Summary
In bacteria, translation-transcription coupling inhibits RNA polymerase (RNAP) stalling. We present evidence suggesting that, upon amino acid starvation, inactive ribosomes promote rather than inhibit RNAP stalling. We developed an algorithm to evaluate genome-wide polymerase progression independently of local noise, and used it to reveal that the transcription factor DksA inhibits promoter-proximal pausing and increases RNAP elongation when uncoupled from translation by depletion of charged tRNAs. DksA has minimal effect on RNAP elongation in vitro and on untranslated RNAs in vivo. In these cases, transcripts can form RNA structures that prevent backtracking. Thus, the effect of DksA on transcript elongation may occur primarily upon ribosome slowing/stalling or at promoter-proximal locations that limit the potential for RNA structure. We propose that inactive ribosomes prevent formation of backtrack-blocking mRNA structures and that, in this circumstance, DksA acts as a transcription elongation factor in vivo.
Keywords: amino acid starvation, genome-wide ChIP-analysis, replication-transcription conflict, transcription elongation, transcription-translation coupling
Introduction
Transcription, translation, and replication occur concurrently on shared DNA/RNA substrates in actively growing bacterial cells. Environmental changes profoundly affect these central dogma processes, making their coordination essential to prevent potential conflicts and to ensure cellular adaptation.
The transcription elongation complex (EC; composed of RNA Polymerase β′βα2ω core subunits, DNA template, and nascent RNA) is the nexus of these processes. To allow coordination, the EC pauses frequently at specific sequences (Ring et al., 1996; Nickels et al., 2004; Chan and Landick, 1989; Kireeva and Kashlev, 2009), upon encountering protein roadblocks (He and Zalkin, 1992), and at DNA lesions (Tornaletti and Hanawalt, 1999). The paused ECs have a strong tendency to reverse translocate RNA and DNA (called backtracking). Extensive backtracking produces arrested ECs that require internal cleavage of the nascent RNA to continue transcription (Nudler et al., 1997; Komissarova and Kashlev, 1997; Shaevitz et al., 2003), and may form barriers to DNA replication that decrease cell viability. The transcription cleavage factors GreA/B, which reactivate arrested ECs (Toulme et al., 2000; Marr and Roberts, 2000), and Rho, which terminates both intragenic and stable RNA transcription (Peters et al., 2009; Peters et al., 2012), help relieve replication-transcription conflicts in vivo (Trautinger et al., 2005; Tehranchi et al., 2010; Washburn and Gottesman, 2011; Dutta et al., 2011) and in vitro (Pomerantz and O'Donnell, 2010).
In addition to GreA/B and Rho, the transcription factor DksA has been implicated in preventing transcription-replication conflicts. DksA protects cells against UV damage (Trautinger et al., 2005), and prevents replication arrest in amino acid starved cells via effects on transcription elongation (Tehranchi et al., 2010). However, DksA is principally known for regulating transcription initiation synergistically with ppGpp through contacts in the RNA polymerase (RNAP) secondary channel (Paul et al., 2004; Lennon et al., 2012) and has only limited effects on transcription elongation in vitro (Furman et al., 2013). DksA binds only weakly to ECs in vitro, and for the minority of ECs affected by DksA in vitro the effects detected can be either inhibitory or stimulatory (Furman et al., 2012; Perederina et al., 2004). This discrepancy between minimal DksA effects on ECs in vitro and DksA suppression of replication-transcription conflicts in vivo is paradoxical. What conditions in vivo account for the strong effects of DksA on ECs? One obvious difference is the presence of nascent RNA-bound ribosomes and coupled transcription-translation in vivo.
Coupling of transcription and translation is maintained both by EC pausing to await for a ribosome to catch up with RNAP (Landick et al., 1985; Proshkin et al., 2010) and by the transcription factor NusG, which binds RNAP through its N-terminal domain and ribosomes through its C-terminal domain (Burmann et al., 2010). After uncoupling of translation and transcription, arrested ECs formed by RNAP backtracking are proposed to block DNA replication and cause double-stranded breaks (Dutta et al., 2011). Amino acid starvation, which stalls translation, induced a massive and disruptive arrest of DNA replication in the absence of DksA (Tehranchi et al., 2010). Translation stalling-triggered formation of arrested ECs was proposed to block replication fork progression in this circumstance. Thus, amino acid starvation may indirectly affect genome integrity via a chain reaction involving the three major information-transfer processes in the cell, by first impacting translation, then transcription, and finally replication (Fig 1A).
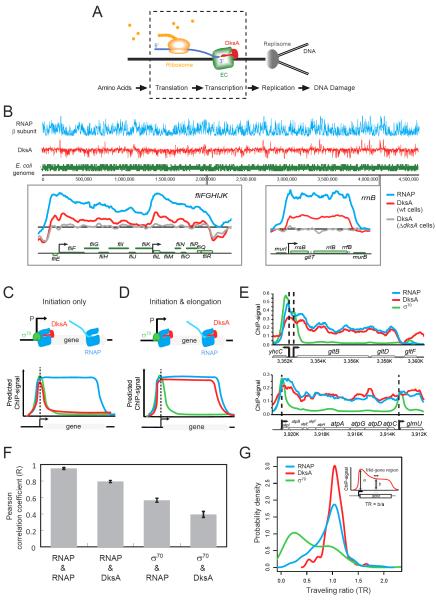
Figure 1. Genome-wide co-localization of DksA with RNAP but not with σ70.
(A) Schematics showing a chain reaction involving translation, transcription and replication. Upon amino acid starvation, which inhibits translation, DksA guards the elongation complex (EC) from blocking DNA replication and consequent DNA damage. The processes enclosed by dashed lines are the focus of this work. (B) ChIP-chip profiles of RNAP β subunit (blue) and DksA (red) from wild-type cells are shown above a plot of genes across the E. coli genome (green). Two expanded regions are shown for RNAP (blue) and DksA (red) signals in wild-type cells and DksA ChIP signals in ΔdksA cells (grey). For this and all subsequent figures, ChIP signals were normalized as log2(IP/input). (C, D, E) Schematic of how ChIP signals reflect the traffic patterns of DksA (red), σ70 (green) and RNAP (blue) if DksA acts during initiation alone (C) or also during elongation (D), and experimental results (E). (F) Pearson correlation coefficient (R) of ChIP signals across the genome between pairs of indicated proteins. Error bars: standard error of the mean (n=3). For all R values, p<2.2e-16. For this and (G), DksA ChIP signals were DksA signals in wild-type cells subtracting signals in ΔdksA cells. (G) Distributions of traveling ratios (TR) of DksA (red), σ70 (green) and RNAP (blue) among the 155 TUs with significant σ70 and DksA enrichments. Inset: TR was defined as the ratio of the mid-gene signal (b) to the promoter-proximal signal (a), averaged over 100bp windows.
To test this idea and to resolve the discrepancy between effects of DksA on transcription elongation in vitro and in vivo, we determined the genome-wide distribution pattern of DksA through its association with RNAP. We also developed a sensitive and broadly applicable algorithm to quantify RNAP progression through transcription units in vivo. Using this algorithm, we determined the effect of DksA on in vivo transcript elongation by comparing wild-type and ΔdksA cells in normal growth conditions and upon amino acid starvation, which uncouples transcription and translation. DksA induced a general but modest amelioration of promoter-proximal stalling in the absence of starvation. A greater effect of DksA on RNAP progression in protein-coding genes was observed after transcription-translation uncoupling, and together with the association of DksA with ECs in vivo, establish that DksA is a bona fide elongation factor (in addition to its role in initiation) and suggest a mechanistic basis for the greater effects of DksA on transcript elongation in vivo than in vitro. In this model, the mRNA-bound ribosome becomes a strong backtrack-promoting factor upon transcription-translation uncoupling because it inhibits mRNA folding, and DksA guards against EC arrest by inhibiting backtracking.
