Abstract
Background
Formoterol has a fast onset of action and can therefore be used to relieve symptoms of asthma. A combination inhaler can deliver formoterol with different doses of inhaled corticosteroid; when used as a reliever both drugs will be delivered more frequently when asthma symptoms increase. This has the potential to treat both bronchoconstriction and inflammation in the early stages of exacerbations.
Objectives
To assess the efficacy and safety of combined inhalers containing both formoterol and an inhaled corticosteroid when used for reliever therapy in adults and children with chronic asthma.
Search methods
We last searched the Cochrane Airways Group trials register in April 2009, and no new studies were found for inclusion in the review.
Selection criteria
Randomised trials in adults and children with chronic asthma, where a combination inhaler containing formoterol and inhaled corticosteroid is compared with fast‐acting beta2‐agonist alone for the relief of asthma symptoms. This should be the only planned difference between the trial arms.
Data collection and analysis
Two review authors independently extracted the characteristics and results of each study. Authors or manufacturers were asked to supply unpublished data in relation to primary outcomes.
Main results
Three trials involving 5905 participants were included. In patients with mild asthma who do not need maintenance treatment, no clinically important advantages of budesonide/formoterol as reliever were found in comparison to formoterol as reliever.
Two studies enrolled patients with more severe asthma who were not controlled on high doses of inhaled corticosteroids (around 700 mcg/day in adults), and had suffered a clinically important asthma exacerbation in the past year. Hospitalisations related to asthma in the two studies comparing budesonide/formoterol for maintenance and relief with the same dose of budesonide/formoterol for maintenance with terbutaline for relief yielded an odds ratio of 0.68 (95% CI 0.40 to 1.16), which was not a statistically significant reduction. In adults there was a reduction in exacerbations requiring oral corticosteroids compared to terbutaline, odds ratio 0.54 (95% CI 0.44 to 0.65), which translates into a number needed to treat over 12 months of 15 (95% CI 13 to 21). The study in children found less serious adverse events with budesonide/formoterol used for maintenance and relief. There was no significant difference in annual growth in children using budesonide/formoterol reliever in comparison to terbutaline.
Authors' conclusions
In mild asthma it is not yet known whether patients who use a budesonide/formoterol inhaler for relief of asthma symptoms derive any clinically important benefits. In more severe asthma, two studies enrolled patients who were not controlled on inhaled corticosteroids, and had suffered an exacerbation in the previous year, and then had their maintenance inhaled corticosteroids reduced in both arms of the study. Under these conditions the studies demonstrated a reduction in the risk of exacerbations that require oral corticosteroids with budesonide/formoterol for maintenance and relief in comparison with budesonide/formoterol for maintenance and terbutaline or formoterol for relief. The incidence of serious adverse events in children was also less using budesonide/formoterol for maintenance and relief in one study, which similarly enrolled children who were not controlled on inhaled corticosteroids, and who had their maintenance inhaled corticosteroids reduced at the start of the study. This study also compared an explorative maintenance dose of budesonide/formoterol that is not approved for treatment.
Keywords: Adolescent; Adult; Child; Humans; Administration, Inhalation; Anti‐Asthmatic Agents; Anti‐Asthmatic Agents/administration & dosage; Asthma; Asthma/drug therapy; Bronchial Diseases; Bronchial Diseases/drug therapy; Bronchodilator Agents; Bronchodilator Agents/administration & dosage; Budesonide; Budesonide/administration & dosage; Chronic Disease; Constriction, Pathologic; Constriction, Pathologic/drug therapy; Drug Combinations; Ethanolamines; Ethanolamines/administration & dosage; Formoterol Fumarate; Randomized Controlled Trials as Topic; Terbutaline; Terbutaline/administration & dosage
Plain language summary
Formoterol and budesonide for the relief of asthma symptoms in adults and children
Combined formoterol and budesonide inhalers can be used for maintenance treatment of asthma and relief of symptoms. Three trials involving 5905 participants were included. We found very little evidence in relation to the use of formoterol and budesonide for relief of symptoms in people with mild asthma, but in people with more severe asthma who had suffered exacerbations in spite of regular treatment with inhaled corticosteroids, we found that reliever formoterol and budesonide compared favourably with terbutaline in reducing asthma exacerbations that required a course of oral corticosteroids. However only a small proportion of the 'severe asthma exacerbations' as defined in the trials led to hospital admissions, and no significant overall benefit has yet been shown for this outcome. In children with asthma that was not controlled with regular inhaled corticosteroids, there were fewer serious adverse events when formoterol and budesonide were used to relieve symptoms as well as for maintenance treatment.
Background
People with asthma take preventer therapy to maintain symptom control, improve lung function and reduce the requirement for emergency care. In response to symptoms, reliever medication in the form of short‐acting beta‐agonists such as salbutamol or terbutaline, or formoterol (a long acting beta‐agonist (LABA) with a fast onset of action), can be used on an 'as needed' basis (BTS 2003). A maintenance combination therapy with formoterol in the combination marketed as 'Symbicort' combines preventer and reliever therapy in a single inhaler, and allows both medications to be increased simultaneously when asthma symptoms increase (SMILE). The pharmacological properties of salmeterol used in the Seretide/Advair combination inhaler give bronchodilation with a slower onset, and it is not licensed for use on an 'as needed' basis. The inclusion of inhaled corticosteroid (ICS) in a reliever inhaler for use during episodes of loss of control requires monitoring for safety, efficacy and assessment of overall ICS dose.
Description of the intervention
In patients whose asthma remains poorly controlled on moderate doses of inhaled corticosteroids (ICS), the addition of a LABA has been shown to compare favourably with placebo (Ni Chroinin 2005) and increasing the dose of ICS (Greenstone 2005), and this is a recommended approach in asthma guidelines (BTS 2003). There are concerns about the impact of LABA on exacerbation rates in children (Bisgaard 2003). Traditionally patients have used an additional short‐acting beta2‐agonist to relieve symptoms (on top of their regular maintenance preventer treatment). Formoterol is a long‐acting beta2‐agonist with fast onset of action (Palmqvist 2001). It is therefore also suitable for use in the relief of asthma symptoms, and when taken as reliever therapy in combination with ICS may have the added advantage of allowing the patient to titrate their ICS treatment according to asthma symptoms.
How the intervention might work
The inflammatory component of chronic asthma can be reduced by regular use of inhaled corticosteroids (Adams 2008), but many patients default from treatment (Sovani 2008). Asthma exacerbations are preceded by many days of worsening symptoms (Tattersfield 1999), but doubling inhaled corticosteroids has not been shown to be effective in preventing exacerbations from developing (FitzGerald 2004; Harrison 2004). Deteriorating symptoms may be an early sign of an increase in underlying inflammation, so including inhaled corticosteroids with formoterol in a reliever inhaler is an alternative approach.
Why it is important to do this review
Concerns have been raised about the use of long‐acting beta2‐agonists in chronic asthma, in particular when used without regular inhaled corticosteroids, in relation to the possible increased risk of severe adverse events such as intubation and asthma‐related death (Salpeter 2006; Walters 2007; Cates 2008; Cates 2008a). This review is needed to identify and summarise the results of clinical trials that compared budesonide/formoterol combination inhaler as reliever therapy with other reliever inhalers.
Objectives
This review has been carried out to assess the efficacy and safety of combined inhalers containing both formoterol and an inhaled corticosteroid, when used for reliever therapy, in adults and children with chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials of parallel group design were included in the review.
Types of participants
Adults and children with a diagnosis of chronic asthma. We accepted trialist‐defined asthma, recording the definition of asthma used in the studies, and the entry criteria. We summarised baseline severity of lung function and persistence of symptoms, and we collected data on pre‐study maintenance therapies. We did not include studies conducted in an emergency department setting.
Types of interventions
Eligible treatment group intervention
This review included studies which assessed the effects of using a combined formoterol and inhaled steroid delivered through a single inhaler device for relief of symptoms. The intervention was considered in three different drug therapy regimens which we analysed separately:
As needed, in addition to regular maintenance therapy with combination inhaled steroid and long‐acting beta‐agonist (i.e. as needed and regular combination therapy).
As needed, in addition to regular maintenance therapy with inhaled steroid only (i.e. as needed combination therapy and regular ICS).
As needed only (i.e. combination therapy used without maintenance treatment).
Eligible control group treatment
The control groups for the studies in this review consisted of a prescribed fast‐acting beta2‐agonist such as terbutaline, salbutamol or formoterol alone, given on an as needed basis. We plan to include future studies which compare reliever therapy with combination formoterol inhalers and other fast‐acting beta2‐agonists combined in a single inhaler with an inhaled corticosteroid.
The maintenance dose of ICS or combination therapy had to be the same in both treatment groups. Fixed dosing had to remain stable within the control limbs of each of the studies. Study duration had to be greater than 12 weeks.
We did not consider studies that compared different combination therapy inhalers (i.e. seretide versus symbicort), or titration of maintenance dosing of combination therapy based on clinical signs and symptoms.
Types of outcome measures
Primary outcomes
Exacerbations of asthma requiring hospitalisation
Exacerbations of asthma requiring oral steroids
Serious adverse events (including mortality and life‐threatening events)
Secondary outcomes
Exacerbations (not otherwise specified)
Clinic spirometry Fixed Expiratory Volume in one second (FEV1)
Diary card morning and evening peak expiratory flow (PEF)
Number of rescue medication puffs required per day
Symptoms
Quality of life
Adverse events
Study withdrawal
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
("single inhaler therapy" or SiT or SMART or relie* or "as need*" or as‐need* or prn or flexible or titrat*) and ((combin* or symbicort or viani) or ((steroid* or corticosteroid* or ICS or budesonide or BUD or Pulmicort or beclomethasone or BDP or becotide) and ("beta*agonist" or "beta*adrenergic agonist" or formoterol or eformoterol or oxis or foradil)))
Searching other resources
We contacted trialists and manufacturers in order to confirm data and to establish whether other unpublished or ongoing studies were available for assessment. We handsearched clinical trials web sites (www.clinicalstudyresults.org; www.clinicaltrials.gov; www.fda.gov) and the clinical trial web sites of manufacturers (www.ctr.gsk.co.uk; www.astrazenecaclinicaltrials.com)
Data collection and analysis
Selection of studies
Following electronic literature searches, two review authors independently selected articles on the basis of title or abstract or both for full text scrutiny. The authors agreed a list of articles which were retrieved, and they subsequently assessed each study to determine whether it was a secondary publication of a primary study publication, and to determine whether the study met the entry criteria of the review.
Data extraction and management
We extracted information from each study for the following characteristics:
Design (description of randomisation, blinding, number of study centres and location, number of study withdrawals). Participants (sample size (N), mean age, age range of the study, baseline lung function, percentage on maintenance ICS or ICS/LABA combination and average daily dose of steroid, entry criteria). Intervention (type and dose of component ICS and LABA, control limb dosing schedule, inhaler device, study duration and run‐in) Outcomes (type of outcome analysis, outcomes analysed)
We extracted data for each of the outcomes considered by the review from the trial publication(s) or from correspondence with trialists or the manufacturer.
