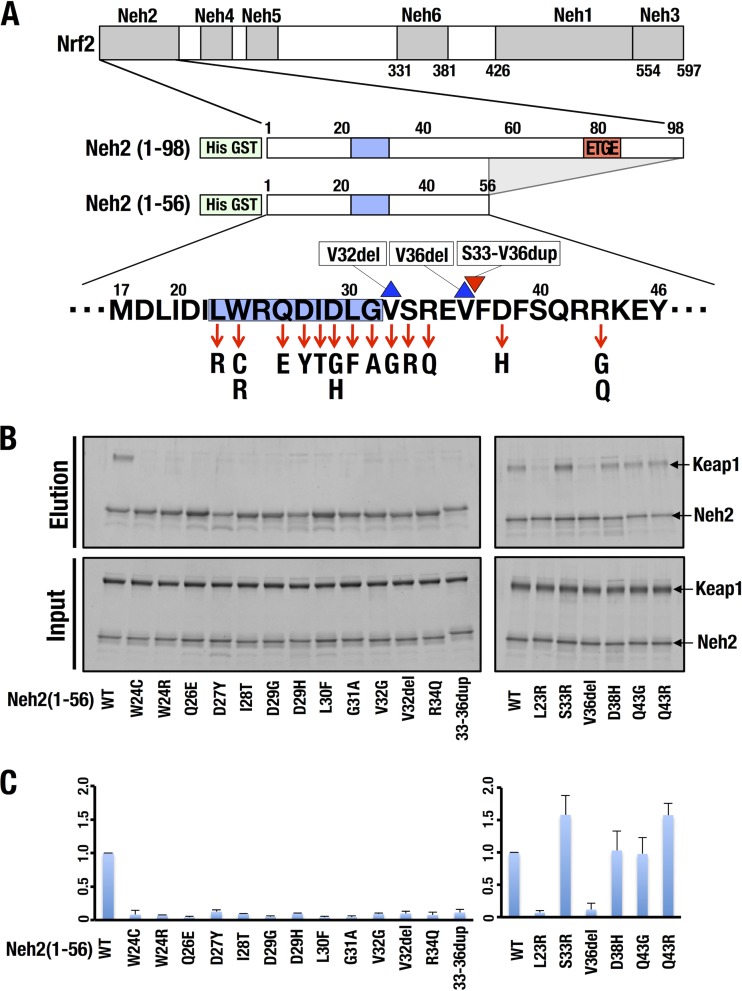
We attempted a closer examination of how the somatic mutations in the DLG motif affect the Keap1-Nrf2 interaction. For this purpose, we constructed His-GST-tagged Neh2(1-56) and mutant constructs thereof harboring 19 of these somatic mutations (Fig. 2A) and conducted GST pulldown assays with purified Keap1-His protein (Fig. 2B). Because the 43rd amino acid residue of Nrf2 differs between humans and mice (R and Q, respectively), we constructed Q43R and Q43G mutant forms of mouse Neh2(1-56) to examine the effects of the R43Q and R43G mutations on human cancer. We found that wild-type (WT) Neh2(1-56) nicely interacted with Keap1 (WT in Fig. 2B). The D38H and Q43G mutant forms retained the same binding activity as the WT, and the S33R and Q43R mutant forms exhibited slightly stronger binding activity than the WT (Fig. 2C, right panel). The potentiated binding activity of human-type Q43R compared to that of mouse Neh2(1-56) implies that the human R43Q mutant form retains slightly decreased binding activity. The binding activity of the Q43R mutant form compared with that of the Q43G mutant form implies that the human R43G mutant form must retain decreased Keap1-binding activity. None of the other 13 mutant forms interacted with Keap1. As for the latter 13 mutant forms, we could not detect even a moderate decrease in the interaction; instead, the interaction was severely disrupted in these 13 mutant forms (Fig. 2C, both panels). This result shows the critical contribution of these 13 amino acids to the Keap1-binding activity of Neh2-DLG. We surmise that the integrity of the elaborate structure of DLG is critical for its interaction with Keap1.
FIG 2.
Somatic mutations encompassing Nrf2-DLG in human cancers impair the binding of Nrf2-DLG and Keap1. (A) Diagrams of cancer-derived mutation constructs of Nrf2-Neh2(1-56) used for a pulldown assay. The blue boxes and red box indicate the classical DLG motif (amino acids Leu23 to Gly31) and the ETGE motif (amino acids Asp77 to Glu82), respectively. The red arrows, blue triangle, and red triangle indicate substitution mutations, a deletion mutation, and an insertion mutation, respectively. (B) A GST pulldown assay with 6×His-GST-tagged WT Neh2(1-56) or mutant forms thereof with purified recombinant Keap1-His. WT Neh2(1-56) or mutant forms thereof and Keap1-His were mixed and incubated with glutathione magnetic beads. The pulled-down complex was eluted with reduced glutathione, subjected to SDS-PAGE, and visualized by fluorescent staining. (C) Quantification of band intensities in panel B by quantitative densitometry of fluorescent staining. Values relative to the intensity of WT Neh2(1-56) are shown. The average results of three independent experiments are shown with standard deviations.
Minimum Keap1-interacting region of the Nrf2-DLG motif.
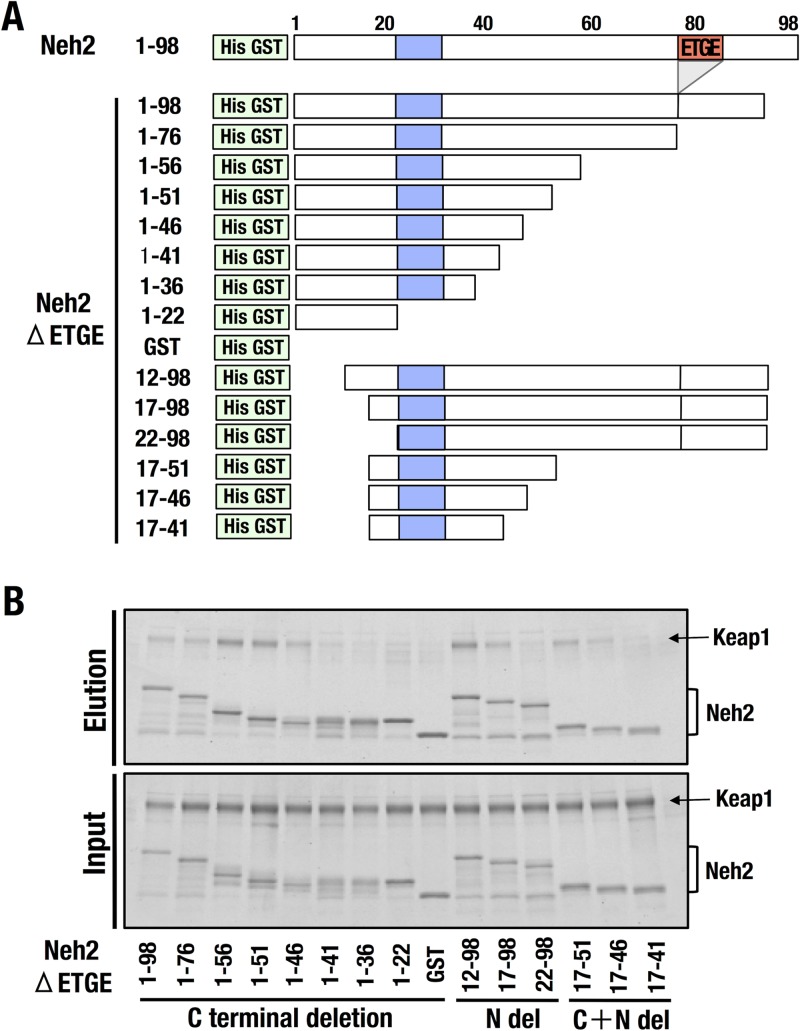
Because the minimum Keap1-interacting region of Nrf2-DLG appears to be larger than the previously described DLG motif (Leu23 to Gly31) (blue boxes in Fig. 3A), we prepared three series of deletion mutant forms of Nrf2-Neh2ΔETGE with His-GST tags (green boxes in Fig. 3A) to delineate the Nrf2-DLG degron interaction interface with Keap1. To this end, we used the Nrf2-Neh2ΔETGE construct (ETGE is shown as a red box) to avoid the ETGE motif interaction with Keap1 (9). The deletion mutant forms used are described in detail in Fig. 3A.
