Introduction
Living tissue–engineered heart valves (TEHV) may circumvent ongoing problems in pediatric valve replacements, offering optimum hemodynamic performance and the potential for growth, remodeling, and self-repair [1]. Although a myriad of external stimuli are available in current bioreactors (e.g., oscillatory flows and mechanical conditioning), there remain significant bioengineering challenges in determining and quantifying parameters that lead to optimal extracellular matrix (ECM) development for the long-term goal of engineering TEHVs exhibiting tissue architecture and functionality equivalent to native tissue. It has become axiomatic that in vitro mechanical conditioning promotes engineered tissue formation, either in organ-level bioreactors or in tissue-level bioreactors with idealized-geometry tissue engineering (TE) constructs [2–5]. However, the underlying mechanisms remain largely unknown. Efforts to date have been largely empirical, but a two-pronged approach involving novel theoretical developments and close-looped designed experiments is necessary to reach a better mechanistic understanding of the cause-effect interplay during incubation.
Materials and Methods
Dynamically conditioned TEHVs involve two disparate characteristic time scales: cellular proliferation and ECM synthesis occur at rates on the order of days to weeks, whereas effective conditioning protocols for growth and development are highly dynamic, with frequencies around 1 Hz. We employ multiscale methods to couple the dissimilar time scales. We describe cellular growth and ECM proliferation with a triphasic system of reaction-advection-diffusion equations governing the biochemical transport and interplay of cells, ECM, and available nutrients. Local transport properties are determined with finite element solutions of the evolving poroelastic TE constructs, with the dynamic flow field of the surrounding fluid resolved by computational fluid dynamics. Two-way coupling in between time scales occurs by up-scaling current transport properties from the poroelastic model into the growth model, and time- and spatially-dependent ECM and cell distributions characterize the evolution of the poroelastic model.
Results and Discussion
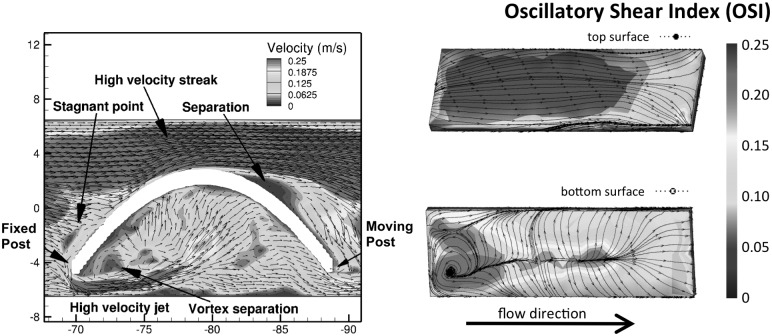
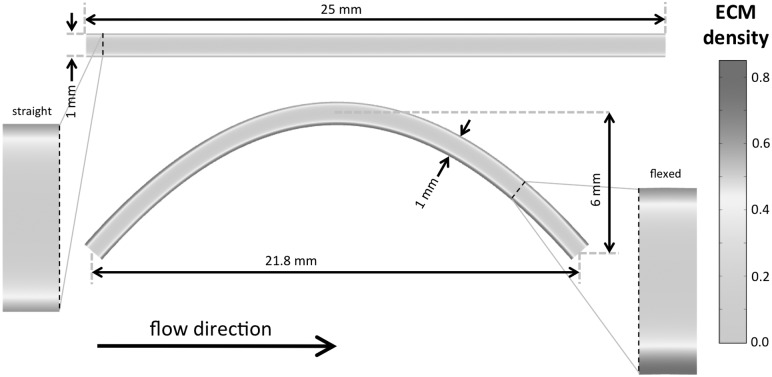
Cellular consumption and transport constraints prevent nutrients from being fully replenished inside static constructs, and cells near the boundaries are able to proliferate faster and synthesize more ECM. Cyclic flexure enhances nutrient transport and leads to homogeneously distributed ECM synthesis and better ECM quality. Cyclic flexure introduces a large degree of oscillatory flows (Fig. 1) and spatial nonhomogeneity in cellular proliferation and ECM production (Fig. 2).