Assessment of risk of bias in included studies
We assessed trial bias protection in the following domains according to whether studies meet the following pre‐specified quality criteria (as met, unmet or unclear, Handbook 2006):
Sequence Generation;
Allocation Concealment;
Blinding of participants and investigators;
Loss to follow up.
Assessment of heterogeneity
We measured statistical variation between combined studies by the I‐squared (I2) statistic (Higgins 2003).
Data synthesis
Data was combined with RevMan 5.0, using a fixed‐effect mean difference (calculated as either a weighted mean difference or a mean difference weighted by generic inverse variance) for continuous data variables, and a fixed‐effect odds ratio for dichotomous variables. For the primary outcome of exacerbations we planned to calculate a number needed to treat to benefit (NNTB) for the different levels of risk as represented by control group event rates over a specified time period (www.nntonline.net).
Subgroup analysis and investigation of heterogeneity
We looked at the data from adults and children separately. Adult studies were considered as those which recruited participants from 18 upwards. Adult and adolescent studies were considered as those which recruit participants from 12 upwards. We considered participants in studies where the upper age limit was 12 years as children, and in studies where the upper age limit was 18 years as children and adolescents.
Sensitivity analysis
Sensitivity analysis was planned on the basis of risk of bias in studies and methods of data analysis (fixed and random‐effects models).
Results
Description of studies
Results of the search
The original search was out in April 2008 and included 199 abstracts and a new search was also carried out in April 2009 which included 30 further abstracts. Thirty six of the original abstracts were potentially relevant and full text articles were obtained. There were 11 citations relating to the three studies included in the review and 25 citations for the 12 studies that were excluded (seeCharacteristics of included studies and Characteristics of excluded studies for full details).
The updated search did not yield any further studies for inclusion; five potentially relevant full text articles were independently assessed by CJC and TL. Stallberg 2008 was excluded because the two comparison arms used different maintenance regimens (either higher dose budesonide/formoterol or adjustable budesonide and formoterol in separate inhalers). Two references were to existing excluded studies (MONO and SALTO) and Riemersma 2008 was excluded as the comparison was with usual care.
Included studies
The three trials (5905 participants) that are included in this review have been considered as four separate data sources, in order to separate the STAY study into children STAY ‐ Children and adults STAY ‐ Adults. However it has not been possible to obtain separate data for adults and children for all outcomes, so for these the overall data has been entered under the label STAY ‐ All ages.
This was a small study on 92 adults with mild asthma who were symptomatic but not taking any inhaled steroids, and had a concentration of nitric oxide in exhaled breath (FeNO) level of more than 20 ppb. The mean pre‐bronchodilator FEV1 was 101% predicted normal. Budesonide/formoterol 200/6 mcg was compared with formoterol 6 mcg as reliever therapy (with no maintenance therapy in either arm) over a 24 week treatment period. The primary outcome for this study was mean change in FeNO, but this was not a pre‐specified outcome for this review so the results are not reported here.
This was a large Multicentre study on 3394 adults and adolescents with moderate to severe persistent asthma who had a history of more than one severe asthma exacerbation in the past year, and remained symptomatic on budesonide/formoterol 200/6 one inhalation twice daily during a two week run‐in. Their mean pre‐bronchodilator FEV1 was 72% predicted and the mean baseline daily ICS level was 755 mcg/day. Fifty nine per cent of participants were also taking LABA before participating in the study. During the 12 month trial period budesonide/formoterol 200/6 mcg one inhalation twice daily was given as maintenance to all patients (half the average dose of ICS used previously); the same inhaler as reliever was compared to formoterol 6 mcg and terbutaline 500 mcg as reliever in the three arms of the study.
The definition of a severe asthma exacerbation in this study included hospitalisation, emergency room (ER) visits or the need for oral steroids for three days or more. The proportion of patients with a severe exacerbation was the primary outcome used for the power calculation in this study.
This was a large Multicentre study on 2419 adults and adolescents with moderate to severe persistent asthma uncontrolled on ICS (400 to 1000 mcg/day) and a history of at least one "clinically important" exacerbation in the past year. Their mean pre‐bronchodilator FEV1 was 73% predicted and the mean baseline daily ICS level was 660 mcg/day. Twenty eight per cent of participants (adults and children) were already taking LABA before participating in the study. To be eligible for randomisation patients had to have used 12 or more inhalations of as‐needed medication during the last 10 days of run‐in, when they were treated with their previous ICS but LABA was withdrawn. During the 12 month trial period budesonide/formoterol 100/6 mcg one inhalation twice daily was given as maintenance to all patients in two arms of the study (one third of the previously prescribed dose of ICS); the same inhaler as reliever was compared to terbutaline 500 mcg as reliever. The third arm of the study did not contribute data to this review as a higher dose of budesonide was used for maintenance with terbutaline as reliever.
The definition of a severe asthma exacerbation in this study included hospitalisation, emergency room (ER) visits, oral steroid treatment, or morning peak flow of 70% or less of baseline on two consecutive days. The primary efficacy variable was time to first severe asthma exacerbation.
This was a subset of data presented from 341 children aged 4 to 11 years in the STAY study. They had moderate to severe persistent asthma uncontrolled on ICS (200 to 500 mcg/day) and a history of at least one "clinically important" exacerbation in the past year. Their mean pre‐bronchodilator FEV1 was 76% predicted and the mean baseline daily ICS level was 315 mcg/day. The proportion of children taking LABA before participating in the study is not reported. To be eligible for randomisation patients had to have used eight or more inhalations of as‐needed medication during the last 10 days of run‐in, when they were treated with their previous ICS but LABA was withdrawn. During the 12 month trial period budesonide/formoterol 100/6 mcg one inhalation in the evening was given as maintenance to all patients in two arms of the study (effectively one third of the previously prescribed dose of ICS); the same inhaler as reliever was compared to terbutaline 500 mcg. The third arm of the study did not contribute data to this review as a higher dose of budesonide was used for maintenance with terbutaline as reliever.
The definition of a severe asthma exacerbation in this study included hospitalisation, emergency room (ER) visits, oral steroid treatment, or morning peak flow of 70% or less of baseline on two consecutive days. For children an increase in inhaled corticosteroids (via a separate inhaler), or other additional treatment were also classified as severe asthma exacerbations. The primary efficacy variable was time to first severe asthma exacerbation.
Excluded studies
Three of these studies (Ind 2002; Richter 2007; Tattersfield 2001) used formoterol alone as reliever therapy, and the other studies (Bousquet 2007; COMPASS; COSMOS; Loukides 2005; Lundborg 2006; Scicchitano 2004; SOLO; Sovani 2008; STEAM) were trials of Single Inhaler Therapy with budesonide/formoterol but used different maintenance treatments as well as comparing different reliever therapy.
Risk of bias in included studies
All the included studies had adequate sequence generation and blinding, drop out rates were not high Figure 1. Although allocation concealment was not well‐reported, it is likely that it was also adequate in view of the sponsored nature of the studies.
1.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Budesonide/formoterol as reliever in mild asthma with no maintenance therapy
One small study in adults (SOMA) has looked at the effect of reliever therapy with budesonide/formoterol 200/6 in comparison with formoterol alone. All patients in the study had a baseline FeNO of at least 20 ppb and this was the main outcome measure for this study (but not a pre‐specified outcome for this review). No studies were found addressing this comparison in children.
Primary outcomes
There were no reported exacerbations requiring oral corticosteroids in this study and no reported hospitalisations. One patient in the formoterol group took 12 reliever inhalations on one day, and further information is awaited from the sponsors as to whether this resulted in treatment with oral corticosteroids (as specified in the trial protocol). Three serious adverse events were reported for the budesonide/formoterol group (migraine, road traffic accident and hydatidiform molar pregnancy), compared to none in the formoterol group.
Secondary outcomes
Reliever inhaler use rose in both groups from baseline levels of 3.9 inhalations/week to 5.7 inhalations per week for budesonide/formoterol and from 3.2 to 5.9 inhalations/week for formoterol alone. The proportion of rescue free days was 52% and 56% respectively. These differences were not statistically significant.
There were no significant between group differences in morning or evening PEF, asthma symptom scores or asthma free days. FEV1 increased from 102.4% to 104.2% predicted with budesonide/formoterol, in comparison to a fall from 99.5% to 99.2% predicted with formoterol. This represents a difference of 2.7% (0.7% to 4.7%), which is statistically significant but is of unproven clinical importance.
Budesonide/formoterol as reliever in asthma with ICS maintenance therapy
No trials were found (in adults or children) where ICS alone was used as maintenance therapy at the same dose in both arms of the trial, with budesonide/formoterol compared to beta2‐agonist alone as reliever.
Budesonide/formoterol as reliever in asthma with ICS and LABA maintenance therapy compared to terbutaline as reliever
Adults
Two studies contribute to this comparison (SMILE; STAY ‐ Adults ). Both studies enrolled patients who were symptomatic on ICS and had a history of previous exacerbations. The SMILE study enrolled patients with a higher previous ICS intake (mean 755 mcg/day) and halved the ICS maintenance by using budesonide/formoterol 200/6 mcg twice daily and compared the same inhaler as reliever to formoterol and terbutaline. In contrast the patients in STAY ‐ Adults had a lower dose of previous ICS (mean 660 mcg/day) and this was reduced by two thirds by using a lower dose of combined inhaler, budesonide/formoterol 100/6 mcg, as twice daily maintenance and reliever compared to the same maintenance with terbutaline reliever. The patients in SMILE had to remain symptomatic on budesonide/formoterol 200/6 mcg twice daily during the run‐in period, whereas those in STAY ‐ Adults had to be symptomatic on their previous ICS dosage without LABA.
Primary outcomes
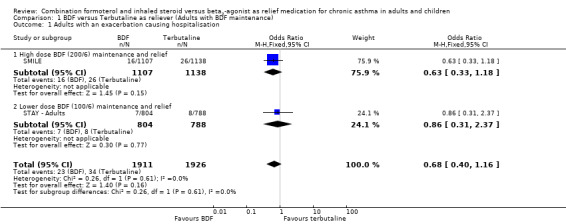
The way that asthma exacerbations have been reported makes full assessment of our primary outcomes impossible without further information. A breakdown of the exacerbation data in both studies has been requested from the sponsors; they have provided information from STAY ‐ Children in relation to the number of patients who had a hospitalisation that was asthma related and additional information from STAY ‐ Adults on patients with one or more courses of oral corticosteroids has now been added. As data on serious adverse events that were asthma related is available from SMILE, we have used this data as a proxy for asthma exacerbations leading to hospital admission, (as almost all serious adverse events are classified as serious due to admission to hospital).
