Abstract
Domestic animal husbandry, a common practice globally, can lead to zoonotic transmission of enteric pathogens. However, this risk has received little attention to date. This systematic review and meta-analysis examines the evidence for an association between domestic exposure to food-producing animals and cases of human diarrhea and specific enteric infections. We performed a systematic review of available literature to examine domestic livestock and poultry as risk factors for diarrhea and applied pre-determined quality criteria. Where possible, we carried out meta-analysis of specific animal–pathogen pairs. We found consistent evidence of a positive association between exposure to domestic food-producing animals and diarrheal illness across a range of animal exposures and enteric pathogens. Out of 29 studies included in the review, 20 (69.0%) reported a positive association between domestic animal exposure and diarrhea. Domestic exposure to poultry revealed a substantial association with human campylobacteriosis (OR 2.73, 95% CI 1.90–3.93). Our results suggest that domestic poultry and livestock exposures are associated with diarrheal illness in humans. Failure to ascertain the microbial cause of disease may mask this effect. Exposure to domestic animals should be considered a risk factor for human diarrheal illness and additional studies may identify potential mitigation strategies to address this risk.
Keywords: Animal husbandry, Diarrhea, Domestic animals, Hygiene, Systematic review
Introduction
Globally, diarrheal diseases kill approximately 1.45 million people per year, and account for 17.4% of infant and 11.9% of early childhood deaths worldwide.1 Diarrheal diseases are also among the leading causes of malnutrition in children under 2 years of age who live in resource-poor settings.2 Among the 2.49 billion disability-adjusted life-years (DALYs) in 2010, 3.6% were attributed to diarrhea across all age groups.3
Zoonotic transmission of infectious diseases is considered to be a key driver in the current emergence and re-emergence of novel diseases,4–6 and contact between animals and humans can also increase the occurrence of pathogens already in common circulation via zoonotic transmission pathways. Diarrheal diseases are caused by the transmission of bacterial, parasitic, or viral enteric organisms to humans through the contamination of water or food sources by feces. Environmental contamination from human feces is the predominant risk factor for human diarrhea,7,8 but zoonotic sources can also be responsible for transmission of diarrheal disease pathogens to humans. Animal feces can contribute to human diarrhea incidence by introducing new zoonotic pathogens that cause diarrheal illness or by increasing transmission of pathogens common to both animals and humans. Several animal hosts are known to be reservoirs of specific diarrheal disease pathogens. For example, poultry animals are associated with transmission of Campylobacter spp.9 and Salmonella spp.9,10 to humans, and ruminants have been identified as the primary animal reservoir for human enterohemorrhagic Escherichia coli (EHEC) O157:H7 infections.9
The presence of domestic livestock and poultry in close proximity to human beings is common throughout the world but particularly prominent in resource-poor countries, where animal husbandry serves as a primary source of income.11 Environmental, cultural, and economic factors lead households to keep livestock and poultry within close range of human living quarters, where the animals may be allowed to roam freely and sleep within the home.12 These conditions increase the potential for fecal contamination by animals within the household environment, and subsequent zoonotic transmission of enteric pathogens harbored by these domestic food-producing animals. In particular, animals have been implicated as a source of fecal contamination of soil.13,14 This is particularly problematic among young children, in whom fecal-oral transmission may be more common during play. Despite evidence of environmental contamination, little attention has been paid to this potential zoonotic source of diarrheal diseases in humans.
In this systematic review and meta-analysis, we examine the evidence for an association between domestic exposure to food-producing animals and both human diarrheal cases and infection by specific enteric pathogens. We also consider if there is evidence of specific animal husbandry and hygiene behaviors that are linked to infection and whether possible interventions exist to reduce exposure to diarrheal pathogens through animals.
Methods
Search strategy and inclusion criteria
We systematically searched the National Library of Medicine/PubMed, ISI/Web of Science and Embase literature databases at any time through to 30 September 2013. The search terms were: ‘Diarrh*’ and ‘Animal Husbandry or Livestock or Cattle or Pig or Poultry or Swine or Chicken or Cow or Sheep or Goat or Dairy or Zoonosis or Feces’ and ‘Household or Domestic or Home or Hygiene’. Additional articles were obtained through hand searches of relevant papers and reviews. For details of the search protocol, see Supplementary Box 1.
Papers were screened by reading titles and abstracts and were included if they examined domestic (or household) livestock and poultry as risk factors for diarrhea. The outcome of interest, diarrhea, was defined broadly as any diarrheal case occurring among any household member (all ages). Specific causal enteric pathogens, if identified, were detailed at a later stage of the analysis. Cross-sectional, cohort and case-control studies were all included. Intervention studies lacking baseline data on the relationship between animal exposure and disease were not considered for inclusion in the meta-analysis because they differed in study design and outcomes measured; there were insufficient intervention studies to consider them independently. Papers were excluded if they related only to: animal pathogens or disease or both; zoonotic potential of domestic pets; zoonotic transmission in the context of an industry or large-scale farming; or human diarrheal illness outside of the context of zoonotic transmission.
The search and review were undertaken by two separate reviewers (LZ and NP). In the case of disagreement, a third reviewer (MF) assessed the article in question to determine its relevance. Relevant articles agreed upon by all reviewers were examined in depth. We applied the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines for epidemiologic reporting of observational studies to gather, assess and report information from studies included in the review.15
Quality assessment and grading methodology
We applied the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE) methodology to assess the quality of evidence and strength of each individual study.16 Studies were graded on the basis of the diagnostic and exposure ascertainment methods used to determine strengths and limitations. Studies were graded favorably if a laboratory assay was used to determine or confirm a diagnosis. We awarded points to studies that limited the potential for recall bias by determining household animal exposures through home visits vs questionnaires. We deducted points if studies failed to control for confounders or address selection or recall bias. A more detailed description of the grading methodology is given in Supplementary Box 2 and Supplementary Table 1. Each study could earn a maximum of 8 points and a minimum of −2 points. Relevant data were extracted from each study by LDZ and cross-checked by MCF.
Statistical analysis
Odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) were reported from each study when available. If a study provided a relative risk (RR) only, we calculated ORs if sample sizes of diarrhea cases and animal exposures were provided. If data were not available in the published article to calculate ORs, we contacted authors of these papers to acquire the appropriate data. Studies were excluded if they did not report ORs, did not provide sufficient data to calculate an OR from an RR, and the author did not respond to a request for data.
