Abstract
Estrogen is a fundamental regulator of the metabolic system of the female brain and body. Within the brain, estrogen regulates glucose transport, aerobic glycolysis, and mitochondrial function to generate ATP. In the body, estrogen protects against adiposity, insulin resistance, and type II diabetes, and regulates energy intake and expenditure. During menopause, decline in circulating estrogen is coincident with decline in brain bioenergetics and shift towards a metabolically compromised phenotype. Compensatory bioenergetic adaptations, or lack thereof, to estrogen loss could determine risk of late-onset Alzheimer’s disease. Estrogen coordinates brain and body metabolism, such that peripheral metabolic state can indicate bioenergetic status of the brain. By generating biomarker profiles that encompass peripheral metabolic changes occurring with menopause, individual risk profiles for decreased brain bioenergetics and cognitive decline can be created. Biomarker profiles could identify women at risk while also serving as indicators of efficacy of hormone therapy or other preventative interventions.
Keywords: adipokine, adipose tissue, aging, Alzheimer’s disease, biomarker, insulin, menopause, metabolism, mitochondria, type 2 diabetes
1. Introduction
Estrogen is a systems-level signaling molecule that regulates and coordinates multiple functions across organs, cells and genes. To achieve this integration, estrogen utilizes a repertoire of receptors and signaling pathways to activate and regulate molecular and genomic responses required for survival at the cellular, organismic and ultimately whole body level. Estrogen integration and coordination of metabolism enables the development of peripheral biomarkers which can serve as reporters of brain bioenergetics, thereby providing early detection of populations at risk for neurodegenerative diseases associated with metabolic dysfunction, such as Alzheimer’s disease. Reviewed herein is estrogen action in the brain and the body with particular emphasis on estrogen regulation of metabolism, and its clinical implications. Throughout, estrogen is used to refer to 17β-estradiol (the predominant estrogen) whereas other types of estrogens are specifically identified and typically are related to formulations of hormone therapies.
2. Estrogen, Estrogen Receptors, and Intracellular Signaling Pathways in the Brain
Estrogens are steroid hormones primarily known for their role in promotion of female sex characteristics and reproductive capability. There are three forms of estrogens in the female body: estrone (E1), estradiol (E2), and estriol (E3). During a woman’s reproductive years, the principal circulating estrogen is 17β-estradiol (E2); importantly, it is also the most potent form of estrogen. In humans, estrogens are produced by the ovaries and adrenal glands, and circulate throughout the body where they have effects on most organ systems, including brain, breast, cardiovascular (heart and vasculature), immune, reproductive (ovaries and uterus), bladder, skin, and bone (Kuiper et al., 1997).
Estrogen can cross the blood-brain barrier, and additionally, the brain can produce estrogen endogenously from cholesterol (Balthazart and Ball, 2006; Garcia-Ovejero et al., 2005; Prange-Kiel et al., 2003; Rune and Frotscher, 2005). Thus, along with its role in female physiology and reproduction, decades of research have established that estrogen is a critical signaling molecule within the brain (Brinton, 2008b). Estrogen receptors (ERs) are widely distributed in the brain, are present on both neurons and glia, and are expressed by both sexes. These receptors are highly evolutionarily conserved, with homologues in all vertebrate species. Estrogen receptors are composed of two general classes: nuclear ERs and membrane embedded/membrane associated ERs (mERs), both of which are present in the brain. There are two isoforms of classical nuclear estrogen receptors: ERα (ESR1) (HUGO Gene Nomenclature Committee, NCBI) and ERβ (ESR2) (HUGO Gene Nomenclature Committee, NCBI), which are functionally distinct and differentially distributed throughout the brain. Coding regions for both ERs are found on chromosome 6 (Menasce et al., 1993). The estrogen nuclear receptors exist initially as monomers, and dimerize prior to translocation to the nucleus, where they regulate transcription. In vitro evidence indicates the potential for heterodimers between ERα and ERβ (Pettersson et al., 1997), although in vivo evidence of this phenomenon remains to be established. In contrast to the nuclear receptors, membrane-associated estrogen receptors are monomers of ERα and ERβ. It is worth noting that in fish, a third nuclear ER has been identified, termed ERγ or ERβa (Halm et al., 2004; Hawkins and Thomas, 2004; Hawkins et al., 2000; Sabo-Attwood et al., 2004). The existence of this third ER in mammals has been investigated (Shughrue et al., 2002), and although no studies have provided concrete evidence for its presence, it would be presumptive to rule it out.
Classical estrogen signaling occurs as a result of the ER translocating to the nucleus, where it binds the estrogen response element (ERE) to regulate gene expression. Additionally, ERα can be alternatively spliced to generate three splice variants (GeneCards, ESR1), and ERβ can be alternatively spliced to generate eleven splice variants (GeneCards, ESR2). Most splice variants have been identified in breast or other cancer cell lines; because of the lack of genomic control in these cell lines, the functionality of splice variants is controversial. In brain, however, splice variants have been detected and have been associated with changes in estrogen responsivity.
2.1 Estrogen Receptors Alpha and Beta: Localization and Splice Variants
In rat and mouse forebrain, ERα shows a wide pattern of distribution (Brinton, 2009; Milner et al., 2001; Mitra et al., 2003; Shughrue, 2004; Shughrue et al., 1997); this is similar to the human brain, where in situ hybridization studies show ERα is distributed throughout the hypothalamus, forebrain, hippocampus (weakly), and amygdala (Mitra et al., 2003; Osterlund et al., 2000b; Ostlund et al., 2003). ERβ is more narrowly distributed, with high concentrations seen primarily in the hippocampus and cerebral cortex both in rodents and humans (Mitterling et al., 2010; Osterlund et al., 2000a; Ostlund et al., 2003; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001). Within the hippocampus, both ERα and ERβ localize to dendritic spines, which are sites of synapse formation that show a high degree of plasticity. (Milner et al., 2005; Milner et al., 2001). ERα and ERβ have both been shown to mediate hippocampal-dependent learning tasks (Spencer et al., 2008); however, signaling through ERα and ERβ leads to differential expression of synaptic proteins, indicating that these two receptors have distinct roles within the hippocampus (Waters et al., 2009). In the rodent midbrain, ERβ is predominantly localized to the substantia nigra, locus coeruleus, and raphe nuclei (Mitra et al., 2003; Shughrue et al., 1997). ERα shows narrower distribution in the midbrain, and is primarily localized to the periaqueductal gray (Mitra et al., 2003; Shughrue et al., 1997). In the hindbrain and cerebellum, most ERα and ERβ immunostaining is within cell nuclei; the cerebellum shows no specific ERα staining, although it does show staining for ERβ (Mitra et al., 2003; Shughrue et al., 1997).
The effect of aging on ERα and ERβ expression and signaling is still a developing area of investigation, but a recent review thoroughly covers what is currently known (Foster, 2012). In short, data suggest that in different areas of the hippocampus, the ERα/ERβ ratio changes with age. In young and middle-aged rats, primates, and humans, ERβ is the dominant ER in the hippocampus, although ERα is present in low quantities. With aging, nuclear ERα localization increases in the dentate gyrus and CA3, but decreases in CA1 (Ishunina et al., 2007). ERα also becomes less sensitive to E2 treatment as animals age; this is in contrast to ERβ, which shows decreased levels with age but remains responsive to E2 treatment (Waters et al., 2011). Clinical studies have shown a linear relationship between Mini Mental State Exam (MMSE) score and ERα β, in the frontal cortex of Alzheimer’s patients (Kelly et al., 2008). The existence of variant isoforms of ERα that may influence cognitive impairment has been proposed (Kelly et al., 2008); this was later observed in a cohort of non-demented elderly (Yaffe et al., 2009). Thus the data show that decreased ERα responsiveness may mediate cognitive impairment and dementia; during aging, although ERβ remains responsive to E2, it is unable to compensate for the loss of ERα.