Results
Genome-wide trafficking of DksA during transcription elongation
To obtain the trafficking pattern of the transcription factor DksA with RNAP throughout the genome, we applied ChIP-chip to exponentially growing E. coli K-12 wild-type (MG1655) cells using polyclonal antibodies against DksA. Although DksA does not directly associate with DNA (Perederina et al., 2004), it can be cross-linked to DNA via its association with RNAP. The specificity of DksA ChIP signals was confirmed by probing ΔdksA cells with an anti-DksA antibody. Comparison of DksA ChIP signals in ΔdksA and wild-type cells revealed obvious enrichments, unambiguously attributable to DksA interaction with RNAP, at a wide range of genes. The DksA associated-genes include those known to be regulated by DksA at the level of transcription initiation, such as ribosomal RNA operons (Paul et al., 2004) and flagella biosynthesis genes (Aberg et al., 2009) (Fig 1B).
Interestingly, we found that DksA was not only enriched at promoter regions but also across entire transcription units. We examined whether DksA is generally enriched beyond promoters by probing the same cross-linked samples with antibodies against the RNAP β subunit and the housekeeping σ factor σ70, and compared their enrichment patterns with that of DksA. If DksA associates with RNAP only during transcription initiation, the DksA ChIP signal should follow the pattern of the σ70 factor, which predominantly localizes to promoter regions (Fig 1C). If DksA indeed also binds to RNAP during elongation, the DksA ChIP signal should mimic that of RNAP (Fig 1D). Examination of the profiles at highly transcribed operons, e.g. gltBDF and atpIBEFHAGDC, revealed that DksA was enriched both at promoters and in downstream regions, similar to RNAP (Fig 1E). As expected for a regulator that decreases the stability of initiation complexes (Paul et al., 2004; Rutherford et al., 2009), the DksA signal was relatively less enriched at promoter regions compared to intragenic regions.
We quantified the correlation between RNAP, σ70, and DksA ChIP signals at all probes (N=378,238) across the genome. As expected, two biological replicates of RNAP ChIP signals were highly correlated (R=0.95), whereas RNAP signals were only modestly correlated with σ70 signals (R= 0.57) (Fig 1F). DksA signals were highly correlated with RNAP signals (R=0.79) but poorly correlated with σ70 signals (R=0.39) (Fig 1F), indicating that the co-localization of DksA and RNAP is a genome-wide feature.
To confirm the intragenic enrichment of DksA, we calculated its traveling ratio (the ratio of the ChIP signal in the mid-region to that in the beginning of a gene), modified from (Reppas et al., 2006; Mooney et al., 2009), to quantify its progression into genes (Fig 1G). Traveling ratios of σ70 were significantly lower than that of RNAP, reflecting the predominant association of σ70 with promoters. Similar to RNAP, DksA had higher traveling ratios, confirming that DksA associates with RNAP during transcription elongation in vivo.
DksA modestly lessens promoter-proximal RNAP stalling/termination in unstarved cells
Next, we directly characterized the effect of DksA on transcription elongation in vivo by comparing the ChIP signals of RNAP in the presence and absence of DksA. We observed modestly increased promoter-proximal enrichment of RNAP ChIP signals at many genes in ΔdksA cells compared to wild-type cells (e.g., Fig 2A), indicating transcription stalling during early elongation or premature termination (Reppas et al., 2006; Mooney et al., 2009) (Fig S1A). To quantify the extent of genome-wide promoter-proximal stalling (or termination), we selected 432 high quality transcription units (TUs) with significant RNAP ChIP signals (Fig S1B, Table S1) and calculated their RNAP traveling ratios (TR) (Fig S1C). We found that TRs of the majority of genes are lower in ΔdksA cells, confirming a genome-wide trend of stronger promoter-proximal stalling (Fig S1D).
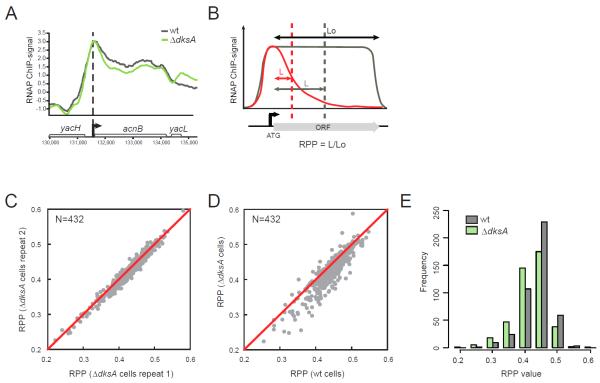
Figure 2. DksA aids genome-wide transcription elongation in vivo.
(A) An example of intragenic RNAP ChIP signals in wild-type (grey) and ΔdksA (green) cells. (B) A schematic of calculating Relative Polymerase Progression (RPP). Dashed lines: the average positions of RNAP across a population of cells, calculated from RNAP ChIP signals over the Lo region. L: the average RNAP progression. RPP equals L/Lo. A lower RPP value (red) indicates more RNAP stalling/termination compared to uninterrupted transcription elongation (grey). (C) Scatter plot comparing the RPP values of 432 selected TUs in ΔdksA cells from two independent replicates. Pearson correlation coefficient R=0.98. (D) Scatter plot comparing RPP values of 432 selected TUs in wild-type and ΔdksA cells. RPP values are averaged from two independent replicates. (E) Histograms of RPP values in wild-type and ΔdksA cells (N=432). See also Fig S1 and Table S1.
TR samples small regions of RNAP signals and has relatively poor sensitivity for detecting RNAP progression changes across the entire gene. Therefore, we developed a new measurement, Relative Polymerase Progression (RPP), to quantify in vivo RNAP processivity robustly. RPP is the median of the RNAP distribution divided by the length of the gene (Fig. 2B) and was calculated by taking advantage of the fact that RNAP ChIP signals reflect the probability densities of RNAP among a population of cells, thereby maximizing the sampling of RNAP distributions (Experimental Procedures, Fig 2B, S1C). Thus an RPP of 0.5 indicates transcription elongation throughout the gene with no loss of RNAP via premature stalling or termination, whereas stalling or termination of RNAP would result in smaller RPP values.
We verified that RPP values of the 432 TUs were highly reproducible between biological replicates (R=0.98) (Fig 2C). In contrast, the RPP values between wild-type and ΔdksA cells were different. Most TUs (356/432; ~82%) had lower RPP values in ΔdksA cells than in wild-type cells (Fig 2D; mean of the differences=0.017, p<2.2e-16, paired t-test), and the distributions of RPP values were also significantly different (Fig 2E; D=0.183, p=1.1e-06, K-S (Kolmogorov-Smirnov) test), indicating modestly increased RNAP stalling or termination in the absence of DksA.