To gain insights into transmission patterns associated with particular pathogens and animal species, we considered the relationships between specific pathogens and animal exposure pairs separately where possible. We stratified all studies by exposure (poultry, swine, ruminants, goats or sheep) and outcome (Campylobacter, EHEC, Cryptosporidium or Giardia infection or diarrhea case with no laboratory confirmation), resulting in 19 strata (Table 1).
Table 1.
Data points obtained for each pathogen–animal exposure stratum in a review of 23 studies of Human diarrhea infections associated with domestic animal husbandry. Data were distributed among 19 strata overall, and pooled estimates calculated for strata that had three or more studies
| Pathogen | Poultry | Swine | Goat/sheep | Ruminant | Animals (unspecified) | Total |
|---|---|---|---|---|---|---|
| Campylobacter spp. | 7 | 1 | 1 | 0 | 1 | 10 |
| EHEC/STEC | 0 | 0 | 1 | 0 | 2 | 3 |
| Unspecified protozoa | 0 | 0 | 0 | 0 | 1 | 1 |
| Cryptosporidium spp. | 1 | 1 | 3 | 1 | 1 | 7 |
| Giardia intestinalis | 1 | 0 | 0 | 1 | 3 | 5 |
| Unspecified pathogen | 0 | 1 | 0 | 3 | 2 | 6 |
| Total | 9 | 3 | 5 | 5 | 10 |
EHEC/STEC: Enterohemorrhagic Escherichia coli/shiga-like toxin-secreting E. coli.
If three or more studies fitted within the same exposure and outcome stratum, we carried out an analysis of heterogeneity and obtained pooled OR estimates. This was ultimately performed for one animal–pathogen pair: ‘Campylobacter–poultry’. Within this stratum, we assessed statistical heterogeneity of results between studies through I2 and Cochran's Q-tests. We also performed a Breslow–Day test to examine heterogeneity of effect size between studies to report alongside our pooled estimates. These tests examine the degree of inconsistency in the results of studies included in meta-analyses.15,17 We evaluated age of participants, study location and hygiene practices as potential contributors to any observed heterogeneity. The analysis yielded evidence of heterogeneity (I2=96.91%) in the pooled stratum, so we used the Mantel–Haenszel random effects method for the meta-analysis as this provides a conservative estimate of the combined effect of animal exposure on reported diarrhea.17,18 All statistics were calculated using Excel 2007 (Microsoft Corp., Redmond, WA, USA) and SAS V.9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Overview of studies included in review
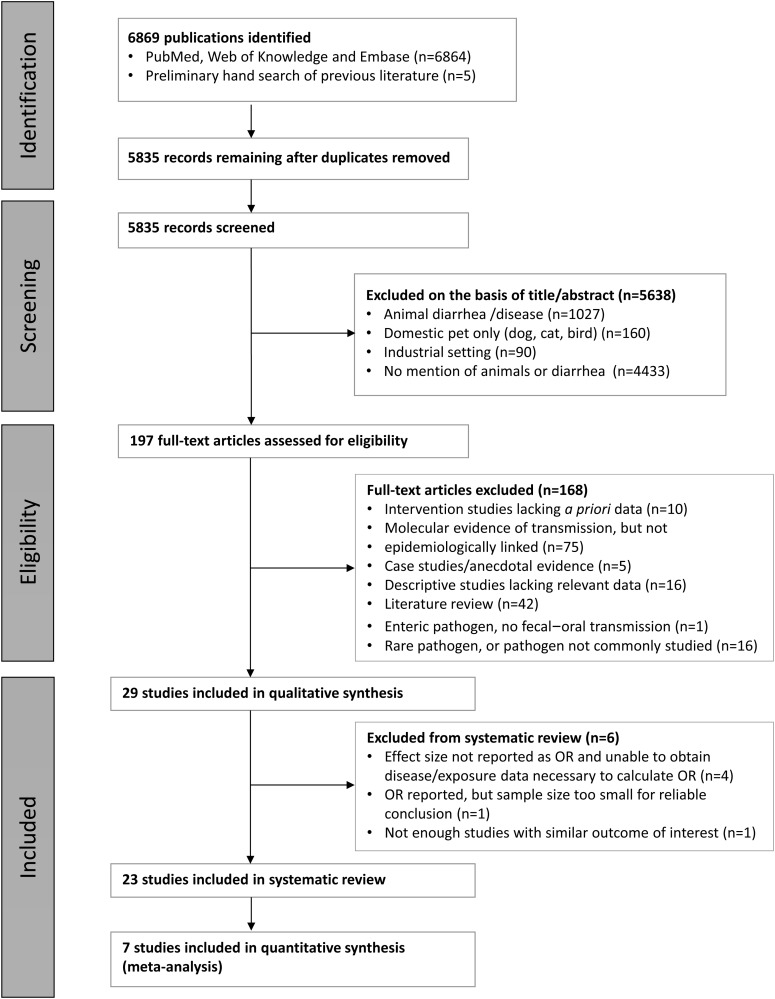
The search criteria yielded 5835 potentially relevant studies after deleting duplicates, of which 5638 were excluded on the basis of title or abstract review or both (Figure 1).19 Of the remaining 197 potentially relevant articles, only 29 studies included sufficient data for inclusion in the final systematic review and meta-analysis. The others were excluded for one or a combination of the following reasons: 10 intervention studies lacked relevant baseline data, 75 described molecular evidence for pathogen transmission between animals and humans but did not relate it epidemiologically, 16 descriptive studies had insufficient or non-generalizable data, and five were case studies or anecdotal reports, and 42 were literature reviews. In addition, one study described zoonotic transmission of an enteric pathogen beyond the fecal–oral transmission pathway and 15 described individual reports of fecal–oral transmission of viruses or rare infectious agents.
Figure 1.
Search procedure for a review of human diarrhea infections associated with domestic animal husbandry. We identified 23 relevant studies with adequate data for inclusion in the systematic review, and seven for inclusion in the meta-analysis.
Of the final 29 relevant articles, 23 were included in the systematic review. Two studies were not included because of the use of a non-comparable effect estimate (incidence density ratios)20,21 or number of diarrheal episodes rather than number of cases reported.22 Only our ‘Poultry exposure / Campylobacter spp. infection’ stratum included enough studies (n=7) with sufficient data to evaluate through meta-analysis. A summary of the studies included in the final review is shown in Table 2.
Table 2.