With aging, there is also an increase in the expression of particular ERα splice variants in the hippocampus that render much of the available ERα non-functional (Ishunina et al., 2007). Interestingly, it has been shown that elderly women are more likely than elderly men to have increased expression of ER α splice variants (Foster, 2012). ERβ the brain (Mott and Pak, 2012). A recent study proposed that one dominant negative splice variant, ERβ2, may mediate differential responses to E2 treatment early and late after ovariectomy (OVX) in rats, such that only if estrogen treatment is initiated early after OVX is it able to prevent induction of the dominant negative ERβ2 variant (Wang et al., 2012). In addition to splice variants, there are several ERα polymorphisms that increase the risk of Alzheimer’s disease (AD) specifically in women, particularly when associated with the APOE ε4 allele (Ryan et al., 2013).
2.2 Membrane-Embedded Estrogen Receptors
In addition to the classical cytoplasmic and nuclear ERs, there are also membrane-embedded ERs, which rapidly initiate intracellular signaling pathways upon exposure to estrogen (Micevych and Dominguez, 2009; Toran-Allerand et al., 2002). These membrane sites of ER action activate both the Src/PI3K and Ras/Raf/MEKK/ERK signaling pathways, leading to activation of CREB, and they have been identified as required for E2-inducible neuroprotection (Levin, 2001; Mannella and Brinton, 2006; Zhao et al., 2005). G protein-coupled receptors that associate with estrogen, such as G-protein coupled estrogen receptor 1 (GPER1; also called GPR30) (HUGO Gene Nomenclature Committee, NCBI), have also been identified (Maggiolini and Picard, 2010). This receptor has a high affinity for E2, and signaling thorough it activates both cAMP/PKA and PI3K/Akt pathways (Maggiolini and Picard, 2010).17β-estradiol binding to ERs activates signaling cascades associated with neuronal survival and function, including MAPK (Arevalo et al., 2012; Nilsen and Brinton, 2003; Singh et al., 2000), PI3K (Brinton, 2008a; Cheskis et al., 2008; Spencer-Segal et al., 2012), and PKC (Cordey et al., 2003). Activation of both the MAPK and PI3K pathways leads to phosphorylation of CREB, which upregulates the transcription of neuronal survival genes including the anti-apoptotic protein Bcl-2 (Nilsen and Brinton, 2003; Nilsen et al., 2006; Pike, 1999; Stoltzner et al., 2001). Further, E2 activation of the PI3K pathway has the potential to simultaneously activate the MAPK, PKC, Ca2+ influx and Akt signaling pathways (Mannella and Brinton, 2006; Simoncini et al., 2000), and this simultaneous activation has been shown to mediate the neuroprotective effect of E2 (Mannella and Brinton, 2006).
Membrane-embedded ERs activate pathways that regulate calcium influx through L-type Ca2+ channels, which in turns activates the PI3K/Src/ERK/CREB cascade (Mannella and Brinton, 2006; Wu et al., 2005). This increases Bcl-2 expression, which potentiates the maximal mitochondrial calcium uptake capacity (Murphy et al., 1996a). The increased mitochondrial uptake of calcium induced by E2 could thus protect neurons against the adverse consequences of excess cytoplasmic calcium. We sought to test this in a series of experiments analyzing calcium dynamics between the cytosolic and mitochondrial compartments (Nilsen and Brinton, 2003). Results showed that E2 treatment led to increased mitochondrial sequestration of [Ca2+]i when neurons were exposed to excitotoxic glutamate, which was paralleled by a decrease in cytoplasmic [Ca2+]i and an increase in expression levels of Bcl-2 (Nilsen and Brinton, 2003). Importantly, despite the increased intramitochondrial calcium load, treatment with E2 was able to preserve mitochondrial respiratory capacity (Nilsen and Brinton, 2003). In neurons derived from the aged brain, E2 sustained calcium homeostasis comparable to the middle-aged level, and prevented transition to the dysregulation associated with aged neurons (Brewer et al., 2006).
2.3 Mitochondrial Estrogen Receptors
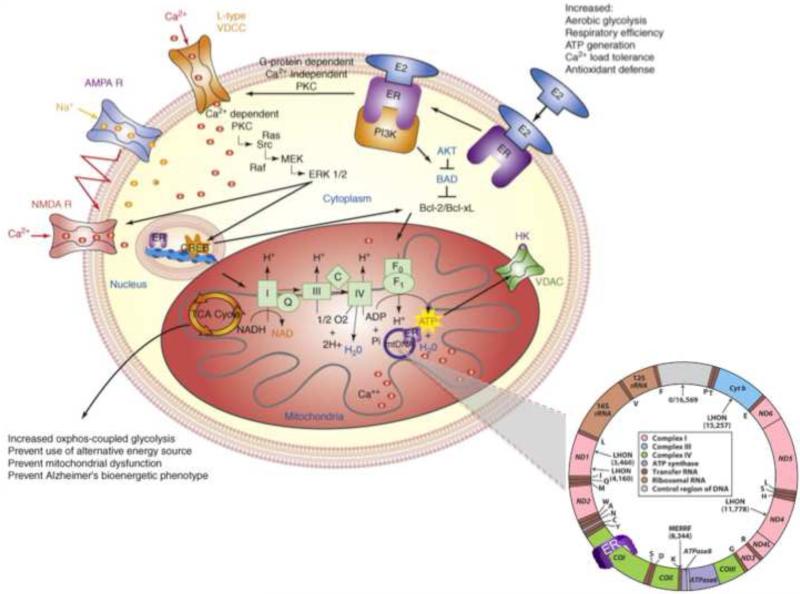
Critically, multiple laboratories have also established the presence of mitochondrial ERs (Irwin et al., 2012; Milner et al., 2005; Mitterling et al., 2010; Stirone et al., 2005; Yager and Chen, 2007; Yang et al., 2004), which emphasizes the role that estrogen plays in regulating cellular bioenergetics (Figure 1). The mitochondrial genome contains DNA sequences that resemble half the palindromic nuclear ERE sequence (Demonacos et al., 1996). In an early experiment, ERα and ERβ were shown to directly bind mitochondrial DNA (mtDNA) in vitro through mitochondrial EREs, and the binding response was increased with exposure to 17β-estradiol (Chen et al., 2004). Further work has demonstrated that ERβ localizes to the mitochondria (Irwin et al., 2012; Simpkins et al., 2008). Considering the predominant location of ERβ in brain mitochondria, it is reasonable to postulate that estrogen directly modulates mitochondrial function via ERβ-mediated regulation of mtDNA transcription (Yang et al., 2004). While mechanisms by which ERs coordinate the complex signaling pathways between the membrane, mitochondria, and nucleus remain to be fully determined (Wagner et al., 2008), it is remarkable that ERs are perfectly positioned to coordinate events at the membrane with events in the mitochondria and nucleus (Figure 1) (Brinton, 2008b, 2009; McEwen et al., 2001; Milner et al., 2005; Milner et al., 2008; Milner et al., 2001; Yang et al., 2004).
Figure 1. Estrogen regulation of intracellular brain metabolism pathways.
Estrogen-induced signaling pathways in hippocampal and cortical neurons converge upon the mitochondria to enhance glucose uptake and metabolism, aerobic glycolysis, tricarboxylic acid cycle (TCA)-coupled oxidative phosphorylation and ATP generation. In parallel, E2 increases antioxidant defense and antiapoptotic mechanisms. Estrogen receptors at the membrane, in mitochondria and within the nucleus are well positioned to regulate coordinated mitochondrial and nuclear gene expression required for optimal bioenergetics.