FIG 3.
Minimum Keap1-interacting region of the Nrf2-DLG motif. (A) Diagrams of the deletion-mutation constructs of Nrf2-Neh2ΔETGE used for the pulldown assay. Blue boxes and red box indicate the DLG motif (amino acids Leu23 to Gly31) and the ETGE motif (amino acids Asp77 to Glu82), respectively. (B) A GST pulldown assay with 6×His-GST-tagged Neh2ΔETGE and deletion mutant forms thereof with purified recombinant Keap1-His. Neh2ΔETGE or deletion mutant forms thereof and Keap1-His were mixed and incubated with glutathione magnetic beads. The pulled-down complex was eluted with reduced glutathione, subjected to SDS-PAGE, and visualized by fluorescent staining.
We first created eight C-terminal side deletion constructs based on Nrf2-Neh2ΔETGE in which Neh2 amino acid (aa) residues 98 to 22 were sequentially removed. For the N-terminal side deletions, we created three constructs in which aa 12 to 22 were sequentially removed (Fig. 3A). Finally, we removed a portion of the N-terminal end, as well as portions of the C-terminal end, yielding constructs encoding aa 17 to 51, 17 to 46, and 17 to 41 of the Neh2 domain. These mutant forms were expressed in E. coli and purified with an Ni-nitrilotriacetic acid column.
To determine the actual and minimal Keap1-binding region of the DLG motif, we used a GST pulldown assay with these three lines of deletion mutant forms of Nrf2-Neh2ΔETGE and purified Keap1-His proteins. The analyses revealed that the C-terminal side deletion up to aa 46 preserved the Keap1-binding activity of the Nrf2-Neh2ΔETGE domain, while deletion to aa 41 significantly reduced this activity (Fig. 3B). Similarly, N-terminal deletion of up to aa 17 retained the Keap1-binding activity, but deletion to aa 22 lost the activity. Finally, the combined deletion analysis revealed that the region spanning Met17 to Tyr46 is essential and sufficient for Keap1 binding (Fig. 3B). We refer to this region as Nrf2-DLG+. The results also indicate that the region corresponding to Met17 to Gln51 retains slightly stronger Keap1-binding activity. Therefore, we designated Met17 to Gln51 as the extended DLG motif of Nrf2 (Nrf2-DLGex) in this study. Compared with the original DLG motif (Leu23 to Gly31) determined in the previous study, we surmise that Nrf2-DLGex recapitulates the true Keap1-binding activity of DLG.
Characterization of the Nrf2-DLGex interaction with Keap1.
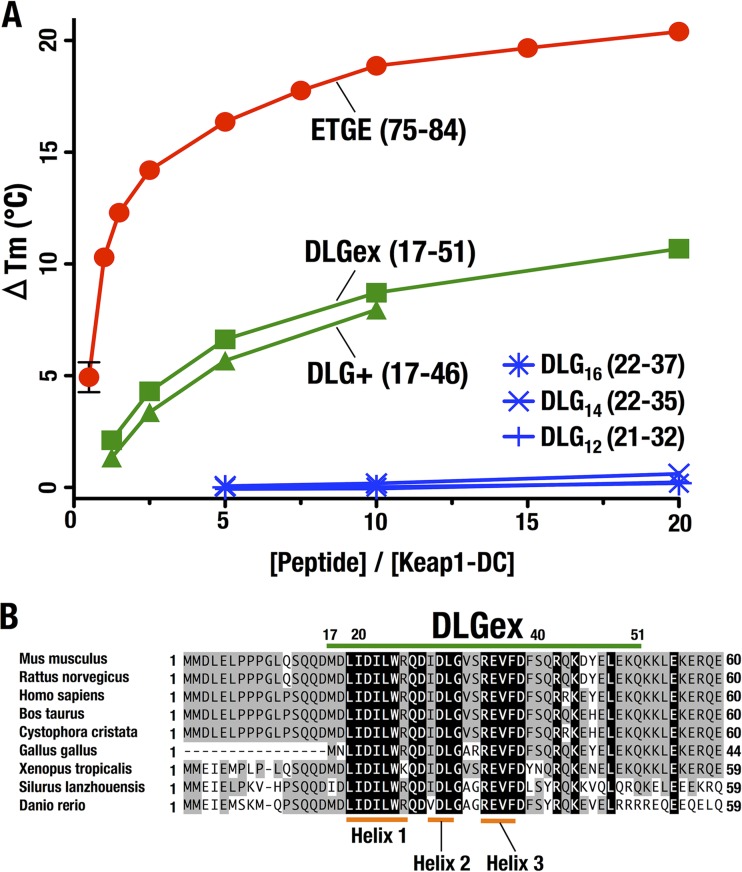
To further characterize the interactions of Nrf2-DLGex and other Nrf2-DLG truncation peptides with Keap1, we performed DSF (35) with various Nrf2 synthetic peptides and the purified Keap1-DC domain. A concentration-dependent thermal shift was observed when Nrf2-DLG+ (green triangles in Fig. 4A) and DLGex (green boxes) were added to Keap1-DC, suggesting that these synthetic peptides bind and stabilize Keap1-DC. Because the Nrf2-DLG+ peptide did not show good solubility, we could measure only up to a molar ratio of 10. No binding was observed when three shorter peptides of the DLG motif (DLG12, DLG14, and DLG16, corresponding to aa residues 21 to 32, 22 to 35, and 22 to 37, respectively) that cover the previously defined DLG motif were used. Of these three DLG-related peptides, DLG12 includes just the previously defined DLG motif. The other two peptides cover a slightly larger area than the previously defined DLG motif. Of note, sequence alignment analyses of the DLG motif and surrounding regions revealed that the Nrf2-DLGex motif is highly conserved among species (Fig. 4B). Thus, these results support our contention that the true DLG motif uses a binding sequence longer than that previously assigned.
FIG 4.
Characterization of the Nrf2-DLGex interaction with Keap1. (A) DSF analysis to determine the Tm of Keap1-DC with various DLG peptides and the ETGE peptide. The horizontal and longitudinal axes indicate peptide concentrations and ΔTms, respectively. ΔTm was generated by subtracting the Tm value without peptide (reference) from the Tm value with each peptide. (B) Alignment of the N-terminal regions of the Nrf2 proteins of various species. Amino acid residues that are conserved completely and partially are shaded black and gray, respectively. Nrf2-DLGex is indicated by the green bar.
Thermodynamic examination of Nrf2-DLGex and Nrf2-ETGE binding to Keap1-DC.