Characteristics of 23 studies examining the association between exposure to food-producing animals and diarrhoea
| Study | Study design and location | Income classa | Quant. review | Pathogen | Population | Subjects (n) | Diagnostic approach (D) |
QC | Exposure assessment (E) |
Other strengths and limitations (O) | Points |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | Assessment method | Exposure ascertainment |
D/E/O | Total | |||||||||
| 38 | Cross-sectional study in Accra, Ghana | LMIC | Yes | Cryptosporidium spp., various pathogens | Children aged <5 years | 227 children | Microscopy and oocyte identification | NS | Interview | NS | NA | 1/0/−1 | 0 |
| 39 | Cross-sectional study in Sana'a City, Yemen | LMIC | Yes | Intestinal protozoa | Outpatients aged 1–80 years with GI complaints at 3 clinical centers | 503 patients | Oocyte identification | NS | Questionnaire | NS | Recall bias not addressed | 1/0/1 | +2 |
| 23 | Cross-sectional study in rural Wisconsin, USA | HIC | Yes | E. coli O157:H7 | Children aged 1–17 years | 215 farm-resident and 396 non-farm resident children | Serology | NS | Population data | NS | Wilcoxon rank-sum used to analyze continuous variable | 2/0/1 | +3 |
| 20 | Cohort study in Port Moresby, Papau New Guinea | LMIC | No | Not specified | Children aged <5 years | 479 children | None | NA | Household visits | Alternate day visits to relate exposure temporally | NA | 0/1/4 | +5 |
| 40 | Cross-sectional study in Quitafine region, Guinea-Bissau | LIC | Yes | Cryptosporidium spp. | Children aged <5 years in 160 households in 8 rural villages | 270 children | Oocyst identification | NS | Household visit | NS | NA | 1/0/0 | +1 |
| 41 | Cohort study in Rahat, Israel | HIC | Yes | Giardia lamblia | Infants followed from birth to 18 months | 238 infants | Antibody serology | NS | Household visits | Weekly interviews to determine health status | Recall bias is minimized | 1/0/5 | +6 |
| 42 | Cohort study in Bangui, Central African Republic | LIC | Yes | Campylobacter spp. | Children born in a maternity ward, followed for 2 years | 111 children | Culture, microscopy | NS | Household visit | Environmental sampling | NA | 1/1/0 | +2 |
| 43 | Case-control study in Lima, Peru | UMIC | Yes | Campylobacter jejuni | Children aged <3 years | 104 cases, 104 controls | Culture, microscopy | NS | Interview | NS | NA | 1/0/1 | +2 |
| 25 | Ismaila province, Egypt | LMIC | No | Cryptosporidium, multiple species | Children aged <10 years | 165 children | RIDA-QUICK, PCR-RFLP | NS | Household visit | NS | Proportion of children exposed vs unexposed not clear | 1/0/4 | +5 |
| 22 | Cross-sectional study in Imo State, Nigeria | LMIC | No | NS | Residents in 5 villages | 4641 residents in dry season, 5920 in wet season | None | NS | Interview | NS | Not clear whether some residents were enrolled in both seasons | 0/0/1 | +1 |
| 26 | Cross-sectional study in Hamadan district, Iran | UMIC | No | Cryptosporidium spp. | 228 participants (all ages) | 228 samples | Ziehl–Neelsen oocyst identification | NS | NS | Household visit with animal sampling | Confounding and bias potential not addressed | 1/0/0 | +1 |
| 24 | Cross-sectional study in Dagoretti, Nairobi, Kenya | LIC | Yes | Cryptosporidium spp. | Participants selected for a prior study analyzing risks and benefits of urban dairying | 300 dairy households, 100 non-dairy neighboring households | NS | NA | Interview | Environmental sampling |
Assessed exposure and risk in context of socioeconomic factors | 0/1/4 | +5 |
| 44 | Cross-sectional study in Gondar, Ethiopia | LIC | Yes | Campylobacter spp. | Diarrheic children aged <5 years who visited teaching hospital | 285 total stool samples | Culture, microscopy, dry spot Campylobacter test | Yes | Interview | NS | Convenience sample; recall bias not addressed | 2/0/1 | +3 |
| 27 | Case-control study in Dhaka, Bangladesh | LIC | No | Non-typhoidal Salmonella | Medical record review of culture +ve hospital patients | 254 cases, 762 controls | Culture and sero-typing | NS | Medical record review | NS | Medical record review may not catch all exposures | 1/0/4 | +5 |
| 45 | Case-control study in Scotland, UK | HIC | Yes | E. coli O157:H7 | EHEC cases diagnosed Oct 1996–March 1999 and up to 4 matched controls | 183 cases, 545 controls | Laboratory report (no method specified) | NS | Phone and mail questionnaires |
NS | Matched controls not found for 36 cases | 1/0/4 | +5 |
| 32 | Cohort study in Bilbeis, Egypt | LMIC | Yes | NS | Newborns followed for first year | 152 infants | Serology/ ELISA | NS | Home visits | NS. | Biweekly home visits to determine diarrhoeal illness | 1/0/4 | +5 |
| 46 | Cohort study in Bilbeis, Egypt | LMIC | Yes | Giardia spp. | Newborns followed for first year | 152 infants | Serology/ ELISA | NS | Home visits | NS | Biweekly home visits to determine diarrheal illness | 1/0/5 | +6 |
| 34 | Cohort study in Bandim II, Bissau, Guinea-Bissau | LIC | Yes | NS | Children aged <4 years in 301 households |
648 children | None | N/A | Interviews | Baseline interview | Weekly interviews for disease incidence to avoid recall bias | 0/0/5 | +5 |
| 33 | Case-control study in 8 villages in Quitafine, Guinea Bissau | LIC | Yes | Cryptosporidium spp. | Children aged <4 years in 160 households | 125 cases, 125 controls | Microscopy | NS | Interviews | Monthly interviews to determine exposures | Weekly interviews for disease incidence to avoid recall bias | 1/1/4 | +6 |
| 47 | Cohort study in Pampas de San Juan, Lima, Peru | UMIC | Yes | Campylobacter spp. | Families with ≥2 free-roaming chickens and ≥2 children aged <5 years, with ≥1 aged <24 months | 423 subjects from 63 families | Culture, microscopy, RAPD, RFLP, serotyping | Yesb | Household visit | NS | NA | 2/1/2 | +5 |
