Abstract
A sperm-specific phospholipase C-zeta (PLCζ) is believed to play an essential role in oocyte activation during mammalian fertilization. Sperm PLCζ has been shown to trigger a prolonged series of repetitive Ca2+ transients or oscillations in oocytes that precede activation. This remarkable intracellular Ca2+ signalling phenomenon is a distinctive characteristic observed during in vitro fertilization by sperm. Previous studies have notably observed an apparent differential ability of PLCζ from disparate mammalian species to trigger Ca2+ oscillations in mouse oocytes. However, the molecular basis and confirmation of the apparent PLCζ species difference in activity remains to be provided. In the present study, we provide direct evidence for the superior effectiveness of human PLCζ relative to mouse PLCζ in generating Ca2+ oscillations in mouse oocytes. In addition, we have designed and constructed a series of human/mouse PLCζ chimeras to enable study of the potential role of discrete PLCζ domains in conferring the enhanced Ca2+ signalling potency of human PLCζ. Functional analysis of these human/mouse PLCζ domain chimeras suggests a novel role of the EF-hand domain in the species-specific differences in PLCζ activity. Our empirical observations are compatible with a basic mathematical model for the Ca2+ dependence of generating cytoplasmic Ca2+ oscillations in mammalian oocytes by sperm PLCζ.
Keywords: phospholipase C, PLCzeta, sperm, fertilization, oocyte activation, male infertility
Introduction
At fertilization, the first event following the fusion of the sperm and oocyte membranes is a series of transient rises in the intracellular-free Ca2+ concentration, termed Ca2+ oscillations. In human and other oocytes, these Ca2+ oscillations persist for several hours after gamete fusion (Miyazaki et al., 1993). Prolonged Ca2+ oscillations have also been observed after intra-cytoplasmic sperm injection (ICSI) in both human and mouse oocytes (Tesarik et al., 1994; Nakano et al., 1997). In all species studied, such Ca2+ oscillations are both necessary and sufficient for the completion of all the events of oocyte activation and early embryonic development (Nomikos et al., 2012). Ca2+ oscillations in mammalian oocytes occur as a result of inositol trisphosphate (IP3)-mediated Ca2+ release from internal stores such as the endoplasmic reticulum (ER) (Miyazaki et al., 1992), with the amplitude, duration and frequency of Ca2+ oscillations being largely species specific (Swann et al., 2006; Nomikos et al., 2012). However, the sperm factor causing the Ca2+ oscillations is not species specific because the injection of human sperm into mouse oocytes can cause Ca2+ oscillations as well as oocyte activation (Vanden Meerschaut et al., 2013). Since it is the Ca2+ oscillations that trigger embryo development, the outcome of such heterologous ICSI has been suggested as a method for evaluating cases of oocyte activation failure after human ICSI (Vanden Meerschaut et al., 2013).
Over the past decade, mounting experimental and clinical evidence suggests that the factor responsible for the initiation of Ca2+ oscillations during mammalian fertilization is a testis-specific isoform of phospholipase C (PLC), PLC-zeta (PLCζ) (Cox et al., 2002; Saunders et al., 2002; Kouchi et al., 2004; Yoon et al., 2008; Heytens et al., 2009; Kashir et al., 2010). Sperm-delivered PLCζ is believed to catalyse phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis within the fertilized oocyte, stimulating the inositol 1,4,5-trisphosphate (InsP3) signalling pathway leading to Ca2+ oscillations (Saunders et al., 2002; Nomikos et al., 2012). The significance of PLCζ in human fertilization has been emphasized by recent clinical studies that have linked abnormal expression, localization and defects in PLCζ protein structure with cases of oocyte activation deficiency and subsequently with male infertility (Yoon et al., 2008; Heytens et al., 2009; Nomikos et al., 2011a; Kashir et al., 2012). Human sperm lacking PLCζ have also been shown to be deficient in causing Ca2+ oscillations in heterologous ICSI with mouse oocytes (Yoon et al., 2008). The clinical potential of PLCζ was also recently highlighted by the use of recombinant human PLCζ protein (Nomikos et al., 2013). It was demonstrated that in a prototype of male factor infertility, microinjection of recombinant human PLCζ could phenotypically rescue failed activation of mouse oocytes expressing dysfunctional PLCζ, leading to efficient blastocyst formation (Nomikos et al., 2013).
Similar to other PLC isoforms, PLCζ demonstrates a typical PLC domain structure with four tandem EF-hand domains at the N-terminus, followed by the characteristic X and Y catalytic domains, which form the active site in all PLC isoforms, and a single C2 domain at the C-terminus (Saunders et al., 2002). Each of the individual PLCζ domains appears to have an essential role in the distinct biochemical characteristics and the unique mode of regulation of this gamete-specific PLC isozyme (Nomikos et al., 2005, 2007, 2011b). A major difference of the sperm PLCζ to somatic cell PLC isoforms is the absence of a pleckstrin homology domain (Saunders et al., 2002). This makes PLCζ the smallest known mammalian PLC with a molecular mass of ∼70 kDa in humans and ∼74 kDa in mice (Cox et al., 2002; Saunders et al., 2002). Notably, there appear to be substantial differences in the relative potency of PLCζ from different species (Swann et al., 2006; Saunders et al., 2007; Cooney et al., 2010; Bedford-Guaus et al., 2011). Previous reports have suggested that it takes nearly >10 times less human PLCζ cRNA than mouse PLCζ cRNA to trigger Ca2+ oscillations in mouse oocytes (Cox et al., 2002). These species differences in PLCζ effectiveness may explain why even ‘dead’ human sperm can still be shown to cause some Ca2+ oscillations in mouse oocytes (Yazawa et al., 2009). However, the expression levels have not been carefully measured in the same set of experiments with both mouse and human PLCζ and so the precise difference in potency between these species is not clear. There have also been no parallel studies of the comparative in vitro enzymatic characteristics of recombinant human and mouse PLCζ. In addition, the domain(s) of human PLCζ that might contribute significantly to the greater potency of human PLCζ are currently unknown.
In the present study, we provide a quantitative and qualitative comparison of the relative potencies of human and mouse PLCζ by using luciferase-tagged fusion proteins, and by using this approach we have determined the specific degree to which human PLCζ is more effective than mouse PLCζ in generating Ca2+ oscillations in mouse oocytes. Recombinant human PLCζ also displayed a higher in vitro PIP2 hydrolysis activity than mouse PLCζ. By preparing human/mouse PLCζ domain ‘swaps’, this has enabled chimeric protein analysis to demonstrate that replacement of the human PLCζ C2 domain with the corresponding mouse C2 domain did not alter the in vitro and in vivo enzymatic properties of human PLCζ. However, exchanging the human for mouse EF-hand domain reduced the Ca2+ oscillation-inducing activity by altering the Ca2+ sensitivity and affinity of human PLCζ for PIP2. Finally, our data suggest that replacement of the human PLCζ XY-linker with the corresponding region of mouse PLCζ dramatically affects the stability of the protein, suggesting a significant role of the XY-linker region in species-specific differences in PLCζ Ca2+ oscillation-inducing activity.