No data was directly available from SMILE in relation to asthma exacerbations leading to hospital admission. Moreover the proportion of severe asthma exacerbations recorded that led to a hospitalisation or ER visit is low in both studies; in SMILE one third of exacerbations fall in this category and in STAY ‐ All ages the proportion is 13% of all severe exacerbations and 19% of those severe exacerbations needing medical intervention. The only current indication of the number of patients who were admitted to hospital for asthma in SMILE comes from asthma‐related serious adverse events, of which there were 16 on budesonide/formoterol and 26 on terbutaline . The sponsors have provided hospitalisation data from STAY ‐ All ages and there were seven on budesonide/formoterol and eight on terbutaline. This is a close match to the asthma‐related serious adverse events, which yielded seven adults on budesonide/formoterol and nine on terbutaline in this category. The pooled results from both studies yield a combined Odds Ratio of 0.68 (95% CI 0.40 to 1.16), Figure 2.
2.

Forest plot of comparison: 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), outcome: 1.1 Adults with an exacerbation causing hospitalisation.
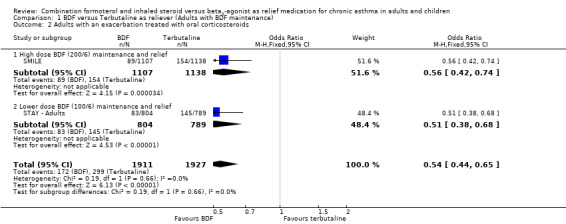
Data on with an asthma exacerbation requiring oral corticosteroids was not originally reported for either study, but has been calculated for SMILE (from Table 2 in the paper publication), by subtracting the number of patients with a hospitalisation or ER visit from the total number that had a severe exacerbation. There was a significant reduction in adults treated with oral corticosteroids in SMILE, and data on this outcome has now been obtained from data on file at AstraZeneca in relation to STAY ‐ Adults. The combined results from these two studies show a reduction in the number of patients needing oral corticosteroids with single inhaler therapy, Odds Ratio 0.54 (95% CI 0.44 to 0.65), Figure 3. This translates into a number needed to treat over 12 months of 15 (95% CI 13 to 21) in order to prevent one patient being treated with oral corticosteroids, Figure 4.
3.
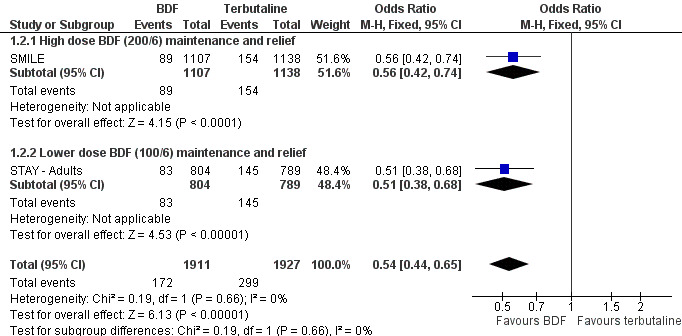
Forest plot of comparison: 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), outcome: 1.2 Adults with an exacerbation treated with oral corticosteroids.
4.
In the control group 16 people out of 100 had asthma exacerbation needing oral steroids over 12 months, compared to 9 (95% CI 8 to 11) out of 100 for the active treatment group using single inhaler therapy compared to terbutaline as reliever over 12 months. NNT(B) 15 (95% CI 13 to 21)
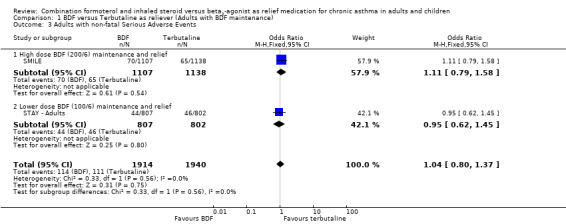
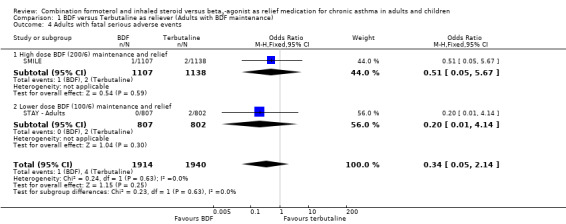
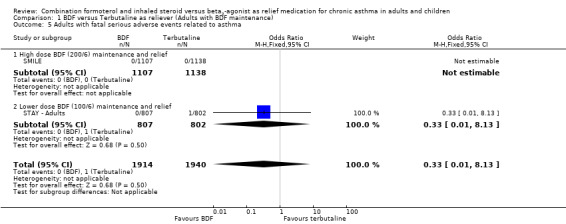
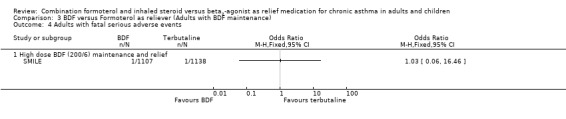
The combined data on adults with all cause serious adverse events from STAY ‐ Adults and SMILE did not show a significant difference between budesonide/formoterol and terbutaline as reliever, Odds Ratio 1.04 (95% CI 0.79 to 1.36), Figure 5. There were four deaths in 1,940 patients on terbutaline from the two studies and one death in 1,914 patients on budesonide/formoterol, giving a pooled Odds Ratio of 0.34 (0.05 to 2.14) for all cause fatal serious adverse events, Figure 6. There were no patients recorded as having an asthma‐related fatal event on budesonide/formoterol and one patient on terbutaline, Figure 7.
5.
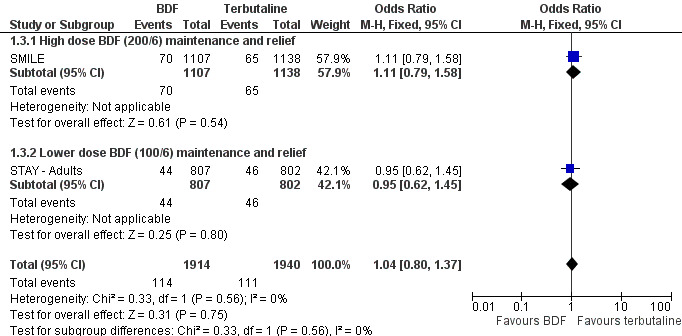
Forest plot of comparison: 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), outcome: 1.3 Adults with non‐fatal Serious Adverse Events.
6.

Forest plot of comparison: 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), outcome: 1.4 Adults with fatal serious adverse events.
7.
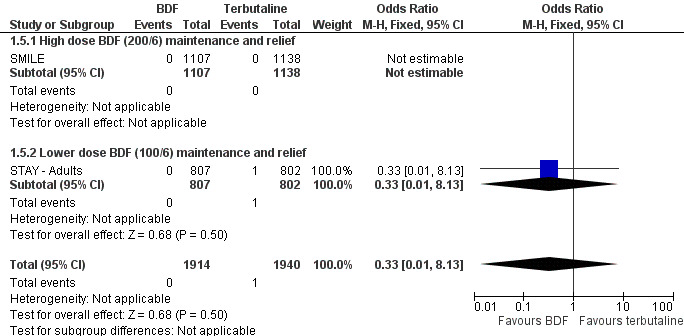
Forest plot of comparison: 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), outcome: 1.5 Adults with fatal serious adverse events related to asthma.
Secondary outcomes
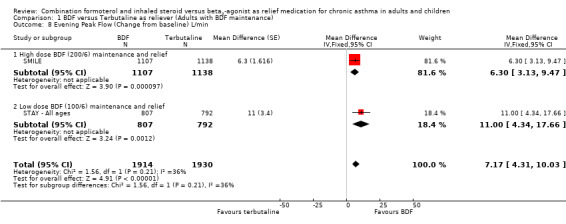
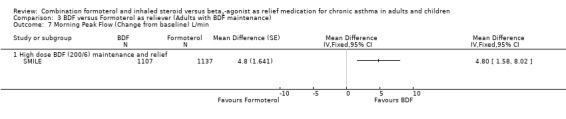
The secondary outcomes were not reported separately for adults in STAY ‐ Adults, so as 87% of the total population were adults and adolescents the combined data for all ages was used (STAY ‐ All ages). With the exception of asthma control days (where no significant difference was seen), all the other secondary outcomes favoured budesonide/formoterol as reliever, but the differences were too small to be clinically significant. The pooled difference in FEV1 for SMILE and STAY ‐ All ages was 0.07 Litres (95% CI 0.05 to 0.09 Litres), Analysis 1.6, and morning peak flow showed a pooled difference of 7.88 Litres/min (95% CI 5.11 to 10.66), Analysis 1.7. The percentage of nights with awakenings fell from 20% in the run‐in period to 9% on budesonide/formoterol reliever and 12% in fixed dose budesonide/formoterol with terbutaline reliever in STAY ‐ All ages. Overall the pooled risk difference was ‐3.16% nights with awakenings (95% CI ‐4.64 to ‐1.69), Analysis 1.10.
1.6. Analysis.
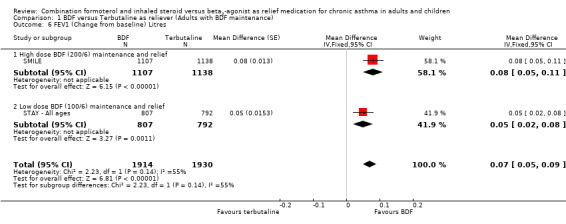
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 6 FEV1 (Change from baseline) Litres.
1.7. Analysis.
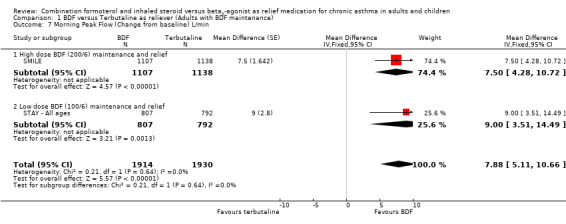
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 7 Morning Peak Flow (Change from baseline) L/min.
1.10. Analysis.

Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 10 Change in % nights with awakenings.
Children
One study included 341 children aged from four to 11 years old (STAY ‐ Children). The children were randomised into three treatment arms, and for the purposes of this review those given budesonide/formoterol 100/6 mcg once daily (in the evening) with the same inhaler to use for relief of symptoms were compared to the same maintenance treatment with terbutaline for symptom relief. The definition of severe exacerbations requiring medical intervention in children was not the same as for adults in this study, but also included an increase in ICS use (via a separate inhaler) and/or additional treatment. Information has been requested from AstraZeneca in order to assess children who had exacerbation by the consequences; separate data on those who were admitted to hospital, attended casualty or received a course of oral steroids are required to do this, for the primary outcome analysis in this review. At present it has only been possible for AstraZeneca to provide data on hospitalisation for asthma.
Primary outcomes
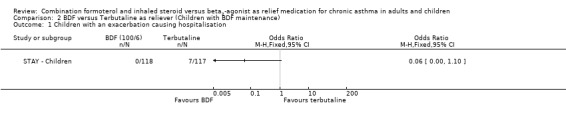
Data provided by AstraZeneca confirmed that there were hospitalisations related to asthma in seven patients on the fixed‐dose combination with terbutaline as reliever, but there were none in patients using budesonide/formoterol as reliever, Figure 8. In addition one child given terbutaline as reliever had an asthma‐related SAE that did not lead to hospitalisation. This reduction in hospitalisation was large, Odds Ratio 0.06 (0.00 to 1.10) but the confidence interval includes the possibility that this could have arisen by chance (as the number of events was small).