3. Estrogen and Brain Bioenergetics
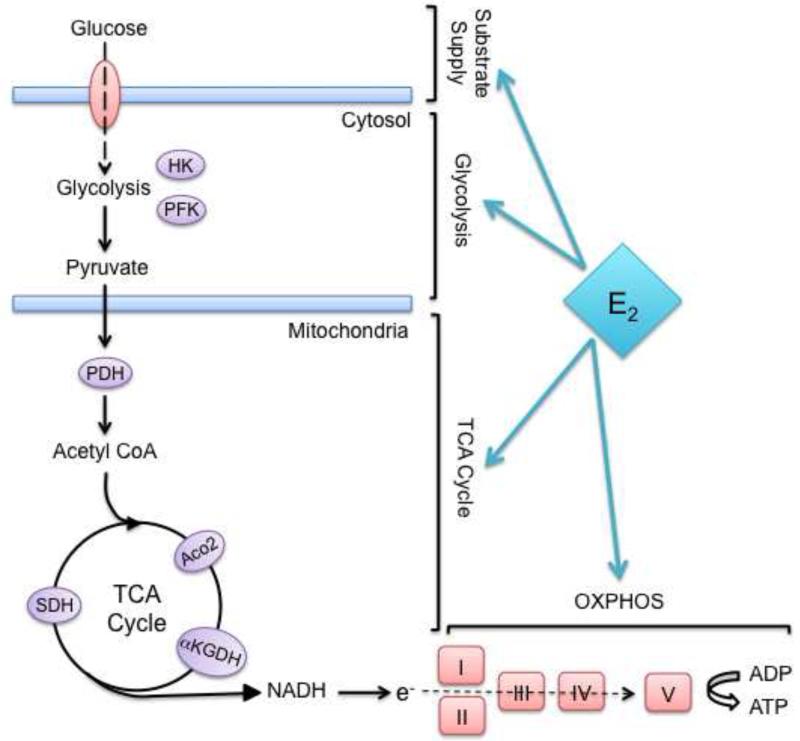
The human brain, despite comprising only 2% of the body’s mass, consumes 20% of the body’s fuel for mitochondrial respiration and ATP generation. Thus the brain is singularly reliant on efficient mitochondrial function, and is at risk for bioenergetic decline if mitochondrial function is impaired. Estrogen has been shown to have beneficial effects on the entire bioenergetic system of the brain from glucose transport into cells to glycolysis, the tricarboxylic citric acid (TCA) cycle, oxidative phosphorylation (OXPHOS), and ATP production (Figure 2) (Brinton, 2008b, 2009).
Figure 2. Estrogen regulation of bioenergetic system.
Estrogen regulates many of the key enzymes involved in mitochondrial bioenergetics, including glucose transporters, hexokinase (HK), pyruvate dehydrogenase (PDH), aconitase (Aco2), alpha ketoglutarate dehydrogenase (aKGDH), succinate dehydrogenase (SDH), and Complexes I, III, and IV of the electron transport chain.
3.1 Estrogen Regulation of Glucose Transport
The family of GLUT receptors mediates glucose transport into the brain. In vivo, glucose transporter-1 (GLUT-1) exists in two glycosylated isoforms: a 55kD isoform that regulates glucose transport from the blood vessels into the brain, and a 45kD isoform that regulates transport from the brain into glia. GLUT-1 is a low-affinity transporter, but is highly sensitive to changes in glucose levels. Its expression levels are closely regulated by glucose availability and demand; in particular, conditions of hypoglycemia lead to increased blood-brain barrier GLUT-1 expression (Carruthers et al., 2009; Simpson et al., 1999). Glucose transporter-3 (GLUT-3) is a high-affinity GLUT isoform that is expressed on neuronal membranes, and allows for the transport of glucose into the neuron. Glucose transporter-4 (GLUT-4) is also expressed on neuronal membranes, and is unique because its translocation from the cytoplasmic compartment to the cell membrane is regulated by insulin. Through this array of transporters, glucose enters specific cellular compartments of the brain. Thus, tracking changes in expression or function of these glucose transporters in each of the cellular compartments of the brain provides a window into understanding adaptations occurring in brain that affect both its metabolic capacity and its relationship to fuels provided from the periphery.
Ovariectomy induces a significant decline in multiple glucose transporters, including GLUT-1, GLUT-3, and GLUT-4 (Cheng et al., 2001; Ding et al., 2013b; Shi and Simpkins, 1997). In both the rodent and the non-human primate, E2 treatment prevents the OVX-induced decline in these glucose transporters (Cheng et al., 2001; Ding et al., 2012; Ding et al., 2013b; Shi and Simpkins, 1997). Recently, our group demonstrated that loss of ovarian hormones with reproductive aging induced a significant decline in brain glucose utilization, which could be attributed to decreased neuronal glucose transporter expression, compromised hexokinase activity, inactivation of the pyruvate dehydrogenase complex, and eventually a functionally significant decrease in mitochondrial bioenergetic function (Ding et al., 2013a).
Estrogen regulation of the insulin-sensitive glucose transporter is particularly interesting, as it requires a simultaneous increase in levels of E2/ER and the insulin/insulin receptor (IR) system. In the rodent brain, coupling between estrogen and insulin involves insulin’s rodent brain homologue insulin growth factor-1 (IGF-1) and its receptor (IGF-1R). The synergistic coupling between ERs and IGF-1R has been extensively investigated (Arevalo et al., 2012; Cardona-Gomez et al., 2002; Garcia-Segura et al., 2010; Garcia-Segura et al., 2000; Mendez and Garcia-Segura, 2006; Mendez et al., 2006). IGF-1R and ERα can form a macromolecular complex to enable downstream signaling functions, such as activation of the PI3K signaling pathway that leads to phosphorylation and activation of Akt (Garcia-Segura et al., 2010; Mendez et al., 2006). The phosphorylated (active) form of Akt has been shown to selectively co-localize with GLUT-4 in IGF-1-expressing neurons, leading to an increase in glucose transport through this regulation of GLUT-4 (Cheng et al., 2000). In the ovariectomized primate referenced above, it was noted that IGF-1R mRNA is concentrated in neurons in a similar distribution to GLUT-3 and -4 (Cheng et al., 2001). The interaction between ER and IGF-1 provides compelling evidence for the coordinated roles of IGF-1, the PI3K to Akt signaling pathway, and ER signaling in estrogen-inducible promotion of neuronal metabolism and neuroprotection.
Interestingly, the above in vitro and in vivo studies correspond well with what has been observed in humans. In patients with AD, low insulin levels, decreased expression of insulin receptors and attenuated insulin signaling are detected in brain regions vulnerable to AD pathology, particularly the hippocampus (Schioth et al., 2012). It is reasonable to hypothesize that attenuated insulin signaling could at least partially account for the memory impairment seen early in the course of the disease. Alleviation of insulin deficits by intranasal insulin administration was found improve cognitive function in preclinical animal models, healthy controls, and patients with Alzheimer’s disease (Benedict et al., 2011). Further analyses suggest that insulin effects can be modified by both sex and APOE genotype, as well as by insulin dose (Claxton et al., 2013).
Soluble amyloid oligomers have been shown to disrupt insulin signaling by causing a loss of insulin receptors (Zhao et al., 2008). In rodents, elevated brain amyloid beta1-42 (Aβ) levels were associated with low circulating IGF-1, whereas increasing serum IGF-1 reduced Aβ burden (Carro et al., 2002). In female rats, IGF-1 gene expression was consistently decreased following both ovariectomy and reproductive senescence (Mao et al., 2012; Zhao et al., 2012). This was paralleled by increased expression of genes involved in Aβgeneration. In addition to its role in promoting insulin/IGF-1 function, estrogen further promotes Aβdegradation and clearance by upregulating insulin-degrading enzyme (IDE) and neprilysin gene and protein expression (Jayaraman et al., 2012; Zhao et al., 2011b).