Materials and Methods
Cloning of PLCζ human–mouse chimeric constructs
The hPLCζ/mEF-luc and hPLCζ/mC2-luc constructs were cloned into pCR3 vector by using a three-step cloning strategy. For hPLCζ/mEF-luc, the EF hands of mouse PLCζ (1–149aa) were amplified from the original cDNA clone (GenBank™ accession number AF435950) by PCR using Phusion polymerase (Finnzymes) and the appropriate primers to incorporate a 5′-KpnI site and a 3′-EcoRI site and cloned into the pCR3 vector. Human PLCζ (143–608aa) was then amplified from the original cDNA clone (GenBank™ accession number AF532185) with the appropriate primers to incorporate a 5′-EcoRI site and a 3′-NotI site in which the stop codon had been removed and cloned into the pCR3-mEF1–149 plasmid. Finally, the firefly (Photinus pyralis) luciferase open reading frame was amplified from pGL2 (Promega) with primers incorporating NotI sites and the product was cloned into the NotI site of the pCR3-mEF1–149-PLCζ143–608 plasmid. For hPLCζ/mC2-luc, human PLCζ (1–481aa) was amplified from the original cDNA clone by PCR using Phusion polymerase (Finnzymes) and with the appropriate primers to incorporate a 5′-EcoRI site and a 3′-EcoRV site and cloned into the pCR3 vector. Mouse PLCζ C2 domain (520–647aa) was then amplified from the original cDNA clone with the appropriate primers to incorporate a 5′-EcoRV site and a 3′-NotI site in which the stop codon had been removed and cloned into the pCR3-hPLCζ1–481 plasmid. Finally, luciferase open reading frame was amplified from pGL2 (Promega) with primers incorporating NotI sites and the product was cloned into the NotI site of the pCR3-hPLCζ1–481-mC2520–647 plasmid. Human PLCζ/mXYlink.-luc construct was cloned into pCR3 vector by using a four-step cloning strategy. First, hPLCζ(1–299aa) was amplified by PCR and with the appropriate primers to incorporate a 5′-KpnI site and a 3′-EcoRI site and cloned into the pCR3 vector. The XY-linker of mouse PLCζ (308–385aa) was then amplified from the original cDNA clone with the appropriate primers to incorporate a 5′-EcoRI site and a 3′- EcoRV site and cloned into the pCR3-hPLCζ1–299 plasmid. Then, hPLCζ(349–608aa) was amplified with the appropriate primers to incorporate a 5′-EcoRV site and a 3′-NotI site and cloned into the pCR3-hPLCζ1–299-mPLCζ308–385 plasmid. Finally, as described for the first two chimeric constructs, luciferase open reading frame was amplified from pGL2 with primers incorporating NotI sites and the product was cloned into the NotI site of the pCR3-hPLCζ1–299-mPLCζ308–385-hPLCζ349–608 plasmid.
All the above chimeric constructs without the C′-terminus luciferase were amplified from the pCR3 vector with the appropriate primers to incorporate a 5′-SalI site and a 3′-NotI site and cloned into the pETMM60 vector to enable bacterial protein expression. Each of the above expression vector constructs was confirmed by dideoxynucleotide sequencing (Prism Big Dye kit; ABI Prism® 3100 Genetic Analyzer, Applied Biosystems, Warrington, UK).
cRNA synthesis
Following linearization of wild-type and chimeric PLCζ plasmids, cRNA was synthesized using the mMessage Machine T7 kit (Ambion) and then was polyadenylated using the poly(A) tailing kit (Ambion), as per the manufacturer's instructions.
Preparation and handling of gametes
Female mice were superovulated and oocytes were collected 13.5–14.5 h after injection of human chorionic gonadotrophin and maintained in droplets of M2 media (Sigma) or H-synthetic oviductal medium enriched with potassium (KSOM) under mineral oil at 37°C. Microinjection of the oocytes was carried out 14.5–15.5 h after the hormone injection. Experimental recordings of Ca2+ or luciferase expression were carried out with mouse oocytes in HEPES-buffered media (H-KSOM) as described previously (Nomikos et al., 2005, 2011b, c, 2013). All compounds were from Sigma unless stated otherwise. All procedures using animals were performed in accordance with the UK Home Office Animals Procedures Act and were approved by the Cardiff University Animals Ethics Committee.
Microinjection and measurement of intracellular Ca2+ and luciferase expression
Mouse oocytes were washed in M2 and microinjected with cRNA diluted in injection buffer (120 mm KCl, 20 mm HEPES, pH 7.4). The volume injected was estimated from the diameter of cytoplasmic displacement caused by the bolus injection. All injections were 3–5% of the oocyte volume. Oocytes were microinjected with the appropriate cRNA, mixed with an equal volume of 1 mm Oregon Green BAPTA dextran (Molecular Probes) in the injection buffer. Oocytes were then maintained in H-KSOM containing 100 µm luciferin and imaged on a Nikon TE2000 or Zeiss Axiovert 100 microscope equipped with a cooled intensified charge-coupled device camera (Photek Ltd., UK). The luminescence (for luciferase expression) and fluorescence (for Ca2+ measurements) from eggs were collected by switching back and forth between two modes on a 10 s cycle (Swann et al., 2009; Nomikos et al., 2011a). These two signals are then displayed as two separate signals over the same time period. The fluorescent light used to measure Ca2+ is shown in relative units because we are essentially interested in the frequency or number of Ca2+ spikes. The luminescence from oocytes was converted into an amount of luciferase using a standard curve that was generated by placing oocytes in a luminometer that had been previously calibrated by microinjection with known amounts of luciferase protein (Sigma) (Nomikos et al., 2005, 2011b, c, 2013; Swann et al., 2009). All live imaging experiments on oocytes were made during a 3-month period.
Protein expression and purification
For NusA-6xHis-fusion protein expression, Escherichia coli [Rosetta (DE3); Novagen], transformed with the appropriate pETMM60 plasmid, was cultured at 37°C until A600 reached 0.6, and protein expression was induced for 18 h, 16°C with 0.1 mM isopropyl β-d-1-thiogalactopyranoside (Promega). Cells were harvested (6000 g for 10 min), resuspended in phosphate-buffered saline (PBS) containing protease inhibitor mixture (EDTA-free; Roche) and sonicated 4 × 15 s on ice. Soluble NusA-6xHisfusion protein was purified on Ni-NTA resin following standard procedures (Qiagen) and eluted with 275 mM imidazole. Eluted proteins were dialysed overnight (10 000 MWCO; Pierce) at 4°C against 4 l of PBS and concentrated with centrifugal concentrators (Sartorius; 10 000 MWCO).
