Abstract
Background
Epidemiological evidence suggests HIV-infected individuals are at increased risk of lung cancer, but no data exist since large CT screening trials routinely exclude HIV-infected participants.
Methods
From 2006–2013, we conducted the first lung cancer screening trial of 224 HIV-infected current/former smokers to assess the CT detection rates of lung cancer. We also used 130 HIV-infected patients with known lung cancer to determine radiographic markers of lung cancer risk using multivariate analysis.
Results
Median age was 48 years with 34 pack-years smoked. During 678 person-years, one lung cancer was found on incident screening. Besides this lung cancer case, eighteen deaths(8%) occurred, but none were cancer-related. There were no interim diagnoses of lung or extrapulmonary cancers. None of the pulmonary nodules detected in 48 participants at baseline were diagnosed as cancer by study end. The heterogeneity of emphysema across the entire lung as measured by CT densitometry was significantly higher in HIV-infected subjects with lung cancer than in those without (p≤0.01). On multivariate regression, increased age, higher smoking pack-years, low CD4 nadir, and increased heterogeneity of emphysema on quantitative CT imaging were all significantly associated with lung cancer.
Conclusions
Despite a high rate of active smoking among HIV-infected participants, only one lung cancer was detected in 678 patient-years. This was probably due to the young age of participants suggesting that CT screening of high-risk populations should strongly consider advanced age as a critical inclusion criterion. Future screening trials in urban American must also incorporate robust measures to ensure HIV patient compliance, adherence, and smoking cessation.
Introduction
HIV-infected smokers are reported to have a higher relative risk of developing lung cancer than the general population, and lung cancer has emerged as the most common and fatal non-AIDS associated malignancy in most western nations [1–10]. After controlling for cigarette smoking, the best epidemiological estimates are that HIV infection increases lung cancer risk by 2.5 fold[11–14]. Since lung cancer increases markedly with age and duration of smoking, lung cancer may become more common and account for even more deaths as HIV-infected patients live longer with highly active antiretroviral therapy (ART).
The high case fatality rate in HIV-associated lung cancer has been shown not to be due to HIV-related causes, but instead, is primarily attributed to an advanced stage of lung cancer presentation in HIV patients[15, 16]. Late lung cancer diagnoses occur even in HIV specialty clinics where frequent chest radiographs evaluating opportunistic pulmonary infections fail to detect lung cancer early[15, 17]. In fact, approximately 130 HIV-infected lung cancer patients have presented to our institution with over 80 percent having late stage disease[15].
Since the 1990s, computed tomography (CT) has been explored as an early detection strategy for lung cancer [18–22] with suggestions that it may allow early stage diagnosis and definitive treatment[23] 37]. The National Lung Cancer CT Screening Trial (NLST) reported a 20% reduction in mortality associated with annual CT screening for older, heavy smokers at high risk for lung cancer[24]. Given the current late stage of presentation of HIV-associated lung cancer, CT screening may have profound implications for improving earlier diagnosis of this high risk group of patients. There are no data, however, to support routine lung cancer screening in HIV-infected smokers because most CT screening studies, including the NLST, excluded their enrollment.
Given that no HIV-infected subjects were enrolled in the NLST, the late stage presentation of HIV-associated lung cancer, and the epidemiological evidence suggesting this population was at particularly high risk for lung cancer, we hypothesized that annual CT screening in HIV-infected smokers may improve early lung cancer detection. From 2006–2013, we initiated a single-armed, prospective, observational study assessing the incidence and stage at diagnosis of lung cancer among HIV-infected smokers undergoing annual CT screening. The primary objective was to determine the prevalence and incidence of lung cancer in HIV-infected smokers. Our secondary objectives were to evaluate the feasibility and adherence to intensive screening in this population, to examine rates of false positive nodule detection, to determine if CT screening could change the stage distribution of HIV-associated cancer to that of an early stage disease, and to determine radiographic markers that may differentiate between HIV-infected smokers with and without lung cancer.