3.2 Estrogen Regulation of Glycolysis
In addition to facilitating glucose transport, estrogen also promotes neuronal aerobic glycolysis. In rodents, E2 increases activity of the glycolytic enzymes hexokinase (soluble and membrane-bound), phosphofructokinase and pyruvate kinase within 4 hours of exposure (Kostanyan and Nazaryan, 1992). Hexokinases are critical enzymes, because they bind the voltage-dependent anion channel (VDAC) on the mitochondrial outer membrane in order to link mitochondrial ATP synthesis to glucose metabolism (Gottlob et al., 2001). This coupling is regulated by Akt, which associates with hexokinase II (HKII) (Miyamoto et al., 2008); HKII activity is then required for the antiapoptotic effect of Akt (Gottlob et al., 2001). E2 acts on this system from multiple angles, both through activating Akt (Mannella and Brinton, 2006; Singh, 2001; Znamensky et al., 2003) and through increasing HKII activity (Kostanyan and Nazaryan, 1992), and thus it is hypothesized that estrogen may play a role in promoting the association of HKII and VDAC in neurons.
3.3 Estrogen Regulation of Mitochondrial Energy Production
A substantial number of the signaling pathways regulated by estrogen converge upon mitochondria (Brinton, 2008a; Mannella and Brinton, 2006; Nilsen and Brinton, 2003, 2004; Nilsen et al., 2006), and the upregulation of glucose transport and glycolysis mediated by estrogen is complemented by its potentiation of mitochondrial bioenergetics. Using proteomic analysis of brain mitochondria from female rats treated with E2, our lab identified several metabolic enzymes whose activity and protein expression levels were regulated by E2, including pyruvate dehydrogenase (PDH), aconitase, and ATP synthase (Nilsen et al., 2007). E2 treatment increased the expression of several PDH subunits (Nilsen et al., 2007), which is important because PDH is the primary regulatory enzyme that links glycolysis to the TCA cycle through the generation of acetyl CoA. Further, in the brain PDH is responsible for directing acetyl-CoA either to the TCA cycle or to be used for acetylcholine synthesis (Holmquist et al., 2007). E2 treatment increased expression of the Complex I β subunit 8 (Irwin et al., 2012). E2 also increased activity (Nilsen et al., 2007; Yao et al., 2012) and expression (Nilsen et al., 2007) of Complex IV (COX), which is consistent with previous findings (Bettini and Maggi, 1992; Stirone et al., 2005). Lastly, E2 treatment led to increased expression of Complex V/ATP synthase F1 subunits α and β (Nilsen et al., 2007). We had previously reported estrogen-induced increases in ATP levels in primary neuronal cultures (Brinton et al., 2000), which coalesces well with results seen in the proteomic analysis. Maximal mitochondrial respiratory rate in neurons and glia was also increased by E2 treatment, and E2 treatment protected against electron transport chain inhibitors (Yao et al., 2012). Thus the results of our analyses show that estrogen produces a coordinated response of many mitochondrial enzymes, leading to optimal glucose metabolism in the brain.
Further studies in our laboratory investigated the contributions of each of the estrogen receptor isoforms, ERα and ERβ, to the promotion of mitochondrial bioenergetics (Irwin et al., 2012). Using the ERα-selective agonist propylpyrazoletriol (PPT), which is 410-fold more selective for ERα than ERβ (Stauffer et al., 2000), and the ERβ-selective agonist diarylpropionitrile (DPN) is 70-fold more selective for ERβ than ERα (Meyers et al., 2001), Irwin et al were able to probe the effects of signaling cascades activated through ERα and ERβ. Hippocampal mitochondrial enzymes that showed increases in protein expression levels after treatment with PPT included PDH subunit E1 and ATP synthase F1 subunit α. DPN upregulated expression levels of COX subunit I, which is mtDNA-encoded. As PPT had no effect on COX subunit I expression, this suggests that mtDNA transcription is regulated by ERβ-dependent mechanisms. COX subunit IV expression and activity were upregulated by both PPT and DPN. This subunit is encoded by nuclear DNA, which suggests that ERα and ERβ are independently capable of upregulating specific mitochondrial proteins. Both agonists also reduced mitochondrial lipid peroxides, consistent with previous findings of estrogen induction of the antioxidants peroxiredoxin 5 and manganese superoxide dismutase (MnSOD) (Nilsen et al., 2007; Yao et al., 2012). Assessment of mitochondrial respiration from the same samples indicated that both PPT and DPN independently regulate mitochondrial function, often with comparable results, leading to enhanced mitochondrial respiration (Irwin et al., 2012). Consistent with the ERβ activation of mitochondrial function, analyses of an ERβ-selective formulation, PhytoSERMS, showed that the formulation induced a significant rise in mitochondrial respiration (Yao et al., 2013; Zhao et al., 2011a). While both ERα and ERβ promote mitochondrial function, targeting ERβ generally results in greater efficacy of mitochondrial respiration (Irwin et al., 2012; Yao et al., 2013).
Estrogen action at the mitochondria extends to protection against Aβ. Estrogen treatment not only increases ATP production in healthy hippocampal neurons, but also sustained ATP generation after the neurons were exposed to Aβ (Brinton et al., 2000). Ovariectomy in mice leads to both a decline in mitochondrial bioenergetics and an elevation in mitochondrial Aβ, but if estrogen treatment is started immediately following ovariectomy, both of these deleterious events are prevented (Yao et al., 2012).
3.4 Estrogen Regulation of Oxidative Stress
A byproduct of oxidative phosphorylation is the production of reactive oxygen species (ROS). During normal (non-pathological) oxidative phosphorylation, mitochondria generate 1-4% incompletely reduced oxygen which can react to form ROS (Cecarini et al., 2007; Sugioka et al., 1988). Electron transport chain complexes I and III have been implicated as the primary sources of ROS (Cadenas and Davies, 2000). Thus, a deficit in complex IV activity without a corresponding decline in activity of complexes I and III - as described in AD patients in Section 4 - could lead to increased ROS leak (Atamna and Frey, 2007). Because mitochondria are the main source of ROS production, they also suffer the greatest oxidative stress when excess ROS are produced. Many components of the mitochondrial bioenergetic network are particularly vulnerable to oxidative stress, e.g., lipid peroxidation of mitochondrial membranes and protein damage on the electron transport chain, which increases the risk of severe mitochondrial impairment that can lead to energetic failure of the cell and/or apoptosis (Blass, 2000; Lin and Beal, 2006; Yao et al., 2004).
In vitro, estrogen has been shown to protect against DNA damage induced by hydrogen peroxide (H2O2) and arachidonic acid (Moor et al., 2004; Tang and Subbiah, 1996). Estrogen also increases expression of several antioxidant enzymes, including peroxiredoxin 5, glutaredoxin and MnSOD (Nilsen and Brinton, 2004; Nilsen et al., 2007). In rodents, ovariectomy led to an increase in lipid peroxides that was prevented by E2 treatment (Irwin et al., 2008; Yao et al., 2012). Estrogen-induced rise in antioxidants and associated reduction in free radicals, and lower oxidative damage to mitochondrial DNA, has been proposed as a mechanism to explain the greater longevity of females relative to males (Borras et al., 2007; Vina et al., 2005; Vina et al., 2006).