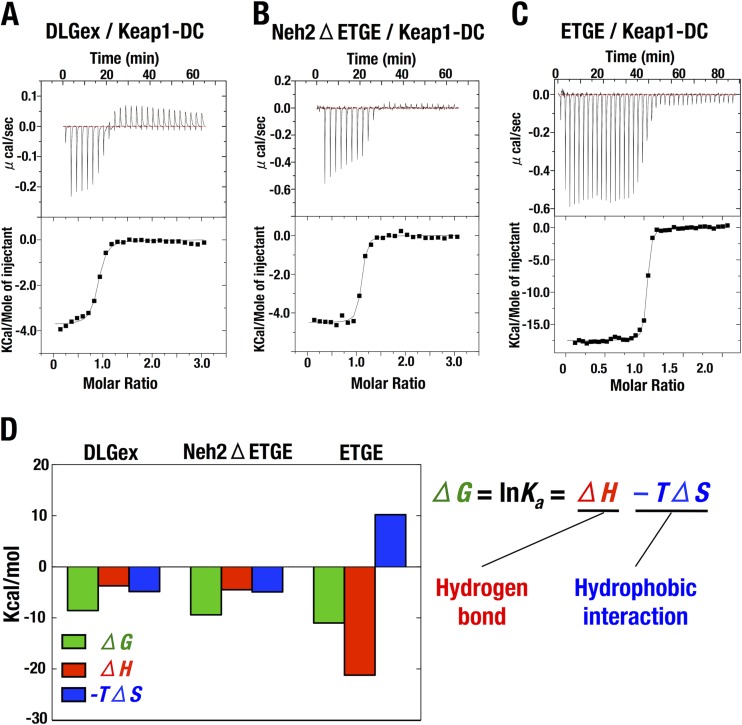
We next conducted a thermodynamic examination to characterize the interactions of Nrf2-DLGex and Nrf2-ETGE with Keap1-DC. For these experiments, the Nrf2-DLGex peptide was used instead of the Nrf2-DLG+ peptide because of the limited solubility of the Nrf2-DLG+ peptide. We found that Nrf2-DLGex and Neh2ΔETGE exhibited comparable thermodynamic parameters in the ITC. This observation was in stark contrast to the thermodynamic parameters of the ETGE peptide (Gln75 to Pro84) (Fig. 5A to C and Table 1). Three helix regions in DLGex clarified by current structural analysis of Keap1-DC-DLGex complex are shown as orange bars.
FIG 5.
Thermodynamic examination of Nrf2-DLGex and Nrf2-ETGE binding to Keap1-DC. (A to C) Representative ITC titration profiles of Keap1-DC with Nrf2-DLGex (A), Neh2ΔETGE (B), and the ETGE peptide (C). (D) Thermodynamic characterization of the modes of Nrf2-DLGex, Neh2ΔETGE, and ETGE peptide binding to Keap1-DC. Bars represent the free-energy change (ΔG), enthalpic component (ΔH), and entropic component (−TΔS). The interrelationships among these parameters are described as ΔG = ΔH − TΔS.
TABLE 1.
Thermodynamic parameters of the binding of DLGex, Neh2ΔETGE, Neh2ΔETGE S40E, and ETGE to the Keap1-DC domaina
| Ligand | n | ka (106 M−1) | ΔH (kcal/mol) | TΔS (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|---|---|---|
| DLGex | 0.86 | 1.9 | −3.7 | 4.8 | −8.5 |
| Neh2ΔETGE | 1.05 | 7.8 | −4.5 | 4.9 | −9.4 |
| Neh2ΔETGE S40E | 1.04 | 4.9 | −3.9 | 5.2 | −9.1 |
| ETGE | 1.02 | 38.0 | −17.9 | −7.53 | −10.4 |
ka is the binding constant. ΔH, ΔS, and ΔG are the changes in binding enthalpy, entropy, and Gibbs energy, respectively. −RTlnKa = ΔH − TΔS, where T and R are the absolute temperature and the gas constant, respectively.
Notably, as shown in Fig. 5D, our analyses revealed that the favorable binding free energy (green bars) of Nrf2-DLGex and Keap1-DC arose from the negative changes in enthalpy (red bars) and entropy (blue bars), whereas the large negative enthalpy contributed mainly to the favorable free-energy change in binding between Nrf2-ETGE and Keap1-DC. It has been known that the favorable enthalpy and entropy changes are attributed primarily to hydrogen bonds and hydrophobic interactions, respectively (36). These distinct thermodynamic parameters of Nrf2-DLGex and ETGE peptide binding to Keap1-DC imply that the interactions of these motifs rely on distinct modes. Both entropic and enthalpic changes contribute to the interaction between Nrf2-DLGex and Keap1-DC. In contrast, interaction between the ETGE motif and Keap1-DC relies heavily on hydrogen bond formation.
No influence of Ser40 phosphorylation on the binding of DLGex to Keap1-DC.
It was reported that phosphorylation of Nrf2 Ser40 by protein kinase C promoted the dissociation of Nrf2 from Keap1 and the consequent activation of Nrf2 in response to oxidative stress (37–40). Because Ser40 was included in Nrf2-DLGex, we were curious about whether the modification of Ser40 has an effect on the association between Nrf2-DLGex and Keap1-DC. To address this point, we extended our ITC experiments with purified Keap1-DC and purified Neh2ΔETGE or its Ser40 mutant form (replaced with glutamate; Neh2ΔETGE-S40E), which mimics phosphorylated Ser40 (Table 1). Unexpectedly, however, we found that the thermodynamic parameters of Neh2ΔETGE-S40E were mostly comparable to those of Neh2ΔETGE. These observations suggest that Ser40 phosphorylation does not affect the interaction between Nrf2-DLGex and Keap1-DC.
Crystal structure of Keap1-DC with long DLG peptides.
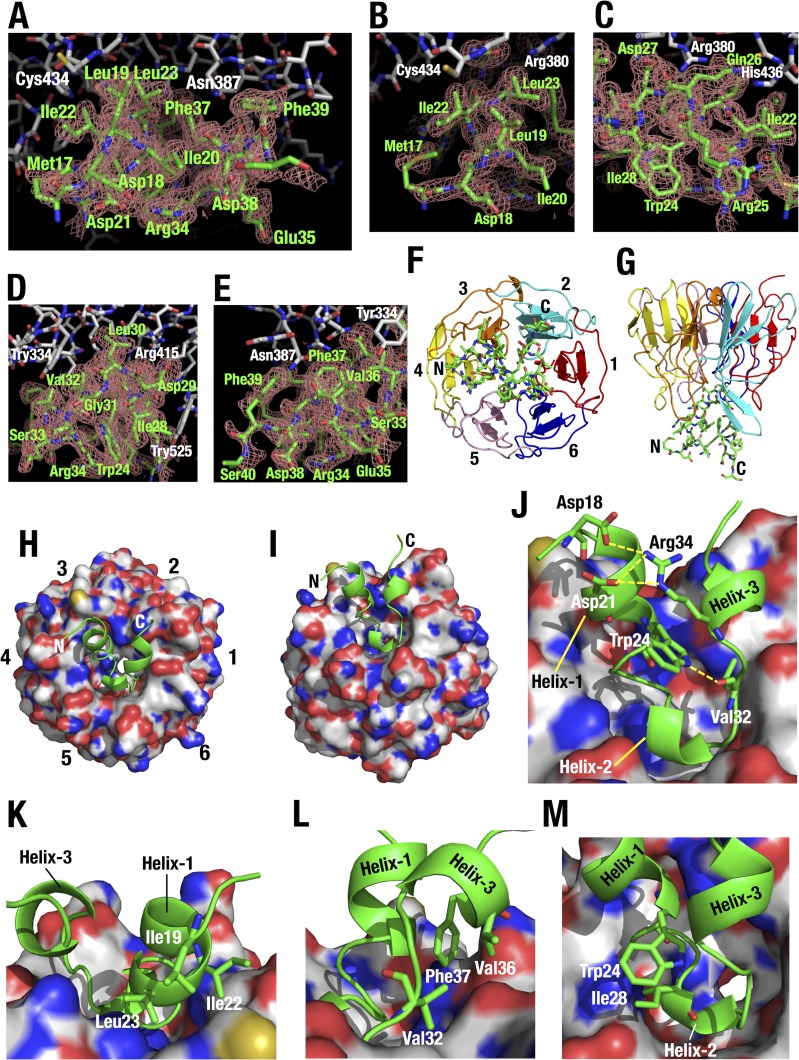
Thermodynamic analyses imply the contribution of hydrophobic interactions to Keap1-DLGex binding. However, details of hydrophobic interactions remain to be elucidated, as the structural basis of Keap1-DLGex binding is not fully understood (9, 41). Therefore, we crystallized Keap1-DC in complex with a long DLG peptide, DLGex(Met17-Gln51), and analyzed the crystal by X-ray diffraction at a 1.6 Å resolution (Table 2). The asymmetric unit contains two heterodimers of Keap1-DC-DLGex (chains A to B and L to M). The overall structures of these two complexes are similar (root mean square deviation [RMSD] range for 286 Cα atoms of 0.11 Å [chains A and L] and for 24 Cα atoms of 0.25 Å [chains B and M]). As the complex involving chain A of Keap1-DC and chain B of DLGex molecules is well defined, we will use it for the discussion below. The electron density difference map clearly indicates the presence of the peptide, and 24 (Met17 to Asp40) of 35 residues were visible in DLGex (Fig. 6A to E). As reported for a previous Keap1-DLG crystal structure with a short DLG peptide (Ile22 to Val36; DLG15) (9, 41) and Keap1-ETGE (42), DLGex binds to Keap1-DC at the bottom surface of the six-bladed β-propeller structure (Fig. 6F to I), which is highly basic and occupied mainly by arginine residues (9, 41).