METHODS
Participants
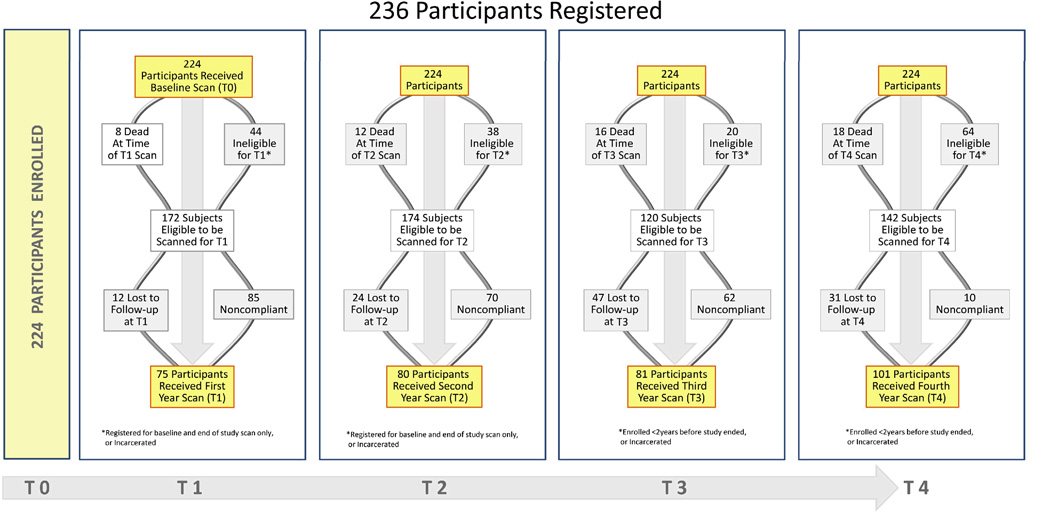
From January 2006 through May 2013, HIV-infected smokers were recruited and followed from HIV outpatient clinics throughout Baltimore City, and from the ALIVE (AIDS Linked to the Intra-Venous Experience) cohort at Johns Hopkins[9]. The study was approved by the Johns Hopkins Institutional Review Board, and all subjects provided informed consent. Eligible participants were seropositive for HIV by ELISA, had no symptoms of a lung malignancy, aged ≥25 years, current or former smokers (quit within 15 years) with ≥20 pack-years of use. Exclusion criteria included chest CT examination 18 months prior to eligibility, pregnancy, history of lung cancer, active respiratory infection, or prior cytotoxic therapy within 6 months. A total of 236 participants were registered. Twelve subjects were excluded due to a CT scan within 18 months of registration leaving 224 participants. Forty-nine participants from the ALIVE study were registered 2010–2011, and consented to undergo baseline and end of study imaging only. All enrolled subjects were unselected, and no preference was given towards recruiting “healthier” smokers.
Patient navigators tracked all study appointments, including contacting subjects prior to appointments, providing minimal financial remuneration for attendance at each visit, and coordinating follow-up study visits with routine clinical care.
Screening
At baseline, smoking habits, general health, occupational, and contact data were recorded, and portable spirometry was performed. Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were measured at each CT screening. Participants were to have a low-dose helical CT scan at baseline (T0) and up to four scans annually (T1-T4). CT screenings were without contrast using a low-dose regimen (120kVp, 50 to 200 mA, 1 to 5mm axial reconstruction, 1.1pitch with collimation of 64×0.6mm) on a single multi-detector scanner (SOMATOM 64; Siemens Medical Solutions, Erlangen, Germany) with daily calibration. Each CT was read independently by two radiologists with inter-observer variability ameliorated through joint discussion. Due to previous work in evaluating CT changes in HIV patients, we presumed that CT screening would yield a high incidence of inflammatory nodules and scarring from previous pulmonary infections in HIV-positive patients[25]. Our protocol thus differed from the current, robust protocols of IELCAP[26] and the NCCN for CT screening[27] in allowing our radiologists to assess non-calcified pulmonary nodules ≥4mm-9mm as suspicious or non-suspicious on an individual basis. Repeat low dose helical CT was recommended at 3 or 6 months for suspicious nodules such as enlarging nodules<7mm diameter or those with other suspicious changes. For nodules ≥10 mm in diameter or enlarging nodules >7mm in diameter, additional diagnostic tests could include CT screening at 3 or 6 months, FDG-PET or Technetium-99m depreotide scintigraphy, or biopsy (percutaneous, bronchoscopic, thoracoscopic or open biopsy).
Vital Status
Participants were contacted semiannually enabling updates on health status, contact information and smoking behavior. The social security numbers of those lost to follow-up were cross referenced with the Social Security Death Index to ascertain vital status. Cause of death was abstracted from the medical record. Current CD4 cell count, nadir CD4 count, HIV viral load, and HIV antiretroviral therapy were obtained from patients, their health care provider, and from medical records.
CT Densitometry of Screening Participants
CT scans were analyzed for emphysema using Pulmonary Workstation 2.0 software (Vida Diagnosis, Iowa City, IA). The program determines lung volumes as well as histogram statistics of all lung pixel attenuation values. Extent of emphysema was estimated by quantifying the percentage of voxels having an attenuation value lower than −910 Hounsfield units (HU). This threshold was chosen empirically due to the thickness of the CT scans in this study, and was validated by analyzing several CT scans over a range of HUs (from −910HU to −1040HU) in 10 HU increments. All lung densitometry measurements were corrected by normalizing to the lung air volume being considered. Of the 224 baseline scans available, 117 were able to be used for CT densitometry calculations. Two investigators independently performed these analyses with any discrepancies resolved by committee.
CT Densitometry of HIV-infected Lung Cancer Patients
From 1989–2012 at the Johns Hopkins Hospital, 130 HIV-infected patients were diagnosed with lung cancer. From these patients, thirty-nine had available archived chest CT scan digital data that could be analyzed quantitatively.
Statistical Analysis
Comparisons of continuous and dichotomous variables between groups were performed with the Student t test (2-tailed) and Χ2 tests, respectively. Multivariable logistic regression models estimated odds ratios with 95% confidence intervals (CIs), and were considered significant for p values <0.05. Statistical analyses were performed with STATA software (Stata Corporation, College Station, Tx).