4. Mitochondrial Relevance to Alzheimer’s Disease
Mitochondria play a critical role in generating energy for the cell, and increasing evidence links mitochondrial dysfunction to age-associated neurodegenerative disorders such as Alzheimer’s disease (Brinton, 2008a; Moreira et al., 2006; Moreira et al., 2010; Mosconi et al., 2011; Mosconi et al., 2009a; Swerdlow and Khan, 2009). Deficits in the activity of several key enzymes involved in mitochondrial energy generation have been described in AD. Decreased PDH activity in post-mortem brain homogenate from AD patients was one of the first deficits described (Perry et al., 1980), and this has since been confirmed by a number of other groups (Sheu et al., 1985; Sorbi et al., 1983; Yates et al., 1990). Microarray analyses also show a downregulation of PDH gene expression in patients with incipient AD, providing further support that a deficit in PDH activity is an early event in AD pathogenesis (Blalock et al., 2004). Activity of α-ketoglutarate dehydrogenase (αKGDH), the rate-limiting enzyme of the TCA cycle, is also deficient in post-mortem brain tissue from patients with AD (Butterworth and Besnard, 1990; Gibson et al., 2000b; Gibson et al., 1988; Mastrogiacoma et al., 1996; Mastrogiacomo et al., 1993). A decline in αKGDH activity is positively correlated with clinical dementia in sporadic (non-genetic) forms of AD, but patients who are APOE ε4 carriers (Gibson et al., 2000a) or carry the Swedish AβPP mutation KM670/671NL (Gibson et al., 1998) show the most reliable correlation between decreased αKGDH activity and degree of dementia. The most consistently documented deficit in mitochondrial enzyme function is decreased activity of COX, the penultimate complex of the electron transport chain. Deficient COX activity has been identified in post-mortem brain tissue (Cottrell et al., 2001; Kish et al., 1992; Maurer et al., 2000; Mutisya et al., 1994; Parker et al., 1994b) as well as platelets (Bosetti et al., 2002; Cardoso et al., 2004; Parker et al., 1990; Parker et al., 1994a; Valla et al., 2006a) and fibroblasts (Curti et al., 1997) from AD patients. As further evidence that a mitochondrial deficit is an early event in AD pathogenesis, the reduction of COX activity has been identified in peripheral tissues from patients with mild cognitive impairment (MCI) (Swerdlow and Kish, 2002; Valla et al., 2006a). Additionally, young adult APOE ε4 carriers without overt AD pathology showed lower COX activity in the posterior cingulate than young adult non-carriers (Valla et al., 2010). Considering that this deficit in COX activity is seen in peripheral tissues and not just in brain, it is unlikely to be a result of neurodegeneration (Swerdlow, 2009). Indeed, the fact that COX deficiency is not restricted to the brain suggests a systemic aspect to AD (Maruszak and Zekanowski, 2011; Swerdlow, 2011).
In the “cybrid model” of AD, neural cultures that lack endogenous DNA (termed ρ0 cells) are fused with platelets that lack a nucleus, creating “cybrids” (Swerdlow et al., 1997). Interestingly, when the cells are fused with platelets from AD patients, they exhibit characteristics that match the findings from clinical AD specimens, including decreased COX function that can be passed down through the cybrid lines (Swerdlow, 2007). This provides evidence that mitochondria from AD patients have particular abnormalities that are carried within mtDNA and are thus heritable, and which may explain the enzyme complex deficiencies seen in individuals with AD.
Multiple in vitro and in vivo preclinical analyses have demonstrated a decline in mitochondrial function prior to the onset of Alzheimer’s histopathological features. Results of these analyses indicate decreased metabolic enzyme expression and activity, decreased cerebral glucose metabolism, increased oxidative stress, and increased mitochondrial Aβ load (Chou et al., 2011; Du et al., 2010; Hauptmann et al., 2009; Nicholson et al., 2010; Silva et al., 2011; Yao et al., 2009). Thus this decline in brain mitochondrial function may serve as a biomarker of AD risk as well as a therapeutic target.
An impairment of mitochondrial bioenergetics and oxidative phosphorylation is often closely associated with increased free radical production and subsequent oxidative damage. As mentioned above in Section 3.4, the consistently documented deficit in complex IV activity which occurs without a corresponding decline in activity of complexes I and III could clog the electron transport chain and lead to increased ROS leak (Atamna and Frey, 2007). Animal models of AD show increased free radical generation and oxidative damage to cellular components prior to the development of pathology (Nunomura et al., 2009; Pratico et al., 2001; Rhein et al., 2009; Wang et al., 2005; Yao et al., 2009). Further, post-mortem analysis of brains from Alzheimer’s patients show markers of increased oxidative stress, including lipid peroxides, 8-oxoguanine, and oxidized amino acids (Gibson and Shi, 2010; Nunomura et al., 2009; Reddy, 2006). Interestingly, an increase in oxidative stress has been demonstrated to increase β-amyloid production in vitro and in vivo (Moreira et al., 2007; Nunomura et al., 2001).
Increasing evidence indicates that mitochondria are direct targets of Aβ. Aβ has been shown to accumulate inside mitochondria, where it interacts with the mitochondrial protein Aβ-binding-alcohol-dehydrogenase (ABAD), resulting in decreased COX activity and increased oxidative stress (Lustbader et al., 2004; Reddy and Beal, 2008; Takuma et al., 2005). In AD cybrids, Aβ-induced toxicity is exacerbated in parallel with mitochondrial dysfunction (Cardoso et al., 2004). At the same time that Aβ exerts its toxicity upon the mitochondria, compromised mitochondrial function - in particular, a decline in mitochondrial bioenergetics and increased levels of ROS - further drives the degenerative process by increasing Aβ result is a vicious cycle in which excessive Aβ accumulation and sustained mitochondrial dysfunction synergistically exacerbate each other, leading to activation of a multitude of neurodegenerative pathways (Cardoso et al., 2004; Silva et al., 2011; Swerdlow et al., 2010; Yao et al., 2011).
5. Estrogen Regulation of Whole-Body Metabolism
In addition to the brain, estrogen activates signaling pathways in nearly every tissue of the body; hence these pathways would be affected by loss of ovarian hormones associated with surgical or natural menopause (Figure 3). Menopause is associated with a significant decline in the ovarian production of both 17β-estradiol, the predominant estrogen during a woman’s reproductive years, and progesterone (Brinton, 2010; Nejat and Chervenak, 2010; Soules et al., 2001). Clinically, menopause is defined by one year of amenorrhea following the final menstrual period. Perimenopause typically begins approximately 2 years before menopause, and it encompasses the early and late transition stages as well as the first year after clinically-defined menopause (Soules et al., 2001). This perimenopausal transition is characterized by widely fluctuating hormone levels (Soules et al., 2001). In most women, the menopausal transition takes about 4 to 5 years (Woods and Mitchell, 2004), with the average age of menopause at 51 years (ESHRE Capri Workshop Group Authors, 2011). After menopause, estradiol levels no longer fluctuate as they did during perimenopause; instead, the ovaries gradually produce declining levels of estradiol and estrone becomes the predominant circulating estrogen (Nejat and Chervenak, 2010). The dysregulation of ovarian hormone secretion characteristic of the menopausal transition is in contrast to the male andropause, in which testosterone levels decrease steadily over a number of years (Ferrari et al., 2013).
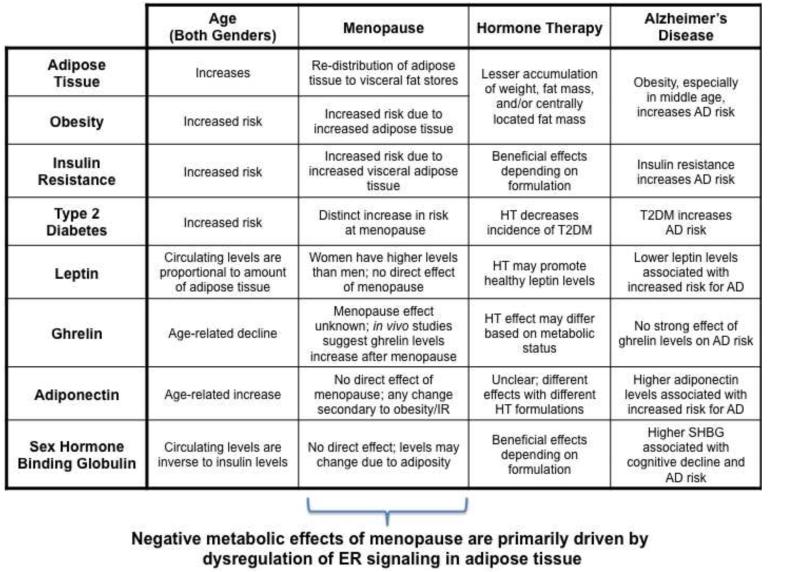
Figure 3. Estrogen regulation of whole-body metabolism.