Assay of PLC activity
PIP2 hydrolytic activity of recombinant PLC proteins was assayed as described previously. The final concentration of PIP2 in the reaction mixture was 220 µM, containing 0.05 µCi of [3H]PIP2. The assay conditions were optimized for linearity, requiring a 10-min incubation of 20 pmol of PLCζ protein sample at 25°C. In assays to determine dependence on PIP2 concentration, 0.05 µCi of [3H]PIP2 was mixed with cold PIP2 to give the appropriate final concentration. In assays examining the Ca2+ sensitivity, Ca2+ buffers were prepared by EGTA/CaCl2 admixture, as described previously (Nomikos et al., 2005, 2011b; 2013).
SDS-PAGE and western blotting
Recombinant proteins were separated by SDS-PAGE as described previously (Nomikos et al., 2011b,; 2013). Separated proteins were transferred onto polyvinylidene difluoride membrane (Immobilon-P; Millipore) using a semi-dry transfer system (Trans-Blot SD; Bio-Rad) in buffer (48 mm Tris, 39 mm glycine, 0.0375% SDS) at 22 V for 4 h. The membrane incubated overnight at 4°C in Tris-buffered saline, 0.1% Tween 20 (TBS-T) containing 5% non-fat milk powder and probed with Penta-His monoclonal antibody (1 : 5000 dilution). Detection of horseradish peroxidase-coupled secondary antibody was achieved using enhanced chemiluminescence detection (ECL; Amersham Biosciences).
Mathematical modelling
To investigate a range of mechanisms that are potentially responsible for the variable action of human and mouse isoforms of PLCζ, we have employed a mathematical modelling approach. The model (outlined in detail in the Appendix) includes minor modifications from the mathatical description previously published in (Theodoridou et al., 2013) and accounts for cytosolic Ca2+ concentrations, Ca2+ concentrations in the ER and cytosolic levels of IP3. In addition, the role of PIP2 hydrolysis by PLCζ has been investigated by using PIP2 concentrations both as an implicit and as an independent variable. The model has been developed to reproduce the characteristic sequence of sperm-induced Ca2+ spikes that are associated with the early stages of oocyte fertilization. The mathematical description of oocyte Ca2+ dynamics has been derived from previously developed models of intracellular Ca2+ dynamics in somatic cells that are based on Ca2+-induced Ca2+ release and IP3-induced Ca2+ release (IICR) from the ER, that have been validated through extensive pharmacological probing (Parthimos et al., 2007; 2009). Numerical simulations were run in parallel on code composed in C++ and MATLAB software (Mathworks).
Results
Ca2+ oscillations induced by human, mouse and human/mouse PLCζ chimeras
To compare the relative potencies of human and mouse PLCζ in generating Ca2+ oscillations within the same batch of mature mouse oocytes and to enable direct and quantitative comparison of relative protein expression within the mouse oocytes, we used luciferase-fusion protein constructs of human and mouse PLCζ that we have characterized in previous studies (Nomikos et al., 2005, 2011b, c; 2013). In addition, in order to investigate the potential importance of human PLCζ domains in any species-specific differences in activity observed for human versus mouse PLCζ, three chimeric plasmids were constructed. To generate these chimeric plasmids, human PLCζ served as the template and then discrete protein domains were individually replaced by the corresponding domain of mouse PLCζ (Fig. 1). The three chimeric constructs comprised human PLCζ containing either the EF-hand domain (hPLCζ/mEF), the C2 domain (hPLCζ/mC2) or the XY-linker region (hPLCζ/mXYlink) from mouse PLCζ (Fig. 1). Similar to the wild-type human and mouse PLCζ constructs, each of the human/mouse PLCζ chimeras was tagged at the C-terminus with luciferase to enable us to verify and quantify their relative expression upon microinjection of the corresponding cRNA into mouse oocytes. As we have previously described, the advantage of this strategy is that enables real-time monitoring of the relative protein expression by luminescence quantification of the recombinant luciferase protein (Swann et al., 2009).
Figure 1.
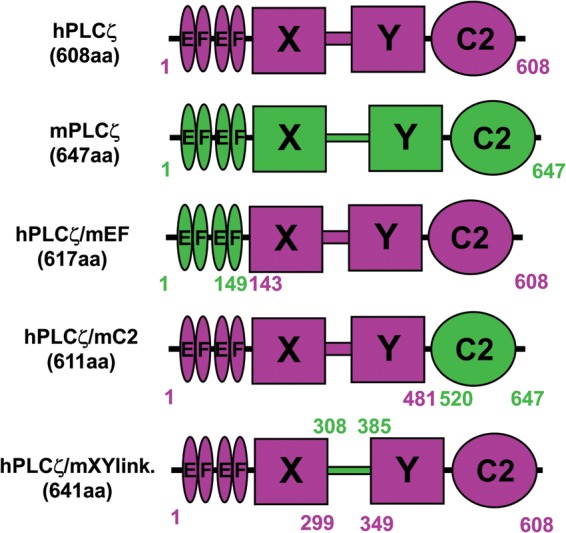
Schematic representation of the domain structure of human PLCζ, mouse PLCζ and human/mouse PLCζ chimeras; hPLCζ, mPLCζ, hPLCζ/mEF, hPLCζ/mC2 and hPLCζ/mXYlink. The various amino acid lengths and respective coordinates are indicated for each construct.
Figure 2 and Table I summarize the results of the wild-type human, mouse and chimeric PLCζ-luciferase cRNA microinjection experiments. Consistent with our previous studies, prominent Ca2+ oscillations (∼20 spikes/2 h) were observed in the mouse PLCζ cRNA-injected oocytes, with the first Ca2+ spike occurring after ∼30 min at a luminescence reading of 0.85 counts per second (cps) and a peak luminescence level of ∼19 cps at the end of the experiment. Microinjection of cRNA encoding human PLCζ also triggered Ca2+ oscillations in the same set of mouse oocytes, but exhibiting a higher frequency to mouse PLCζ (∼46.2 spikes/2 h), although we had microinjected 20-fold less cRNA for human compared with mouse PLCζ, resulting in a peak luminescence level of 7.6 cps for human PLCζ. Interestingly, the first Ca2+ spike with human PLCζ was detected at a mean luminescence of 0.17 cps, ∼20 min post-cRNA microinjection. These data show that human PLCζ is more effective than mouse PLCζ in triggering Ca2+ oscillations. Judging by the number of Ca2+ spikes (in 2 h) per unit of expression (in cps), then human PLCζ (6.08) can be seen to be about six times more effective at causing Ca2+ oscillations than mouse PLCζ (1.05). If we use the threshold for initiating Ca2+ oscillations then human PLCζ (0.17 cps) is five times more effective than mouse PLCζ (0.85 cps).
Figure 2.