RESULTS
Participant Characteristics
We recruited 224 asymptomatic HIV smokers for CT screening (Table 1). At study entry, the median age was 48 years, 90% were black, and 58% had a history of injecting drugs. Most were current smokers (89%) with a median of 35 pack-years smoked. Most had previously received ART. These 224 screened participants were dissimilar demographically to 130 HIV-associated lung cancer patients previously diagnosed at our institution. with the latter being more immunocompromised with higher viral counts, and having more obstructive lung disease(Supplemental Table 1). The age distribution of the screened participants has a bimodal, normal age distribution around a median of 48 years old (Supplemental Figure 2).
Table 1.
Baseline Characteristics of HIV-infected Individuals enrolled in the Lung Cancer Detection by CT Screening Study (N = 224)a
| Characteristics | No. Subjects (%) |
|---|---|
| Age, median [IQR], yrs | 48 [44–53] |
| Gender (M/F) | 161/63 |
| Race | |
| Blacks | 201 (90) |
| Whites | 22 (10) |
| Hispanic or Latino | 1 (0.5) |
| Smoking Status | |
| Former | 25 (11) |
| Current | 199 (89) |
| Never | 0 (0) |
| Pack-years smoked, median [IQR], yrs | 34 [31–36] |
| History of Marijuana Use | 90 (40) |
| History of Cocaine Use | 65 (29) |
| IVDUb | 129 (58) |
| Hepatitis Cc | 114 (54) |
| TB skin test positived | 44 (21) |
| STDe | 84(44) |
| AZTf | 143 (68) |
| CD4 nadir, median [IQR], cells/mm3 g | 179 [61–332] |
| CD4 cell count, median [IQR], cells/mm3 h | 400 [217–568] |
| Viral load <400 cells/mm3 i | 123 (59.1) |
| FEV1, median [IQR],% Predicted | 85 [70–101] |
| FVC, median [IQR], % Predicted | 88 [74–101] |
| FEV1/FVC, median [IQR], | 81 [73–91] |
| Highest Educational Level Attainedj | |
| Middle school | 65 (52) |
| High School | 40 (32) |
| College Degree | 21 (17) |
| Annual Incomek | |
| <$ 8,000 | 63 (72) |
| $ 8,000 to $14,999 | 12 (14) |
| $ 15,000 to $24,999 | 10 (12) |
| $ 25,000 to $49,999 | 2 (3) |
Abbreviations: IQR, Interquartile Range; IVDU, Intravenous Venous Drug User; TB, Tuberculosis; STD, Sexually Transmitted Disease; AZT, Azidothymidine.
Some subjects had missing demographic data as noted.
n = 222,
n = 213,
n = 210,
n = 189,
n = 209,
n = 200,
n = 187,
n = 207,
n = 126,
n = 87
Adherence to Screening
Over 70% of those eligible received both a baseline CT scan and a CT scan in the final year of the study (Fig. 1).The total length of follow-up was 678 patient-years with the median length of follow-up being 3.2 years. After baseline scanning, 44% of eligible patients received a T1 scan, 46% had a T2 scan, 68% received a T3 scan, and fully 71% returned for a final T4 scan (Fig 1.). Participation in each annual screening was hindered by regular changes in residence and frequent alterations in contact information. Out of 5 possible scans for all 224 participants, 18 (8%) received only one scan, 103 (46%) had two scans, 44 (20%) had three scans, 39 (17%) had four scans, and 20 (9%) received all five scans.
Fig 1.
Flow diagram of HIV smokers enrolled in the lung cancer screening study by year of study participation. (Title: Flow-chart of Registered and Enrolled HIV smokers)
Screening Results
Forty-eight nodules, 32 at baseline and 16 during incident screening, were detected during the study and followed (Supplemental Table 2). The majority of the 48 nodules were solid; ground-glass consistency represented about 30%. Only 25% of nodules were larger than 1 cm in diameter (Supplemental Table 3). None of these nodules were found to be malignant during subsequent examinations. Of the 48 nodules, 38 were judged not to be suspicious by the radiologists. These included 14/38 thought to be due to chronic inflammation such as from fungal or granulomatous disease while 24/38 were thought to have non-inflammatory etiologies such as active infection, scarring from previous infection, or hamartomas. Ten participants with suspicious nodules underwent further CT imaging. Two received a PET scan, and only one had a bronchoscopic biopsy. No participant received surgery due to a false positive screening.
Although no subject had an interim diagnosis of lung cancer, one non-small cell carcinoma (NSCLC) of advanced staged (stage 3B) was detected on incident, first year screening after baseline. The baseline screening CT scan of this patient was at the time not thought to be clinically significant, since the image showed mild hilar adenopathy typical of HIV patients, but the time of the first annual T1 screening, there was clear evidence of interval growth in this patient’s hilar mass. The patient elected not to have treatment and died 4 months after diagnosis. There were 18 other deaths; all due to causes other than lung cancer. Sixteen of these patients had known CD4 counts at the time of death with 40% (7/16) of the patients having a CD4<200cells/mm3. Of these seven participants, three died of pneumonia and respiratory failure, two of cancer (tonsillar and pancreatic), and one of encephalitis and renal failure, each.”