| 48 | Case-control study in England (country-wide) | HIC | Yes | E. coli O157:H7 | Patients with positive culture at Public Health Laboratory Service labs | 369 cases, 511 controls | NS (from laboratory reports) | NS | Mailed questionnaires | NS | Controls were not matched | 1/0/2 | +3 |
| 49 | Cross-sectional study in Jordan Valley, Jordan | UMIC | Yes | NS | Children aged 0–13 years | 197 children | Routine stool analysis | NS | Interviews | Assistants rated cleanliness of home | NA | 0/0/1 | +1 |
| 50 | Cross-sectional study in Goiania, Goias State, Brazil | UMIC | Yes | Giardia lamblia | Children aged 2 weeks–10 years | 445 | Serology and microscopy | NS | Questionnaire | NS | Convenience sample | 1/0/1 | +2 |
| 51 | Case-control study throughout Australia | HIC | Yes | Campylobacter spp. | All aged ≥5 years | 881 cases, 883 controls | Culture | NS | Telephone interview | NS | Potential for recall bias | 1/0/2 | +3 |
| 52 | Case-control study in Alvsborg County, Sweden | HIC | Yes | Campylobacter jejuni, Campylobacter coli | County residents with culture-positive campylobacteriosis | 101 cases, 198 controls | Microscopy | NS | Phone interview | NS | NA | 1/0/2 | +3 |
| 53 | Case-control study in Queensland, Australia | HIC | Yes | Campylobacter jejuni, Campylobacter coli | Children aged 0–35 months | 81 cases, 144 controls | Pathology laboratory reports | NS | Questionnaire | NS | Recall bias not addressed for cases | 1/1/1 | +3 |
| 21 | Cross-sectional study in Khanh Hoa Province, Vietnam | LMIC | No | NS | Children aged <5 years at 2 area hospitals for diarrhea | 353 525 individuals, in 75 828 households | None | NA | Census data | NS | NA | 0/0/3 | +3 |
| 54 | Case-control study in Colorado, USA | HIC | Yes | Cryptosporidium spp. | State residents with stool-positive cryptosporidiosis | 47 cases, 92 matched controls | Microscopy, PCR for speciation | Yesb | Telephone questionnaires | NS | Recall bias not addressed | 2/1/2 | +5 |
| 55 | Cross-sectional study in Oromia region, Ethiopia | LIC | Yes | Cryptosporidium spp., Giardia duodenalis | Children in randomly selected households from 2 districts | 384 children | Ziehl–Neelsen oocyst identification |
Yesc | Household visit | Visual inspection | Well-described sampling method | 2/0/4 | +6 |
a Income classifications based on World Bank 2013 income classifications by country.
b Multiple confirmatory assays.
c Many quality checks.
HIC: high-income country; LIC: low income country; LMIC: lower middle income country; UMIC: upper middle income country; NS: not specified; QC: quality control; RIDA-QUICK: norovirus test.
Most papers addressed bacterial pathogens (Campylobacter spp.: 10 articles; EHEC: three articles); and protozoal pathogens (Cryptosporidium spp.: seven articles; Giardia intestinalis: five articles). The remaining six articles did not specify a particular pathogen. Studies assessing specific exposures examined the impact of exposure to domestic poultry (nine studies), swine (three studies), goats and sheep (five studies) and ruminants (five studies). Ten studies did not specify a particular animal of interest, but addressed the broader impact of domestic animal exposure overall. The largest number of studies were carried out in Africa (eight in the Sub-Saharan and four in the Middle East/Northern region), and other regions represented included South America (three studies), Asia (two studies) Oceania (three studies), North America (two studies), and Europe (three studies).
All but one of the 23 studies included in our analysis incorporated water, sanitation and hygiene (WASH) indicators in their analysis. Fifteen studies incorporated indicators for clean water access, six included access to a clean toilet or latrine and eight incorporated food preparation hygiene and food consumption. Because each study controlled for different socioeconomic and WASH indicators, unadjusted estimates were used in our analyses.
Study characteristics and integrity
Approximately equal numbers of cross-sectional, case-control, and cohort studies were included (Table 2). Twenty-three studies incorporated diagnostic approaches (serology, microscopy, or DNA-based methods) to confirm the etiologic agent. Twenty-one studies used some form of interview to gather exposure information and nine incorporated household visits. Researchers in four of the studies that incorporated household visits visited sufficiently often to ascertain the timing of disease status and animal exposures. The grade assigned to each category of assessment (diagnostics, exposure ascertainment and study design/data analysis) is listed with each study on Table 2. Studies that incorporated biological diagnostic assays and mitigated the potential for recall bias for exposures and outcomes through household visits were graded more favorably than studies that did not incorporate these measures. Additional details on point assignment are provided in Supplementary Box 2. On our scale of –2 (worst quality) to +8 (best quality), the mean score was 3.5.
Associations between animal exposure and diarrheal illness
Twenty out of 29 studies (69%) included in our overall analysis reported a significant association between domestic animal husbandry and human diarrheal disease (Figure 2). Among studies that specified causal pathogens, 20 out of 21 (95%) reported a significant positive association between animal exposure and diarrhea. Of the eight studies that did not find an association, six studies (75%) specified neither a causal pathogen nor a diarrheal cause.
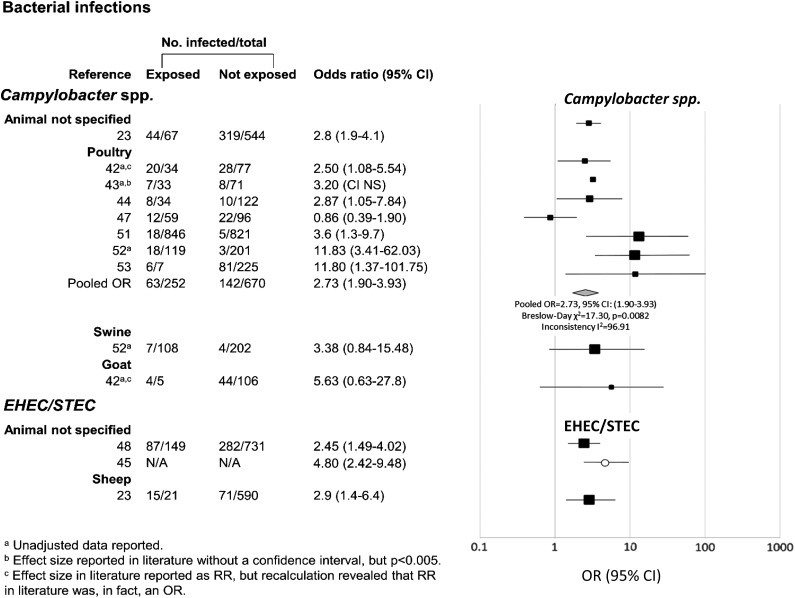
Figure 2.
