Each year, approximately 250,000 surgical procedures are performed to repair or to replace cardiac valves [1], and of these, approximately 50,000 are aortic valve replacement operations. Despite decades of development, many of the limitations of cardiac valve prostheses remain consequences of the fluid dynamics generated by the replacement valve [1]. To enable cross-validation studies that allow for detailed comparisons of experimental and computational results, we are developing fluid-structure interaction (FSI) models of the dynamics of aortic valve prostheses mounted in a ViVitro Systems, Inc. pulse duplicator apparatus. Such an experimentally validated computational model promises both to facilitate the design of novel valve prostheses, and also to assist in the regulatory process, by providing access to detailed spatially- and temporally-resolved flow data that are challenging to obtain via experimental approaches.
Our numerical approach to FSI is based on the immersed boundary (IB) method [2]. The structural dynamics are described in Lagrangian form using a material coordinate system, whereas the momentum of the fluid-solid system and the viscosity and incompressibility of the fluid are described in Eulerian form using fixed Cartesian physical coordinates. Lagrangian and Eulerian variables are coupled by integral transforms with Dirac delta function kernels. When the equations are discretized, the singular delta function is replaced by a regularized version of the delta function. See Peskin [2] for details.
To treat rigid body dynamics within the framework of the IB method, we adopt an approach similar to that recently developed by Kim and Peskin [3], in which the continuous equations are:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
in which are physical coordinates, are material coordinates, and are time-dependent mappings from material coordinates to current coordinates, is the Eulerian velocity field, is the Eulerian pressure field, and are equivalent Eulerian and Lagrangian force densities, is the fluid density, is the fluid viscosity, and is the three-dimensional Dirac delta function. The force field acts to impose the rigidity constraint
in which and are respectively the linear and angular velocity of the structure and is the radius vector. In the limit , the constraint is imposed exactly; for finite , the constraint is only approximately satisfied. The dynamics of and are determined from the requirement that the time rate of change of the “excess” linear and angular momentum (i.e., in excess of the momentum accounted for by the momentum equation (1)) be proportional to the net force and net torque acting on the body.
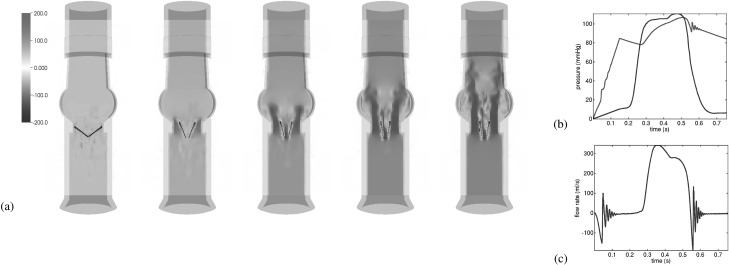
Geometrical models of the aortic section of the ViVitro pulse duplicator and of a St. Jude Regent valve were created using SolidWorks (Dassault Systèmes SolidWorks Corp., Waltham, MA). These geometrical models were meshed using CUBIT (Sandia National Laboratory, Albuquerque, NM). To drive flow through the valve, we impose a physiological left ventricular pressure waveform, and characteristic downstream compliance and resistance to mimic the response of the systemic circulation. Simulations used the IBAMR software [4]. Initial simulation results are shown in Fig. 1, demonstrating that realistic flow rates can be obtained using this model under realistic driving and loading conditions. We are presently fine tuning this model in preparation for validation studies.
Fig. 1.
(a) Opening dynamics, (b) imposed driving pressure and computed loading pressure, and (c) computed flow rate
Acknowledgment
We acknowledge discussions with A. P. S. Bhalla, A. Donev, D. M. McQueen, and C. S. Peskin, and funding from AHA award 10SDG4320049, NIH award HL117063, and NSF awards DMS 1016554 and OCI 1047734.
Contributor Information
Boyce E. Griffith, Leon H. Charney Division of Cardiology, Department of Medicine, New York University School of Medicine; Department of Mathematics, Courant Institute of Mathematical Sciences, New York University, e-mail: boyce.griffith@nyumc.org.
Vittoria Flamini, Department of Mechanical and Aerospace Engineering, Polytechnic Institute of New York University.
Abe DeAnda, Department of Cardiothoracic Surgery, New York University School of Medicine.
Lawrence Scotten, ViVitro Systems, Inc..
References
- [1]. Yoganathan, A. P. , He, Z. M. , and Jones, S. C. , 2004, “Fluid Mechanics of Heart Valves”, Annu. Rev. Biomed. Eng., 6, pp. 331–36210.1146/annurev.bioeng.6.040803.140111 [DOI] [PubMed] [Google Scholar]
- [2]. Peskin, C. S. , 2002, “The Immersed Boundary Method”, Acta. Numer., 11, pp. 479–51710.1017/S0962492902000077 [Google Scholar]
- [3]. Kim, Y. , and Peskin, C. S. , “A New Penalty Immersed Boundary Method for a Rigid Body,” (submitted). [Google Scholar]
- [4].“IBAMR: An Adaptive and Distributed-Memory Parallel Implementation of the Immersed Boundary (IB) Method,” 2013, IBAMR Users Google Group, http://ibamr.googlecode.com