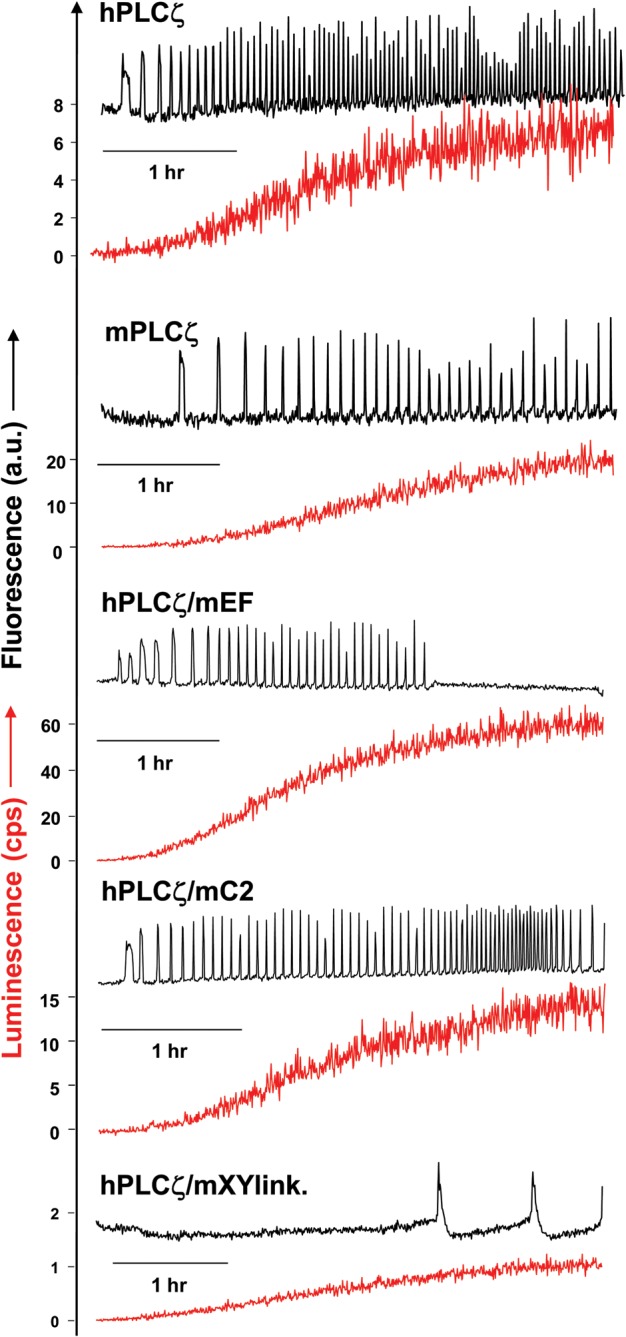
Expression of human PLCζ, mouse PLCζ and human/mouse PLCζ chimeras in unfertilized mouse oocytes. Fluorescence and luminescence recordings reporting the Ca2+ changes (black traces; Ca2+) and luciferase expression [(red traces; Lum, in counts per second (cps)], respectively, in unfertilized mouse oocytes following microinjection of cRNA encoding luciferase-tagged hPLCζ, mPLCζ, hPLCζ/mEF, hPLCζ/mC2 and hPLCζ/mXYlink.
Table I.
Expression of microinjected cRNA encoding luciferase-tagged human PLCζ, mouse PLCζ and human/mouse PLCζ chimeras.
| PLC protein | Ca2+ oscillations in the first 2 h (spikes/2 h) | Peak lumin. (cps) | Time to first spike (min) | Lumin. at first spike (cps) | No. of mouse eggs |
|---|---|---|---|---|---|
| hPLCζ | 46.2 ± 5.40 | 7.60 ± 1.28 | ∼20 | 0.17 ± 0.03 | 11 |
| mPLCζ | 20.2 ± 1.46 | 18.8 ± 0.73 | ∼30 | 0.85 ± 0.09 | 10 |
| hPLCζ/mEF | 32.4 ± 1.90 | 51.50 ± 3.16 | ∼25 | 1.42 ± 0.18 | 12 |
| hPLCζ/mC2 | 49.1 ± 2.89 | 14.6 ± 0.89 | ∼20 | 0.32 ± 0.03 | 17 |
| hPLCζ/mXYlink. | 2.5 ± 0.40 | 0.77 ± 0.03 | ∼130 | 0.60 ± 0.03 | 28 |
Ca2+ oscillation-inducing activity (Ca2+ spike number in 2 h) and luciferase luminescence levels (peak luminescence and luminescence at first spike) are summarized for mouse oocytes microinjected with each of the PLC-luciferase constructs (see Fig. 1A). Values are mean ± SEM.
Microinjection of cRNA corresponding to hPLCζ/mC2 chimera induced Ca2+ oscillations in oocytes with a similar frequency to wild-type human PLCζ (∼49.1 spikes/2 h versus 46.2 spikes/2 h), with the first Ca2+ spike occurring (similar to human PLCζ) after ∼20 min at a luminescence reading of 0.32 cps (Fig. 2, Table I). In contrast, microinjection of oocytes with cRNA corresponding to hPLCζ/mEF chimera showed a reduced frequency of Ca2+ oscillations compared with wild-type human PLCζ (∼32.4 spikes/2 h versus 46.2 spikes/2 h), even though this chimeric protein was expressed at ∼7-fold higher levels compared with wild-type human PLCζ, showing a peak luminescence level of 51.5 cps (versus 7.6 cps). The first Ca2+ spike was detected ∼25 min post-injection at a luminescence reading of 1.42 cps, which is ∼8-fold higher than for wild type. Interestingly, microinjection of cRNA corresponding to the hPLCζ/mXYlink chimera showed very low protein expression (peak luminescence of 0.77 cps) even when 100-fold more cRNA was injected compared with human PLCζ. The hPLCζ/mXYlink chimera induced very low frequency Ca2+ oscillations (∼2.5 spikes/2 h) with the first Ca2+ spike occurring after ∼130 min at a luminescence reading of 0.60 cps.
These data indicate that the wild-type human PLCζ is five to six times more effective in inducing Ca2+ oscillations compared with wild-type mouse PLCζ, even in a heterologous set of oocytes. In contrast to this intrinsic difference in wild-type activity, we found that substitution of the human PLCζ C2 domain with the corresponding structure from mouse PLCζ did not alter the Ca2+ oscillation-inducing activity. However, substitution of the human PLCζ EF-hand domain for that from mouse PLCζ significantly reduced the Ca2+ oscillation-inducing activity. Finally, replacement of the human PLCζ XY-linker with the corresponding region of mouse PLCζ appears to dramatically affect the expression levels of the chimeric PLCζ in mouse oocytes.