Incidental Findings
Of the 224 patients with T0 imaging, 189 participants (84%) had incidental abnormal intrathoracic findings other than suspicious pulmonary nodules. The most commonly observed intrathoracic abnormalities were emphysematous changes in 69 (37%), pneumonia in 69 (37%) and CT-evidence of coronary artery disease in 58 (31%) participants (Supplemental Table 4). Extrathoracic disease was evident in 40% of patients with the majority being renal and hepatic abnormalities. There was a low prevalence of incidental findings that prompted further investigations in the chest and abdomen occurring in 1% and 7%, respectively. Moreover, no extrapulmonary lesions suspicious for cancer were identified.
CT Densitometry
Given the high rate of emphysema detected incidentally and emphysema’s high predictive value for lung cancer, we performed CT densitometry analyses of HIV-infected subjects with and without lung cancer. Of the 224 HIV-infected subjects with baseline CT scans, 117 (all without lung cancer) had scans suitable for densitometry. Densitometry analyses of the 117 scans from the CT screening study were compared to 39 scans from HIV-infected patients with known lung cancer diagnosed at our institution. These two groups were dissimilar in age, smoking, the use of azidothymidine and pulmonary function tests (Supplemental Table 5).
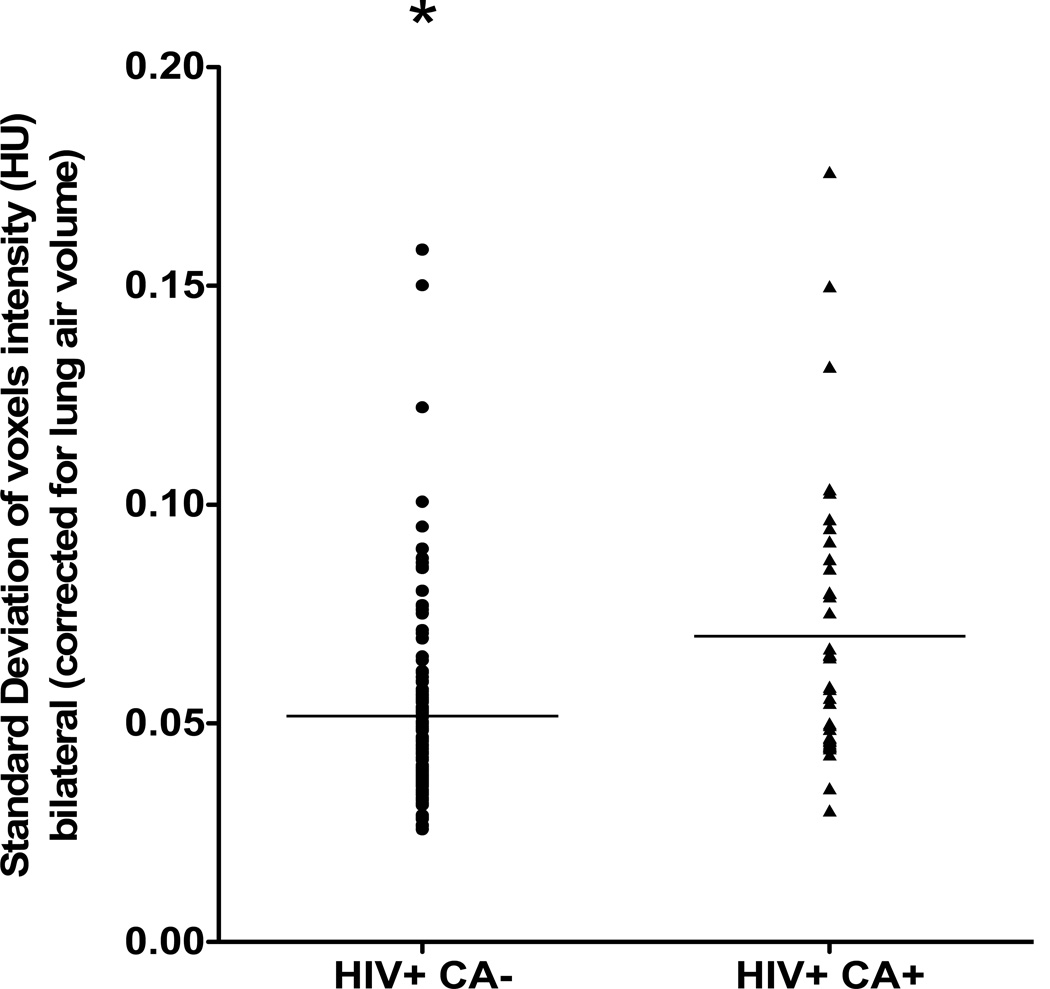
In order to assess the degree of heterogeneity of bilateral emphysematous changes.in these patients, we measured the variability in voxel intensity across both lung fields in those with and without cancer. The standard deviation of voxel intensity, corrected for lung air volume, was significantly higher in those HIV subjects with lung cancer versus those without lung cancer (p=0.0001, Fig 2).
Fig 2.