8.

Forest plot of comparison: 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), outcome: 2.1 Children with an exacerbation causing hospitalisation.
The number of children with exacerbations requiring oral corticosteroids were not reported in the paper, although the number of days on treatment was less with budesonide/formoterol as reliever (32 versus 320 days, descriptive results only).
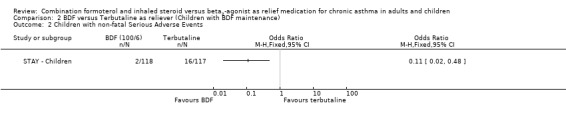
Children with serious adverse events of any cause were significantly less with budesonide/formoterol as reliever, (two events in contrast to 16 events with fixed dose budesonide/formoterol and terbutaline as reliever, odds ratio 0.11 (95% CI 0.02 to 0.48), Figure 9. No fatal events were reported in children. It should also be pointed out that the maintenance dose of budesonide/formoterol used with terbutaline as reliever was only 100/6 mcg daily, which the sponsors regard as an experimental dose and not approved for treatment.
9.

Forest plot of comparison: 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), outcome: 2.3 Children with non‐fatal Serious Adverse Events.
Secondary outcomes
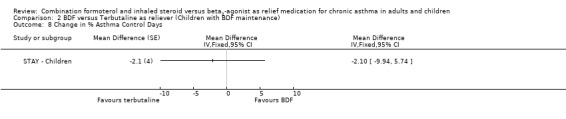
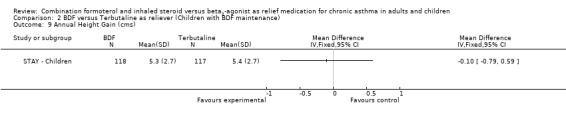
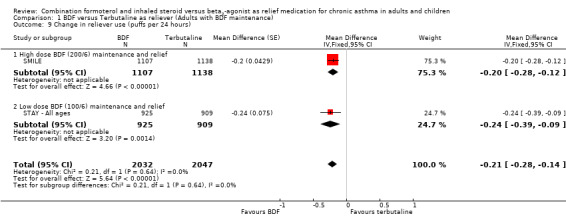
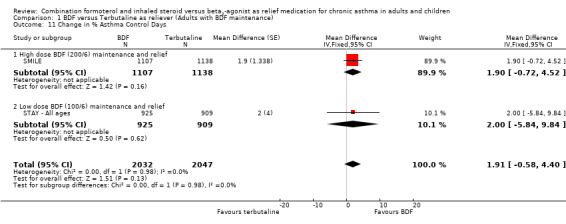
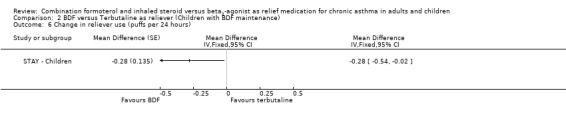
In contrast to the adults, the improvements seen in lung function in this trial were not significant (Analysis 2.4; Analysis 2.5). The use of relievers fell significantly more in the group using budesonide/formoterol as reliever, from an average of 1.6 inhalations per day at baseline to 0.58 per day with budesonide/formoterol and 0.76 per day in the terbutaline reliever group, a reduction of ‐0.28 puffs per day (95% CI ‐0.54 to ‐0.02). Change in percentage nights with awakenings is reported as a significant reduction in the adjusted mean difference in favour of budesonide/formoterol. However this represents 2.4% nights with awakenings on budesonide/formoterol and 4.4% on terbutaline, and the baseline difference between groups is the same size (12.8% and 10.8% respectively), Analysis 2.7. Asthma control days (Analysis 2.8) and annual growth (Analysis 2.9), did not show significant differences between budesonide/formoterol and terbutaline.
2.4. Analysis.
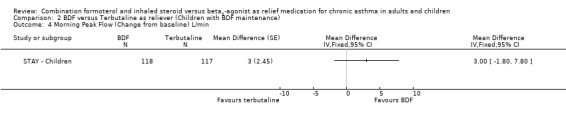
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 4 Morning Peak Flow (Change from baseline) L/min.
2.5. Analysis.

Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 5 Evening Peak Flow (Change from baseline) L/min.
2.7. Analysis.
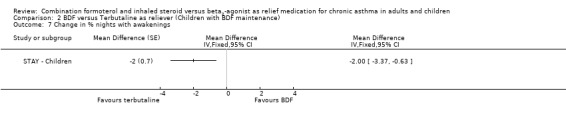
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 7 Change in % nights with awakenings.
2.8. Analysis.
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 8 Change in % Asthma Control Days.
2.9. Analysis.
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 9 Annual Height Gain (cms).
Budesonide/formoterol as reliever in asthma with ICS and LABA maintenance therapy compared to formoterol as reliever
Only the SMILE study in adults contributed to this outcome, as budesonide/formoterol as reliever was compared to formoterol in one of the arms of this trial.
Primary outcomes
As there was no separate reporting of asthma exacerbations leading to hospitalisation in the paper, we used serious adverse events relating to asthma as a measure of hospitalisation. There were 16 patients in the budesonide/formoterol arm in comparison to 23 in the formoterol arm, odds ratio 0.71 (95% CI 0.37 to 1.35), Figure 10.
10.

Forest plot of comparison: 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), outcome: 3.1 Adults with an exacerbation causing hospitalisation.
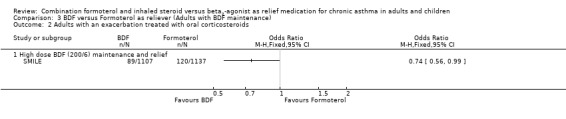
Asthma exacerbations requiring oral corticosteroids were not reported but could be calculated by subtracting the number of patients with admissions/ER visits from the total number with exacerbations. This represented a significant reduction using budesonide/formoterol as reliever from 120 to 89 patients, odds ratio 0.74 (95% CI 0.56 to 0.99), Figure 11 .
11.

Forest plot of comparison: 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), outcome: 3.2 Adults with an exacerbation treated with oral corticosteroids.
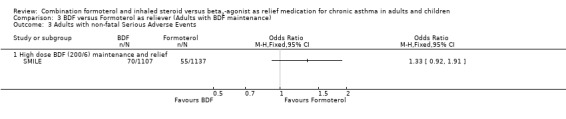
Non‐fatal serious adverse events relating from any cause were not significantly different, budesonide/formoterol 70 participants and formoterol 55 participants, odds ratio 1.33 (95% CI 0.92 to 1.91), Figure 12. There were was one fatal serious adverse event on budesonide/formoterol and one on formoterol, neither was reported as due to asthma.
12.

Forest plot of comparison: 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), outcome: 3.3 Adults with non‐fatal Serious Adverse Events.
Secondary outcomes
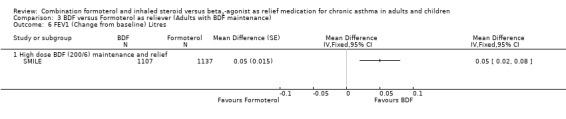
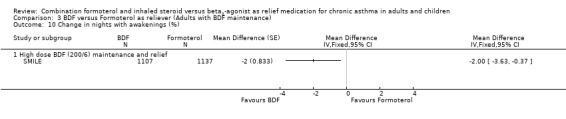
The results for lung function, reliever use and night awakenings were very similar to the findings when budesonide/formoterol was compared to terbutaline, with clinically small, but statistically significant advantages for budesonide/formoterol as reliever. SeeAnalysis 3.6 to Analysis 3.10. Again the difference in asthma control days was not statistically significant Analysis 3.11.
3.6. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 6 FEV1 (Change from baseline) Litres.
3.10. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 10 Change in nights with awakenings (%).
3.11. Analysis.

Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 11 Change in Asthma Control Days (%).
Discussion
Summary of main results
In patients with mild asthma who do not need maintenance treatment, no clinically important advantages of budesonide/formoterol as reliever were found in comparison to formoterol as reliever.
In the two studies on patients with more severe asthma, who had suffered a recent exacerbation in spite of quite high doses of inhaled corticosteroids, a lower maintenance ICS dose (in the form of regular budesonide/formoterol), and budesonide/formoterol as reliever was compared to formoterol or terbutaline as reliever. Hospital admissions make up a small proportion of those reported as having a severe asthma exacerbation, and the combined results yield a non‐significant pooled odds ratio of 0.68 (95% CI 0.40 to 1.16). The use of oral corticosteroids was significantly lower in SMILE (both in comparison to formoterol or terbutaline as reliever), but comparative data from STAY ‐ All ages are not currently available. Serious adverse events were not significantly different in adults, but in children there were significantly less serious adverse events in the group using budesonide/formoterol as maintenance and reliever, in comparison to those on budesonide/formoterol maintenance and terbutaline as reliever. However the maintenance dose of budesonide/formoterol used with terbutaline as reliever was only 100/6 mcg daily, which the sponsors regard as an experimental dose and not approved for treatment.
There was no significant difference in annual growth in children using budesonide/formoterol reliever in comparison to terbutaline in STAY ‐ Children.
Overall completeness and applicability of evidence
The application of the results of SMILE, STAY ‐ Adults and STAY ‐ Children should be in the light of the patients enrolled in these trials, who all had suffered an asthma exacerbation in the past year, were not controlled on quite high doses of inhaled corticosteroids, and then were all treated with a lower dose of maintenance inhaled corticosteroid treatment. Data for our primary outcome were rarely available in a format usable for meta‐analysis as dichotomous data, nor were exacerbation types sub‐divided sufficiently to give a complete overview of exacerbations from the studies we included. There remains some uncertainty as to how complete our numerical analyses are.
Quality of the evidence
All the included studies were double blind. Although allocation concealment was not clearly reported, we concluded that the included studies were unlikely to have suffered from selection bias, as they were carried out by the sponsors for regulatory purposes.
Potential biases in the review process
Reporting of serious adverse events was not uniform across the studies, and the use of a composite outcome for exacerbations with no separate reporting of hospital admissions for asthma makes the possibility of publication bias a concern. A report on the manufacturer's controlled trial web site was only found for STAY ‐ All ages and STAY ‐ Children.
Agreements and disagreements with other studies or reviews
The use of budesonide/formoterol as a reliever therapy for asthma is subject to product license, and this is currently restricted; for example use as reliever is restricted to adults over 18 years in the British National Formulary (issue 55).
Authors' conclusions
Implications for practice.