Enzymatic characterization of human, mouse and human/mouse PLCζ chimeras
Wild-type mouse PLCζ and all three human/mouse chimeric PLCζ constructs were subcloned into the pETMM60 vector and purified as NusA-6xHis-tagged fusion proteins. We have recently demonstrated that NusA is an effective fusion protein partner for human PLCζ, significantly increasing the expression of soluble PLCζ protein in E. coli, as well as enhancing the stability of the purified protein over time (Nomikos et al., 2013). Expression of active recombinant human PLCζ protein has also been reported by other groups (Kashir et al., 2011; Yoon et al., 2012). Following expression of NusA-PLCζ fusion proteins in E. coli and purification by Ni-NTA affinity chromatography, the recombinant proteins were characterized. Figure 3 shows NusA-tagged wild-type human and mouse PLCζ, as well as the three PLCζ chimeras analysed by SDS-PAGE (upper panel) and immunoblot detection with penta-His mouse monoclonal antibody (lower panel). The major protein band with mobility corresponding to the predicted molecular mass for each construct was observed for all except the hPLCζ/mXYlink chimera. These major bands were also confirmed by the penta-His antibody after immunoblot analysis (Fig. 3; lower panel). Interestingly, in the case of hPLCζ/mXYlink chimera the major band appeared to be ∼103 kDa instead of the expected ∼133 kDa, suggesting protein degradation due to the mouse XY-linker region resulting from instability of the purified recombinant protein. This protein instability might explain the very low expression levels of this chimera in mouse oocytes.
Figure 3.
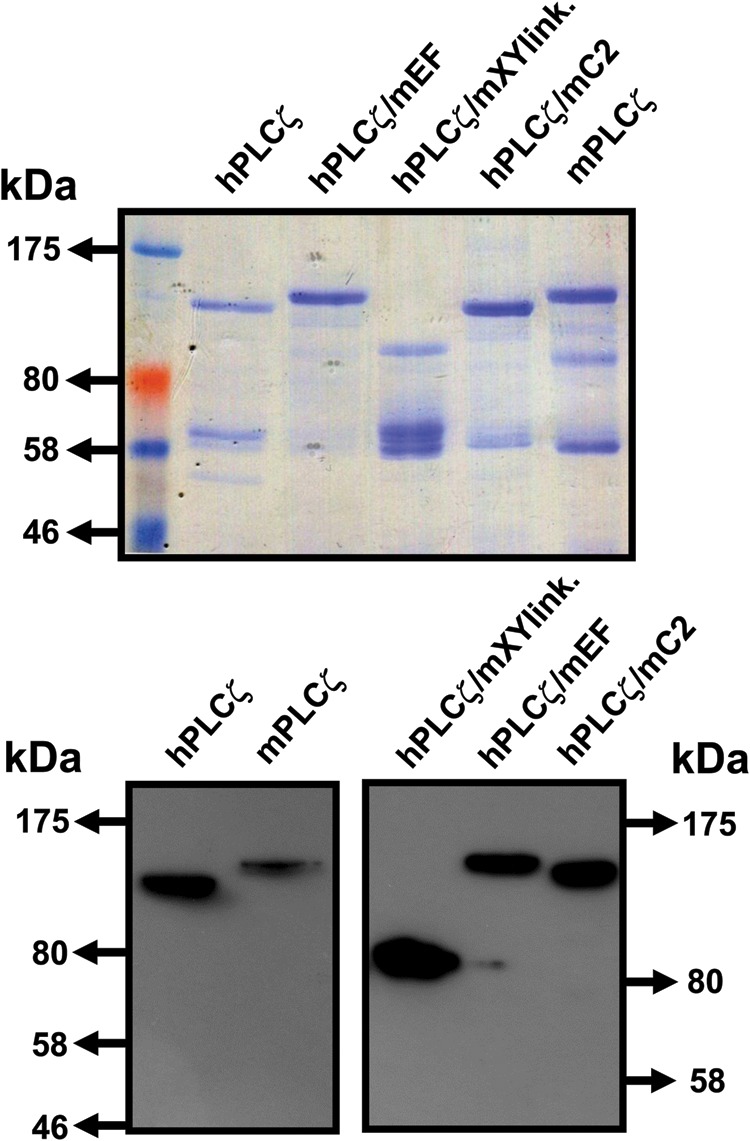
Expression and purification of recombinant NusA-6xHis-tagged human, mouse and human/mouse PLCζ chimeric proteins. Affinity-purified, NusA-6His-PLC fusion proteins (1 µg) were analysed by 7.5% SDS-PAGE followed by either Coomassie Brilliant Blue staining (upper panel) or immunoblot analysis using the Penta-His monoclonal antibody (1:5000 dilution) (lower panel).
The specific PIP2 hydrolytic enzyme activity for each recombinant protein was determined by the standard [3H]PIP2 hydrolysis assay. The histogram of Fig. 4A and Table II summarizes the enzyme specific activity values obtained for each recombinant protein. The enzymatic activities of wild-type human and mouse PLCζ are in agreement with our previous observations revealing that human PLCζ exhibits a ∼76% higher specific activity than mouse PLCζ (978 ± 34 versus 556 ± 29 nmol/min/mg). The specific activity for hPLCζ/mC2 chimera was similar to the specific activity of wild-type human PLCζ (970 ± 42 versus 978 ± 34 nmol/min/mg), in contrast with hPLCζ/mEF chimera that showed a reduced enzymatic activity (∼20%) compared with human PLCζ (782 ± 33 versus 978 ± 34 nmol/min/mg). In accord with the poor activity observed in oocytes, the hPLCζ/mXYlink chimera did not exhibit in vitro enzymatic activity.
Figure 4.
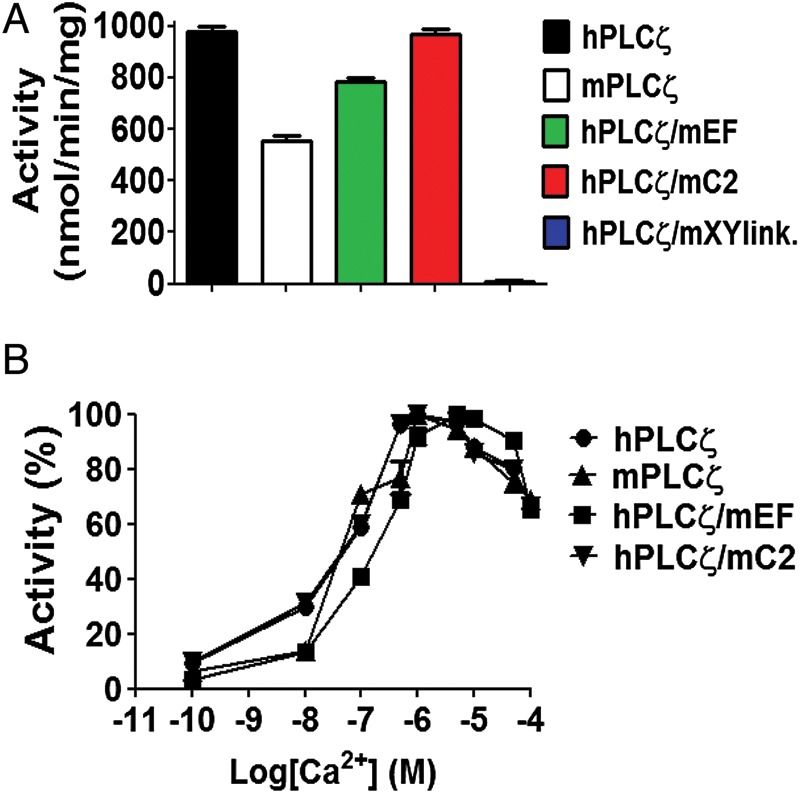
In vitro enzymatic properties of human/mouse PLCζ chimeras. (A) [3H]PIP2 hydrolysis activities of the purified NusA-6His-PLC fusion proteins (20 pmol), n = 3 ± SEM, determined using two different preparations of recombinant protein and with each experiment performed in duplicate. In control experiments with NusA, there was no specific PIP2 hydrolysis activity observed (data not shown). (B) Effect of varying [Ca2+] on the normalized activity of NusA-6His-tagged, human and mouse PLCζ and human/mouse PLCζ chimeric fusion proteins. For these assays n = 3 ± SEM, determined using two different preparations of recombinant protein and with each experiment performed in duplicate.