Heterogeneity of emphysema of 39 HIV-infected patients with lung cancer and 117 HIV-infected smokers without lung cancer as measured by the variability (standard deviation) in voxel intensities, corrected for lung air volume. The standard deviation was significantly higher in those HIV subjects with lung cancer versus those without lung cancer (p=0.0001).
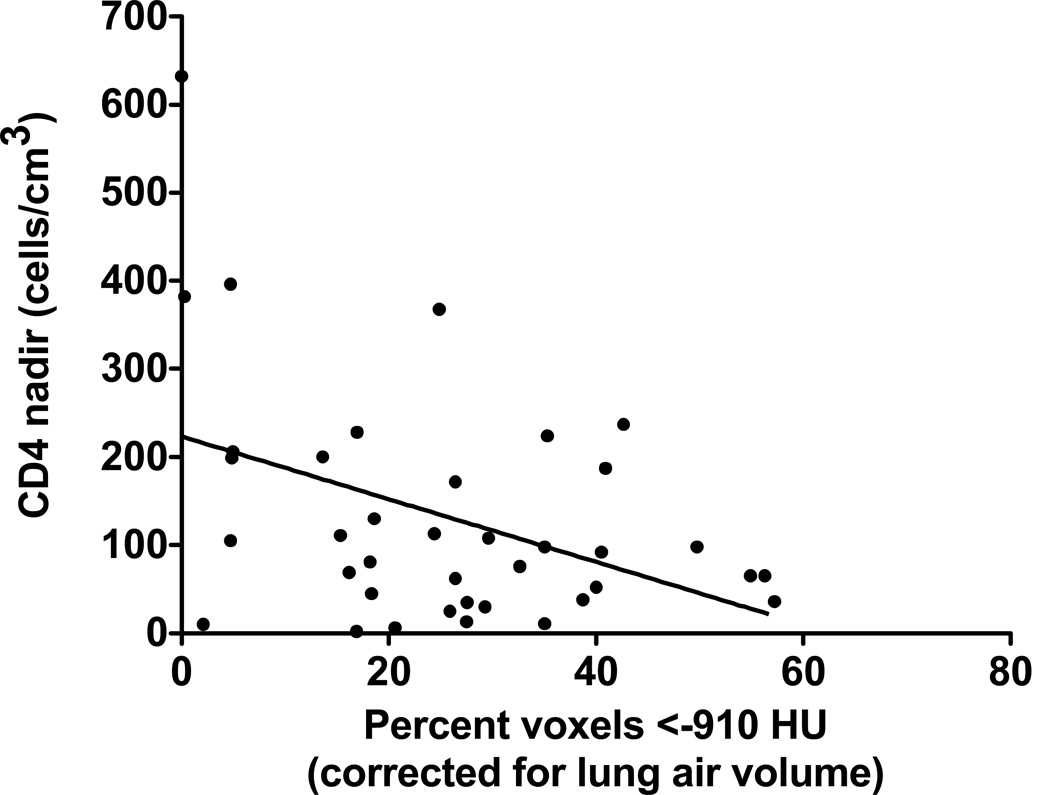
Since decreased innate immunity has been associated with emphysema both preclinically and clinically[28, 29], we investigated whether there were differences in the association of nadir CD4 counts and CT densitometry changes in HIV-infected subjects with and without lung cancer. With lower nadir CD4 counts in HIV subjects with lung cancer, there was a significant increase in emphysematous changes by CT densitometric scoring (p<0.001, Fig 3). This inverse correlation was not observed in HIV subjects without lung cancer (p=0.25). Moreover, since only one HIV patient with lung cancer out of 39 patients had a nadir CD4 count above 400 cells/mm3, the threshold of nadir CD4 may represent a clinical biomarker to identify HIV smokers at increased risk for lung cancer.
Fig 3.
Inverse correlation between CD4 counts in cells/mm3 and the percentage of voxels with attenuation less than −910HU (corrected for lung air volume) in 38 HIV-infected patients with lung cancer. Only one HIV-infected individual with lung cancer had a CD4 count >400 cells/mm3.
Using our 117 HIV-positive patients from our screening cohort and our 39 known HIV-positive lung cancer patients, we performed a multivariate logistic regression assessing clinical and radiographic risk factors associated with lung cancer in HIV-infected patients. Increased age, higher pack-years of cigarette smoking, low CD4 count nadir, and increased heterogeneity of emphysema on CT imaging were all significantly associated with lung cancer in HIV patients (Table 2).
Table 2 .
Adjusted Odds Ratio in 140 HIV individuals (117 HIV-positive Smokers and 39 HIV-positive lung cancer patients) of Having Lung Cancer based on Clinical and Radiographic Characteristics.*
| Odds Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|
| Clinical Characteristics | |||
| Increasing Agei | 1.08 | 1.01–1.15 | 0.02 |
| Increasing Pack-yearsii | 1.09 | 1.04–1.15 | <0.0001 |
| Decreasing CD4 Nadiriii | 1.006 | 1.002– 1.01 | 0.006 |
| Increased SD/TLViv | 1.23 | 1.03–1.47 | 0.02 |
Logistic regression model includes subject,
age in years,
pack-years cigarette smoking history,
CD4 nadir counts (continuous),
SD/TLV (Standard deviation of voxel intensities corrected by total bilateral lung air volumes), and the percentage of voxels less than −910HU corrected by total lung volume.