Amino acid starvation strongly promotes RNAP stalling within genes
In unstarved cells, coupling of transcription to translation minimizes pausing and premature stalling/termination of transcription (Landick et al., 1985; Proshkin et al., 2010), and thus could reduce effects of DksA on transcript elongation. Therefore, we characterized the effect of amino acid starvation, which stalls translation, on the genome-wide RNAP distribution. Cells were grown to mid-exponential phase and treated with the nonfunctional serine analog serine hydroxamate (SHX), a standard treatment of amino acid starvation (Durfee et al., 2008; Tehranchi et al., 2010), to deplete charged tRNASer, and RNAP ChIP signals were compared with untreated samples (Fig 3A). RNAP signals exhibited enrichment patterns largely in agreement with known effects of DksA on transcription, e.g., decreasing transcription of rRNA operons upon starvation in wild-type cells but not ΔdksA cells (Lemke et al., 2011) (data not shown). Interestingly, we observed strong enrichment of RNAP at the SOS regulon (e.g., umuD in Fig 3B) in starved ΔdksA cells, but not in wild-type cells, agreeing with our prior observation that the SOS response was strongly induced by SHX treatment of ΔdksA cells (Tehranchi et al., 2010).
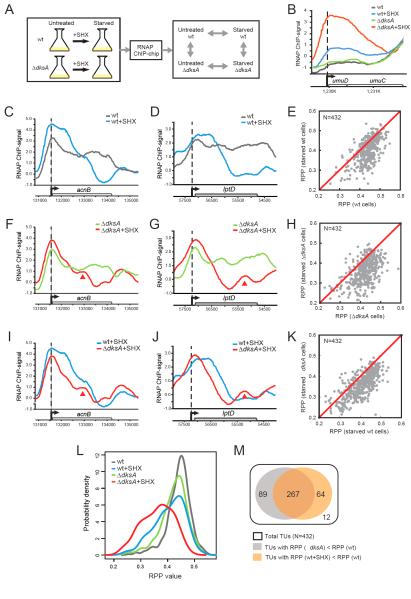
Figure 3. Effect of amino acid starvation on transcription elongation.
(A) RNAP ChIP signals were compared between samples in the presence or absence of DksA, with or without SHX to deplete charged tRNAser. (B) RNAP ChIP signals on a representative SOS operon (umuDC) in untreated (grey) and starved (blue) wild-type cells, untreated (green) and starved (red) ΔdksA cells. (C-K) RNAP ChIP signals were compared between indicated samples. Examples were shown for acnB (C, F, I) and lptD (D, G, J) genes. Red arrows: intragenic RNAP peaks in starved ΔdksA cells. Scatter plots comparing RPPs of 432 TUs between indicated samples were shown in (E, H, K). RPP values were averaged from two independent replicates. (L) Distributions of RPP values in untreated (grey) and starved (blue) wild-type cells, and untreated (green) and starved (red) ΔdksA cells. (M) Venn diagram showing the effective spectrum of ΔdksA and starvation on transcription elongation. White: 432 total TUs; grey: 356 TUs with lower RPPs in ΔdksA than in wild-type cells; orange: 331 TUs with lower RPPs in starved than untreated wild-type cells. See also Fig S2 and Table S2.
Although the genome-wide effects of SHX treatment on general transcription have been shown previously (Durfee et al., 2008; Tehranchi et al., 2010), its effects on transcription elongation have not been examined. We observed strongly elevated transcription stalling or termination upon SHX-treatment in both wild-type and ΔdksA cells (Fig 3C-H). Upon starvation of wild-type cells (e.g., Fig 3C-D), 331 out of 432 (~77%) selected TUs exhibited lower RPP values (Fig 3E; mean of the differences=0.035, p<2.2e-16), reflected in RPP distributions (Fig 3L; D=0.296, p<2.2e-16). The reduction of RPP upon starvation was stronger in ΔdksA cells (e.g., Fig 3F-G), where 376 out of 432 (~87%) TUs had lower RPP values upon starvation (Fig 3H; mean of the difference=0.062, p<2.2e-16), and the distribution of RPP values was strongly altered (Fig 3L; D=0.444, p<2.2e-16). Intriguingly, the difference is much subdued when Traveling Ratio, instead of RPP, was used as a proxy of RNAP processivity (Fig S1F-I), confirming that RPP is a more robust measurement for transcription stalling, and that SHX-induced transcription stalling takes place beyond promoter-proximal region.
DksA strongly prevents genome-wide transcription stalling when translation is inhibited
Direct comparison of starved ΔdksA and starved wild-type cells confirmed that DksA greatly aided RNAP progression upon starvation (e.g., Fig 3I-J). Most TUs (374/432; ~87%) exhibited lower RPP values in starved ΔdksA cells (Fig 3K; mean of the difference=0.044, p<2.2e-16). The distribution of RPP values was also significantly changed (Fig 3L; D=0.299, p<2.2e-16). In addition to increased transcription stalling/termination in starved ΔdksA cells, we also observed enriched intragenic RNAP peaks at distal sites from promoters (indicated by arrows in Fig 3F-G, 3I-J). This may reflect appearance of sites of slow transcript elongation, arrays of arrested ECs in the middle of TUs, or both (Fig S1A).
Although decrease in RPP values indicates altered transcription elongation in ΔdksA cells, it is conceivable that the change of RNAP occupancy at some promoters in ΔdksA cells (Paul et al., 2004; Paul et al., 2005; Lemke et al., 2009; Lemke et al., 2011) can indirectly affect transcription elongation and RPP values. To rule out the possibility that DksA affects RPP values only indirectly via altering transcription initiation, we examined a subset of TUs for which DksA does not affect initiation, i.e., for which σ 70 ChIP signals were similar between starved ΔdksA and wild-type cells (e.g., acnB gene, Fig S2A). A majority of TUs (278/432) passed this additional criterion (Table S2). Consistent with results obtained for all 432 TUs, we found lower RPP values in ΔdksA cells compared to wild-type cells for these 278 TUs, and further reduction of RPP values upon amino acid starvation (Fig S2B-D). This result strongly supports the hypothesis that DksA reduces transcription stalling independently of its effect on initiation.
DksA and amino acid starvation affected transcription elongation of a broad and overlapping spectrum of genes (Fig 3M). Nearly all selected TUs (420/432) exhibited elevated transcription stalling either in the absence of dksA or upon SHX treatment, of which 64% (267/420) TUs were affected by both DksA and SHX.
DksA protects viability synergistically with transcription-translation coupling
We found the effect of DksA on transcription stalling is restricted to protein-coding genes. The RPP values of untranslated genes (rRNA, tRNA and noncoding RNA) were not generally affected by loss of DksA, by amino acid starvation, or by both ΔdksA and starvation combined (Fig 4A-D). Thus, both promotion of RNAP stalling by amino-acid starvation and its suppression by DksA appear to require the presence of ribosomes on the nascent mRNA. Based on these observations, we propose that during translational stress induced by charged tRNA depletion, stalled ribosomes may enhance RNAP stalling, whereas DksA appears to guard against RNAP stalling in protein-coding genes on a genomic scale.
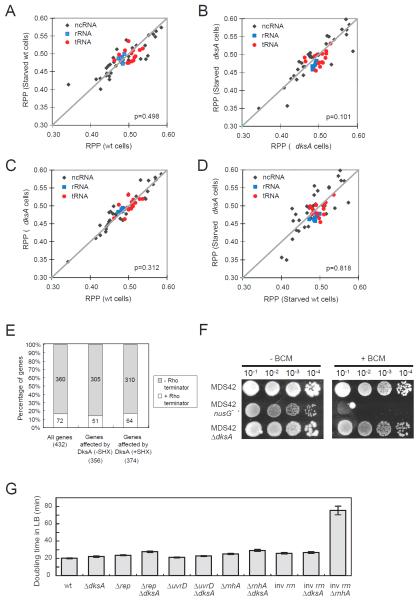
Figure 4. DksA does not affect transcription elongation of untranslated genes.