In mild asthma it is not yet known whether patients who use a budesonide/formoterol inhaler for relief of asthma symptoms derive any clinically important benefits. In more severe asthma, two studies enrolled patients who were not controlled on inhaled corticosteroids, and had suffered an exacerbation in the previous year, and then had their maintenance inhaled corticosteroids reduced in both arms of the study. Under these conditions the studies demonstrated a reduction in the risk of exacerbations that require oral corticosteroids with budesonide/formoterol for maintenance and relief in comparison with budesonide/formoterol for maintenance and terbutaline or formoterol for relief. The incidence of serious adverse events in children was also less using budesonide/formoterol for maintenance and relief in one study, which similarly enrolled children who were not controlled on inhaled corticosteroids, and who had their maintenance inhaled corticosteroids reduced at the start of the study. This study also compared an explorative maintenance dose of budesonide/formoterol that is not approved for treatment.
Implications for research.
More research is needed in the use of budesonide/formoterol for symptom relief in patients with less severe asthma, particularly in those patients who take low doses of inhaled corticosteroids. Asthma exacerbations reporting should include patients with hospital admissions and those treated with oral corticosteroids, the composite of these outcomes, and transparent reporting of serious adverse events.
Feedback
Study generalisability and interpretational issues, 9 March 2009
Summary
The patients recruited to the studies were randomised to receive lower doses of ICS than they had been taking prior to study entry, and would do poorly compared to those that received reliever/ICS. The control groups in both the SMILE and STAY studies are essentially set up to fail. In our opinion, this critical point about the inappropriate treatment given to the control group (although briefly mentioned in the review) was not emphasized enough.
The SMILE and STAY studies are really attempting to see if using ICS for relief is worthwhile as both treatment arms get reliever medications; the only difference is that one group receives ICS and the other does not.
Did the authors confirm that patients who were hospitalized did not receive oral steroids as well? Did the authors confirm that exacerbations were counted properly? If a patient was first hospitalized for an exacerbation, and then later had another exacerbation not requiring hospitalization (a subsequent event), was the latter counted and reported? And if so was this accounted for in the current review?
The authors state that selection bias was unlikely as trials were all double‐blinded. As we understand it, double‐blinding limits reporting/assessment/concomitant treatment bias and it is allocation concealment procedures that limit selection bias. With regard to implications for research we do not feel that use of a combination reliever in patients with less severe asthma would be of clinical value. If patients only require low dose ICS or no ICS then why expose them to the potential for adverse events of corticosteroids even if it is through intermittent use?
The serious adverse event findings did not receive enough emphasis. The suggestion that severe asthmatics derived a benefit from reliever/ICS would only be true if serious adverse events rates were lower for this group compared to the formoterol alone patients. In other words, the reduction in exacerbations requiring oral steroids should have correlated with a reduction in serious adverse events, if the combination produces a net benefit, but it did not. A more appropriate conclusion would have been that no overall health benefit was proven in adults with severe asthma despite the potential reduction in exacerbations requiring steroids.
Reply
We address these comments on a point by point basis as follows:
We agree that the study populations recruited were randomised to receive a lower dose of steroid than they had previously been treated with. We have stated this more clearly in the abstract and conclusions of the review, although we have not restated this in the discussion since we feel that this is clearly emphasized already.
The corticosteroid is co‐delivered with formoterol and compared with terbutaline in most of the studies we included. It is true that both groups of participants receive reliever medications, but essentially the studies are assessing the combination of ICS with a long‐acting beta‐agonist with a fast onset against a short‐acting beta‐agonist alone.
This point refers to the first published version of the review. Since the review was published, we have received data on OCS‐treated exacerbations from the sponsors of the studies and analysed these data as per protocol. The data provided by the sponsors enabled us to add these findings as binary data for both OCS‐treated exacerbations and hospital admission in December 2008 (see History of review in the What's new section). The results have been presented as patients with at least one hospital admission or course of oral steroids (as described in the protocol for the review); this is to avoid unit of analysis errors which can arise when multiple events in a single patient are counted as if they came from separate patients.
We have clarified that our judgement of selection bias is based on sequence generation and allocation concealment (not on double blinding).
We have made the SAE data in the review available for users to make up their own minds about the benefits and risks of treatment. We disagree that we should conclude overall benefit in favour of combination therapy only when SAEs favour this form of therapy. Our primary outcomes included oral steroid use, which favoured combination therapy, and SAEs which did not indicate that either form of relief therapy had an advantage over the other.
We thank Dr Tejani for these comments.
Contributors
Aaron Tejani & Vivian Yih
What's new
| Date | Event | Description |
|---|---|---|
| 10 April 2013 | Amended | Acknowledgements updated |
| 15 July 2009 | Amended | Correction to contributors who made the comment posted in March 2009 |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 21 April 2009 | New search has been performed | New search carried out in April 2009 but no new studies found for inclusion |
| 9 March 2009 | Feedback has been incorporated | Comment submitted relating to study generalisability and interpretational issues. Changes made to emphasis on some of the results, and rebuttal included in the review. |
| 11 December 2008 | Amended | Additional data incorporated in relation to patients with at least one course of oral corticosteroids in STAY ‐ Adults. The conclusions are unchanged as the results are very similar to the same outcome from SMILE that were already included in the review. |
| 14 March 2008 | Amended | Converted to new review format. |
| 14 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Susan Hansen and Elizabeth Stovold of the Cochrane Airways Group for their assistance in searching for trials and obtaining the abstracts and full reports. We also thank Roman Jaeschke and Robyn Von Maltzahn for help in obtaining data on asthma‐related hospitalisations and serious adverse events from AstraZeneca in relation to STAY ‐ Adults and STAY ‐ Children.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. BDF versus Terbutaline as reliever (Adults with BDF maintenance).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adults with an exacerbation causing hospitalisation | 2 | 3837 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.16] |
| 1.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.18] |
| 1.2 Lower dose BDF (100/6) maintenance and relief | 1 | 1592 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.31, 2.37] |
| 2 Adults with an exacerbation treated with oral corticosteroids | 2 | 3838 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.65] |
| 2.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.42, 0.74] |
| 2.2 Lower dose BDF (100/6) maintenance and relief | 1 | 1593 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.38, 0.68] |
| 3 Adults with non‐fatal Serious Adverse Events | 2 | 3854 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.80, 1.37] |
| 3.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.58] |
| 3.2 Lower dose BDF (100/6) maintenance and relief | 1 | 1609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.45] |
| 4 Adults with fatal serious adverse events | 2 | 3854 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.05, 2.14] |
| 4.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.67] |
| 4.2 Lower dose BDF (100/6) maintenance and relief | 1 | 1609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.14] |
| 5 Adults with fatal serious adverse events related to asthma | 2 | 3854 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.13] |
| 5.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Lower dose BDF (100/6) maintenance and relief | 1 | 1609 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.13] |
| 6 FEV1 (Change from baseline) Litres | 2 | 3844 | Mean Difference (Fixed, 95% CI) | 0.07 [0.05, 0.09] |
| 6.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Mean Difference (Fixed, 95% CI) | 0.08 [0.05, 0.11] |
| 6.2 Low dose BDF (100/6) maintenance and relief | 1 | 1599 | Mean Difference (Fixed, 95% CI) | 0.05 [0.02, 0.08] |
| 7 Morning Peak Flow (Change from baseline) L/min | 2 | 3844 | Mean Difference (Fixed, 95% CI) | 7.88 [5.11, 10.66] |
| 7.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Mean Difference (Fixed, 95% CI) | 7.5 [4.28, 10.72] |
| 7.2 Low dose BDF (100/6) maintenance and relief | 1 | 1599 | Mean Difference (Fixed, 95% CI) | 9.0 [3.51, 14.49] |
| 8 Evening Peak Flow (Change from baseline) L/min | 2 | 3844 | Mean Difference (Fixed, 95% CI) | 7.17 [4.31, 10.03] |
| 8.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Mean Difference (Fixed, 95% CI) | 6.30 [3.13, 9.47] |
| 8.2 Low dose BDF (100/6) maintenance and relief | 1 | 1599 | Mean Difference (Fixed, 95% CI) | 11.0 [4.34, 17.66] |
| 9 Change in reliever use (puffs per 24 hours) | 2 | 4079 | Mean Difference (Fixed, 95% CI) | ‐0.21 [‐0.28, ‐0.14] |
| 9.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Mean Difference (Fixed, 95% CI) | ‐0.2 [‐0.28, ‐0.12] |
| 9.2 Low dose BDF (100/6) maintenance and relief | 1 | 1834 | Mean Difference (Fixed, 95% CI) | ‐0.24 [‐0.39, ‐0.09] |
| 10 Change in % nights with awakenings | 2 | 4079 | Mean Difference (Fixed, 95% CI) | ‐3.16 [‐4.64, ‐1.69] |
| 10.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Mean Difference (Fixed, 95% CI) | ‐2.6 [‐4.28, ‐0.92] |
| 10.2 Low dose BDF (100/6) maintenance and relief | 1 | 1834 | Mean Difference (Fixed, 95% CI) | ‐5.0 [‐8.04, ‐1.96] |
| 11 Change in % Asthma Control Days | 2 | 4079 | Mean Difference (Fixed, 95% CI) | 1.91 [‐0.58, 4.40] |
| 11.1 High dose BDF (200/6) maintenance and relief | 1 | 2245 | Mean Difference (Fixed, 95% CI) | 1.9 [‐0.72, 4.52] |
| 11.2 Low dose BDF (100/6) maintenance and relief | 1 | 1834 | Mean Difference (Fixed, 95% CI) | 2.0 [‐5.84, 9.84] |
1.1. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 1 Adults with an exacerbation causing hospitalisation.
1.2. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 2 Adults with an exacerbation treated with oral corticosteroids.
1.3. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 3 Adults with non‐fatal Serious Adverse Events.
1.4. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 4 Adults with fatal serious adverse events.
1.5. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 5 Adults with fatal serious adverse events related to asthma.
1.8. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 8 Evening Peak Flow (Change from baseline) L/min.
1.9. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 9 Change in reliever use (puffs per 24 hours).
1.11. Analysis.
Comparison 1 BDF versus Terbutaline as reliever (Adults with BDF maintenance), Outcome 11 Change in % Asthma Control Days.
Comparison 2. BDF versus Terbutaline as reliever (Children with BDF maintenance).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children with an exacerbation causing hospitalisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Children with non‐fatal Serious Adverse Events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 FEV1 (Change from baseline) Litres | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Morning Peak Flow (Change from baseline) L/min | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Evening Peak Flow (Change from baseline) L/min | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Change in reliever use (puffs per 24 hours) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Change in % nights with awakenings | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 Change in % Asthma Control Days | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Annual Height Gain (cms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 1 Children with an exacerbation causing hospitalisation.
2.2. Analysis.
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 2 Children with non‐fatal Serious Adverse Events.
2.3. Analysis.

Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 3 FEV1 (Change from baseline) Litres.
2.6. Analysis.
Comparison 2 BDF versus Terbutaline as reliever (Children with BDF maintenance), Outcome 6 Change in reliever use (puffs per 24 hours).