Table II.
In vitro enzymatic properties of NusA-6xHis-tagged human PLCζ, mouse PLCζ and human/mouse PLCζ chimeras.
| Recombinant PLC protein | PIP2 hydrolytic enzyme activity (nmol/min/mg) | Ca2+-dependence EC50 (nM) | Km (µM) |
|---|---|---|---|
| hPLCζ | 978 ± 34 | 49 | 75 |
| mPLCζ | 556 ± 29 | 43 | 99 |
| hPLCζ/mEF | 782 ± 33 | 148 | 475 |
| hPLCζ/mC2 | 970 ± 42 | 40 | 89 |
| hPLCζ/mXYlink. | 10 ± 4 | – | – |
Summary of specific enzyme activity, Km and EC50 values of Ca2+-dependent enzyme activity for PIP2 hydrolysis, determined by non-linear regression analysis (GraphPad Prism 5) for the NusA-6xHis-fusion PLC proteins (see Fig. 3).
To investigate the effect of single domain replacement on the Ca2+ sensitivity of PLCζ enzyme activity, we assessed the ability of wild-type human, mouse and chimeric PLCζ proteins to hydrolyse [3H]PIP2 at different Ca2+ concentrations ranging from 0.1 nM to 0.1 mM. The resulting EC50 value for human PLCζ (49 nM), mouse PLCζ (43 nM) and hPLCζ/mC2 (40 nM) were near identical (Fig. 4B, Table II). However, replacement of EF hands from human PLCζ with those from mouse PLCζ increased the EC50 value of human PLCζ by ∼3-fold (148 nM). To compare the enzyme kinetics of wild-type human and mouse PLCζ and the three PLCζ chimeras, the Michaelis-Menten constant, Km was calculated for each construct (Table II). The Km values obtained for human PLCζ (75 µM), mouse PLCζ (99 µM) and the hPLCζ/mC2 (89 µM) chimera were quite similar. In contrast, the Km for hPLCζ/mEF chimera was ∼6.3-fold (475 µM) higher than that of human PLCζ, suggesting that replacement of the EF hand of human from that of mouse PLCζ reduces the in vitro affinity for PIP2.
Modelling of Ca2+ oscillations induced by human and mouse PLCζ
The above data show that human PLCζ is more active than mouse PLCζ both in vitro and in intact oocytes. However, the differences in ability to cause Ca2+ oscillations is more dramatic (5- to6-fold) than that for maximum enzymatic activity (∼2-fold). We employed a mathematical model of IP3-induced Ca2+ oscillations to understand this apparent discrepency. The distinguishing properties that we examined were the Ca2+-dependence EC50 values, PIP2 hydrolytic activities and Kms for PIP2 for human and mouse PLCζ (Table II). However, the main difference is that the intrinsic hydrolytic activity for human PLCζ is almost twice that of mouse PLCζ. Experimental estimates summarized in Tables I and II have been employed as parametric values in Equations (1–3) (Appendix), while a full list of coefficient/parameter values is provided in Table III. Numerical simulations generated under these assumptions produce sustained Ca2+ oscillations associated with the specific action of human and mouse PLCζ (Fig. 5A). In each of the two cases, we mimicked the effect of increasing expession of PLCζ with time as a sigmoidal, in agreement with luminescence traces presented in Fig. 1B. Simulated traces presented in Fig. 5A closely match the Ca2+ oscillations recorded in mouse oocytes (Fig. 2) both in terms of oscillatory Ca2+ frequency (Table I) and in terms of the protein expression level associated with onset of oscillations (Table I). Importantly, the earlier onset of oscillatory activity for human PLCζ followed by an earlier frequency saturation underlines the potential for even higher activity of the human versus mouse PLCζ (possibly up to a 6-fold potency ratio). These simulations demonstrate that the different enzymatic properties we observe for the recombinant human versus mouse PLCζ can explain the difference in the frequency of Ca2+ oscillations we see in mouse oocytes.
Table III.
Description of parameters used in Equations (1–4) of the mathematical model and their numerical values.
| Parameter | Description | Units |
|---|---|---|
| A | Ca2+ influx through non-specific cation channels | 0.035 µM/s |
| D | Rate constant for Ca2+ extrusion by the ATPase pump | 0.64 µM(1−q)/s |
| q | Exponent for x dependence of Ca2+ extrusion | 2 |
| xd | Half-point of Ca2+ extrusion ATPase activation sigmoidal | 1 µM |
| B | ER uptake rate constant | 17.8 µM/s |
| xb | Half-point of the ER ATPase activation sigmoidal | 4.4 µM |
| n | Hill coefficient for x dependence of ER uptake | 4 |
| C | InsP3-mediated release from InsP3-sensitive stores (IICR) | 278 µM/s |
| yc | Half-point of the IICR Ca2+ efflux sigmoidal | 8.9 µM |
| m | Hill coefficient for y dependence of IICR | 2 |
| xca | Half-point of the IICR Ca2+ activation sigmoidal | 0.9 µM |
| pa | Hill coefficient for Ca2+ activation of IICR | 2 |
| xci | Half-point of the IICR Ca2+ inactivation sigmoidal | 1.2 µM |
| pi | Hill coefficient for Ca2+ inactivation of IICR | 5 |
| pc | Half-point of the IICR InsP3 activation sigmoidal | 0.1 µM |
| k | Hill coefficient for InsP3 activation of IICR | 2 |
| L | Leak from SR rate constant | 0.001/s |
| PLCζ-h | Vmax of human PLCζ | 0.035 µM/s |
| PLCζ-m | Vmax of mouse PLCζ | 0.018 µM/s |
| PIP2 | Concentration of PIP2 | 1.5 µM |
| Km-h | Michaelis–Menten constant for human PLCζ | 0.099 µM |
| Km-m | Michaelis–Menten constant for mouse PLCζ | 0.075 µM |
| j | Hill coefficient for PLC activation of PIP2 hydrolysis | 1 |
| xp | Half-point of the Ca2+ activation sigmoidal of InsP3 production | 0.05 µM |
| i | Hill coefficient for Ca2+ activation of InsP3 production | 4 |
| r | Rate of InsP3 decay | 0.44/s |
Figure 5.