(A-D) Scatter plots comparing RPPs of 55 TUs that encode for non-coding RNAs (including rRNAs (N=7), tRNA (t=14) and other non-coding RNAs (N=34)) between indicated samples. RPP values were averaged from two independent replicates. p-value = 0.498 (A), 0.101 (B), 0.312 (C) and 0.818 (D), paired student’s t-test. (E) TUs in which DksA affects transcription elongation were not enriched with Rho-dependent terminators. (F) Sensitivity of ΔdksA cells and nusG mutants to Rho inhibitor bicyclomycin (BCM). MDS42-based cells were grown in LB liquid medium to mid-exponential phase; 5 μl of serially diluted culture was spotted on LB agar with 25 μg/ml BCM and incubated at 37°C for 2 days. See also Fig S3. (G) Doubling times of indicated strains grown in LB broth at 37°C for genetic interaction analysis. Error bars: standard error of the mean (n=3-4). See also Table S3.
As independent tests of the hypothesis that DksA targets ribosome-stalling-induced effects on transcription, we used chemogenetic and other genetic approaches to test the relationship of DksA action to translation and to several other pathways known or hypothesized to affect ECs at non-coding genes. First, we examined the transcription termination factor Rho, which plays crucial functions in dissociating ECs of antisense RNA and stable RNA (Peters et al., 2012; Peters et al., 2009) and also promotes genome integrity (Washburn and Gottesman, 2011). We found that genes where ECs were arrested in ΔdksA cells were not enriched with established Rho-dependent terminators (Fig 4E), suggesting that the lowered RPP values in ΔdksA cells were not due to Rho-dependent early termination. Furthermore, loss of DksA did not render cells more sensitive or resistant to a Rho-inhibitor bicyclomycin (BCM) (Zwiefka et al., 1993) (Fig 4F, S3). Thus DksA and Rho do not exhibit strong genetic interactions.
Second, inversion of rRNA operons (inv rrn) was found to block replication in the absence of several auxiliary helicases (encoded by rep, uvrD and dinG) or the R-loop-removing RNase HI (encoded by rnhA) (Boubakri et al., 2010). R-loops (RNA-DNA hybrids) that occur during transcription of rRNA are proposed to block replication and to induce DNA breaks (Gomez-Gonzalez et al., 2009). The absence of RNase H1 (ΔrnhA) caused a strong synthetic growth defect with inversion of rrnBE operons (Fig 4G), confirming a previous observation that R-loop formation and head-on transcription of rrn operons to replication result in synergistic effects on viability (Boubakri et al., 2010). However, the absence of dksA did not result in significant synergistic growth defects with the inversion of rrnBE operons, with rnhA, rep or uvrD mutants during exponential-phase cell growth in rich medium (Fig 4G, Table S3), confirming that DksA does not promote viability by regulating transcription elongation or termination of rRNA.
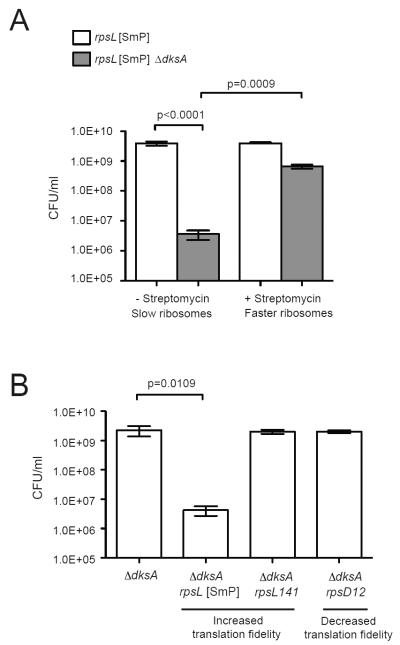
We found that the strongest genetic interaction occurred between dksA and the major mechanism for minimizing RNAP backtracking in protein-coding genes: transcription-translation coupling (Proshkin et al., 2010; Burmann et al., 2010). Deletion of dksA decreased the plating efficiency of an rpsL mutant strain (rpsL[SmP]) (Fig 5A) in which the translation rate is decreased (Siller et al., 2010; Ruusala and Kurland, 1984). The loss of viability was rescued by addition of streptomycin (Fig 5A), which restores the translation rate of the rpsL mutant to wild-type level and thus prevents RNAP backtracking by ribosome-assisted forward translocation of ECs (Dutta et al., 2011). Although rpsL[SmP] also affects translation fidelity, its effect on viability in the absence of dksA was unrelated to translation fidelity, as viability was not affected by rpsL141 and rpsD12 mutations (Fig 5B) with hyperaccurate or lower translation fidelity (ribosome ambiguity) phenotypes, respectively (Zaher and Green, 2010). These results support an indispensable function for DksA in facilitating transcription elongation during conditions that uncouple ribosome translocation from transcription, such as amino acid starvation.
Figure 5. DksA prevents loss of viability due to slow ribosome translocation.
(A) Plating efficiencies of rpsL[SmP] and rpsL[SmP] ΔdksA cells grown in the presence or absence of 50 μg/ml streptomycin. Error bar: standard deviation (n=4). (B) Plating efficiencies of ΔdksA cells carrying three different ribosomal mutations that alter translation accuracy. The rpsD12 mutant has increased translation errors and the rpsL[SmP] and rpsL141 mutations result in improved translational fidelity. Error bar: standard deviation (n=3).
Discussion
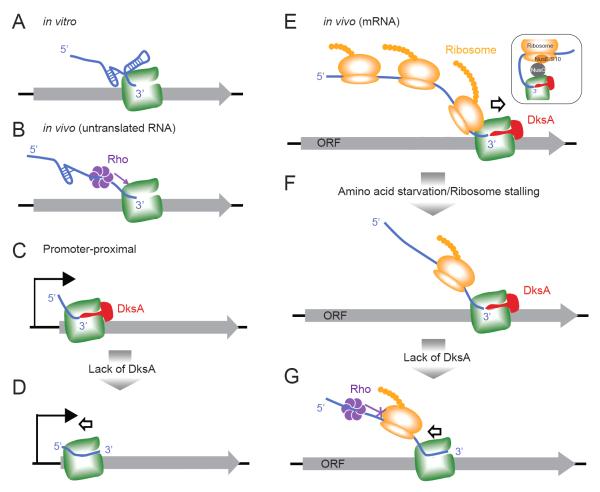
In this study, we provide genome-scale evidence that DksA guards transcription elongation against the deleterious effects of charged tRNA depletion or other events that affect translation. DksA exhibits a similar genomic localization pattern as RNAP, suggesting it associates at least transiently with RNAP during elongation. DksA inhibits genome-wide RNAP stalling, whereas ribosome stalling exacerbates RNAP stalling. As a consequence, ΔdksA cells are hypersensitive to inhibition of ribosome translocation. Based on these results, we propose a model in which DksA prevents the formation of arrested ECs on mRNA (Fig 6). In unstarved cells, promoter-proximal regions are most prone to RNAP stalling and DksA reduces this stalling (Fig 6C-D). Amino acid starvation impairs translation elongation, which will uncouple translation and transcription, promote backtracking of RNA and DNA through RNAP, and thus increase RNAP stalling at mRNA (Fig 6E-G). We propose that stalling of ribosomes on nascent RNAs not only eliminates the backtrack-inhibiting effect of active ribosomes (Proshkin et al., 2010), but also converts ribosomes into backtrack-promoting entities that inhibit RNA structure formation in the segments of nascent RNA involved in backtracking. In this way, translational changes due to amino acid starvation can be transmitted to influence transcription elongation globally. DksA appears to prevent backtracking because deletion of dksA exacerbates EC arrests. Our results thus indicate that DksA facilitates transcription elongation and becomes especially important when inhibition of translation increases the risk of EC arrest.