FIG 6.
Crystal structure of Keap1-DC with the long DLGex peptide. (A to E) Overall (A) and closeup (B to E) views of the interface between Keap1 (white) and DLGex (green). The Fo-Fc omit map of the DLGex peptide is contoured at 2.0 σ. DLGex was omitted from the calculation. (F to I) Overall structure of Keap1-DC (shown as a ribbon model [F and G] and a surface model [H and I]) in complex with the Nrf2 DLGex peptide (Met17 to Gln51) (shown as a stick model [F and G] and a ribbon model [H and I]). The six blades in the β-propeller structure are numbered 1 to 6 (F and H) and are distinguished by separate colors (F and G). (J to M) Closeup view of the DLGex peptide bound to Keap1-DC. (J) Intramolecular electrostatic interactions in DLGex are shown as yellow broken lines. (K to M) Intramolecular hydrophobic interactions in DLGex among the side chains of Ile19, Ile22, and Leu23 (K), among the side chains of Val32, Val36, and Phe37 (L) and between the side chains of Trp24 and Ile28 (M).
DLGex possesses three helices, helix 1 (Leu19 to Arg25), helix 2 (Ile28 to Leu30), and helix 3 (Arg34 to Phe37) (Fig. 6J). The sequences of these helix regions are especially conserved among species (Fig. 4B). Helix 1 is an α-helix, while helix 2 and helix 3 are short 310 helices. This DLGex structure is strikingly different from that of ETGE, which has a β-hairpin structure (42).
Helix 1 and helix 3 are stabilized by intramolecular hydrophobic interactions among side chains of Leu19, Ile22, and Leu23 (Fig. 6K) and among side chains of Val32, Val36, and Phe37 (Fig. 6L), respectively. Helix 1 and helix 3 are bundled by intramolecular salt bridges between side chains of Asp18/Asp21 and that of Arg34. The side chain of Trp24 in helix 1 generates the hydrogen bond with the main-chain carbonyl group of Val32 (Fig. 6J) and the hydrophobic interaction with the side chain of Ile28 (Fig. 6M). DLGex forms a U shape through these interactions. The resulting conformation generated by intramolecular hydrophobic and electrostatic interactions allows the residues of DLGex to interact firmly with the counterpart residues of Keap1-DC.
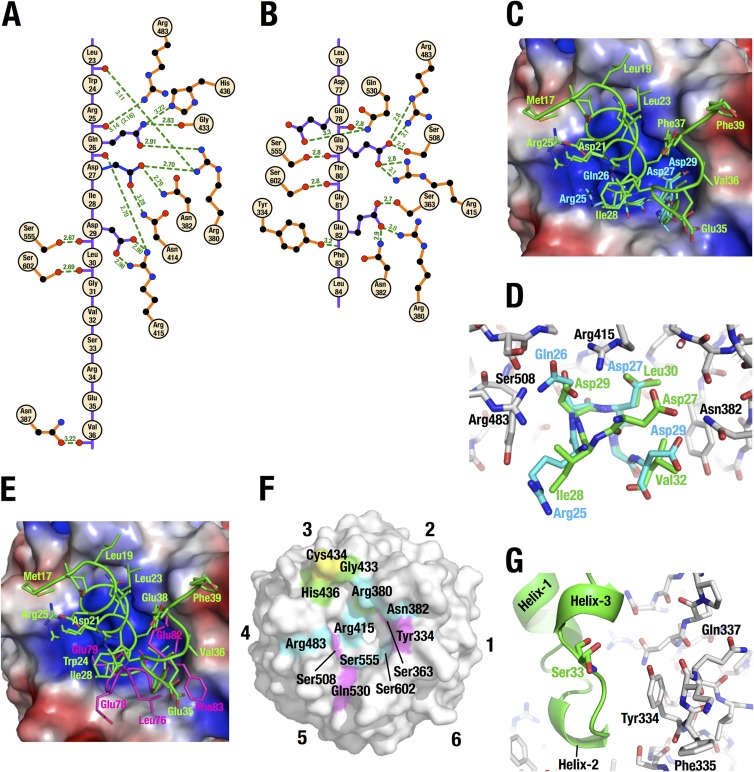
The DLGex peptide generates 14 potential intermolecular electrostatic interactions between Keap1 and the DC domain (Fig. 7A). Gln26 of DLGex interacts with multiple residues, including His436, Gly433, Arg380, and Arg415, of Keap1. The Nε2 atom of Gln26 supplies hydrogen bonds to the side chain of His436 and the main-chain carbonyl group of Gly433 of Keap1. The Oε1 atom of Gln26 supplies hydrogen bonds to the side chain of Arg380 of Keap1. In addition to side chain interaction, the main-chain carbonyl group of Gln26 interacts electrostatically with the side chain of Arg415 of Keap1. Asp27 of DLGex supplies hydrogen bonds to the side chain of Arg380, Asn382, and Asn414. Asp29 of DLGex interacts with Arg415 and Ser555. The side chain of Asp29 makes a salt bridge and firmly interacts with Arg415 of Keap1-DC. In addition, the main-chain carbonyl group of Asp29 also interacts electrostatically with Ser555. The main-chain carbonyl groups of Leu23, Arg25, Leu30, and Val36 of DLGex supply hydrogen bonds to the side chains of Arg380, Arg483, Ser602, and Asn387, respectively. Arg25 of DLGex consists of multiple conformers in the current crystal structure, and Arg25 appears to be flexible in DLGex bound to Keap1-DC. In sharp contrast to the electrostatic interactions, we could not detect any contributions of intermolecular hydrophobic interactions to Keap1-DLGex binding.
FIG 7.
Structural characteristics of the Keap1-DC and DLGex peptide interaction. (A and B) Intermolecular hydrogen bonds between Keap1-DC and Nrf2-DLGex (A) and between Keap1-DC and Nrf2-ETGE (PDB accession number 1X2R) (B). Hydrogen bonds (green broken lines) and their distance (in angstroms) are displayed. As Arg25 of DLGex consists of multiple conformers, the distance between the lower-population conformer of Arg25 and Arg483 of Keap1 is displayed in parentheses. (C and E) Superimposition of the Keap1-DLGex peptide complex with the Keap1-short DLG peptide (Ile22 to Val36; DLG15) complex (accession number 2DYH) (C) or with the Keap1-ETGE complex (PDB accession number 1X2R) (E). The backbone and side chains of bound peptides are shown as tubes and sticks, respectively. The DLGex, DLG15, and ETGE peptides are green, cyan, and magenta, respectively. The electrostatic surface potential of the bottom surface of Keap1 was calculated by PYMOL. Red and blue coloring indicates negative and positive charges, respectively. (D) Closeup view of the binding interfaces in panel C. Amino acid residues of Keap1-DC, DLGex, and DLG15 are shown as white, green, and cyan sticks, respectively. (F) Amino acid residues involved in the intermolecular interactions with Nrf2-DLGex, Nrf2-ETGE, and both are green, magenta, and cyan, respectively. Cysteine residues are yellow. (G) Closeup view around Nrf2-Ser33. DLGex is shown as a ribbon model. Amino acid residues of Keap1 and Nrf2-Ser33 are shown as white and green sticks, respectively.