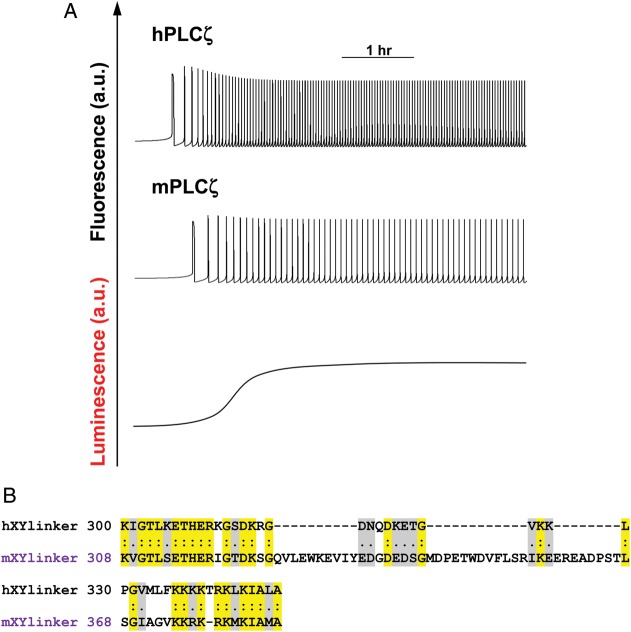
(A) Ca2+ oscillations in a mouse oocyte were simulated using a mathematical model (Equations 1–3, Appendix) based upon the enzymatic properties of human PLCζ (top panel) and mouse PLCζ (middle panel). Simulations were performed under a sigmoidal increase of PLCζ expression (bottom panel). (B) Clustal alignment of human and mouse sperm PLCζ XY-linkers. Identical amino acids are shown in shaded yellow boxes and conservative substitutions in grey.
Discussion
A growing body of evidence supports the assertion that sperm-specific PLCζ is the molecule that stimulates cytoplasmic Ca2+ oscillations at fertilization, triggering all the early events of embryo development in many mammalian species (Cox et al., 2002; Saunders et al., 2002; Nomikos et al., 2012; Swann and Lai, 2013). No other sperm-specific molecule has been shown to trigger Ca2+ oscillations in mammalian oocytes. Despite recent advances in the PLCζ field, which has helped us to understand the role of this sperm-derived enzyme at fertilization and its clinical potential, as a therapeutic intervention and a prognostic indicator of oocyte activation deficiency, there are still many aspects of PLCζ regulatory mechanisms that need to be addressed.
There are significant species-specific differences in the relative potency of PLCζ from different species (Swann et al., 2006; Saunders et al., 2007; Cooney et al., 2010; Bedford-Guaus et al., 2011). In the present study, we have compared quantitatively and qualitatively the relative potencies of human and mouse PLCζ to induce Ca2+ oscillations in unfertilized mouse oocytes (Fig. 2). Human PLCζ caused a higher frequency of Ca2+ oscillations even when expressed at much lower levels than mouse PLCζ. This quantitatively confirms that human PLCζ is more effective in generating Ca2+ oscillations in mouse oocytes than the mouse PLCζ. This remarkable difference can largely be explained by the differences in the in vitro enzymatic properties of these proteins (Fig. 3) to hydrolyse PIP2. In our in vitro studies, the purified human PLCζ exhibited a ∼76% higher specific activity than mouse PLCζ (978 ± 34 versus 556 ± 29 nmol/min/mg), however, the comparable values for EC50 and Km (see Fig. 4 and Table II) suggest a similar Ca2+ sensitivity and affinity for PIP2 for these two PLCζ isozymes. This is consistent with a mathematical model of Ca2+ oscillations (Fig. 5) incorporating the different enzymatic properties of human and mouse PLCζ which generates a similar difference in the frequency of Ca2+ oscillations to that we observe empirically in mouse oocytes.
Our chimeric analysis approach showed that replacement of the human PLCζ C2 domain with its mouse counterpart had minimal effect on the in vitro enzymatic properties of human PLCζ and consequently its potency to elicit Ca2+ oscillations in unfertilized mouse oocytes. In contrast, replacement of the human PLCζ EF-hand domain with that of mouse PLCζ resulted in a slightly reduced enzymatic activity, but caused a ∼3-fold decrease in the Ca2+ sensitivity of human PLCζ, as well as a ∼6-fold increase in the Km value for PIP2, suggesting reduced substrate affinity of the enzyme. These results correlate with the reduced Ca2+ oscillation-inducing activity of the hPLCζ/mEF chimera and suggest that the EF-hand domains are not only responsible for the Ca2+ sensitivity of PLCζ but might also contribute to the enzyme substrate affinity. The most dramatic effect was observed after the replacement of the human XY-linker with the corresponding region of mouse PLCζ. This substitution led to poor expression levels of hPLCζ/mXYlink possibly due to significant degradation of unstable recombinant protein (Fig. 3), which would be consistent with the low expression levels of this chimera in mouse oocytes (see Fig. 2, Table I). We have previously proposed that an unstructured positively charged cluster within the XY-linker region of PLCζ may help anchor the protein to biological membranes through electrostatic interactions with the negatively charged PIP2 (Nomikos et al., 2007; 2011c). Interestingly, the amino acid sequence of the XY-linker region of PLCζ is poorly conserved among species (Saunders et al., 2007). Notably, the XY-linker of human PLCζ is shorter in length and more positively charged than the mouse PLCζ XY-linker, showing only 34.2% sequence identity and 18% similarity (see Fig. 5B). The significance of this XY-linker diversity is still unclear, but our data suggest that this variation may contribute to the different rates of PIP2 hydrolysis and relative potency in inducing Ca2+ oscillations for these two species' PLCζ isoform.
The full explanation of why human PLCζ is more active than the mouse requires further investigation. However, we can speculate as to why the human sperm may contain a more intrinsically active enzyme. Human and mouse sperm are different in shape but not greatly different in size, and although the amount of PLCζ has not been quantified in human sperm it may be similar to that of mouse sperm (Saunders et al., 2002). If this is the case, then the human sperm faces a greater challenge in activating the oocyte. The sperm-derived PLCζ is assumed to distribute evenly into the oocyte cytoplasm after gamete fusion. However, the volume of the human oocyte is about five times larger than that of the mouse oocyte, so human PLCζ may have to trigger oscillations at much lower concentrations. The current study suggests that this problem may be overcome by human PLCζ having at least a 5-fold greater ability to trigger Ca2+ oscillations. Such species-dependent variation in PLCζ potency may enable a precise tuning of the effective ‘dose’ of PLCζ delivered by the sperm, which is adjusted to match the size of the recipient oocyte.
Authors' roles
M.N., G.N., K.S. and F.A.L. devised the project strategy, D.P. prepared the mathematical model, M.N. and F.A.L. designed the experiments, which were performed by M.N., K.E., M.T., B.L.C. and L.B., M.N., K.S. and F.A.L. prepared the manuscript.