Estrogen receptors are expressed throughout peripheral systems involved in metabolism. Estrogen affects adipose tissue distribution and risk of obesity, insulin resistance and risk of diabetes, and concentration of the adipokines leptin, ghrelin, and adiponectin. Loss of estrogen at menopause leads to significant changes in many of these systems, which can be stabilized with the use of hormone therapy. Adverse metabolic profiles, e.g. type 2 diabetes and metabolic syndrome, can increase risk of developing Alzheimer’s disease.
5.1 Estrogen, Adiposity, and Obesity
Adiposity is a measure of the amount of adipose tissue (fat deposition) in the body. Adipose tissue is preferentially stored in the form of subcutaneous adipocytes, but if energy intake is too high, the body will store fat as intra-abdominal visceral adipose tissue. This visceral fat is associated with most of the health risks of adiposity (Jensen, 2008). As adiposity increases, an individual’s risk of developing insulin resistance, diabetes, hypertension, and cardiovascular disease also increases (Pi-Sunyer, 2002; Poirier et al., 2006). Specifically, intra-abdominal obesity is associated with the greatest risk of all of these diseases independent of total body adiposity (Despres, 1993; Pouliot et al., 1992).
Adipose tissue has a different pattern of distribution in men and women. Age-matched men tend to accumulate a greater amount of abdominal fat relative to premenopausal women, who accumulate fat in a metabolically healthier gluteo-femoral pattern that is promoted by estrogen (Carr, 2003; Krotkiewski et al., 1983). After menopause, when estrogen levels drop, women experience a general increase in weight (Davis et al., 2012a; Pimenta et al., 2013) as well as a redistribution of adipose tissue leading to increased abdominal fat deposition (Bjorkelund et al., 1996; Toth et al., 2000; Zamboni et al., 1992). Importantly, the increased abdominal fat in postmenopausal women tends to be visceral and not subcutaneous fat (Lovejoy et al., 2008). This effect is also seen in healthy pre-menopausal women treated with gonadotropin-releasing hormone (GnRH) agonists (Revilla et al., 1998; Yamasaki et al., 2001). Thus men have an unhealthier adiposity profile compared to women before menopause, but after menopause the prevalence of intra-abdominal adiposity rises significantly in women.
Estrogen receptors are present in adipose tissue, indicative of the potential for estrogen regulation of adipocyte function (Mayes and Watson, 2004; Pallottini et al., 2008; Pedersen et al., 1996). ERα and ERβ may differentially mediate estrogen’s effects on adipose tissue, with ERα predominantly regulating adipose homeostasis via growth and proliferation of adipocytes, and ERβ regulating sex-specific distribution of adipose tissue (Pallottini et al., 2008). Estrogen signaling pathways mediated through ERα have been shown to upregulate α2A-adrenergic receptor expression in subcutaneous but not visceral adipose tissue (Pedersen et al., 2004). The α2A-adrenergic receptor controls anti-lipolytic pathways, promoting the accumulation of adipose tissue. Thus E2 signaling can bias adipose distribution towards the “healthier” subcutaneous fat depots versus unhealthy visceral fat deposition (Pallottini et al., 2008). Differential ERα and ERβ effects on adiposity have been investigated using ERα knockout (αERKO) mice, which develop severe intra-abdominal obesity (Heine et al., 2000). This provides evidence that ERα positively regulates adipose homeostasis and metabolism, whereas in the absence of ERα, unregulated signaling through ERβ promotes an unhealthy adipose phenotype (Naaz et al., 2002).
Sex dimorphisms in the distribution of ERα and ERβ in adipose tissue have been reported. Mature human adipocytes express both ERα and ERβ mRNA; expression of ERα is identical between the sexes whereas mRNA levels of ERβ are higher in women (Dieudonne et al., 2004). In human adipocytes isolated from subcutaneous and visceral fat depots from men and premenopausal women, exposure to E2 resulted in upregulated ERα mRNA levels in both adipocyte types in men, but only subcutaneous adipocytes in women. In men, E2 exposure did not affect ERβ mRNA levels, but in women ERβ mRNA was upregulated - again, only in subcutaneous adipocytes (Dieudonne et al., 2004). This suggests that men are more predisposed towards ERα-dominant adipose regulation, whereas women have a more balanced ratio of ERα and ERβ adipose regulation before menopause, which could explain sex-specific patterns of adipose tissue distribution. After menopause, however, a shift in the ERα/ERβ ratio towards greater ERβ signaling could mediate the postmenopausal increase in weight (Tomicek et al., 2011).
The issue of postmenopausal hormone therapy (HT) and changes in weight has been the subject of controversy. A meta-analysis published in 2000 indicated no difference in postmenopausal weight gain between women taking and not taking HT, but had insufficient data to assess HT effects on body-mass index (BMI), waist-hip ratio, or fat mass (Norman et al., 2000). However, a more recent meta-analysis conducted by the Endocrine Society reported that HT was associated with less accumulation of weight, fat mass, and/or centrally located fat mass (Santen et al., 2010). The 2000 meta-analysis was potentially influenced by its use of the term “weight” at menopause, there are large changes in both the distribution of body fat and the proportions of fat to non-fat mass, all of which can affect weight in different ways (Santen et al., 2010). Because increases in adiposity can be subcutaneous or visceral, women may experience a gain in weight, but their overall adipose profile could be healthier. Consistent with this postulate, HT use is associated with decreased abdominal/visceral fat (Haarbo et al., 1991; Salpeter et al., 2006). Additionally, it may be that some types of HT lead to greater weight gain than others (O’Sullivan et al., 1998), and weight changes may be less predictable in already obese women (Santen et al., 2010). Overall, in non-overweight and non-obese women, results from the Endocrine Society meta-analysis indicate that postmenopausal HT protects against weight gain, and also promotes less adipose tissue deposition in visceral fat stores (Santen et al., 2010).
Adiposity exists along a continuum, and a high degree of adiposity is referred to as obesity. Recent prevalence estimates show that approximately 70% of men and 62% of women in the US are either overweight (BMI > 25) or obese (BMI > 30); although rates of obesity are comparable between the sexes, more women fall into the category of extreme obesity (BMI > 40) (Ogden et al., 2006). This is a critical problem because obesity, specifically visceral obesity, is correlated with a large number of adverse cardiovascular outcomes. It is an established risk factor for coronary heart disease, which explains why premenopausal women have a lower risk of heart disease than age-matched men, but an increased risk after menopause (ESHRE Capri Workshop Group Authors, 2011; ESHRE Capri Workshop Group, 2006). Obesity is also well recognized as the primary risk factor for both insulin resistance (a pre-diabetic condition) and type 2 diabetes (Ahima, 2009; Pi-Sunyer, 2002).