Discussion
This observational study is the first to evaluate CT screening for lung cancer in HIV smokers. Despite 89% of our cohort being active smokers and the epidemiological evidence of a two-fold increase in lung cancer incidence in HIV-infected versus non-HIV- infected individuals[1–9, 30–34], we found only one incident cancer over 678 patient-years using up to five annual CT screenings. Even with the limitation of a small sample size, this is a low rate of lung cancer detection despite our appropriate targeting of a community, epidemiologically, at high risk for lung cancer[35–41]. Although selection bias in recruiting “healthier” HIV smokers is a remote possibility, the more plausible explanation for this low detection rate is the cohort’s young median age of 48 years. The normal, Gaussian age distribution shows that this recruitment around a median age of 48 years old most likely reflects the range of ages of HIV-positive patients who sought care at our outpatient clinics and does not suggest selection bias in recruitment. We mistakenly hypothesized that the low immunosurveillance associated with HIV infection would be the most powerful risk factor for lung cancer in our cohort, and underestimated the significant contribution of advanced age as a risk factor. In 2003, when our trial was initially designed, the median patient age of all HIV-positive patients in our HIV outpatient clinic was 42.4 years. In 2013, with the widespread use of ART, the median age of all outpatient HIV-positive patients has risen to 52.9 years.. Indeed, in most CT screening trials involving non-HIV subjects, 55 years old is the minimum age of eligibility for study participation, and in the original ELCAP CT screening study published in 1999 by Henschke et al. which showed a 2.7% prevalent lung cancer detection rate, the one thousand participants had a median age of 67 years[36]. Prospective cohort studies have also shown a strong association between advanced age and increased risk of lung cancer in HIV-infected subjects[42]. The low rate of HIV-infected lung cancer in this trial supports the null hypothesis, and the importance of advanced age to lung cancer incidence, even in communities at high risk for lung cancer. As more CT screening programs increasingly develop algorithms to target high risk populations of smokers, our negative study suggests that the contribution of advanced age as a significant risk factor should not be ignored.
We found that non-calcified nodules ≥4mm in diameter were common in smokers who were HIV-positive, with the majority of them (38/48) interpreted as non-suspicious by our radiologists due to active infection such as tuberculosis and pneumonia, scarring from previous infection, or granulomatous disease. Only 10/48 nodules were thought to be suspicious by the radiologists and all participants with these nodules returned for subsequent imaging. These subsequent images all showed definitively that the lesions were not malignant, sometimes even showing nodule regression.
Over 80% of the screened cohort had additional intrathoracic CT abnormalities, including a third with diffuse coronary artery disease, a finding noted previously[43]. Similar to CT screening trials in non-HIV patients, however, incidental abnormalities requiring further diagnostic work-up were few[44]. Such a high rate of additional intrathoracic findings is an interesting observation as it begs the question whether abnormal CT changes in a HIV-positive and HIV-negative patient require similar follow-up, or whether HIV-positive patients on ART have medication induced changes to the lung and other organs that are new entities that may raise the false-positive rate, and cause potential harm to the patient if aggressively pursued.
But, the most frequently observed intrathoracic abnormalities were emphysematous changes to the lung parenchyma. We endeavored to quantify these emphysematous abnormalities to assess whether they may prove to differentiate an even higher risk subpopulation of HIV smokers. We did this by comparing 117 participants from our CT screening study with 39 HIV-positive patients with CT scans who had previously been diagnosed at Johns Hopkins. There is a suggested association between bullous disease and cannabis usage[45], but the causal link is not established[46]. In this study, however, there was no difference in the cannabis usage between the 117 participants and the 39 HIV-positive lung cancer patients. Interestingly, there was a significant correlation between decreasing nadir CD4 counts and increasing degrees of CT-determined emphysema in those with lung cancer. Since only one HIV patient with lung cancer had a nadir CD4 count >400 cells/mm3, our data suggest that a certain threshold of nadir CD4 counts for CT screening eligibility may target those HIV smokers at particularly high risk for emphysema mediated CT densitometry changes. This could also be explained, however, by the possibility that our lung cancer patients with HIV, who were older and smoked more, may have presented for care later from an immune standpoint, with delayed use of ART. However, an increasingly robust literature suggests the significant dose-response relationship of decreasing HIV-induced immunity, often measured by CD4 counts, and the increasing risk of non-AIDS defining malignancies[47–49]. Finally, our multivariate analysis, between the 39 patients with lung cancer previously diagnosed at Johns Hopkins and those 117 patients without the disease from our screening study, also identified low CD4 nadir as a risk factor for lung cancer, along with increased age, higher pack-years cigarette smoking, and an enhanced heterogeneous pattern of emphysema on CT scanning. Injury and inflammation are known to be pivotal in the non-uniformity of emphysema in the lung[50], and a dysfunctional immune response in HIV subjects may have accentuated the upper lobe predominant emphysema observed in HIV subjects.