Funding
This work was supported by a Wellcome Trust grant 080701. K.E. and M.T. hold research scholarships supported by the Libyan Government and NCSR Demokritos, respectively.
Conflict of interest
All authors declare that no conflict of interest exists.
Acknowledgement
We thank Junaid Kashir for helpful comments on the manuscript.
Appendix
The system of coupled differential equations employs cytosolic-free [Ca2+], [Ca2+] in the ER and [InsP3] in the cytosol as independent parameters. Individual ionic fluxes and chemical reactions contributing to these variables are described below.
Cytosolic-free Ca2+
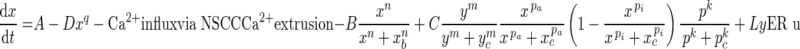 |
(1) |
[Ca2+] in the sarcoplasmic reticulum
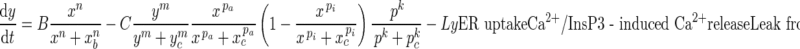 |
(2) |
[InsP3] in the cytosol
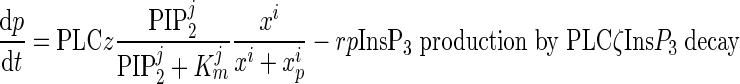 |
(3) |
All parameters and coefficients used in Equations (1–4) are described in Table III.
References
- Bedford-Guaus SJ, McPartlin LA, Xie J, Westmiller SL, Buffone MG, Roberson MS. Molecular cloning and characterization of phospholipase C zeta in equine sperm and testis reveals species-specific differences in expression of catalytically active protein. Biol Reprod. 2011;85:78–88. doi: 10.1095/biolreprod.110.089466. [DOI] [PubMed] [Google Scholar]
- Cooney MA, Malcuit C, Cheon B, Holland MK, Fissore RA, D'Cruz NT. Species-specific differences in the activity and nuclear localization of murine and bovine phospholipase C zeta 1. Biol Reprod. 2010;83:92–101. doi: 10.1095/biolreprod.109.079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M, et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCζ) in spermatozoa from infertile men. Hum Reprod. 2009;24:2417–2428. doi: 10.1093/humrep/dep207. [DOI] [PubMed] [Google Scholar]
- Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16:690–703. doi: 10.1093/humupd/dmq018. [DOI] [PubMed] [Google Scholar]
- Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, Ruas M, Tee ST, Heindryckx B, Galione A, et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod. 2011;26:3372–3387. doi: 10.1093/humrep/der336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, Heindryckx B, Deane CM, De Sutter P, Fissore RA, et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod. 2012;27:222–231. doi: 10.1093/humrep/der384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S. Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol Hum Reprod. 1997;3:1087–1093. doi: 10.1093/molehr/3.12.1087. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem. 2005;280:31011–31018. doi: 10.1074/jbc.M500629200. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Mulgrew-Nesbitt A, Pallavi P, Mihalyne G, Zaitseva I, Swann K, Lai FA, Murray D, McLaughlin S. Binding of phosphoinositide-specific phospholipase C-zeta (PLC-zeta) to phospholipid membranes: potential role of an unstructured cluster of basic residues. J Biol Chem. 2007;282:16644–16653. doi: 10.1074/jbc.M701072200. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Elgmati K, Theodoridou M, Calver BL, Cumbes B, Nounesis G, Swann K, Lai FA. Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP2 hydrolysis activity of sperm PLCzeta. Biochem J. 2011a;434:211–217. doi: 10.1042/BJ20101772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Elgmati K, Theodoridou M, Georgilis A, Gonzalez-Garcia JR, Nounesis G, Swann K, Lai FA. Novel regulation of PLCζ activity via its XY-linker. Biochem J. 2011b;438:427–432. doi: 10.1042/BJ20110953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Elgmati K, Theodoridou M, Calver BL, Nounesis G, Swann K, Lai FA. Phospholipase Czeta binding to PtdIns(4,5)P2 requires the XY-linker region. J Cell Sci. 2011c;124:2582–2590. doi: 10.1242/jcs.083485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Swann K, Lai FA. Starting a new life: sperm PLC-zeta mobilizes the Ca2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays. 2012;34:126–134. doi: 10.1002/bies.201100127. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Yu Y, Elgmati K, Theodoridou M, Campbell K, Vassilakopoulou V, Zikos C, Livaniou E, Amso N, Nounesis G, et al. Phospholipase Cζ rescues failed oocyte activation in a prototype of male factor infertility. Fertil Steril. 2013;99:76–85. doi: 10.1016/j.fertnstert.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthimos D, Haddock RE, Hill CE, Griffith TM. Dynamics of a three-variable nonlinear model of vasomotion: comparison of theory and experiment. Biophys J. 2007;93:1534–1556. doi: 10.1529/biophysj.107.106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthimos D, Edwards DH, Griffith TM. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. Am J Physiol. 2009;277:H1119–H1144. doi: 10.1152/ajpheart.1999.277.3.H1119. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Swann K, Lai FA. PLCzeta, a sperm-specific PLC and its potential role in fertilization. Biochem Soc Symp. 2007;74:23–36. doi: 10.1042/BSS0740023. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium. 2013;53:55–62. doi: 10.1016/j.ceca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Swann K, Saunders CM, Rogers NT, Lai FA. PLCζ(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Swann K, Campbell K, Yu Y, Saunders C, Lai FA. Use of luciferase chimaera to monitor PLCzeta expression in mouse eggs. Methods Mol Biol. 2009;518:17–29. doi: 10.1007/978-1-59745-202-1_2. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9:511–518. doi: 10.1093/oxfordjournals.humrep.a138537. [DOI] [PubMed] [Google Scholar]
- Theodoridou M, Nomikos M, Parthimos D, Gonzalez-Garcia JR, Elgmati K, Calver BL, Sideratou Z, Nounesis G, Swann K, Lai FA. Chimeras of sperm PLCζ reveal disparate protein domain functions in the generation of intracellular Ca2+ oscillations in mammalian eggs at fertilization. Mol Hum Reprod. 2013;19:852–864. doi: 10.1093/molehr/gat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Meerschaut F, Leybaert L, Nikiforaki D, Qian C, Heindryckx B, De Sutter P. Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum Reprod. 2013;28:87–98. doi: 10.1093/humrep/des368. [DOI] [PubMed] [Google Scholar]
- Yazawa H, Yanagida K, Hayashi S, Sato A. The oocyte activation and Ca2+ oscillation-inducing abilities of mouse and human dead (sonicated) spermatozoa. Zygote. 2009;17:175–184. doi: 10.1017/S0967199408005157. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, et al. Human sperm devoid of PLC zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Eum JH, Lee JE, Lee HC, Kim YS, Han JE, Won HJ, Park SH, Shim SH, Lee WS, et al. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod. 2012;27:1768–1780. doi: 10.1093/humrep/des092. [DOI] [PubMed] [Google Scholar]