Structural characteristics of Keap1-DC and DLG long-peptide interaction.
We compared the previous Keap1-DLG crystal structure with a short DLG peptide, DLG15 (9, 41), with the current Keap1-DLGex crystal structure. We found that the main chain of DLGex shows nearly the same configuration as that of DLG15 (Fig. 7C). The interactions of main-chain carbonyl groups of Gln26 and Asp27 with Ser555 and Ser602 in DLG15 show the same structure as those of the corresponding amino acids Asp29 and Leu30 of DLGex. However, closer examination revealed that the amino acids positions of DLGex and DLG15 are significantly shifted. It should be noted that in the previous analysis, the positions of Gln26, Asp27, and Asp29 of the DLG15 peptide were mislocated to the positions of Asp29, Leu30, and Val32 of DLGex, respectively (Fig. 7D). Although the previous report stated that Gln26 of DLG15 was wedged between Arg415 and Arg483 of Keap1 and generated hydrogen bonds with both residues, we found in this study that corresponding amino acid Asp29 of DLGex makes a salt bridge and firmly interacts with only Arg415 of Keap1 (Fig. 7A and D).
On the other hand, comparison of the crystal structure of Keap1-DLGex with that of Keap1-ETGE (42) revealed that while amino acid residues of Keap1 involved in the interaction with DLGex partially overlap those for ETGE binding (Fig. 7A and B), there is a striking difference between the binding interface for DLGex and that for ETGE. DLGex contacts a much wider surface (780 Å2) of the Keap1-DC bottom area than ETGE does (550 Å2) (Fig. 7F). The area of the binding surface of Keap1-DC and DLGex or ETGE is calculated from the solvent-accessible surface area (ASA) of Keap1-DC, DLGex, and ETGE and those of the complexes by the following equation: binding surfaceDLGex or ETGE = [(ASAKeap1 + ASADLGex or ETGE) − ASADLGex-Keap1 complex or ETGE-Keap1 complex]/2.
The binding interface for DLGex spans the region from blade 3 with Gly433 and His436 to blade 6 with Ser555 and Ser602 over the basic patch composed of Arg380, Arg415, and Arg483 around the central cavity of Keap1-DC. DLGex almost reaches Cys434, suggesting that the interaction between DLGex and Keap1-DC is disrupted by the modification of Cys434 with 8-nitro-cGMP (43). In contrast, the binding interface for ETGE is limited to the area between the basic patch and blade 6.
Structural implications of somatic mutations encompassing DLGex.
Our present mutational analyses have demonstrated that most of the mutations encompassing DLG disrupt Keap1-DLGex binding severely, while some of them retain the same or slightly altered binding activity (Fig. 2C). The structure-function relationships of these mutant forms are intriguing. The replacement of Leu30 with Phe appears to cause steric hindrance by a bulky side chain. The replacement of Gly31 with alanine also may cause steric hindrance. Since residues Gln26, Asp27, and Asp29 of DLGex form intermolecular electrostatic interactions with the respective amino acid residues of Keap1 by using their side chains (Fig. 7A), it seems easy to reconcile the fact that their replacement with different amino acids affects the interaction between DLGex and Keap1.
In contrast, most of the amino acid mutations are found to affect the intramolecular interactions within Nrf2 DLGex. Using their side chains, Trp24 interacts with the main-chain carbonyl group of Val32 and Arg34 forms intramolecular electrostatic interactions with side chains of Asp18/Asp21. Therefore, their replacement disrupts the conformation to bind to Keap1 (Fig. 6J). Deletion of Val32 also disrupts the intramolecular association between Asp18/Asp21 and Arg34 by shifting the position of Arg34 (Fig. 6J). The replacement of Trp24 and Ile28 also disrupts the appropriate conformation of DLGex for Keap1 binding, as Trp24 also forms intramolecular hydrophobic interaction with Ile28 to put helix 2 into the optimal position for Keap1 binding (Fig. 6M). Since Leu23 forms intramolecular hydrophobic interactions to form helix 1 (Fig. 6K), their substitution mutations disrupt helix 1. Since the side chains of Val32, Val36, and Phe37 form intramolecular hydrophobic interactions to form helix 3 (Fig. 6L), the replacement of Val32, the duplication of Ser33-Val36, and the deletion of Val36 disrupt helix 3, which is essential for Keap1 binding.
On the other hand, we found that substitution mutation of Glu38 retains the same binding activity. We envisage that Glu38 of DLGex is located far from the binding surface and seems not to be involved in intermolecular interactions and intramolecular interactions. Ser33 of DLGex consists of multiple conformers in the current crystal structure, and Ser33 appears to be flexible in DLGex bound to Keap1-DC. We surmise that the replacement of Ser33 with Arg increases Keap1 binding activity by generating an additional hydrogen bond with Oη of Tyr334 of Keap1-DC (Fig. 7G). Collectively, our current crystal structure interprets well the loss of function and gain of function of the mutations encompassing DLGex. It is noteworthy that most of the cancer somatic mutations encompassing DLGex disrupt intramolecular interactions, giving rise to loss of the structural integrity necessary for Keap1 binding. These results further support our contention that Keap1-DLGex binding is more fragile and prone to disruption than Keap1-ETGE binding as the latch site.
Kinetic analyses of DLGex and Nrf2-ETGE binding to Keap1-DC.
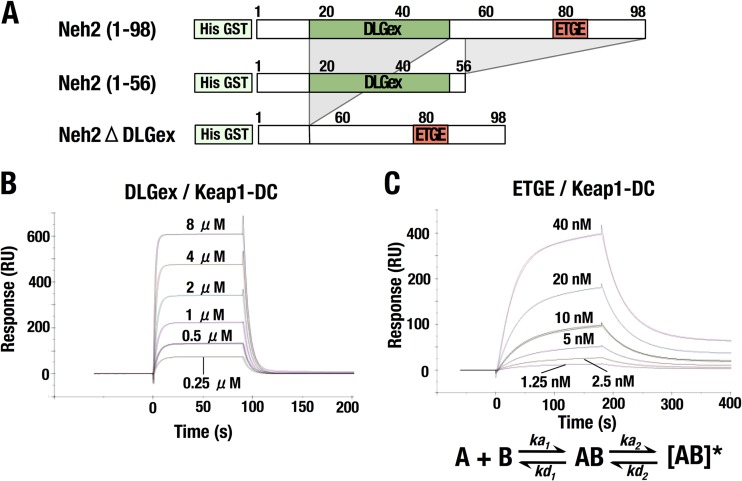
We finally wished to verify the differences in the binding modes by exploiting a distinct approach. To this end, we compared the kinetics of the binding of Nrf2-DLGex and Keap1-DC with those of Nrf2-ETGE and Keap1-DC by using SPR (Fig. 8 and Table 3). The two mutant proteins we prepared for this purpose are Neh2(1-56), including Nrf2-DLGex, and Nrf2ΔDLGex, lacking aa 17 to 51 and including Nrf2-ETGE (Fig. 8A).
FIG 8.
Kinetic analyses of DLGex and Nrf2-ETGE binding to Keap1-DC. (A) Diagrams of Neh2(1-56) and Neh2ΔDLGex used for kinetic analyses. Neh2(1-56) and Neh2ΔDLGex contain the DLGex motif and the ETGE motif, respectively. (B and C) SPR sensorgrams showing the association and dissociation of the DLGex motif and the Keap1-DC domain (B) and of the ETGE motif and the Keap1-DC domain (C). RU, resonance units.