Outcomes of multiple studies demonstrate that obesity is associated with an increased risk of dementia, including late onset AD. Increased BMI in middle-aged populations (Kivipelto et al., 2005; Whitmer et al., 2008; Whitmer et al., 2005) and older populations (Gustafson et al., 2003) is predictive of higher risk of all forms of dementia. In the Baltimore Longitudinal Study of Aging, both men and women showed associations between mid-life increases in BMI and higher incidence of AD (Beydoun et al., 2008). Measurements of waist to hip ratio in middle-aged and younger elderly individuals have also been shown to robustly correlate with higher risk of late-onset AD (Luchsinger et al., 2011a; Luchsinger et al., 2007a). There are some reports of no association between BMI and AD risk (Stewart et al., 2005) or of low BMI being related to AD risk (Nourhashemi et al., 2003); however, those results are likely affected by the age group being studied. Most research indicates that elevated BMI in middle age is related to an increased risk of dementia, while such association diminishes in elderly populations (Fitzpatrick et al., 2009; Luchsinger, 2008).
5.2 Estrogen and Insulin Resistance
Insulin is pivotal to maintaining and sustaining glucose metabolism in the periphery and in the brain (Cholerton et al., 2011; Craft, 2007; De La Monte, 2012). Insulin resistance is a state in which the tissues that require blood glucose have a diminished response to insulin, and the subsequent reduced clearance of glucose from blood feeds back onto the pancreas to increase secretion of insulin to induce glucose uptake (Luchsinger, 2008). Insulin resistance is primarily caused by obesity, particularly visceral obesity (Luchsinger et al., 2011b). Susceptibility to developing insulin resistance is known to increase with age (Carr, 2003; Iozzo et al., 1999), but there is less evidence that sex differentially affects development of insulin resistance. Fasting insulin (Poehlman et al., 1995; Razay et al., 2007) and glucose (Dallongeville et al., 1995; Lynch et al., 2002) levels have been shown to rise as estrogen levels decrease during the menopausal transition, but this is proposed as a secondary response to the change in body fat distribution and not a direct effect of estrogen decline (Wing et al., 1992; Wing et al., 1991). One study suggests women may have differential insulin metabolism after menopause, with postmenopausal women producing less insulin, but eliminating it more slowly (Walton et al., 1993).
The effects of estrogen signaling through ERs on insulin production have been studied in vivo using aromatase knockout mice (ArKO), which lack the enzyme responsible for conversion of androgens to estrogens (Jones et al., 2000). These animals develop insulin resistance by one year of age; as in humans, this is likely due to increased visceral fat. Larger fat depots lead to greater release of free fatty acids, which has the potential to disturb insulin dynamics in the liver (Pallottini et al., 2008). A similar development of insulin resistance in conjunction with an increase in fat mass is seen in ERα knockout (αERKO) mice (Bryzgalova et al., 2006; Heine et al., 2000; Manrique et al., 2012). Ovariectomy provides a good model of postmenopausal insulin resistance: loss of ovarian hormones induces an increase in body weight along with increased plasma glucose levels and decreased plasma insulin response to glucose, and this can be reversed through treatment with estrogen after ovariectomy (Bailey and Ahmed-Sorour, 1980; Ding et al., 2012; Zhu et al., 2013). Still, in these models, the challenge remains to distinguish between primary insulin resistance mediated by loss of estrogen and insulin resistance that develops secondary to adipose deposition.
In human clinical research, the majority of studies have focused on the impact of hormone therapy on incidence of type 2 diabetes (T2DM) as opposed to insulin resistance, although several studies have investigated the effect of HT on insulin resistance. A meta-analysis of studies of HT and insulin resistance indicated that postmenopausal HT significantly reduced levels of insulin resistance (as measured by the homeostatic assessment of insulin resistance (HOMA-IR) score) (Salpeter et al., 2006). Interestingly, two studies in non-human primates found that specific hormone formulations may change estrogen’s beneficial effects on insulin resistance; in these primates, a conjugated equine estrogen (CEE) formulation decreased body mass and HOMA-IR, whereas addition of the progestin medroxyprogesterone acetate (MPA) significantly increased body weight, fat mass, and HOMA-IR (Shadoan et al., 2003; Shadoan et al., 2007).
Insulin can cross the blood-brain barrier (Park, 2001) and insulin receptors are expressed throughout the brain with particularly high receptor densities in the hippocampus and entorhinal cortex (Burns et al., 2011). Insulin resistance in non-diabetic (or pre-diabetic) individuals is associated with increased risk of cognitive impairment and dementia (Craft, 2005; Xu et al., 2007; Yaffe et al., 2004). Further, hyperinsulinemia as a response to insulin resistance has been associated with increased risk of AD in multiple cross-sectional and longitudinal studies (Kuusisto et al., 1997; Luchsinger et al., 2004; Peila et al., 2004; Razay and Wilcock, 1994; Stolk et al., 1997). In a recent study, hyperinsulinemia a decade or more before death correlated with presence and severity of amyloid plaques upon autopsy (Matsuzaki et al., 2010). Additionally, HOMA-IR negatively correlates with hippocampal volume in cognitively normal middle-aged women (Rasgon et al., 2011). Within the brain, insulin may lead to decreased clearance of Aβ by competing for the insulin-degrading enzyme (Farris et al., 2003). Insulin has also been shown to increase tau phosphorylation (Park, 2001).
Early preclinical analyses indicated that a decreased cerebral metabolic rate of glucose uptake (CMRglu) is evident in rodent models of diabetes (Garris et al., 1984; Vannucci et al., 1997). These findings have also been shown in humans: higher insulin resistance was associated with decreased CMRglu in the same regions that show hypometabolism in AD (Baker et al., 2011). This was replicated in a later study in which higher fasting glucose in nondiabetic individuals was associated with decreased CMRglu in AD-affected brain regions (Burns et al., 2013). These findings support earlier analyses in humans indicating that a decrease in the cerebral metabolic rate of glucose uptake is one of the earliest events in the pathogenesis of AD (Mosconi et al., 2006; Reiman et al., 1996b). Collectively, preclinical and clinical data provide evidence of a link between development of insulin resistance and increased risk of AD, and suggest that insulin resistance could be a biomarker of risk of AD or preclinical AD.
5.3 Estrogen and Diabetes
The association between ovarian hormone loss and development of dysregulation of glucose homeostasis dates back decades. In preclinical analyses, ovariectomy resulted in increased plasma glucose levels, which was prevented by estrogen treatment (Bailey and Ahmed-Sorour, 1980; El Seifi et al., 1981). Estrogen regulation of insulin and glucose homeostasis is mediated through ERα receptors, which - in addition to their expression pattern in adipose tissue -are also expressed in the liver, pancreatic beta cells, and skeletal muscle (Meyer et al., 2011; Sutter-Dub, 2002). This allows for coordination of signals regulating metabolic homeostasis in response to hormonal milieu. ERα in pancreatic beta cells has been proposed to regulate insulin production in vivo, such that it has an anti-diabetic effect (Le May et al., 2006; Wong et al., 2010). Consistent with these findings, αERKO mice show impaired glucose tolerance and increased insulin resistance (Ribas et al., 2010). Several studies indicate a key role of mERs in regulating pancreatic beta cell function (Nadal et al., 2004; Sutter-Dub, 2002). Collectively, preclinical analyses indicate a role for estrogen in sustaining insulin and glucose homeostasis that underlies a healthy metabolic profile.
However, the relationship between estrogen levels and diabetes following menopause is complicated. Several studies have shown that, in women, higher endogenous estrogen levels after menopause are associated with greater risk of developing insulin resistance and diabetes (Ding et al., 2007; Kalish et al., 2003; Oh et al., 2002). An increase in diabetes risk factors was also seen in early studies using high-estrogen HT (Wynn et al., 1979; Wynn and Doar, 1966). However, the Women’s Health Initiative (WHI) (Margolis et al., 2004), Heart and Estrogen/Progestin Replacement Study (Kanaya et al., 2003) and the Nurses’ Health Study (Manson et al., 1992) all reported that women taking HT had fewer cases of incident diabetes. It should be noted that most of these large epidemiological studies used combination hormone therapy (CEE + MPA), so it is also unclear how well the results generalize to other HT regimens. Several recent prospective cohort studies have also found reduced incidence of new-onset diabetes in women taking HT (de Lauzon-Guillain et al., 2009; Pentti et al., 2009); this effect was much stronger for women taking oral HT vs. transdermal HT (de Lauzon-Guillain et al., 2009) and for women who had long-term HT exposure (Pentti et al., 2009). In the WHI trial, women with larger waist circumference measurements showed a greater beneficial effect of HT on decreasing diabetes risk (Margolis et al., 2004). An unresolved issue is whether HT has a direct effect on diabetes risk through regulation of pancreatic ERα signaling, or an indirect effect through decreasing obesity.