Despite patient navigators and remuneration for continued participation, this study is limited by few eligible subjects returning for all five scans. Longitudinal engagement in regular HIV care in U.S. urban settings also is a limitation to effective antiretroviral treatment[51]. A recent report of 22,984 adult HIV outpatients receiving care in the U.S. between 2001 to 2009 indicated that only 20.4% of HIV outpatients were retained as patients on a continual basis without interruption or loss to follow-up[52]. Urban HIV cohorts with a high prevalence of polysubstance abuse are especially vulnerable to poor compliance and follow-up rates[53, 54]. Many of our participants returned for a final CT screening at study’s end with over 70% of eligible HIV subjects completing at least a baseline and final CT scan. This suggests that only 14% of our original cohort were truly lost to follow-up, and the majority of participants were grossly non-compliant.
Since only one lung cancer was detected, we were unable to investigate the secondary endpoint concerning whether CT screening changes the stage distribution of NSCLC in screened HIV patients versus historic controls. The NLST suggests that stage distribution may change in non-HIV individuals, but the aggressiveness of NSCLC in the HIV-infected patient makes this an open question.
Given the results of this pilot screening study, considerable thought must be given concerning the execution of any large screening study in this high-risk population especially given the many other factors that could make a case against lung cancer screening in such persons; such as “over diagnosis bias[55]”, competing mortality over a course of screening, more aggressive cancer types, faster interval progression of cancers, and the personal anxiety, financial burden as well as the morbidity due to the work-up of false positive tests,. At the very minimum, we believe that until the median age of HIV smokers increases, the rate of detection by helical CT of HIV-associated lung cancers will remain low. Advanced age and length of exposure to cigarette smoking are strong risk factors for lung cancer, and most CT screening studies use age above 55 years as an important eligibility criterion. Our identification of biologic and radiographic markers in HIV smokers to define an even higher subpopulation of high-risk individuals may allow algorithms to determine lung cancer risk more effectively, individualize the frequency of subsequent scans, reduce false positives, and limit the costs of future lung cancer screening trials. Given the high rate of active smokers in a HIV community and the epidemiological data, as the median age of HIV-infected individuals surpasses 55 years in the U.S., perhaps a far larger study enrolling older HIV-positive smokers may answer some of the initial questions we raise here. However, for such a study to be feasible in an urban American HIV cohort plagued by polysubstance abuse, considerable measures to ensure patient compliance, adherence, and smoking cessation must ensue.
Supplementary Material
Acknowledgements
Catherine DeAngelis M.D., M.P.H for comments on study design, Allison Brooker and Genevieve Pridham for technical assistance, Nimisha Thuluvath, Sue Lee, and Eli Luong for patient accrual and follow-up, Mary Missouri for administrative guidance at the Johns Hopkins General Clinical Research Clinic.
Supported in part by the NIH Grants 5K23CA117820-01, CAP50 CA058184, P30-AI42855, 5M01 RR002719-21, R01DA04334 and R01 HL090483
Footnotes
Trial Registration: Screening for Lung Cancer in the HIV Patient. NCT01748136 http://clinicaltrials.gov/show/NCT01748136
REFERENCES
- 1.Bower M, et al. HIV-related lung cancer in the era of highly active antiretroviral therapy. Aids. 2003;17(3):371–375. doi: 10.1097/00002030-200302140-00011. [DOI] [PubMed] [Google Scholar]
- 2.Clifford GM, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 3.Frisch M, et al. Association of cancer with AIDS-related immunosuppression in adults. Jama. 2001;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 4.Parker MS, et al. AIDS-related bronchogenic carcinoma: fact or fiction? Chest. 1998;113(1):154–161. doi: 10.1378/chest.113.1.154. [DOI] [PubMed] [Google Scholar]
- 5.Serraino D, et al. Cancer incidence in a cohort of human immunodeficiency virus seroconverters. HIV Italian Seroconversion Study GrouCancer. 1997;79(5):1004–1008. doi: 10.1002/(sici)1097-0142(19970301)79:5<1004::aid-cncr17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24(9):1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 7.Engels EA, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 8.Patel P, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kirk GD, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaberg EC, et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 116(23):5507–5516. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadranel J, et al. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax. 2006;61(11):1000–1008. doi: 10.1136/thx.2005.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels EA, et al. Elevated incidence of lung cancer among HIV-infected individuals. Journal Of Clinical Oncology: Official Journal Of The American Society Of Clinical Oncology. 2006;24(9):1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 13.Shiels MS, et al. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr. 55(4):510–515. doi: 10.1097/QAI.0b013e3181f53783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk GD, Merlo CA. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 8(3):326–332. doi: 10.1513/pats.201009-061WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brock MV, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43(1):47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 16.Hooker CM, et al. Human immunodeficiency virus infection as a prognostic factor in surgical patients with non-small cell lung cancer. Ann Thorac Surg. 93(2):405–412. doi: 10.1016/j.athoracsur.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazot M, et al. Computed tomographic diagnosis of bronchogenic carcinoma in HIV-infected patients. Lung Cancer (Amsterdam, Netherlands) 2000;28(3):203–209. doi: 10.1016/s0169-5002(99)00124-5. [DOI] [PubMed] [Google Scholar]