TABLE 3.
Kinetic constants for the binding of Neh2(1-56) or Neh2ΔDLGex to Keap1-DCa
| Constant | Neh2(1-56) ligand, 1:1 binding model | Neh2ΔDLGex ligand, two-state reaction model |
|---|---|---|
| ka(ka1) (M−1 · s−1, 104) | 6.1 ± 0.82 | 345 ± 22 |
| kd(kd1) (s−1, 10−1) | 1.96 ± 0.29 | 2.82 ± 0.28 |
| ka2 (M−1 · s−1, 10−3) | 1.20 ± 0.10 | |
| kd2 (s−1, 10−4) | 1.22 ± 0.50 | |
| kD (nM) | 3199 ± 74 | 7.31 ± 2.59 |
| χ2 | 28.6 ± 2.2 | 1.85 ± 0.29 |
All standard deviations are derived from triplicate runs. ka is the association rate, and kd is the dissociation rate. ka1 and kd1 are the association and dissociation rates, respectively, in the first step of the two-state reaction model. ka2 and kd2 are the association and dissociation rates, respectively, in the second step of the two-state reaction model. kD is the dissociation constant (kD = kd/ka).
The kinetic data for the binding of Neh2(1-56) and Keap1-DC fit a 1:1 Langmuir binding model well (Fig. 8B and Table 3). On the basis of the observed association rate constant (ka was 6.1 × 104 M−1 s−1) and dissociation rate constant (kd was 0.196 s−1), we concluded that the binding of Nrf2-DLGex to Keap1-DC has fast association and dissociation modes.
In contrast, the binding of Neh2ΔDLGex, representing Nrf2-ETGE, to Keap1-DC did not fit a 1:1 Langmuir binding model well, which had a large value for the goodness of fit (χ2 = 317) (data not shown). The goodness of fit was significantly improved (χ2 = 1.85) when we adopted a two-state binding model (Fig. 8C and Table 3) (44, 45), implying that the binding of Nrf2-ETGE and Keap1-DC involves a sequential two-step process. In the first step, ka1 was 3.45 × 106 M−1 s−1 and kd1 was 0.282 s−1. The first binding step is characterized by fast association and dissociation. In the second step, ka2 was 1.20 × 10−3 M−1 s−1 and kd2 was 1.22 × 10−4 s−1. This second binding step is characterized by slow reactions. We surmise that Nrf2-ETGE binds to Keap1-DC in a transient conformation in the first step and that the binding state is slowly transferred to the fully optimized conformation for the stable complex in the second step.
DISCUSSION
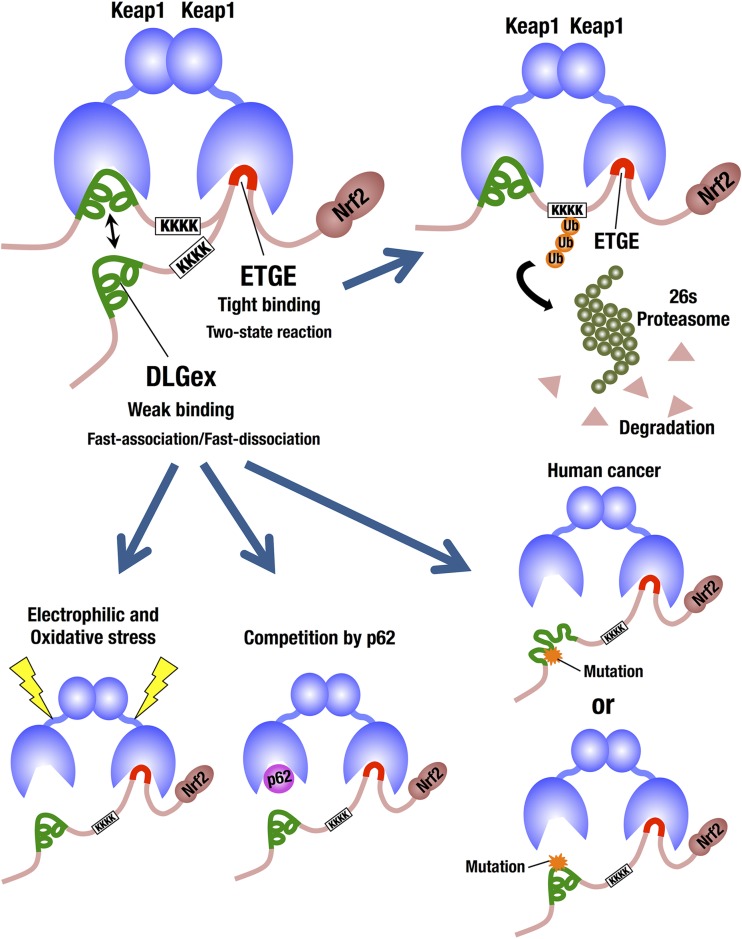
Two motifs in the Neh2 domain of Nrf2 bind individually to the DC domain of Keap1; one is the low-affinity DLG motif, and the other is the high-affinity ETGE motif. The difference in binding affinity between these two motifs supports the two-site hinge-and-latch binding model for the electrophilic and oxidative stress sensing by Keap1 (5). The classical DLG motif corresponds to Leu23 to Gly31 (7), and the related DIDLID element corresponds to Met17 to Val32 (8). However, on the basis of the recent somatic mutation analyses of many human cancer cells, we have noticed that the actual DLG motif may be much more extended than these classical DLG motifs. Therefore, in this study, we determined the minimum Keap1-binding sequence of the DLG motif and found that the motif is much longer than that previously defined. We have defined the DLGex motif that corresponds to Met17 to Gln51, containing both the classical DLG motif and the DIDLID element. The DLGex motif covers a much larger sequence (35 aa) than the minimum Keap1-binding sequence for ETGE (9 aa; Leu76 to Leu84) (46). We have also successfully clarified the crystal structure of the Keap1-DC-DLGex peptide (Met17 to Gln51) at a 1.6 Å resolution. DLGex possesses a complicated helix structure, which interprets well the human-cancer-derived loss-of-function and gain-of-function mutations in DLGex. The modes of DLGex and ETGE binding to Keap1 are quite distinct from each other; Keap1-DLGex binding is characterized thermodynamically as both enthalpy and entropy driven and kinetically as fast on and fast off. In contrast, ETGE-Keap1 binding is characterized as purely enthalpy driven and seems to involve a two-state reaction that leads to a stable conformation. These results support the notion that Keap1-DLGex binding is disrupted much more easily than Keap1-ETGE binding, conforming to a hinge-and-latch mechanism (Fig. 9).
FIG 9.
Schematic representation of the distinctive modes of Nrf2 binding to Keap1. We have determined that the DLGex motif corresponds to Met17 to Gln51 (green lines) and covers a much larger sequence (35 aa) than the minimum Keap1-binding sequence of ETGE (9 aa; red lines). Importantly, the binding modes of DLGex and ETGE are quite distinct from each other. Our present Keap1-DLGex complex crystal structure reveals that DLGex possesses a complicated helix structure, which interprets well the human-cancer-derived loss-of-function and gain-of-function mutations in DLGex. Of note, most of the mutations disrupt intramolecular interactions, which gives rise to the abrogation of their Keap1-binding activity. Keap1-DLGex binding is characterized as fast-on and fast-off binding that allows disruptor proteins, such as p62, to intercalate easy. In contrast, the binding of the ETGE motif to the DC domain most properly fits a two-state reaction model, and this two-step binding process of ETGE ensures Keap1-ETGE binding a more stable conformation and contributes to the efficient ubiquitination and degradation of Nrf2. Thus, the DLGex motif consists of efficient sensor machinery acting as a latch that easily dissociates from Keap1-DC upon detecting electrophilic and oxidative stress.