Associations between exposure to domestic food-producing animals and enteric infections with Campylobacter spp. and enterohemorrhagic shiga toxin-producing Escherichia coli (EHEC/STEC). Data have been separated by specific type of exposure and pathogen of interest. ORs are represented by rectangles of different sizes, according to the grading weight given to each study. Horizontal lines represent 95% CIs. Open circle indicates study for which discrete counts were lacking, but which included ORs. Squares increase in size as study weight (1/s2) increases.
Three of the studies included in our final qualitative analysis included animal exposure as the primary exposure of interest;23–25 one of these focused on the risk of animal exposure in relation to various socioeconomic factors.24 Animal exposure tended to be analyzed alongside various socioeconomic and water and sanitation indicators.
Of the 29 studies that we included in our qualitative synthesis, only 23 included OR estimates or data from which ORs could be calculated. Our pooled random effects analysis for the relationship between poultry exposure and Campylobacter infection revealed an OR of 2.73 (95% CI: 1.90–3.93). There was significant heterogeneity in effect size between studies (Breslow–Day χ2=17.30, p=0.0082). In addition, analysis of heterogeneity of results between the six studies within the ‘poultry/Campylobacter’ stratum yielded an I2 value of 96.91%, indicating considerable heterogeneity in effect between studies. Despite this, we still performed a meta-analysis as the direction of effect was consistent. It is worth noting that Mantel–Haenszel techniques do not necessarily account for heterogeneity, but that our analysis did describe the average effect of domestic poultry exposure on Campylobacter infection found between studies.
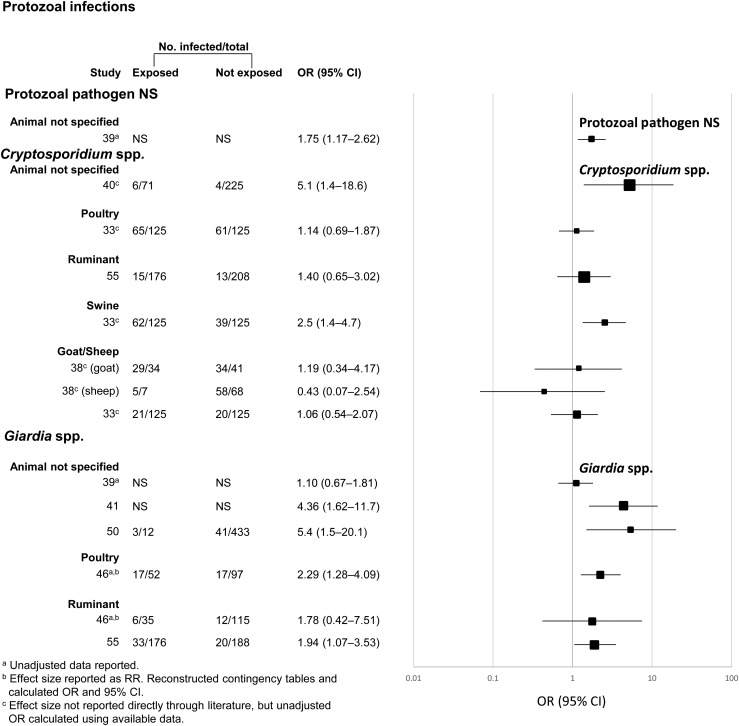
While the other animal–pathogen pairs had an insufficient number of studies for quantitative meta-analysis, some trends emerged. Despite having distinct exposure categories (unspecified animals and sheep), the three studies in the EHEC/STEC disease category all had positive associations with animal exposure (Figure 2). Similarly, four out of five studies examining Giardia spp. infection were significantly positively associated with domestic animal exposure (Figure 3). On the other hand, only two out of five studies yielding a significant positive relationship between domestic animal exposure and diarrheal illness attributable to Cryptosporidium spp. (Figure 3).
Figure 3.
Meta-analysis examining the association between exposure to livestock and infection with Cryptosporidium spp. and Giardia intestinalis. Data have been separated by specific type of exposure and pathogen of interest. ORs are represented by rectangles of different sizes, according to the grading weight given to each study. Horizontal lines represent 95% CIs. Squares increase in size as study weight (1/s2) increases.
Four out of the six studies deemed ineligible for our quantitative review did not report the effect size as an OR or relevant data from which we could calculate an OR. The results of these studies were therefore not comparable to the other results. One study reported an OR based on two cases and the results were deemed unreliable.26 Despite these limitations, the results of these studies are worth discussing. In households where animals were present, there was a significant increased risk of diarrheal illness in a cohort study in Papau New Guinea (incidence density ratio [IDR]=1.69, 95% CI: 1.32–2.19).20 In Egypt, Cryptosporidium infections were significantly more likely to be detected in children who had contact with animals in their household (87.3%, 95% CI: 76.4–94.3%) than in children who had no contact (12.7%, 5.7–23.6%).25 A cross-sectional study in Imo State, Nigeria found a significant protective effect linked with animal exposure (IDR=0.8, 95% CI: 0.7–0.9).21 In Vietnam, children <5 years old who were hospitalized with diarrhea exhibited no association between animal exposure and diarrheal disease in spatial analysis, even in areas where livestock were most dense (15 747 animals/km2) (RR: 1.00, 95% CI: 0.84–1.20).21 Another study in Iran found no significant association between Cryptosporidium infection and contact with domestic livestock (OR=0.44, 95% CI: 0.03–7.13); however, this study yielded only two total Cryptosporidium cases out of 228 subjects, so these results should be interpreted with care. Finally, a case-control study in Bangladesh found that there was no significant relationship between having animals in the home and non-typhoidal Salmonella (NTS) infection (OR=1.03, 95% CI: 0.78–1.37),27 but as this was the only study that included NTS infection as an outcome, it was not eligible for quantitative synthesis with other studies that focused on bacterial outcomes.