Figure 6. Model of opposing effects of DksA and stalled ribosome on transcription elongation.
(A, B) In the absence of translation in vivo or in vitro, RNA secondary structure prevents backtracking sterically (A). In vivo, Rho can be recruited to stalled ECs through the naked RNA and dislodge ECs (B). (C, D) During early transcription elongation at promoter-proximal regions, before significant upstream RNA structure forms to prevent backtracking, DksA modestly facilitates RNAP progression. (E) During in vivo mRNA transcription, the trailing ribosome prevents RNA/DNA backtracking on the ECs, by sequestering the mRNA, possibly aided by association of the ribosome and RNAP via NusG (inset; Burmann et al., 2010). (F-G) Upon amino acid starvation, translation is inhibited, allowing transcription stalling because uncoupling of transcription and translation frees unstructured segments of RNA that can backtrack through RNAP (the arrow indicates movement of RNAP relative to DNA; in vivo DNA must backtrack through a stationary RNAP that is constrained against rotation by the tethered ribosome). In this situation, DksA becomes crucial to maintain transcription elongation (F). In the absence of DksA, RNAP backtracks as it is no longer protected by the trailing ribosome or by RNA secondary structure, and unable to be released by Rho-dependent termination (G).
RPP reveals promoter-distal RNAP arrest upon ribosome stalling
Our genomic results establish that DksA interacts with ECs throughout most if not all TUs during active elongation and generally suppresses transcription stalling. To quantify genome-wide RNAP progression, we developed a robust algorithm (RPP). RPP integrates RNAP ChIP signals across an entire gene to calculate its average position within a population of cells (Fig S1C), and has two advantages over Traveling Ratio (TR), which only takes ChIP signals at the beginning and mid-gene into calculation: (1) RPP is more resistant to local noise of ChIP signals; (2) RPP can reveal the effect on polymerase elongation across the entire gene, whereas TR mostly reflects polymerase progression before mid-gene.
In unstarved cells, DksA promotes RNAP progression mainly at promoter-proximal regions, and the effect of DksA is equally evident with both RPP and TR (Fig 2, S1). Interestingly, RPP showed a great advantage over TR in revealing the effect of amino acid starvation on transcription elongation (Fig S1E-I), suggesting that RNAP is arrested beyond promoter-proximal regions and DksA strongly prevents RNAP arrest throughout the gene upon ribosome uncoupling. Strong RNAP arrest happens only in protein-coding regions (Fig 4), suggesting that inactive ribosomes, rather than preventing RNAP arrest in vivo, actually facilitate RNAP arrest. The functional synergy between DksA and the trailing ribosome in promoting viability (Fig 5) confirms that DksA prevents the deleterious effects of stalled ribosomes on RNAP in vivo.
Inactive ribosome promotes EC arrests
The significant effect of DksA on transcript elongation when translation is uncoupled is puzzling given the minimal and variable effects observed in vitro. At first glance, in vitro transcription in the absence of ribosomes would appear to have a similar potential for RNA/DNA backtracking as the in vivo situation when translation halts and ECs extrude nascent RNA not immediately fed into ribosomes. In fact, these situations differ significantly because the entire nascent RNA is free to fold in vitro (Fig 6A), whereas a short segment of RNA opens between the ribosome and RNAP when translation is uncoupled with transcription in vivo (Fig 6F-G). Nascent RNA folding is known to block backtracking (Zamft et al., 2012; Tadigotla et al., 2006), which may partially explain lack of significant backtracking in transcription assays in vitro and in stable RNA transcripts in vivo (Fig 6A). The lack of significant upstream RNA on promoter-proximal ECs is thought to be one component in promoter-proximal pausing/stalling of ECs (Klopper et al., 2010; Ujvari et al., 2002), which is partially prevented by DksA in unstarved cells (Fig 6C-D). Consistent with this idea, DksA inhibits transcription arrest associated with backtracking at promoter-proximal sites in vitro (Perederina et al, 2004), even though it has variable or no effects on in vitro arrest/pausing where RNA folding effects come into play (Perederina et al, 2004; Furman et al., 2012). We propose that uncoupling of translation from transcription during starvation generates a much greater potential for RNA/DNA backtracking and thus a greater potential for DksA action than evident in in vitro assays lacking ribosomes. The RNA segment that opens between the uncoupled ribosome and EC in vivo has a much lower potential to form stable RNA structures which inhibit backtracking than the unconstrained RNA transcripts that form in vitro. It will be of significant interest to study the mechanistic basis for EC control by DksA using in vitro coupled transcription/translation systems (Castro-Roa and Zenkin, 2012).
Interactions of DksA with other general mechanisms of transcription regulation
DksA is structurally similar to other transcription elongation factors that act through the secondary channel of RNAP: GreA, GreB, Rnk and Gfh1 (Perederina et al., 2004). Gre factors stimulate the transcript cleavage activity of RNAP to reactivate backtracked/arrested RNAP (Furman et al., 2012). Gre factors can bind to RNAP more strongly in the absence of DksA (Aberg et al., 2008). DksA and Gre can have opposite roles both in gene regulation (Vinella et al, 2012) and in conferring resistance to preformed DNA lesions (data not shown). DksA should stimulate backtracking by inhibiting Gre factor binding if DksA were inert in its own effect on elongation complexes. However, we see the opposite from our RNAP ChIP analysis, that loss of DksA promotes RNAP stalling, similar to what was observed for loss of Gre factors in E. coli (RL, data not shown) and B. subtilis (Kusuya et al, 2011). This is in agreement with our prior observation that DksA and Gre factors synergistically prevent replication-transcription conflict (Tehranchi et al, 2010). We propose that DksA may displace Gre factors and lessen the cleavage of backtracked RNA, while at the same time itself prevents backtracking. Thus the two systems may be complementary or competitive, depending on the steps in transcription (initiation or elongation) and on physiological circumstances.
Although our results support a role of DksA in preventing RNAP backtracking in vivo rather than reactivating backtracked ECs, the molecular mechanism remains unclear. One likely possibility is that DksA, by occupying the secondary channel of RNAP, sterically prevents entry of the 3’ end of the transcript into the secondary channel during backtracking (Perederina et al, 2004). DksA could also inhibit backtracking by its interaction with the trigger loop, bridge helix, or both; DksA appears to interact with both parts of RNAP during transcription initiation (Rutherford et al., 2009; Lennon et al., 2012).
Amino acid starvation induces (p)ppGpp. (p)ppGpp not only extensively regulates transcription initiation at promoters but is also proposed to modulate transcription elongation rate directly (Kingston et al., 1981; Vogel et al., 1992; Vogel and Jensen, 1994). (p)ppGpp induction is likely to account for the decreased RNAP progression in wild-type cells upon SHX treatment, while dksA deletion further decreases RNAP progression (Fig 3I-K). Thus, during transcript elongation, (p)ppGpp and DksA appear to have opposing effects, in contrast to their mostly concordant effects on transcription initiation. However, (p)ppGpp effects on ECs may be complex - (p)ppGpp slows RNAP elongation to maintain transcription-translation coupling (Vogel et al., 1992) and also destabilizes stalled RNA polymerase arrays (Trautinger et al., 2005). We have previously shown that DksA protects replication during amino acid starvation even without (p)ppGpp-induction (Tehranchi et al., 2010), supporting a model that the function of DksA on elongation does not require (p)ppGpp accumulation.