There are somatic mutations in the KEAP1 and NRF2 genes in various human cancer cells that cause constitutive NRF2 activation and promote cancer malignancy (19–22). Notably, all of the somatic mutations in the NRF2 gene reside within or near the sequence encoding the DLG and ETGE motifs and introduce substitution mutations of amino acid residues. While these mutations are believed to disturb the proper binding of Nrf2 and Keap1, out of the 35 mutations reported for the DLG motif (as summarized in Fig. 1A), only 2, W24C and L30F, were shown experimentally to compromise its association with Keap1 (19). Therefore, we have systematically examined mutations within or near the DLG motif and produced 19 mutations (including W24C and L30F) encompassing the motif. We found that 15 mutant forms severely disrupted binding to Keap1, implying that the actual DLG motif consists of a much wider region than was previously assigned, and defined a new DLGex motif covering the 35-aa region.
One of the salient findings of this study is the identification of the difference between the thermodynamic profiles of the DLGex and ETGE associations with the Keap1 DC domain. While binding of the ETGE motif to the DC domain is purely enthalpy driven, binding of the DLGex motif is both enthalpy and entropy driven. Considering the fact that favorable enthalpy and entropy changes are attributable primarily to the hydrogen bonds and hydrophobic interactions, respectively (36), we surmised that the contribution of electrostatic interactions should be larger for the binding of the ETGE motif than for the binding of the DLGex motif. On the contrary, the contribution of hydrophobic interactions should be larger for the binding of DLGex than for the binding of the ETGE motif.
However, to our surprise, we could not detect any intermolecular hydrophobic interactions in the Keap1-DLGex complex structure. A favorable entropy change was attributable fundamentally to burial of the apolar surface, releasing ordered water molecules surrounding the hydrophobic group (36). DLGex bound to Keap1 contains many more hydrophobic amino acid residues on the binding surface, such as Leu23, Ile28, Leu30, Val32, Val36, and Phe37, than ETGE does. We surmise that the favorable entropy change in Keap1-DLGex binding may be generated by burial of the apolar surface of DLGex.
The other salient finding of this study is that the long DLG peptide, DLGex(Met17-Gln51), possesses a complicated helix structure. In contrast, the short DLG peptide DLG15 and ETGE possess a simple β-hairpin conformation (9, 41, 42). DLGex possesses three helices, which are stabilized by intramolecular hydrophobic interactions and bundled by intramolecular electrostatic and hydrophobic interactions. The complicated conformation achieved through these intramolecular noncovalent interactions allows the amino acid residues of DLGex to interact firmly with the counterpart residues of Keap1-DC. Actually, many human somatic mutations related to intramolecular interaction, such as L23R, W24C, W24R, I28T, V32G, V32del, R34Q, V36del, and 33-36dup, disrupt Keap1-DLGex binding. We found that most of the mutations disrupt the intramolecular interactions important for Keap1-DLGex binding.
The main chains of DLGex show nearly the same configuration as that of DLG15 (Ile22 to Val36) in the previous Keap1-short DLG peptide crystal structure. However, our present analyses also show that amino acid positions of DLG15 (9, 41) were mislocated by three amino acids. We surmise that DLG15 did not bind completely to Keap1-DC, as DLG15 lacks amino acid residues Asp18 and Asp21, which are essential to the formation of intermolecular electrostatic interactions. Similarly, the DLG nonapeptide (LDIDLGVFL) used by McMahon et al. (47) seems to be insufficient for Keap1 binding.
We found that the binding surface of DLGex is much wider than that of ETGE and is quite different from that of ETGE, as shown in Fig. 7F. Intriguingly, Cys434, a target for S-guanylation by 8-nitro-cGMP (43), resides in close proximity to the binding surface of DLGex but far from that of ETGE, suggesting that the modification of Keap1-C434 may disrupt Keap1-DLGex binding specifically, conforming to a hinge-and-latch mechanism.
The binding of the Keap1 DC domain and the Nrf2 DLGex motif shows fast association and dissociation kinetics. We surmise that the rapid association-dissociation nature of the DLGex motif with the DC domain enables the prompt response of the Keap1-Nrf2 system in reaction to electrophilic and oxidative stress. As shown in Fig. 9, this kinetic nature also permits the other proteins, such as autophagy chaperone p62 (26, 48), to compete with DLGex for Keap1 binding.
In contrast, the binding of the Keap1 DC domain and Nrf2-ETGE most properly fit a two-state reaction model. The first binding step is characterized by fast association and dissociation, and the second binding step is characterized by slow reactions. We surmise that this two-step binding process of ETGE gives Keap1-ETGE binding a more stable conformation and contributes to the efficient ubiquitination and degradation of Nrf2. Thus, these two distinct kinetic features appear to be critically important for the sensing of electrophilic and oxidative stress and strongly support the hinge-and-latch mechanism (5). In contrast, one group reported kinetic data for the binding of ETGE with Keap1-DC that fit a 1:1 Langmuir binding model well (46), and the result sharply contradicts our present results. Because the short ETGE peptide was immobilized directly on the sensor chip in that experiment, we speculate that strong steric hindrance might hamper the two-state reactions.
It has been reported that Ser40 within the DLGex motif is phosphorylated by protein kinase C and that this promotes the dissociation of Nrf2 from Keap1 (37–40). However, in our analysis with an Nrf2 S40E mutant that mimics the phosphorylation of Ser40, Ser40 phosphorylation does not affect the interaction between the DLGex motif and the DC domain of Keap1. Showing very good agreement with this result, Ser40 in DLGex of Nrf2 is located far from the binding surface and helix structure in our crystal structure, so that phosphorylation of Nrf2 Ser40 seems not to affect Keap1-DLGex binding.
A “conformational cycling model” has been proposed for Keap1 inactivation in which a Nrf2 inducer, such as sulforaphane, strengthens Keap1-DLGex binding and enfeebles Keap1 activity (49). While our present data do not support or deny this model, we surmise that the fragile helix structure of DLGex and the fast-on/fast-off nature of Keap1-DLGex binding strongly support our contention that the disruption of Keap1-DLGex binding by stressors is much easier to achieve than that of Keap1-ETGE binding. Therefore, our present results rather support a hinge-and-latch model in which Keap1-ETGE acts as a hinge and Keap1-DLGex acts as a latch (5). In summary, we demonstrate that the DLGex binding to Keap1 is distinct from ETGE motif binding to Keap1 in thermodynamic, kinetic, and structural profiles. These unique features of the DLGex motif establish the molecular and structural basis that enables the prompt response of the Keap1-Nrf2 system to oxidative and electrophilic stress.
ACKNOWLEDGMENTS
This work was supported through funding from JSPS KAKENHI grants 24249015 (M.Y.) and 24112009 (T.M.), the Naito Foundation (M.Y.), the Takeda Scientific Foundation (M.Y.), and the Core Research for Evolutional Science and Technology from the JST (M.Y.). This work was performed with synchrotron beamline BL44XU at SPring-8 under the Cooperative Research Program of the Institute for Protein Research, Osaka University (proposal 2013B6852).
We thank Tatsuro Iso and Yoshiko Izumi for their kind help and the Biomedical Research Core of the Tohoku University Graduate School of Medicine for their technical support. We also thank Masanori Sato and Yusuke Uchibori for their help with the preparation of the expression vectors and recombinant proteins and Hozumi Motohashi for helpful discussions.