Fig. 1.
Velocity flow field and distribution of oscillatory shear index on construct surfaces (Re = 1376)
Fig. 2.
Spatial distribution of ECM (nondimensional) in a cross-section of the nondeformed and flexed TEHV leaflet constructs. Highly oscillatory flows enhance oxygen transport, cell growth, and matrix production in the bottom surface of the flexed scaffold.
Conclusions
The ability to quantify and predict cell proliferation and ECM production (outputs) in response to diverse controllable stimuli (inputs) is of the utmost importance for successful clinical development of all TE applications. Starting from this preliminary modeling step, our future objective is to improve our modeling capabilities towards a useful and robust TE design tool, supported by carefully designed experiments, accounting for multiple inputs available to the tissue engineer and predicting their effect and impact on the evolving TE construct.
Acknowledgment
Funding by the National Institutes of Health (HL-068816 and HL-089750 to M.S.S. and HL-07262 to F.S.) and the Minnesota Supercomputing Institute.
Contributor Information
João S. Soares, Institute for Computational Engineering and Sciences, University of Texas at Austin, Austin, TX 78712
Fotis Sotiropoulos, Saint Anthony Falls Laboratory, Department of Civil Engineering, University of Minnesota, Minneapolis, MN 55414.
Michael S. Sacks, Institute for Computational Engineering and Sciences, Department of Biomedical Engineering, University of Texas at Austin, Austin, TX 78712.
References
- [1]. Stock, U. A. , Vacanti, J. P. , Mayer, Jr., J. E. , and Wahlers, T. , 2002, “Tissue Engineering of Heart Valves—Current Aspects,” Thorac. Cardiovasc. Surg., 50, pp. 184–19310.1055/s-2002-32406 [DOI] [PubMed] [Google Scholar]
- [2]. Ramaswamy, S. , Gottlieb, D. , Engelmayr, Jr., G. C. , Aikawa, E. , Schmidt, D. E. , Gaitan-Leon, D. M. , Sales, V. L. , Mayer, Jr., J. E. , and Sacks, M. S. , 2010, “The Role of Organ Level Conditioning on the Promotion of Engineered Heart Valve Tissue Development In Vitro Using Mesenchymal Stem Cells,” Biomaterials, 31, pp. 1114–112510.1016/j.biomaterials.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Engelmayr, Jr., G. C. , Rabkin, E. , Sutherland, F. W. , Schoen, F. J. , Mayer, Jr., J. E. , and Sacks, M. S. , 2005, “The Independent Role of Cyclic Flexure in the Early In Vitro Development of an Engineered Heart Valve Tissue,” Biomaterials, 26, pp. 175–18710.1016/j.biomaterials.2004.02.035 [DOI] [PubMed] [Google Scholar]
- [4]. Engelmayr, Jr., G. C. , Sales, V. L. , Mayer, Jr., J. E. , and Sacks, M. S. , 2006, “Cyclic Flexure and Laminar Flow Synergistically Accelerate Mesenchymal Stem Cell-Mediated Engineered Tissue Formation: Implications for Engineered Heart Valve Tissues,” Biomaterials, 27, pp. 6083–609510.1016/j.biomaterials.2006.07.045 [DOI] [PubMed] [Google Scholar]
- [5]. Engelmayr, Jr., G. C. , Soletti, L. , Vigmostad, S. C. , Budilarto, S. G. , Federspiel, W. J. , Chandran, K. B. , Vorp, D. A. , and Sacks, M. S. , 2008, “A Novel Flex-Stretch-Flow Bioreactor for the Study of Engineered Heart Valve Tissue Mechanobiology,” Ann. Biomed. Eng., 36, pp. 700–71210.1007/s10439-008-9447-6 [DOI] [PMC free article] [PubMed] [Google Scholar]