Comparison 3. BDF versus Formoterol as reliever (Adults with BDF maintenance).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adults with an exacerbation causing hospitalisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 High dose BDF (200/6) maintenance and relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adults with an exacerbation treated with oral corticosteroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 High dose BDF (200/6) maintenance and relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adults with non‐fatal Serious Adverse Events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 High dose BDF (200/6) maintenance and relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adults with fatal serious adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 High dose BDF (200/6) maintenance and relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adults with fatal serious adverse events related to asthma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 High dose BDF (200/6) maintenance and relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 FEV1 (Change from baseline) Litres | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6.1 High dose BDF (200/6) maintenance and relief | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Morning Peak Flow (Change from baseline) L/min | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7.1 High dose BDF (200/6) maintenance and relief | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Evening Peak Flow (Change from baseline) L/min | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8.1 High dose BDF (200/6) maintenance and relief | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Change in reliever use (puffs per 24 hours) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9.1 High dose BDF (200/6) maintenance and relief | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change in nights with awakenings (%) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10.1 High dose BDF (200/6) maintenance and relief | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in Asthma Control Days (%) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 11.1 High dose BDF (200/6) maintenance and relief | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 1 Adults with an exacerbation causing hospitalisation.
3.2. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 2 Adults with an exacerbation treated with oral corticosteroids.
3.3. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 3 Adults with non‐fatal Serious Adverse Events.
3.4. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 4 Adults with fatal serious adverse events.
3.5. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 5 Adults with fatal serious adverse events related to asthma.
3.7. Analysis.
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 7 Morning Peak Flow (Change from baseline) L/min.
3.8. Analysis.
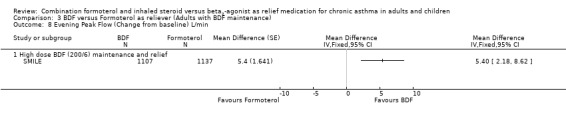
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 8 Evening Peak Flow (Change from baseline) L/min.
3.9. Analysis.
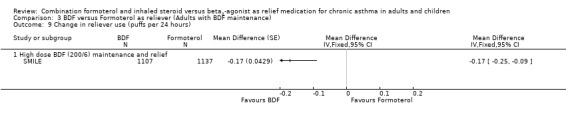
Comparison 3 BDF versus Formoterol as reliever (Adults with BDF maintenance), Outcome 9 Change in reliever use (puffs per 24 hours).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
SMILE.
| Methods | Study Design: Randomised, double‐blind, parallel group study over a 12 month period in 289 centres in 20 countries (between April 2003 and Dec 2004). | |
| Participants | Population: 3394 asthmatic adults aged 12 years or more with asthma previously on ICS and symptomatic on budesonide/formoterol 200/6 twice daily during 2 week run‐in, with more than one exacerbation in the past year. The maintenance treatment was budesonide/formoterol for all patients and terbutaline, formoterol and budesonide/formoterol were compared as reliever therapy. Adults Mean age: 42 years. FEV172% predicted pre bronchodilator. Mean ICS dose at enrolment 755 mcg/day. Hospital admission for asthma in the past year: unknown proportion. Course of oral steroids for asthma in past year: unknown proportion. Inclusion Criteria: Outpatients aged 12+ years, clinical diagnosis of asthma with ICS for at least 3 months, and steady dose for at least 4 weeks. More than one severe asthma exacerbation in the past 12 months was required. Prebronchodilator FEV1 of 50‐100% predicted normal value and at least 12% reversibility following Terbutaline. To be included patients had to need at rescue inhalations for 5 or more of the last 7 days of run‐in. Adults using ten or more rescue inhalations in a single day or with oral steroid course during previous 4 weeks were not included. |
|
| Interventions | 1. Budesonide/formoterol 200/6 mcg twice daily [400 mcg budesonide/day], and as needed (one maintenance and one relief Turbuhaler) 2. Budesonide/formoterol 200/6 mcg twice daily [400 mcg budesonide/day], and Formoterol 6 mcg as needed (one maintenance and one relief Turbuhaler) 3. Budesonide/formoterol 200/6 mcg twice daily [400 mcg budesonide/day], and Terbutaline 500 mcg as needed (one maintenance and one relief Turbuhaler) Maximum of 10 as needed inhalations could be used per day before contacting the investigator. |
|
| Outcomes | Primary outcome ‐ time to first severe exacerbations. Secondary outcomes included number of severe exacerbations, number of days with hospitalisation, ER room visit or both, and days of oral steroid use were recorded. Exacerbation Definition: Severe ‐ Deterioration in asthma requiring hospital or emergency room treatment, or oral steroids (for 3 days or more). Severe exacerbations did not include change in PEF . Mild exacerbation day ‐ defined as PEF 80% or less of baseline this was the average of 10 days before randomisation, relief medication 2 or more inhalations above baseline, (not awakenings due to asthma). Mild exacerbation defined as 2 consecutive mild exacerbation days using the same criteria. |
|
| Notes | SAE data given (70,55,65) of these (16,23,26) were related to asthma. Deaths given for whole trial (1,1,2) Funding source ‐ AstraZeneca. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by an independent person |
| Allocation concealment (selection bias) | Unclear risk | At each centre, eligible patients were sequentially assigned a randomisation code by the investigator from the computer‐generated list. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. All as‐needed study medication was given by identical turbuhalers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and Dropouts: 2989/3374 (88%) completed the study |
SOMA.
| Methods | Study Design: Randomised, double‐blind, parallel group study over 24 weeks. In the 14 days of run‐in patients had to use a reliever on one to five of the last ten days to be eligible. | |
| Participants | Population: 92 asthmatic adults with intermittent mild asthma needing a maximum of two doses of reliever medication per week in the preceding months, with FeNO of at least 20 ppb at enrolment or randomisation. There was no maintenance treatment and formoterol and budesonide/formoterol were compared as reliever therapy. Adults Mean age: 36 years. FEV1101% predicted pre bronchodilator. Mean ICS dose at enrolment zero mcg/day. Inclusion Criteria: Outpatients aged 15+ years, clinical diagnosis of asthma with no ICS for at least 3 months, and no regular maintenance treatment. Prebronchodilator FEV1 of over 80% predicted normal value and at least 12% reversibility following terbutaline or salbutamol, or documented fall in FEV1 of at least 12% after exercise. PEF had to demonstrate at least 15% variability in 2 or more days over 2 weeks, or 15% rise post bronchodilator. To be included patients had to need rescue inhalations for 1 to 5 of the last 10 days of run‐in. |
|
| Interventions | 1. Budesonide/formoterol 200/6 mcg twice as reliever (one Turbuhaler). No maintenance therapy. 2. Formoterol 6 mcg as reliever (one Turbuhaler). No maintenance therapy. Maximum of 12 as needed inhalations could be used per day before contacting the investigator. Patients taking maximum reliever were treated with oral prednisolone and removed from the study if more asthma medication was needed. |
|
| Outcomes | Primary outcome ‐ mean change in FeNO. Secondary outcomes included asthma symptom scores, asthma‐free days, PEF, FEV1 and number of inhalations of study drug. No mention is made with regard to courses of oral prednisolone in each group. | |
| Notes | SAE data given (0,3), none of these were related to asthma. Funding source ‐ AstraZeneca. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation at each study centre was performed in balanced blocks using a computer programme |
| Allocation concealment (selection bias) | Unclear risk | no details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77/93 (83%) completed the study |
STAY ‐ Adults.
| Methods | Study Design: Randomised, double‐blind, parallel group study over a 12 month period in 246 centres in 22 countries (between Jan 2001 and Jan 2003). In the 14‐18 day run‐in patients used pre‐study ICS with terbutaline for symptom relief (LABA had to be discontinued at least 3 days before run‐in). | |
| Participants |
Adult Population: 2419 asthmatic adults aged 12 years or more with asthma uncontrolled on ICS (400‐1000 mcg/day) and a history of at least one "clinically important" exacerbation in the past year. The maintenance ICS dose was cut to about one third with additional Budesonide/formoterol (SiT) compared to terbutaline for relief. Adults Mean age: 40 years. FEV173% predicted pre bronchodilator. Mean ICS dose at enrolment 660 mcg/day. Hospital admission for asthma in the past year: unknown proportion. Course of oral steroids for asthma in past year: unknown proportion. Inclusion Criteria: Aged 12‐80 years, with a constant dose of ICS (400‐1000 mcg/day) at least 3 months. Prebronchodilator FEV1 of 60‐90% predicted normal value and at least 12% reversibility following Terbutaline. To be included patients had to need at least 12 rescue inhalations in the last 10 days of run‐in. Adults using ten or more rescue inhalations in a single day or with an exacerbation during run‐in were not randomised. |
|
| Interventions | 1. Budesonide/formoterol 100/6 mcg twice daily [200 mcg budesonide/day] and as needed (one maintenance and one relief Turbuhaler) 2. Budesonide/formoterol 100/6 mcg twice daily [200 mcg budesonide/day] and Terbutaline as needed (one maintenance and one relief Turbuhaler) 3. Budesonide 400 mcg twice daily [800 mcg budesonide/day] and Terbutaline as needed (one maintenance and one relief Turbuhaler) Maximum of 10 as needed inhalations could be used per day before contacting the investigator. |
|
| Outcomes |
Primary outcome ‐ time to first severe exacerbations. Secondary outcomes included number of severe exacerbations, time to mild exacerbations, number of mild exacerbations, symptom free days, QOL scores. No particular variable was chosen to assess safety. Exacerbation Definition: Severe ‐ Deterioration in asthma requiring hospital or emergency room treatment, or oral steroids (or other additional treatment) or morning PEF 70% or less of baseline on two consecutive days. Severe exacerbations requiring medical intervention were analysed separately . Mild exacerbation day ‐ PEF 80% or less of baseline, relief medication 2 or more inhalations above baseline, or awakenings due to asthma. Mild exacerbation defined as 2 consecutive mild exacerbation days using the same criteria. |
|
| Notes | SAE data (44,46,42) in the adult population; deaths given for whole trial (0,2,1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation scheme |
| Allocation concealment (selection bias) | Unclear risk | Eligible patients were randomised in balanced blocks by allocating patient numbers in consecutive order. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. All study medication was given by turbuhalers. Maintenance and as needed medication distinguished by the colour of the label and its wording. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and Dropouts: 2039/2419 (84%) completed the study |
STAY ‐ All ages.
| Methods | see STAY ‐ Adults and STAY ‐ children | |
| Participants | Combined data on Children and Adults aged 4 to 60 years with a history of one or more exacerbations in the past year. | |
| Interventions | see STAY ‐ Adults and STAY ‐ children | |
| Outcomes | see STAY ‐ Adults and STAY ‐ children | |
| Notes | Data presented for adults and children. P values based on ANOVA and changes from baseline calculated from Tables 1 and 2 in the primary publication. Standard Errors estimated from P values. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | see STAY ‐ Adults and STAY ‐ children |
| Allocation concealment (selection bias) | Unclear risk | see STAY ‐ Adults and STAY ‐ children |
| Blinding (performance bias and detection bias) All outcomes | Low risk | see STAY ‐ Adults and STAY ‐ children |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | see STAY ‐ Adults & STAY ‐ children |
STAY ‐ Children.