Footnotes
Published ahead of print 23 December 2013
REFERENCES
- 1.Motohashi H, O'Connor T, Katsuoka F, Engel JD, Yamamoto M. 2002. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294:1–12. 10.1016/S0378-1119(02)00788-6 [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Yamamoto M. 2006. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 46:113–140. 10.1016/j.advenzreg.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 3.Taguchi K, Motohashi H, Yamamoto M. 2011. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140. 10.1111/j.1365-2443.2010.01473.x [DOI] [PubMed] [Google Scholar]
- 4.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. 2006. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol. Chem. 387:1311–1320 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. 2004. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24:7130–7139. 10.1128/MCB.24.16.7130-7139.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes JD, Itoh K, Yamamoto M. 2005. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch. Biochem. Biophys. 433:342–350. 10.1016/j.abb.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 8.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. 2004. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 279:31556–31567. 10.1074/jbc.M403061200 [DOI] [PubMed] [Google Scholar]
- 9.Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M. 2007. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 27:7511–7521. 10.1128/MCB.00753-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. 2006. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol. Cell. Biol. 26:2887–2900. 10.1128/MCB.26.8.2887-2900.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogura T, Tong KI, Mio K, Maruyama Y, Kurokawa H, Sato C, Yamamoto M. 2010. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc. Natl. Acad. Sci. U. S. A. 107:2842–2847. 10.1073/pnas.0914036107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki T, Shibata T, Takaya K, Shiraishi K, Kohno T, Kunitoh H, Tsuta K, Furuta K, Goto K, Hosoda F, Sakamoto H, Motohashi H, Yamamoto M. 2013. Regulatory nexus of synthesis and degradation deciphers cellular Nrf2 expression levels. Mol. Cell. Biol. 33:2402–2412. 10.1128/MCB.00065-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. 2013. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 33:2996–3010. 10.1128/MCB.00225-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi G, Johnson JA. 2012. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat. CNS Drug Discov. 7:218–229. 10.2174/157488912803252023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. 2003. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis 24:461–467. 10.1093/carcin/24.3.461 [DOI] [PubMed] [Google Scholar]
- 16.Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. 2004. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 64:6424–6431. 10.1158/0008-5472.CAN-04-1906 [DOI] [PubMed] [Google Scholar]
- 17.Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. 2013. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 73:4158–4168. 10.1158/0008-5472.CAN-12-4499 [DOI] [PubMed] [Google Scholar]
- 18.Ohkoshi A, Suzuki T, Ono M, Kobayashi T, Yamamoto M. 2013. Role of Keap1-Nrf2 system in upper aerodigestive tract carcinogenesis. Cancer Prev. Res. 6:149–159. 10.1158/1940-6207.CAPR-12-0401-T [DOI] [PubMed] [Google Scholar]
- 19.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. 2008. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl. Acad. Sci. U. S. A. 105:13568–13573. 10.1073/pnas.0806268105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. 2010. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox Signal. 13:1627–1637. 10.1089/ars.2010.3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. 2010. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol. Cancer Ther. 9:336–346. 10.1158/1535-7163.MCT-09-0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata T, Kokubu A, Saito S, Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y, Kushima R, Kiyono T, Yamamoto M. 2011. NRF2 Mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia 13:864–873. 10.1593/neo.11750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki H, Hikosaka Y, Okuda K, Kawano O, Moriyama S, Yano M, Fujii Y. 2010. NFE2L2 gene mutation in male Japanese squamous cell carcinoma of the lung. J. Thorac. Oncol. 5:786–789. 10.1097/JTO.0b013e3181db3dd3 [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Sonobe M, Menju T, Nakayama E, Mino N, Iwakiri S, Nagai S, Sato K, Miyahara R, Okubo K, Hirata T, Date H, Wada H. 2010. Mutations in Keap1 are a potential prognostic factor in resected non-small cell lung cancer. J. Surg. Oncol. 101:500–506. 10.1002/jso.21520 [DOI] [PubMed] [Google Scholar]
- 25.Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, Sasano H, Nakagawa T, Satoh K, Tanaka N, Kubo H, Motohashi H, Yamamoto M. 2012. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 103:760–766. 10.1111/j.1349-7006.2012.02216.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. 2013. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 51:618–631. 10.1016/j.molcel.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 27.Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307–326. 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 28.Vagin A, Teplyakov A. 2010. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66:22–25. 10.1107/S0907444909042589 [DOI] [PubMed] [Google Scholar]
- 29.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60:2126–2132. 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 30.Murshudov GN, Vagin AA, Dodson EJ. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53:240–255. 10.1107/S0907444996012255 [DOI] [PubMed] [Google Scholar]
- 31.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y, Ju Y, Lin D, Wang Z, Huang Y, Zhang S, Wu C, Jiao S. 2012. Mutation of the Nrf2 gene in non-small cell lung cancer. Mol. Biol. Rep. 39:4743–4747. 10.1007/s11033-011-1266-4 [DOI] [PubMed] [Google Scholar]
- 33.Cho HY. 2013. Genomic structure and variation of nuclear factor (erythroid-derived 2)-like 2. Oxid. Med. Cell. Longev. 2013:286524. 10.1155/2013/286524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA. 2011. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39:D945–D950. 10.1093/nar/gkq929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niesen FH, Berglund H, Vedadi M. 2007. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2:2212–2221. 10.1038/nprot.2007.321 [DOI] [PubMed] [Google Scholar]
- 36.Leavitt S, Freire E. 2001. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11:560–566. 10.1016/S0959-440X(00)00248-7 [DOI] [PubMed] [Google Scholar]
- 37.Huang HC, Nguyen T, Pickett CB. 2002. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 277:42769–42774. 10.1074/jbc.M206911200 [DOI] [PubMed] [Google Scholar]
- 38.Huang HC, Nguyen T, Pickett CB. 2000. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. U. S. A. 97:12475–12480. 10.1073/pnas.220418997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloom DA, Jaiswal AK. 2003. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 278:44675–44682. 10.1074/jbc.M307633200 [DOI] [PubMed] [Google Scholar]
- 40.Niture SK, Jain AK, Jaiswal AK. 2009. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-δ-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J. Cell Sci. 122:4452–4464. 10.1242/jcs.058537 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Padmanabhan B, Tong KI, Kobayashi A, Yamamoto M, Yokoyama S. 2008. Structural insights into the similar modes of Nrf2 transcription factor recognition by the cytoplasmic repressor Keap1. J. Synchrotron Radiat. 15:273–276. 10.1107/S090904950705114X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. 2006. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell 21:689–700. 10.1016/j.molcel.2006.01.013 [DOI] [PubMed] [Google Scholar]
- 43.Fujii S, Sawa T, Ihara H, Tong KI, Ida T, Okamoto T, Ahtesham AK, Ishima Y, Motohashi H, Yamamoto M, Akaike T. 2010. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J. Bol. Chem. 285:23970–23984. 10.1074/jbc.M110.145441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlsson R, Fält A. 1997. Experimental design for kinetic analysis of protein-protein interactions with surface plasmon resonance biosensors. J. Immunol. Methods 200:121–133. 10.1016/S0022-1759(96)00195-0 [DOI] [PubMed] [Google Scholar]
- 45.Lipschultz CA, Li Y, Smith-Gill S. 2000. Experimental design for analysis of complex kinetics using surface plasmon resonance. Methods 20:310–318. 10.1006/meth.1999.0924 [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Inoyama D, Kong AN, Beamer LJ, Hu L. 2011. Kinetic analyses of Keap1-Nrf2 interaction and determination of the minimal Nrf2 peptide sequence required for Keap1 binding using surface plasmon resonance. Chem. Biol. Drug Des. 78:1014–1021. 10.1111/j.1747-0285.2011.01240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. 2006. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 281:24756–24768. 10.1074/jbc.M601119200 [DOI] [PubMed] [Google Scholar]
- 48.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. 2010. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12:213–223. 10.1038/ncb2021 [DOI] [PubMed] [Google Scholar]
- 49.Baird L, Llères D, Swift S, Dinkova-Kostova AT. 2013. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. U. S. A. 110:15259–15264. 10.1073/pnas.1305687110 [DOI] [PMC free article] [PubMed] [Google Scholar]