Rho is a key factor for removing ECs from intragenic and stable RNA regions. However, DksA-dependent protection takes place mostly on different genes than Rho-dependent termination (Fig 4E). Further, there are no significant synergistic or epistatic interactions between DksA and Rho in promoting viability (Fig 4F, S3). In vitro, DksA does not affect Rho-dependent termination (Furman et al., 2012). Finally, Rho-dependent termination is also proposed to prevent generations of excessive R-loops from transcription (Leela et al., 2013) and we did not observe significant genetic interactions between dksA and rnhA (Fig 4G). The lack of strong interaction between DksA and Rho is expected, as the major function of Rho is to target untranslated RNAs (Fig 6B) and its accessibility requires ~80 nucleotides of C-rich unstructured nascent RNA segments (rut) (Peters et al., 2012). On the other hand, DksA mostly affects ECs in promoter-proximal regions (Fig 6C-D) or during translated mRNA synthesis (Fig 6E-G) where Rho has limited accessibility (Figure 6G).
Implications for replication-transcription conflicts
The combined effect of DksA and the trailing ribosome on transcription elongation likely underlies their synergistic impact on viability. Loss of viability in the absence of DksA upon ribosome stalling could be due to altered gene expression caused by changes in transcription initiation/elongation, due to elevated replication-transcription conflicts, or both. The intrinsic interference between replication and transcription exists genome-wide and is highly susceptible to nutritional stresses in the absence of DksA (Tehranchi et al., 2010). Our results support a model in which DksA directly aids transcription elongation to coordinate the replication and transcription machineries to prevent generation of DNA lesions by replication blockage. It remains unclear whether replication forks are blocked physically by ECs or by altered topological states of the chromosome (Rovinskiy et al., 2012), and whether replication fork blockage takes place specifically in protein-coding regions or at other locations such as the rrn operons. The directionality of transcription and replication may also have different consequences. In E. coli, head-on transcription increases DNA replication stalling (Boubakri et al., 2010), and co-directional collisions result in double-strand breaks on a plasmid reporter (Dutta et al., 2011).
Amino-acid depletion is just one of many physiological or environmental changes that can alter translation, and that may in turn be transmitted to influence transcription genome-wide via the mechanism of ribosome-stalling-induced backtracking. Thus, we suggest that DksA may have a role in guarding transcription in other cellular states, which also merit investigation.
Experimental Procedures
Strains and growth conditions
All E. coli strains used are derivatives of MG1655 or MDS42 (listed in Table S4). Deletion mutants were constructed by P1 phage transduction from the Keio collection (Baba et al., 2006). Unless indicated, cells were grown at 37°C with vigorous shaking at 250 rpm.
ChIP-chip
ChIP-chip assays were performed as previously described (Mooney et al., 2009; also see supplemental information for detailed experimental procedures). Wild type and ΔdksA cells were grown in MOPS medium with 0.2% glucose, leucine, isoleucine, valine, glycine, phenylalanine, threonine (40 μg/ml) based on the polyauxotrophy of ΔdksA cells (Brown et al., 2002) and uracil (50 μg/ml) to mid-log phase at 37°C with vigorous shaking. Cultures were split into two flasks and one was treated with SHX (0.5 mg/ml) for 20 min. Formaldehyde was added to 1% and incubated at 37 °C for 5 min before quenching with ice-cold glycine (100 mM). Cells were harvested, washed with ice-cold PBS and then lysed. DNA was sheared by sonication followed by treatment with RNase A. RNAP crosslinked to DNA was immunoprecipitated using antibodies against RNAP β subunit (NT63 monoclonal antibodies, Neoclone W0002), σ70 subunit (monoclonal antibodies, Neoclone W0004), or DksA (rabbit polyclonal antisera, a kind gift from Diana Downs). Enriched ChIP DNA and input DNA were amplified by ligation-mediated PCR (LM-PCR), labeled and hybridized to a Nimblegen tiling array. ChIP signals (log2(IP/input)) were associated with genome coordinates and smoothed by calculating the rolling average within a 300bp window to eliminate the bias due to different hybridization efficiency of probes.
Correlation of DksA with RNAP and σ70 ChIP signals
To confirm the specificity of DksA ChIP signals, we probed ΔdksA cells with the anti-DksA antibody and subtracted the DksA ChIP signals of ΔdksA cells (background) from that of wild-type cells. The subtracted DksA ChIP signals from all probes across the genome were compared to that of RNAP or σ 70 by calculating the Pearson correlation coefficient using R programming language (R Core Team, 2012, http://www.R-project.org). Resulting coefficient values were averaged from 2-3 independent biological replicates.
Calculation of traveling ratio
The traveling ratio was defined as the ratio of ChIP signal in the mid-region of an ORF to that in the beginning (100bp window size), modified from (Mooney et al., 2009). To avoid the interference from non-specific signals, we confined this analysis to a set of genes (N=155) with a σ70 peak at the promoter, and with higher DksA ChIP signals in wild-type cells than in ΔdksA cells.
Calculation of RPP
An RPP (Relative Polymerase Progression) value was defined to quantify the extent of RNAP progression into each individual transcription unit (TU). Each RNAP ChIP signal was associated with a genome position corresponding to the midpoint of the probe (Pi). The expected position of RNAP for each TU, averaged among a population of cells (), was calculated as the weighted average of Pi within a pre-defined region (Lo).
The weights used in computing this average were RNAP ChIP signals, based on the hypothesis that ChIP signals are proportional to the probability of RNAP at each probe location. The following equation was applied:
The average progression of RNAP (L) in each TU was calculated using equation (2), where PATG indicates the genomic coordinate of the first nucleotide of the start codon:
Lo was defined as the entire first open reading frame for TUs where RNAP ChIP signal showed only one peak in the promoter-proximal region. For the rest of TUs where additional RNAP peaks exist in the first open reading frame, Lo was defined from the first nucleotide of the start codon to the nucleotide with the lowest ChIP signal between the promoter-proximal peak and the second peak downstream of the promoter.
Finally, RPP was calculated by:
To select high-quality transcription units (TUs) for RPP analysis, the ChIP signals of RNAP across the genome were compared between different samples. 432 TUs (Table S1) were selected based on the criteria that (1) Only protein-coding genes are included; (2) TUs had significant RNAP ChIP signals compared to the cryptic bglB gene (student’s t-test, p<0.05) (Defez and De Felice, 1981; Mooney et al., 2009), implicating active transcription; (3) RNAP ChIP signals within the TU are not interfered by convergent transcription or dominant ChIP signals from adjacent TUs on the chromosome; (4) TUs are longer than 400 bp, allowing differentiation of transcription elongation from initiation.
To further narrow down the RPP analysis from the 432 TUs to a subset of TUs with no effect of DksA on transcription initiation, RNAP and σ 70 ChIP signals were normalized by using the normalize.quantiles function in the R package affy. 278 TUs (Table S2) were selected based on the additional criterion that the range of σ 70 ChIP signal peaks (obtained from two independent replicates) for starved wild-type and starved ΔdksA samples overlap.