| Methods | Study Design: Randomised, double‐blind, parallel group study over a 12 month period in 41 centres in 12 countries (between Jan 2001 and Jan 2003). In the 14‐18 day run‐in patients used pre‐study ICS with terbutaline for symptom relief (LABA had to be discontinued at least 3 days before run‐in). | |
| Participants |
Children in Study: 341 asthmatic children aged 4‐11 years with asthma uncontrolled on ICS (200‐500 mcg/day) and a history of at least one "clinically important" exacerbation in the past year. Mean age: 8 years. Mean morning PEF: 220 L/min. FEV176% predicted pre bronchodilator. Mean ICS dose at enrolment 315 mcg/day. Hospital admission for asthma in the past year: unknown proportion. Course of oral steroids for asthma in past year: unknown proportion. Inclusion Criteria: Aged 4‐11 years, with a constant dose of ICS (200‐500 mcg/day) at least 3 months. Prebronchodilator FEV1 of 60‐100% predicted normal value and at least 12% reversibility following Terbutaline. To be included patients had to need at least 8 rescue inhalations in the last 10 days of run‐in. Children using seven or more rescue inhalations in a single day or with an exacerbation during run‐in were not randomised. |
|
| Interventions | 1. Budesonide 100 mcg (80 mcg delivered dose) and Formoterol 4.5 mcg in the evening and as needed (one maintenance and one relief Turbuhaler) 2. Budesonide 100 mcg (80 mcg delivered dose) and Formoterol 4.5 mcg in the evening and Terbutaline as needed (one maintenance and one relief Turbuhaler) 3. Budesonide 400 mcg (320 mcg delivered dose) in the evening and Terbutaline as needed (one maintenance and one relief Turbuhaler) |
|
| Outcomes |
Primary outcome ‐ time to first severe exacerbations. Secondary outcomes included number of severe exacerbations, time to mild exacerbations, number of mild exacerbations, symptom free days, QOL scores. No particular variable was chosen to assess safety. Exacerbation Definition: Severe ‐ Deterioration in asthma requiring hospital or emergency room treatment, or oral steroids (or an increase in ICS or other additional treatment) or morning PEF 70% or less of baseline on two consecutive days. Severe exacerbations requiring medical intervention were analysed separately . Mild exacerbation day ‐ PEF 80% or less of baseline, relief medication 2 or more inhalations above baseline, or awakenings due to asthma. Mild exacerbation defined as 2 consecutive mild exacerbation days using the same criteria. |
|
| Notes | Adverse Events:SAE data given (2,16,5) of these (0,7,2) were related to asthma. Change from baseline nights with awakenings were the same in both groups, P value in the paper not used as it related to post treatment levels not changes. No SD data published in the paper with respect to growth comparing budesonide/formoterol to Terbutaline as reliever, so SD calculated from the other comparisons presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation scheme |
| Allocation concealment (selection bias) | Unclear risk | Eligible patients were randomised in balanced blocks by allocating patient numbers in consecutive order. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. All study medication was given by turbuhalers. Maintenance and as needed medication distinguished by the colour of the label and its wording. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 309/341 (91%) completed the study |
FEV1: Forced expiratory volume in one second
ICS: Inhaled corticosteroids
LABA: Long acting beta‐agonist
PEF: Peak expiratory flow
SAE: Serious Adverse Event
SD: Standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balanzat 2004 | Overview of three existing trials |
| Bousquet 2007 | Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high‐dose salmeterol/fluticasone |
| COMPASS | Different doses of Symbicort used for maintenance |
| COSMOS | Different maintenance regimen in each arm |
| D5890C00003 | SiT compared to higher dose maintenance regimen |
| Ind 2002 | Formoterol v Terbutaline as reliever |
| Jenkins 2007 | budesonide/formoterol dose adjustment with FeNO (not used as reliever) |
| Jonkers 2006 | Single dose study |
| Loukides 2005 | Maintenance with symbicort 200/6 mcg bd in SiT group and Budesonide 200 mcg with Formoterol 12 mg bd with formoterol reliever |
| Lundborg 2006 | Higher dose maintenance therapy in the arm using formoterol as reliever |
| MONO | SiT compared to conventional best treatment |
| NCT00235911 | Comparison of SiT with usual care |
| NCT00252863 | SiT compared to current best practice |
| Richter 2007 | Formoterol not combination therapy as reliever |
| Riemersma 2008 | SiT compared to usual care |
| SALTO | SiT compared to conventional best practice |
| Scicchitano 2004 | SiT compared to higher dose budesonide |
| SOLO | SiT compared to conventional best practice (different maintenance regimen) |
| Sovani 2008 | SiT compared to budesonide (different maintenance regimen) |
| Stallberg 2008 | SiT compared to adjustable maintenance separate budesonide and formoterol inhalers or higher dose combination budesonide/formoterol inhaler |
| STEAM | SiT compared to higher dose maintenance therapy |
| STYLE | SiT compared to conventional best practice |
| Tattersfield 2001 | Formoterol versus Terbutaline as reliever |
bd: twice daily
FeNO:‐ exhaled nitric oxide
SiT: Single inhaler therapy
Differences between protocol and review
The review authors have included studies with no maintenance therapy, (but not combined the results with other studies of patients on maintenance ICS). Formoterol with ICS is the focus of this review, so salmeterol has been removed from the search terms, as it is not used for reliever therapy. The primary outcome has been modified to patients with exacerbations (rather than rates) that require hospitalisation or courses of oral steroids. All‐cause serious adverse events (fatal and non‐fatal) have been added as a further primary outcome.
Contributions of authors
CJC: Conception of the idea, study selection and data collection, statistical analysis, and co‐writing of the review. TL: Discussion of concepts, study selection and data collection and co‐writing of the review.
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR, UK.
NIHR program grant for CJC
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
SMILE {published and unpublished data}
- Rabe F, Atienza T, Magyar P, Larsoon P, Jorup C, Lalloo G. A new combination therapeutic approach challenging the current dogma of using inhaled corticosteroids as maintenance only to control asthma. European Respiratory Journal 2006;28(Suppl 50):666s. [Google Scholar]
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double‐blind study. Lancet 2006;938(9537):744‐53. [DOI] [PubMed] [Google Scholar]
SOMA {published and unpublished data}
- Haahtela T, Tamminen K, Malmberg LP, Zetterstrom O, Karjalainen J, Yla‐Outinen H, et al. Formoterol as needed with or without budesonide in patients with intermittent asthma and raised NO levels in exhaled air: A SOMA study. European Respiratory Journal 2006;28(4):748‐55. [DOI] [PubMed] [Google Scholar]
- Haahtela T, Tamminen K, Malmberg P, Karjalainen J, Yia‐Outinen H, Zetterstrom O, et al. As‐needed treatment with a b2‐agonist/ corticosteroid combination in mild intermittent asthma (SOMA). European Respiratory Journal 2005;26(Suppl 45):Abstract No. 1722. [Google Scholar]
STAY ‐ Adults {published and unpublished data}
- Bateman ED, Palmqvist M, Juniper EF, Zhu Y, Ekstrom T. Single inhaler therapy with budesonide/formoterol has superior efficacy to fixed‐dose budesonide/formoterol or a higher dose of budesonide alone. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004.
- Bruce SA, Scherer YK. Maintenance and symptom relief with budesonide plus formoterol reduced severe asthma exacerbations. Evidence‐Based Nursing 2005;8(3):78. [DOI] [PubMed] [Google Scholar]
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Budesonide and formoterol in mild persistent asthma compared with doubling the dose of budesonide ‐ a cost‐effectiveness analysis. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:517s.
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Economic results of adding formoterol to budesonide in mild persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:331s.
- O'Byrne PM. Acute asthma intervention: Insights from the STAY study. Journal of Allergy and Clinical Immunology 2007;119(6):1332‐6. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory & Critical Care Medicine 2005;171(2):129‐36. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Godard P, Pistolesi M, Ekstrom T. Single inhaler therapy with budesonide/formoterol improves asthma control compared with fixed dosing with budesonide/formoterol or a higher dose of budesonide alone [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:Poster J93.
- SD‐039‐673. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler® as Single Therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). http://www.astrazenecaclinicaltrials.com 2006.
STAY ‐ All ages {published and unpublished data}
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory & Critical Care Medicine 2005;171(2):129‐36. [DOI] [PubMed] [Google Scholar]
STAY ‐ Children {published and unpublished data}
- Bisgaard H, Hultquist C. Budesonide/formoterol for maintenance and as needed ‐ a new approach to asthma management in children [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1060. [Google Scholar]
- Bisgaard H, Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy ‐ a new strategy in pediatric asthma. Chest 2006;130(6):1733‐43. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM. Acute asthma intervention: Insights from the STAY study. Journal of Allergy & Clinical Immunology 2007;119(6):1332‐6. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory & Critical Care Medicine 2005;171(2):129‐36. [DOI] [PubMed] [Google Scholar]
- SD‐039‐673. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler® as Single Therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). http://www.astrazenecaclinicaltrials.com 2006.
References to studies excluded from this review
Balanzat 2004 {published data only}
- Balanzat A, Centanni S, Palmqvist M, Rabe K. Budesonide/formoterol single inhaler therapy reduces over reliance on rapid acting reliever medication [Abstract]. European Respiratory Journal 2004;24(Suppl 48):344s. [Google Scholar]
Bousquet 2007 {unpublished data only}
- Astrazeneca (D5890C00002). Efficacy and safety of Symbicort®Turbuhaler®160/4.5 mcg/inhalation, two inhalations twice daily plus as‐needed compared with Seretide™ Diskus™ 50/500 mcg/inhalation, one inhalation twice daily plus terbutaline Turbuhaler 0.4 mg/inhalation as‐needed ‐ a 6‐month, randomised, double‐blind, parallel‐group, active controlled, multinational phase IIIB study in adult and adolescent patients with persistent asthma. AstraZeneca Clinical Trials Register 2006, issue www.astrazenecaclinicaltrials.com (accessed 21/02/2008).
- Bousquet J, Boulet L‐P, Peters MJ, Magnussen H, Quiralte J, Martinez‐Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high‐dose salmeterol/fluticasone. Respiratory Medicine 2007;101(12):2437‐46. [DOI] [PubMed] [Google Scholar]
COMPASS {published and unpublished data}
- AstraZeneca. SYM/050/DEC2007. Data on File.