- 19.Friedman PJ. Imaging studies in emphysema. Proceedings Of The American Thoracic Society. 2008;5(4):494–500. doi: 10.1513/pats.200708-128ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brock MV, et al. Prognostic importance of promoter hypermethylation of multiple genes in esophageal adenocarcinoma. Clin Cancer Res. 2003;9(8):2912–2919. [PubMed] [Google Scholar]
- 21.deTorres JP, et al. Assessing the Relationship Between Lung Cancer Risk and Emphysema Detected on Low-Dose CT of the Chest. Chest. 2007;132(6):1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado F, et al. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 138(6):1295–1302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 23.Henschke CI, et al. Survival of patients with stage I lung cancer detected on CT screening. The New England Journal Of Medicine. 2006;355(17):1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 24.Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishman JE, et al. Bronchogenic carcinoma in HIV-positive patients: findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1995;164(1):57–61. doi: 10.2214/ajr.164.1.7998569. [DOI] [PubMed] [Google Scholar]
- 26.Henschke CI, et al. Guidelines for the use of spiral computed tomography in screening for lung cancer. Eur Respir J Suppl. 2003;39:45s–51s. doi: 10.1183/09031936.03.00405103. [DOI] [PubMed] [Google Scholar]
- 27. NCCN.org. NCCN Guidelines for the use of spiral computed tomography in screening for lung cancer. NCCN Clinical Practice Guidelines in Oncology. 2014
- 28.Zhang X, et al. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116(11):3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SW, et al. The association of down-regulated toll-like receptor 4 expression with airflow limitation and emphysema in smokers. Respir Res. 2012;13(1):106. doi: 10.1186/1465-9921-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allardice GM, et al. Incidence of malignant neoplasms among HIV-infected persons in Scotland. Br J Cancer. 2003;89(3):505–507. doi: 10.1038/sj.bjc.6601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dal Maso L, et al. Lung cancer in persons with AIDS in Italy, 1985–1998. Aids. 2003;17(14):2117–2119. doi: 10.1097/01.aids.0000088179.01779.fd. [DOI] [PubMed] [Google Scholar]
- 32.Grulich AE, et al. Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. Aids. 2002;16(8):1155–1161. doi: 10.1097/00002030-200205240-00009. [DOI] [PubMed] [Google Scholar]
- 33.Herida M, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21(18):3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 34.Seaberg EC, et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010;116(23):5507–5516. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swensen SJ, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165(4):508–513. doi: 10.1164/ajrccm.165.4.2107006. [DOI] [PubMed] [Google Scholar]
- 36.Henschke CI, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 37.Nawa T, et al. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest. 2002;122(1):15–20. doi: 10.1378/chest.122.1.15. [DOI] [PubMed] [Google Scholar]
- 38.Sobue T, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol. 2002;20(4):911–920. doi: 10.1200/JCO.2002.20.4.911. [DOI] [PubMed] [Google Scholar]
- 39.Sone S, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84(1):25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Infante M, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180(5):445–453. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 41.Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guiguet M, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 43.Lai S, et al. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165(6):690–695. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs PC, et al. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. J Comput Assist Tomogr. 2008;32(2):214–221. doi: 10.1097/RCT.0b013e3181585ff2. [DOI] [PubMed] [Google Scholar]
- 45.Gill A. Bong lung: regular smokers of cannabis show relatively distinctive histologic changes that predispose to pneumothorax. Am J Surg Pathol. 2005;29(7):980–982. doi: 10.1097/01.pas.0000157998.68800.cb. [DOI] [PubMed] [Google Scholar]
- 46.Tan C, Hatam N, Treasure T. Bullous disease of the lung and cannabis smoking: insufficient evidence for a causative link. J R Soc Med. 2006;99(2):77–80. doi: 10.1258/jrsm.99.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reekie J, Mocroft A, Engsig F. 16th Conference on retrovirus and opportunistic infections. Montreal: 2009. Relationship between current level of Immunodeficiency and non-AIDS defining malignancies. Abstract 860a. [Google Scholar]
- 48.Monforte A, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22(16):2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marin B, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23(13):1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuder RM, et al. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc. 2006;3(8):673–679. doi: 10.1513/pats.200605-124SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westergaard RP, et al. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS. 2013 doi: 10.1097/QAD.0b013e328363bff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleishman JA, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60(3):249–259. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arici C, et al. Factors associated with the failure of HIV-positive persons to return for scheduled medical visits. HIV Clin Trials. 2002;3(1):52–57. doi: 10.1310/2XAK-VBT8-9NU9-6VAK. [DOI] [PubMed] [Google Scholar]
- 54.Giordano TP, et al. Patients referred to an urban HIV clinic frequently fail to establish care: factors predicting failure. AIDS Care. 2005;17(6):773–783. doi: 10.1080/09540120412331336652. [DOI] [PubMed] [Google Scholar]
- 55.Patz EF, Jr, et al. Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.