RPP values of 432 (or 278) selected TUs from two samples were compared by scatter plots (by plot function in R) and estimated probability density curves (by density function in R). A paired student’s t-test (by t.test function in R) was used to calculate statistical significance of the difference between RPP values from different samples. The Kolmogorov–Smirnov (K-S) test was used to compare RPP distributions from different samples, with D value representing the maximum difference between the empirical distribution functions of two samples (by ks.test function in R).
Analysis of genetic interaction
Growth curves of wild-type and mutant cells were obtained in 100 μl LB in 96-well plates at 37°C, using a BioTek Synergy 2 Microplate Reader to record optical density at 600 nm until cells reached stationary phase. The doubling time (T) was calculated from the mid-exponential growth phase and the fitness (W) of a strain with gene x deleted was defined as the ratio of doubling time of the wild type (Twt) to that of the deletion strain (Tx), i.e. Wx=Twt/Tx (St Onge et al., 2007). Genetic interaction between genes x and y was determined based on whether the fitness of double mutant (Wxy, i.e. Twt/Txy) is significantly different (student t-test) from the fitness predicated for non-interaction gene pairs (Wx * Wy).
If Wxy = Wx * Wy, the genetic effects of x and y are independent.
If Wxy < Wx * Wy, the genetic effects of x and y are synergistic.
Supplementary Material
Highlights.
Stalled ribosomes promote genome-wide transcription stalling
DksA prevents transcription arrest upon ribosome stalling
RPP is a sensitive and robust measurement for polymerase elongation in vivo
DksA modulates both transcription initiation and elongation in bacteria
Acknowledgments
We thank Benedicte Michel, Max Gottesman, Robert Washburn, Jonathan Dworkin and Diana Downs for generous gifts of strains and reagents. We also thank Rick Gourse, Wilma Ross, Ido Golding and the Wang and Landick labs for suggestions and critical reading of the manuscript. This work was supported by NIH grants DP2OD004433 and GM084003 (JDW), GM038660 (RL) and GM088653 (CH). YZ was supported, in part, by a predoctoral fellowship from CPRIT (RP101499).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg A, Fernandez-Vazquez J, Cabrer-Panes JD, Sanchez A, Balsalobre C. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J Bacteriol. 2009;191:3226–3236. doi: 10.1128/JB.01410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg A, Shingler V, Balsalobre C. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol Microbiol. 2008;67:1223–1241. doi: 10.1111/j.1365-2958.2008.06115.x. [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006. doi: 10.1038/msb4100050. 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubakri H, de Septenville AL, Viguera E, Michel B. The helicases DinG, Rep and UvrD cooperate to promote replication across transcription units in vivo. EMBO J. 2010;29:145–157. doi: 10.1038/emboj.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Gentry D, Elliott T, Cashel M. DksA affects ppGpp induction of RpoS at a translational level. J Bacteriol. 2002;184:4455–4465. doi: 10.1128/JB.184.16.4455-4465.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmann BM, Schweimer K, Luo X, Wahl MC, Stitt BL, Gottesman ME, Rosch P. A NusE:NusG complex links transcription and translation. Science. 2010;328:501–504. doi: 10.1126/science.1184953. [DOI] [PubMed] [Google Scholar]
- Castro-Roa D, Zenkin N. In vitro experimental system for analysis of transcription-translation coupling. Nucleic Acids Res. 2012;40:e45. doi: 10.1093/nar/gkr1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CL, Landick R. The Salmonella typhimurium his operon leader region contains an RNA hairpin-dependent transcription pause site. Mechanistic implications of the effect on pausing of altered RNA hairpins. J Biol Chem. 1989;264:20796–20804. [PubMed] [Google Scholar]
- Defez R, De Felice M. Cryptic operon for beta-glucoside metabolism in Escherichia coli K12: genetic evidence for a regulatory protein. Genetics. 1981;97:11–25. doi: 10.1093/genetics/97.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour YS, Wesenberg GE, Tritt AJ, Glasner JD, Perna NT, Mitchell JC, Donohue TJ. chipD: a web tool to design oligonucleotide probes for high-density tiling arrays. Nucleic Acids Res. 2010;38:W321–W325. doi: 10.1093/nar/gkq517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T, Hansen AM, Zhi H, Blattner FR, Jin DJ. Transcription profiling of the stringent response in Escherichia coli. J Bacteriol. 2008;190:1084–1096. doi: 10.1128/JB.01092-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA Polymerase Backtracking to Genome Instability in E. coli. Cell. 2011;146:533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R, Sevostyanova A, Artsimovitch I. Transcription initiation factor DksA has diverse effects on RNA chain elongation. Nucleic Acids Res. 2012;40:3392–3402. doi: 10.1093/nar/gkr1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R, Tsodikov OV, Wolf YI, Artsimovitch I. An insertion in the catalytic trigger loop gates the secondary channel of RNA polymerase. J Mol Biol. 2013;425:82–93. doi: 10.1016/j.jmb.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Felipe-Abrio I, Aguilera A. The S-Phase checkpoint is required to respond to R-loops accumulated in THO mutants. Mol Cell Biol. 2009 doi: 10.1128/MCB.00402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Zalkin H. Repression of Escherichia coli purB is by a transcriptional roadblock mechanism. J Bacteriol. 1992;174:7121–7127. doi: 10.1128/jb.174.22.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston RE, Nierman WC, Chamberlin MJ. A direct effect of guanosine tetraphosphate on pausing of Escherichia coli RNA polymerase during RNA chain elongation. J Biol Chem. 1981;256:2787–2797. [PubMed] [Google Scholar]
- Kireeva ML, Kashlev M. Mechanism of sequence-specific pausing of bacterial RNA polymerase. Proc Natl Acad Sci U S A. 2009;106:8900–8905. doi: 10.1073/pnas.0900407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopper AV, Bois JS, Grill SW. Influence of secondary structure on recovery from pauses during early stages of RNA transcription. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:030904. doi: 10.1103/PhysRevE.81.030904. [DOI] [PubMed] [Google Scholar]
- Komissarova N, Kashlev M. Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3’ end of the RNA intact and extruded. Proc Natl Acad Sci U S A. 1997;94:1755–1760. doi: 10.1073/pnas.94.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuya Y, Kurokawa K, Ishikawa S, Ogasawara N, Oshima T. Transcription Factor GreA Contributes to Resolving Promoter-Proximal Pausing of RNA Polymerase in Bacillus subtilis Cells. J Bacteriol. 2011;193:3090–3099. doi: 10.1128/JB.00086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R, Carey J, Yanofsky C. Translation activates the paused transcription complex and restores transcription of the trp operon leader region. Proc Natl Acad Sci U S A. 1985;82:4663–4667. doi: 10.1073/pnas.82.14.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leela JK, Syeda AH, Anupama K, Gowrishankar J. Rho-dependent transcription termination is essential to prevent excessive genome-wide R-loops in Escherichia coli. Proc Natl Acad Sci U S A. 2013;110:258–263. doi: 10.1073/pnas.1213123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke JJ, Durfee T, Gourse RL. DksA and ppGpp directly regulate transcription of the Escherichia coli flagellar cascade. Mol Microbiol. 2009;74:1368–1379. doi: 10.1111/j.1365-2958.