Among the 10 intervention studies identified, the only specific intervention evaluated by more than one study in the literature was corralling of poultry.12,28–30 One study revealed that children in households with corralled poultry were more likely to have Campylobacter-associated diarrhea than those who lived in households with free-roaming poultry (0.57 episodes per year vs 0.27 episodes per year, p=0.006).29 Another studied revealed that defecation by non-corralled chickens led to increased fecal contamination and increased feces-to-mouth episodes among children under 5 years old.28 Two other studies examined attitudes to and the social acceptability of poultry corralling, but not the impact of corralling on diarrheal infection.12,30
Discussion
This review shows that zoonotic transmission of enteric pathogens in the domestic setting is common, particularly in the context of water contamination by animal excreta. We found consistent evidence of a positive association between domestic food-producing animal exposure and diarrheal illness across a range of animal exposures and diarrheal disease pathogens (Figures 2–4). Of the 29 studies included in the full qualitative review, 21 reported a positive association for at least one animal-pathogen pair and only two studies revealed a negative association, or potential protective effect.22,24 In the one stratum for which we had sufficient studies to carry out a meta-analysis, poultry exposure more than doubled the odds of Campylobacter spp. infection. Domestic food-producing animals therefore appear to contribute to enteric pathogen transmission, and zoonotic infection should be considered an important contributor to diarrheal illness. To our knowledge, this is the first systematic review and meta-analysis of the relationship between domestic animal husbandry and diarrheal-related pathogens.
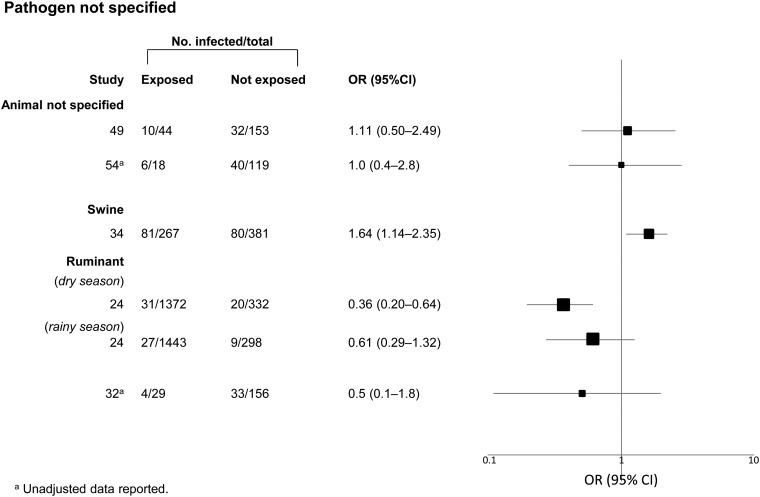
Figure 4.
Meta-analysis examining the association between exposure to livestock and diarrheal illness with no specified etiology. Data have been separated by specific type of exposure and pathogen of interest. ORs are represented by rectangles of different sizes, according to the grading weight given to each study. Horizontal lines represent 95% CIs. Squares increase in size as study weight (1/s2) increases.
While the overall weight of evidence supported a positive association between domestic food-producing animals and diarrheal disease, we observed heterogeneity in both strength of association and effect size. This could be attributable to factors that differ between the studies, including study design, study location, population sampled, housing and community conditions, hygiene and sanitation, age of the population, nature of the animal exposure and survey methods. The strength of evidence for zoonotic transmission was strongest among studies that elucidated and confirmed a microbial cause of diarrhea through laboratory methods. There were five studies for which no pathogen was specified, and four of these studies found no association between animal exposure and disease. This may indicate the importance of confirming a microbial cause of disease in future studies that examine zoonotic transmission. The limited available data and heterogeneity of effect size between studies, in combination with the strength of the associations observed across studies in the systematic review, highlights the need for more research in this area, especially studies that ascertain the microbial cause of diarrhea.
Our search used three common databases, and was supplemented with hand-searching through the bibliographies of the relevant articles we identified. Despite the strengths of our search methods, this review was limited by the number of relevant articles available. Among the 29 studies included in the qualitative review, only 23 articles could be used for our systematic review, of which only seven studies in the domestic poultry exposure/Campylobacter stratum could be subjected to meta-analysis.
In addition to the limited number of articles available for review, we found consistent limitations in the studies we included. On a scale of –2 (worst quality) to +8 (best quality), the mean score for study quality was only 3.5. Most studies used questionnaires or interviews or both to ascertain exposures. Often, questionnaires or interviews were also used to record disease incidence. This practice, while common and relatively simple to implement, may introduce recall and/or interviewer bias.31 Only in three studies were frequent home visits used to ascertain exposure or disease and thus relate the two temporally;32–34 this method largely eliminates the risk of recall bias and is recommended for future studies. Several more studies used convenience rather than random sampling, which may contribute to selection bias. The included studies may have been subject to publication bias; however, most of the studies we included assessed animal exposure as an ancillary factor. As animal exposure was the primary or sole exposure of interest in only two22,24 of these studies, the risk of publication bias was probably minimal.
This review identified several gaps in the available literature on the link between domestic animal husbandry practices and transmission of enteric pathogens. In addition to the 29 relevant articles we found, another 75 articles revealed compelling molecular evidence that identical bacterial and protozoal strains existed in both domestic animals and humans; however, as these data were not epidemiologically linked, they were outside the scope of this review. Molecular epidemiological evidence of transmission, such as that reported in Helmy et al.,25 would offer additional insights into disease transmission across species.
Our review revealed the need to identify animal husbandry practices that might limit zoonotic transmission. All three studies that evaluated poultry corralling, despite being conducted by different researchers, were performed in and around Lima, Peru, and had inconclusive results. We found no intervention studies that assessed measures to limit exposures to ruminant, goat, sheep and swine feces. There is considerable evidence of the part played by improvements in water, sanitation, and hygiene (WASH) in reducing diarrheal morbidity and mortality,35–37 and WASH interventions may mitigate pathogen exposure from domestic food-producing animals, but this link has not been adequately explored. No study in this review focused on WASH as a means of limiting disease transmission from animals.
Conclusions
Domestic animal husbandry is critical for the development of communities and economic viability of families, especially in developing countries, and the results of this review should not be taken to suggest cessation of domestic animal husbandry. However, it is important to understand that raising food-producing animals in domestic environments is not without human health risks. More comprehensive research is needed on specific behaviors surrounding animal husbandry that may affect transmission of pathogens between animals and humans; this would facilitate the design and implementation of measures to reduce animal exposure in the domestic environment. Further WASH research should incorporate animals as potential sources of contamination. Clarification of behaviors and community attitudes toward various husbandry practices will help us understand how risk of transmission develops within a household, and how interventions can best be directed. Particularly useful would be reports of observed and self-reported behaviors of humans and animals near the home, presence and state of corralling structures for animals, and individual approaches toward various animal husbandry practices.