- Bleecker ER, Postma DS, Lawrance R, Meyers DA, Ambrose H, Goldman M. Effect of polymorphisms in the beta2‐adrenergic receptor gene (ADRB2) on response to long‐acting beta2‐agonist (LABA) therapy. Journal Allergy and Clinical Immunology 2007;119(2):523. [Google Scholar]
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Current Medical Research and Opinion 2007;23(8):1867‐78. [DOI] [PubMed] [Google Scholar]
- Kuna P, Peters MJ, Buhl R. Budesonide/formoterol as maintenance and reliever therapy reduces asthma exacerbations a higher maintenance dose of budesonide/versus formoterol or salmeterol/fluticasone. European Respiratory Journal 2006;28(Suppl 50):205s. [Google Scholar]
- Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martinez‐Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. International Journal of Clinical Practice 2007;61(5):725‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, Wiren A, Kuna P. Budesonide/formoterol (B/F) as maintenance and relief for asthma improves efficacy and is cost saving versus higher maintenance dose of B/F or salmeterol/fluticasone (S/F) [Abstract]. European Respiratory Journal 2006;28(Suupl 50):214s. [Google Scholar]
- Price D, Wiren A, Kuna P. Cost‐effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy 2007;62(10):1189‐98. [DOI] [PubMed] [Google Scholar]
COSMOS {published data only}
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Current Medical Research and Opinion 2007;23(8):1867‐78. [DOI] [PubMed] [Google Scholar]
- D'Urzo A, Vogeimeier C, Jaspal M, Merino JM, Boulet S. Symbicort (budesonide/formoterol) for both maintenance and relief reduces the exacerbation burden compared with titration of seretide (salmeterol/fluticasone) in patients with asthma, a real life stud. American Thoracic Society International Conference; May 20‐25; San Diego, California. 2005:Poster G24.
- Johansson G, Andreasson EB, Larsson PE, Vogelmeier CF. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics 2006;24(7):695‐708. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, D'Urzo A. Maintenance plus as‐needed budesonide/formoterol vs salmeterol/fluticasone in a real‐life setting. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 2770. [Google Scholar]
- Vogelmeier C, D'Urzo A, Jaspal M, Merino JM, Johansson G, Boulet S. Symbicort for both maintenance and relief reduces exacerbations compared with a titration of Seretide (Advair) in patients with asthma: a real life study. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:Poster F67.
- Vogelmeier C, D'Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: An effective asthma treatment option?. European Respiratory Journal 2005;26(5):819‐28. [DOI] [PubMed] [Google Scholar]
D5890C00003 {published data only}
- AstraZeneca (D5890C00003). A comparison of the control of asthma inflammation provided by Symbicort turbuhaler 160/4.5 mcg/inhalation bid plus as‐needed versus symbicort turbuhaler 320/9 mcg/inhalation bid plus Pulmicort turbuhaler 400 mcg/dose bid plus terbutaline turbuhaler 0.4 mg/inhalation as‐needed. www.clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00244608 (Accessed 22 February 2008).
Ind 2002 {published data only}
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. [DOI] [PubMed] [Google Scholar]
Jenkins 2007 {published data only}
- Jenkins CR, Marks GB, Gibson PG, Wark PAB, Thien FC, Belousova EG, et al. A randomised controlled trial of two algorithms for maintaining asthma control on long‐acting bronchodilators (LABA) and inhaled corticosteroids (ICS). Thoracic Society of Australia and New Zealand Annual Scientific Meeting, 25‐28 March 2007, Auckland. 2007:Abstract TP044.
Jonkers 2006 {published data only}
- Jonkers RE, Bantje TA, Aalbers R. Onset of relief of dyspnoea with budesonide/formoterol or salbutamol following methacholine‐induced severe bronchoconstriction in adults with asthma: a double‐blind, placebo‐controlled study. Respiratory Research 2006;7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loukides 2005 {published data only}
- Loukides S, Papageorgiou M, Karokis A, Zervas E, Christodoulopoulou A, Papageorgiou N, et al. Single inhaler therapy (SiT) with budesonide/formoterol (BUD/FUM) is effective in asthma control. European Respiratory Journal. 2005:http://www.ersnet.org/ers/lr/browse/default.aspx (accessed 22 February 2008).
Lundborg 2006 {published data only}
- Astrazeneca (LD‐039‐0003). An open, randomized, parallel‐group, multicentre, phase IIIB study to evaluate the efficacy of Symbicort® Turbuhaler® single inhaler therapy (SiT), given as a low maintenance dose once or twice daily plus as needed, compared to a higher maintenance dose of Symbicort Turbuhaler given twice daily plus Oxis® Turbuhaler® as needed during 24 weeks in asthmatic patients (LD‐039‐0003). AstraZeneca Clinical Trials Register 2006, issue http://www.astrazenecaclinicaltrials.com/ncmprint.aspx?type=article¶m=516829 (accessed 21st February 2008).
- Lundborg M, Wille S, Bjermer L, Tilling B, Lundgren M, Telg G, et al. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: An efficacy and cost‐effectiveness study. Current Medical Research & Opinion 2006;22(5):809‐21. [DOI] [PubMed] [Google Scholar]
MONO {published data only}
- AstraZeneca (D5890L00008). MONO: Symbicort single inhaler therapy and conventional best standard treatment for the treatment of persistent asthma in adolescents and adults. clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00242411 (Accessed 12 April 2006).
- Soes‐Petersen U, Dahl R, Kava T, Lei Y, Dam N. Budesonide/formoterol maintenance and reliever therapy compared with conventional best sound treatment [Abstract]. European Respiratory Society Annual Congress, Berlin, Germany, October 4‐8 2008:[P3508].
NCT00235911 {unpublished data only}
- AstraZeneca (BN‐00S‐0011). Symbicort single inhaler therapy for asthma in a general practice setting. www.clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00235911 (Accessed 22 February 2008).
NCT00252863 {unpublished data only}
- Worth H. DESOLO ‐ SiT Peri‐Launch: a comparison of symbicort single inhaler therapy and conventional best practice for the treatment of persistent asthma in adults. clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00252863 (Accessed 22nd February 2008).
Richter 2007 {published data only}
- Richter K, Hartmann U, Metzenauer P, Magnussen H. Randomised trial comparing as‐needed versus regular treatment with formoterol in patients with persistent asthma. Respiratory Medicine 2007;101(3):467‐75. [DOI] [PubMed] [Google Scholar]
Riemersma 2008 {published data only}
- Riemersma RA, Postma DS, Molen T. Budesonide/formoterol maintenance and reliever therapy for asthma in general practice [Abstract]. American Thoracic Society International Conference, May 16‐21, 2008, Toronto 2008:A612[#A29].
SALTO {published data only}
- AstraZeneca (D5890L00009). SALTO ‐ symbicort single inhaler therapy use in adolescent adults and adults with persistent asthma. www.clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00290264 (Accessed 22 February 2008).
- Louis R, Joos G, Michiels A, Vandenhoven G, Schepper I, Duquenne V. Budesonide/formoterol maintenance and reliever therapy compared to conventional best practice in the treatment of persistent asthma [Abstract]. European Respiratory Journal 2007; Vol. 30, issue Suppl 51:616s [P3617].
Scicchitano 2004 {published data only}
- Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centann S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Current Medical Research & Opinion 2004;20(9):1403‐18. [DOI] [PubMed] [Google Scholar]
SOLO {published data only}
- Sears MR, Boulet L‐P, Laviolette M, FitzGerald J Mark, Bai TR, et al. Budesonide/formoterol maintenance and reliever therapy: impact on airway inflammation in asthma. European Respiratory Journal 2008:Epub: doi: 10.1183/09031936.00104007. [DOI] [PubMed]
- Sears R, Boulet L‐P, Laviolette M, FitzGerald JM, Bai R, SmiljanicGeorijev N, et al. Budesonide/formoterol maintenance and reliever therapy for asthma compared to conventional best practice a randomised real life study. European Respiratory Journal 2006;28(Suppl 50):613s. [Google Scholar]
Sovani 2008 {published data only}
- Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekström T, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?. British Journal of General Practice 2008;58(546):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stallberg 2008 {published and unpublished data}
- AstraZeneca. Symbicort and Health Economics in a Real Life Evaluation – SHARE – A randomised, open‐label, parallel‐group, multicentre study to assess the. http://www.astrazenecaclinicaltrials.com/ (accessed 21 April 2008).
- Stallberg B, Ekstrom T, Neij F, Olsson P, Skoogh BE, Wennergren G, et al. A real‐life cost‐effectiveness evaluation of budesonide/formoterol maintenance and reliever therapy in asthma. Respiratory Medicine 2008;102(10):1360‐70. [DOI] [PubMed] [Google Scholar]
- Stallberg B, Lofdahl CG, Skoogh BE, Wennergren G, Olsson P, Neij F, et al. Budesonide/formoterol maintenance and reliever therapy effective treatment at a lower cost [Abstract]. European Respiratory Society Annual Congress, Berlin, Germany, October 4‐8. 2008:[4424].
STEAM {published data only}
- Astrazenca (SD‐039‐0667). Efficacy and Safety of Symbicort® Turbuhaler® as Single Therapy in Patients with Mild to Moderate Asthma ‐ STEAM (SD‐039‐0667). Astrazeneca Clinical Trials Register 2005:http://www.astrazenecaclinicaltrials.com (accessed 20th February 2008).
- Rabe KF, Pizzichini E, Stallberg B, Romero S, Balanzat AM, Atienza T, et al. Budisonide/formoterol in a single inhaler for maintenance and relief in mild‐to‐moderate asthma: A randomized, double‐blind trial. Chest 2006;129:245‐56. [DOI] [PubMed] [Google Scholar]
STYLE {published data only}
- AstraZeneca (D5890L00014). STYLE ‐ symbicort single inhaler therapy vs. conventional therapy in treatment of persistent asthma. www.clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00252824 (Accessed 22 February 2008).
Tattersfield 2001 {published data only}
- Berggren F, Ekström T. A cost‐effectiveness study comparing the as‐needed use of formoterol (Oxis) and terbutaline (Bricanyl) in patients with moderate to severe asthma. Respiratory Medicine 2001;98(9):753‐8. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Löfdahl CG, Postma DS, Eivindson A, Schreurs AGM, Rasidakis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2008
- Adams NP, Bestall JC, Lasserson TJ, Jones PW, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [PubMed] [Google Scholar]
Bisgaard 2003
BTS 2003
- British Thoracic Society. British Guideline on Asthma Management. Thorax 2003;58(Suppl 1):1‐83. [Google Scholar]
Cates 2008
- Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events (Cochrane Review). Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2008a
- Christopher CJ, Cates MJ, Lasserson TJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006923.pub2] [DOI] [PubMed] [Google Scholar]
FitzGerald 2004
- FitzGerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax 2004; Vol. 59, issue 7:550‐6. [0040‐6376: (Print)] [DOI] [PMC free article] [PubMed]
Greenstone 2005
- Greenstone IR, Ni Chroinin MN, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005533] [DOI] [PubMed] [Google Scholar]
Handbook 2006
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 6. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 14 February 2008). Chichester: John Wiley & Sons Ltd, 2006. [Google Scholar]
Harrison 2004
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] [DOI] [PubMed] [Google Scholar]
Palmqvist 2001
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology & Therapeutics 2001;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(14):904‐12. [DOI] [PubMed] [Google Scholar]
Tattersfield 1999
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory & Critical Care Medicine 1999;160(2):594‐9. [DOI] [PubMed] [Google Scholar]
Walters 2007
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
www.nntonline.net [Computer program]
- Cates C. Visual Rx. Cates C, 2001.