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke JJ, Sanchez-Vazquez P, Burgos HL, Hedberg G, Ross W, Gourse RL. Direct regulation of Escherichia coli ribosomal protein promoters by the transcription factors ppGpp and DksA. Proc Natl Acad Sci U S A. 2011;108:5712–5717. doi: 10.1073/pnas.1019383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon CW, Ross W, Martin-Tumasz S, Toulokhonov I, Vrentas CE, Rutherford ST, Lee JH, Butcher SE, Gourse RL. Direct interactions between the coiled-coil tip of DksA and the trigger loop of RNA polymerase mediate transcriptional regulation. Genes Dev. 2012;26:2634–2646. doi: 10.1101/gad.204693.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr MT, Roberts JW. Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol Cell. 2000;6:1275–1285. doi: 10.1016/s1097-2765(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Davis SE, Peters JM, Rowland JL, Ansari AZ, Landick R. Regulator trafficking on bacterial transcription units in vivo. Mol Cell. 2009;33:97–108. doi: 10.1016/j.molcel.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels BE, Mukhopadhyay J, Garrity SJ, Ebright RH, Hochschild A. The sigma 70 subunit of RNA polymerase mediates a promoter-proximal pause at the lac promoter. Nat Struct Mol Biol. 2004;11:544–550. doi: 10.1038/nsmb757. [DOI] [PubMed] [Google Scholar]
- Nudler E, Mustaev A, Lukhtanov E, Goldfarb A. The RNA-DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell. 1997;89:33–41. doi: 10.1016/s0092-8674(00)80180-4. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Berkmen MB, Gourse RL. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci U S A. 2005;102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perederina A, Svetlov V, Vassylyeva MN, Tahirov TH, Yokoyama S, Artsimovitch I, Vassylyev DG. Regulation through the secondary channel--structural framework for ppGpp-DksA synergism during transcription. Cell. 2004;118:297–309. doi: 10.1016/j.cell.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Peters JM, Mooney RA, Grass JA, Jessen ED, Tran F, Landick R. Rho and NusG suppress pervasive antisense transcription in Escherichia coli. Genes Dev. 2012;26:2621–2633. doi: 10.1101/gad.196741.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Mooney RA, Kuan PF, Rowland JL, Keles S, Landick R. Rho directs widespread termination of intragenic and stable RNA transcription. Proc Natl Acad Sci U S A. 2009;106:15406–15411. doi: 10.1073/pnas.0903846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz RT, O’Donnell M. Direct restart of a replication fork stalled by a head-on RNA polymerase. Science. 2010;327:590–592. doi: 10.1126/science.1179595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328:504–508. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppas NB, Wade JT, Church GM, Struhl K. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Mol Cell. 2006;24:747–757. doi: 10.1016/j.molcel.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Yarnell WS, Roberts JW. Function of E. coli RNA polymerase sigma factor sigma 70 in promoter-proximal pausing. Cell. 1996;86:485–493. doi: 10.1016/s0092-8674(00)80121-x. [DOI] [PubMed] [Google Scholar]
- Rovinskiy N, Agbleke AA, Chesnokova O, Pang Z, Higgins NP. Rates of gyrase supercoiling and transcription elongation control supercoil density in a bacterial chromosome. PLoS Genet. 2012;8:e1002845. doi: 10.1371/journal.pgen.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Lemke JJ, Vrentas CE, Gaal T, Ross W, Gourse RL. Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase. J Mol Biol. 2007;366:1243–1257. doi: 10.1016/j.jmb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Villers CL, Lee JH, Ross W, Gourse RL. Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 2009;23:236–248. doi: 10.1101/gad.1745409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusala T, Kurland CG. Streptomycin preferentially perturbs ribosomal proofreading. Mol Gen Genet. 1984;198:100–104. doi: 10.1007/BF00328707. [DOI] [PubMed] [Google Scholar]
- Shaevitz JW, Abbondanzieri EA, Landick R, Block SM. Backtracking by single RNA polymerase molecules observed at near-base-pair resolution. Nature. 2003;426:684–687. doi: 10.1038/nature02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller E, DeZwaan DC, Anderson JF, Freeman BC, Barral JM. Slowing bacterial translation speed enhances eukaryotic protein folding efficiency. J Mol Biol. 2010;396:1310–1318. doi: 10.1016/j.jmb.2009.12.042. [DOI] [PubMed] [Google Scholar]
- St Onge RP, Mani R, Oh J, Proctor M, Fung E, Davis RW, Nislow C, Roth FP, Giaever G. Systematic pathway analysis using high-resolution fitness profiling of combinatorial gene deletions. Nat Genet. 2007;39:199–206. doi: 10.1038/ng1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadigotla VR, O Maoileidigh D, Sengupta AM, Epshtein V, Ebright RH, Nudler E, Ruckenstein AE. Thermodynamic and kinetic modeling of transcriptional pausing. Proc Natl Acad Sci U S A. 2006;103:4439–4444. doi: 10.1073/pnas.0600508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranchi AK, Blankschien MD, Zhang Y, Halliday JA, Srivatsan A, Peng J, Herman C, Wang JD. The transcription factor DksA prevents conflicts between DNA replication and transcription machinery. Cell. 2010;141:595–605. doi: 10.1016/j.cell.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S, Hanawalt PC. Effect of DNA lesions on transcription elongation. Biochimie. 1999;81:139–146. doi: 10.1016/s0300-9084(99)80046-7. [DOI] [PubMed] [Google Scholar]
- Toulme F, Mosrin-Huaman C, Sparkowski J, Das A, Leng M, Rahmouni AR. GreA and GreB proteins revive backtracked RNA polymerase in vivo by promoting transcript trimming. EMBO J. 2000;19:6853–6859. doi: 10.1093/emboj/19.24.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautinger BW, Jaktaji RP, Rusakova E, Lloyd RG. RNA polymerase modulators and DNA repair activities resolve conflicts between DNA replication and transcription. Mol Cell. 2005;19:247–258. doi: 10.1016/j.molcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ujvari A, Pal M, Luse DS. RNA polymerase II transcription complexes may become arrested if the nascent RNA is shortened to less than 50 nucleotides. J Biol Chem. 2002;277:32527–32537. doi: 10.1074/jbc.M201145200. [DOI] [PubMed] [Google Scholar]
- Vinella D, Potrykus K, Murphy H, Cashel M. Effects on growth by changes of the balance between GreA, GreB, and DksA suggest mutual competition and functional redundancy in Escherichia coli. J Bacteriol. 2012;194:261–273. doi: 10.1128/JB.06238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel U, Jensen KF. Effects of guanosine 3’,5’-bisdiphosphate (ppGpp) on rate of transcription elongation in isoleucine-starved Escherichia coli. J Biol Chem. 1994;269:16236–16241. [PubMed] [Google Scholar]
- Vogel U, Sorensen M, Pedersen S, Jensen KF, Kilstrup M. Decreasing transcription elongation rate in Escherichia coli exposed to amino acid starvation. Mol Microbiol. 1992;6:2191–2200. doi: 10.1111/j.1365-2958.1992.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Washburn RS, Gottesman ME. Transcription termination maintains chromosome integrity. Proc Natl Acad Sci U S A. 2011;108:792–797. doi: 10.1073/pnas.1009564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher HS, Green R. Hyperaccurate and error-prone ribosomes exploit distinct mechanisms during tRNA selection. Mol Cell. 2010;39:110–120. doi: 10.1016/j.molcel.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamft B, Bintu L, Ishibashi T, Bustamante C. Nascent RNA structure modulates the transcriptional dynamics of RNA polymerases. Proc Natl Acad Sci U S A. 2012;109:8948–8953. doi: 10.1073/pnas.1205063109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiefka A, Kohn H, Widger WR. Transcription termination factor rho: the site of bicyclomycin inhibition in Escherichia coli. Biochemistry. 1993;32:3564–3570. doi: 10.1021/bi00065a007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.