Supplementary data
Supplementary data are available at Transactions Online (http://trstmh.oxfordjournals.org/).
Acknowledgments
Authors' contributions: MCF conceived the study; MCF, KL, NPM and LDZ designed the study protocol; LDZ and NPM carried out the literature review; LDZ analyzed and interpreted the data; LDZ wrote the manuscript; MCF and KL critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. MCF and LDZ are the guarantors of the paper.
Funding: Research reported in this paper was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda, MD, USA [Award Number K01AI103544]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant RL. Policy and Practice. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Coker R, Rushton J, Mounier-Jack S, et al. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect Dis. 2011;11:326–31. doi: 10.1016/S1473-3099(10)70312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christou L. The global burden of bacterial and viral zoonotic infections. Clin Microbiol Infect. 2011;17:326–30. doi: 10.1111/j.1469-0691.2010.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones KE, Patel NG, Levy M, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–3. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byers KE, Guerrant RL, Farr BM, editors. Fecal-oral transmission. In: Thomas JC, Webber DJ, editors. Epidemiologic Methods for the Study of Infectious Diseases. Oxford: Oxford University Press; 2001. pp. 228–48. [Google Scholar]
- 8.Curtis V, Cairncross S, Yonli R. Review: Domestic hygiene and diarrhoea–pinpointing the problem. Trop Med Int Health. 2000;5:22–32. doi: 10.1046/j.1365-3156.2000.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Jay J, Loessner MJ, Golden DA. Modern Food Microbiology, 7th edn. Heidelberg: Springer; 2005. pp. 61–91. [Google Scholar]
- 10.Gaffga NH, Barton Behravesh C, Ettestad PJ, et al. Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N Engl J Med. 2012;366:2065–73. doi: 10.1056/NEJMoa1111818. [DOI] [PubMed] [Google Scholar]
- 11.Sansoucy R, Jabbar MA, Ehui S, Fitzhugh H. Keynote Paper. The contribution of livestock to food security and sustainable development. In: Wilson RT, Ehui S, Mack S, editors. Nairobi, Kenya: Food and Agriculture Organization/International Livestock Research Institute; 1995. Proceedings of the Joint FAO/ILRI Roundtable on Livestock Development Strategies for Low Income Countries, ILRI, Addis Ababa, Ethiopia, 27 February–2 March 1995. [Google Scholar]
- 12.Harvey SA, Winch PJ, Leontsini E, et al. Domestic poultry-raising practices in a Peruvian shantytown: implications for control of Campylobacter jejuni-associated diarrhea. Acta Trop. 2003;86:41–54. doi: 10.1016/s0001-706x(03)00006-8. [DOI] [PubMed] [Google Scholar]
- 13.Chacín-Bonilla L, Barrios F, Sanchez Y. Epidemiology of Cyclospora cayetanensis infection in San Carlos Island, Venezuela: strong association between socio-economic status and infection. Trans R Soc Trop Med Hyg. 2007;101:1018–24. doi: 10.1016/j.trstmh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Pickering AJ, Julian TR, Marks SJ, et al. Fecal contamination and diarrheal pathogens on surfaces and in soils among Tanzanian households with and without improved sanitation. Environ Sci Technol. 2012;46:5736–43. doi: 10.1021/es300022c. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Oxman AD. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapter 9: Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JPT, Altman DG, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available: www.cochrane-handbook.org . [Google Scholar]
- 18.Montori V, Hatala R, Ioannidis J. Making sense of variability in study results: magnitude of heterogeneneity statistical tests. In: Guyatt G, Rennie D, Meade MO, Cook DJ, et al., editors. JAMA Users' Guides to the Medical Literature. Part F. Summarizing the Evidence. Columbus, OH: McGraw-Hill Education; 2008. Chapter 20.3. [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukenya GB, Nwokolo N. Compound hygiene, presence of standpipe and the risk of childhood diarrhea in an urban settlement of Papua-New-Guinea. Int J Epidemiol. 1991;20:534–9. doi: 10.1093/ije/20.2.534. [DOI] [PubMed] [Google Scholar]
- 21.Thiem VD, Schmidt WP, Suzuki M, et al. Animal livestock and the risk of hospitalized diarrhoea in children under 5 years in Vietnam. Trop Med Int Health. 2012;17:613–21. doi: 10.1111/j.1365-3156.2012.02969.x. [DOI] [PubMed] [Google Scholar]
- 22.Huttly SR, Blum D, Kirkwood BR, et al. The epidemiology of acute diarrhoea in a rural community in Imo State, Nigeria. Trans R Soc Trop Med Hyg. 1987;81:865–70. doi: 10.1016/0035-9203(87)90055-1. [DOI] [PubMed] [Google Scholar]
- 23.Belongia EA, Chyou PH, Greenlee RT, et al. Diarrhea incidence and farm-related risk factors for Escherichia coli O157:H7 and Campylobacter jejuni antibodies among rural children. J Infect Dis. 2003;187:1460–8. doi: 10.1086/374622. [DOI] [PubMed] [Google Scholar]
- 24.Kimani VN, Mitoko G, McDermott B, Grace D, Ambia J, Kiragu MW, et al. Social and gender determinants of risk of cryptosporidiosis, an emerging zoonosis, in Dagoretti, Nairobi, Kenya. Trop Anim Health Prod. 2012;44:17–23. doi: 10.1007/s11250-012-0203-4. [DOI] [PubMed] [Google Scholar]
- 25.Helmy YA, Krücken J, Nöckler K, et al. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet Parasitol. 2013;31;193:15–24. doi: 10.1016/j.vetpar.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Jafari R, Maghsood AH, Fallah M. Prevalence of cryptosporidium infection among livestock and humans in contact with livestock in Hamadan District, Iran, 2012. J Res Health Sci. 2013;13:86–9. [PubMed] [Google Scholar]
- 27.Leung DT, Das SK, Malek MA, et al. Non-typhoidal Salmonella gastroenteritis at a diarrheal hospital in Dhaka, Bangladesh, 1996–2011. Am J Trop Med Hyg. 2013;88:661–9. doi: 10.4269/ajtmh.12-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquis GS, Ventura G, Gilman RH, et al. Fecal contamination of shanty town toddlers in households with non-corralled poultry, Lima, Peru. Am J Public Health. 1990;80:146–9. doi: 10.2105/ajph.80.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberhelman RA, Gilman RH, Sheen P, et al. An intervention-control study of corralling of free-ranging chickens to control Campylobacter infections among children in a Peruvian Periurban shantytown. Am J Trop Med Hyg. 2006;74:1054–9. [PubMed] [Google Scholar]
- 30.Martinez L, Collazo G, Cabrera L, et al. Free-ranging chickens in households in a periurban shantytown in Peru-attitudes and practices 10 years after a community-based intervention project. Am J Trop Med Hyg. 2013;89:229–31. doi: 10.4269/ajtmh.12-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87–91. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
- 32.Mahmud MA, Hossain MM, Huang DB, et al. Sociodemographic, environmental and clinical risk factors for developing persistent diarrhoea among infants in a rural community of Egypt. J Health Popul Nutr. 2001;19:313–9. [PubMed] [Google Scholar]
- 33.Mølbak K, Aaby P, Højlyng N, et al. Risk factors for Cryptosporidium diarrhea in early childhood: A case-control study from Guinea-Bissau, West Africa. Am J Epidemiol. 1994;139:734–40. doi: 10.1093/oxfordjournals.aje.a117064. [DOI] [PubMed] [Google Scholar]
- 34.Mølbak K, Jensen H, Ingholt L, et al. Risk factors for diarrheal disease incidence in early childhood: a community cohort study from Guinea-Bissau. Am J Epidemiol. 1997;146:273–82. doi: 10.1093/oxfordjournals.aje.a009263. [DOI] [PubMed] [Google Scholar]
- 35.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 37.Fewtrell L, Kaufmann RB, Kay D, et al. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 38.Adjei AA, Armah H, Rodrigues O, et al. Cryptosporidium spp., a frequent cause of diarrhea among children at the Korle-Bu Teaching Hospital, Accra, Ghana. Jpn J Infect Dis. 2004;57:216–9. [PubMed] [Google Scholar]
- 39.Alyousefi NA, Mahdy MAK, Mahmud R, Lim YAL. Factors Associated with High Prevalence of Intestinal Protozoan Infections among Patients in Sana'a City, Yemen. PLoS One. 2011:6. doi: 10.1371/journal.pone.0022044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carstensen H, Hansen HL, Kristiansen HO, Gomme G. The epidemiology of cryptosporidiosis and other intestinal parasitoses in children in southern Guinea-Bissau. Trans R Soc Trop Med Hyg. 1987;81:860–4. doi: 10.1016/0035-9203(87)90054-x. [DOI] [PubMed] [Google Scholar]
- 41.Coles CL, Levy A, Dagan R, et al. Risk factors for the initial symptomatic Giardia infection in a cohort of young Arab-Bedouin children. Ann Trop Paediatr. 2009;29:291–300. doi: 10.1179/027249309X12547917869041. [DOI] [PubMed] [Google Scholar]
- 42.Georges-Courbot MC, Cassel-Beraud AM, Gouandjika I, et al. A cohort study of enteric Campylobacter infection in children from birth to two years in Bangui (Central African Republic) Trans R Soc Trop Med Hyg. 1990;84:122–5. doi: 10.1016/0035-9203(90)90402-z. [DOI] [PubMed] [Google Scholar]
- 43.Grados O, Bravo N, Black RE, Butzler JP. Paediatric Campylobacter diarrhoea from household exposure to live chickens in Lima, Peru. Bull World Healh Org. 1988;66:369–74. [PMC free article] [PubMed] [Google Scholar]
- 44.Lengerh A, Moges F, Unakal C, Anagaw B. Prevalence, associated risk factors and antimicrobial susceptibility pattern of Campylobacter species among under five diarrheic children at Gondar University Hospital, Northwest Ethiopia. BMC Pediatr. 2013:82. doi: 10.1186/1471-2431-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locking ME, O'Brien SJ, Reilly WJ, et al. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol Infect. 2001;127:215–20. doi: 10.1017/s0950268801006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmud MA, Chappell C, Hossain MM, et al. Risk factors for development of first symptomatic Giardia infection among infants of a birth cohort in rural Egypt. Am J Trop Med Hyg. 1995;53:84–8. [PubMed] [Google Scholar]
- 47.Oberhelman RA, Gilman RH, Sheen P, et al. Campylobacter transmission in a Peruvian shantytown: A longitudinal study using strain typing of Campylobacter isolates from chickens and humans in household clusters. J Infect Dis. 2003;187:260–9. doi: 10.1086/367676. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien SJ, Adak GK, Gilham C. Contact with farming environment as a major risk factor for Shiga toxin (Vero cytotoxin)-producing Escherichia coli O157 infection in humans. Emerg Infect Dis. 2001;7:1049–51. doi: 10.3201/eid0706.010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okour AM, Al-Ghazawi Z, Gharaibeh M. Diarrhea among children and the household conditions in a low-income rural community in the Jordan Valley. Jordan Med J. 2012;46:108–17. [Google Scholar]
- 50.Pereira MD, Atwill ER, Barbosa AP. Prevalence and associated risk factors for Giardia lamblia infection among children hospitalized for diarrhea in Goiania, Goias State, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:139–45. doi: 10.1590/s0036-46652007000300002. [DOI] [PubMed] [Google Scholar]
- 51.Stafford RJ, Schluter PJ, Wilson AJ, et al. Population-attributable risk estimates for risk factors associated with Campylobacter. Emerg Infect Dis. 2008;14:895–901. doi: 10.3201/eid1406.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Studahl A, Andersson Y. Risk factors for indigenous campylobacter infection: a Swedish case-control study. Epidemiol Infect. 2000;125:269–75. doi: 10.1017/s0950268899004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenkate TD, Stafford RJ. Risk factors for Campylobacter infection in infants and young children: a matched case-control study. Epidemiol Infect. 2001;127:399–404. doi: 10.1017/s0950268801006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valderrama AL, Hlavsa MC, Cronquist A, et al. Multiple risk factors associated with a large statewide increase in cryptosporidiosis. Epidemiol Infect. 2009;137:1781–8. doi: 10.1017/S0950268809002842. [DOI] [PubMed] [Google Scholar]
- 55.Wegayehu T, Adamu H, Petros B. Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in North Shewa Zone, Ethiopia. BMC Infect Dis. 2013:419. doi: 10.1186/1471-2334-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.