Better concordance exists between outcomes of preclinical and clinical analyses of dysregulated glucose metabolism and cognitive function. Diabetes is strongly linked to cognitive impairment in both preclinical and clinical studies. Rats with streptozotocin-induced diabetes have deficits in learning and memory (Baydas et al., 2003; Kucukatay et al., 2007; Lupien et al., 2003; Stranahan et al., 2008; Tiwari et al., 2009; Ye et al., 2011). Both pigs and rats with combined diabetes and hypercholesterolemia show increased blood-brain barrier permeability and increased Aβ plaque deposition (Acharya et al., 2013). Diabetic animal models also show decreased insulin uptake into the brain and consequent reduced levels of neuronal insulin (Banks et al., 1997; Baskin et al., 1985; Kaiyala et al., 2000).
Persons with diabetes have a high risk of cognitive dysfunction, including amnestic MCI (Luchsinger et al., 2007b; Solfrizzi et al., 2004) and all types of dementia (Brayne et al., 1998; Brismar et al., 2007; Northam et al., 2006; Ott et al., 1999; Ristow, 2004; Stewart and Liolitsa, 1999). One study reported that diabetes was only related to risk of vascular dementia (VaD) (MacKnight et al., 2002), whereas others have reported that diabetes is associated with increased risk of developing both AD and VaD, but that the relative risk for VaD is greater than that for AD (Luchsinger et al., 2005; Luchsinger et al., 2001; Yoshitake et al., 1995). However, the majority of studies have found diabetes to be related to a higher risk of AD, particularly late-onset AD (Arvanitakis et al., 2004; Cheng et al., 2011; Leibson et al., 1997; Luchsinger et al., 2005; Peila et al., 2002). Two recent meta-analyses identified 15 separate studies in which the association between AD and diabetes was investigated, and concluded that diabetes is an independent risk factor for AD (Vagelatos and Eslick, 2013; Williams et al., 2010). The presence of diabetes in patients who are newly diagnosed with AD is also related to greater baseline cognitive impairment and more rapid progression of AD (Sanz et al., 2011).
The clear association between type 2 diabetes and risk of dementia emphasizes the importance of strategies that reduce the risk of developing insulin resistance and dysregulation of glucose homeostasis in both the periphery and the brain. Thus, clearly establishing the factors that determine efficacy of estrogen or hormone therapy to reduce the risk of type 2 diabetes has the potential to greatly impact both the incidence of both type 2 diabetes and associated dementias.
5.4 Estrogen and Leptin
Leptin is an adipokine secreted by adipose tissue that has a strong effect on regulating energy intake and expenditure (Brennan and Mantzoros, 2006). Circulating levels of leptin are directly proportional to the amount of adipose tissue in the body. Leptin functions through binding leptin receptors in the hypothalamus, where it produces both an acute inhibition of appetite due to food intake, and a more long-term inhibition based on the body’s fat stores (Brennan and Mantzoros, 2006). Absence of leptin or genetic knockout of the leptin receptor leads to extreme obesity, as demonstrated by the ob/ob mouse. Diseases such as obesity and metabolic syndrome are associated with chronically high leptin levels (Brennan and Mantzoros, 2006); conversely, following a low-fat diet decreases circulating leptin (Dubuc et al., 1998). Both pre- and post-menopausal women have higher leptin levels than men, which may be due to endogenous estrogen levels or adipose tissue distribution (Dedeoglu et al., 2009; Rosenbaum et al., 1996; Saad et al., 1997). In premenopausal women, leptin levels correlate with plasma levels of estrogen; this correlation disappears after menopause, when it is confounded by a rise in obesity (Hong et al., 2007).
Together with leptin receptors, ERα and ERβ are expressed in the hypothalamus, although expression of ERα is greater than ERβ (Brown et al., 2010). There is evidence that changing estrogen levels across the estrus cycle regulate leptin receptor expression at the mRNA level, likely through an ERE located on the leptin receptor gene (Bennett et al., 1999). The result of this regulation is higher sensitivity to leptin when estrogen levels are higher (Brown et al., 2010). Crosstalk between leptin receptors and ERs leads to activation of the Stat3/Erk pathway (Fusco et al., 2010; He et al., 2012). Shp2, a nonreceptor tyrosine phosphatase, has been proposed to mediate this cross-talk between leptin receptors and ERs (He et al., 2012). Analyses show that Shp2 associates with ERα, and that it is through this association that leptin and estrogen synergistically activate the Erk signaling pathway. The interaction between leptin and estrogen signaling pathways provides a mechanistic rationale for the observation that postmenopausal women may have a reduced response to leptin once estrogen levels decrease, which could lead to the increased obesity seen after menopause.
There is no consensus as to whether leptin levels increase, stay the same, or even decrease after menopause, and each of these has been reported by multiple clinical studies (reviewed by (Dedeoglu et al., 2009)). This is likely due to age differences between the populations studied in each trial. One group showed that total body fat and subcutaneous fat were more predictive of leptin levels than visceral fat; as the menopausal transition is typically associated with a redistribution of adipose tissue from subcutaneous to visceral fat stores, this could account for decreased leptin after menopause (Dua et al., 1996). However, menopause is also associated with more weight gain, which could lead to an increase in leptin levels.
Studies using rat ovariectomy models showed that after OVX, rats retain the sensitivity to estrogen signaling effects on leptin, which is promising for studies of hormone therapy (Machinal et al., 1999). Clinical studies have shown that women on HT typically have both better maintenance of weight and healthier leptin levels (which could refer to an increase or decrease in leptin depending on overall metabolic status), although it is unclear which of these the principal driving force - or, again, if the driving forces are bidirectional (Dedeoglu et al., 2009 ; Di Carlo et al., 2004). However, other studies show that once BMI is corrected for, the association between HT and healthier leptin levels disappears (Bednarek-Tupikowska et al., 2006; Gower et al., 2000).
A typical feature of AD is decreased BMI over the course of the disease. One hypothesis is that this could be due to a disruption in energy homeostasis; thus, some recent studies have investigated whether leptin levels are associated with AD. In vitro and in vivo, leptin has a multitude of beneficial effects. A recent review summarized much of the preclinical research on leptin as it relates to AD; critically, apart from leptin’s effects in the hypothalamus, there is a significant amount of research showing that leptin has neurogenic and neuroprotective actions in the hippocampus (Paz-Filho et al., 2010). In vitro, leptin activates the AMPK signaling pathway (Greco et al., 2011), which is critical for stimulation of neuronal energy production, either through glucose or lipid metabolism (Salminen et al., 2011). Further, there is also evidence that Aβ production and tau phosphorylation can be mediated through the AMPK pathway (Greco et al., 2009a; Greco et al., 2009b; Greco et al., 2008; Salminen et al., 2011). Additionally, leptin decreased the amount of neurodegeneration caused by Aβ (Perez-Gonzalez et al., 2011). Similar effects were seen both in the CRND8 mouse model of AD (Greco et al., 2010), and in rabbits (Marwarha et al., 2010), where treatment with leptin decreased both amyloid burden and phosphorylated tau in the hippocampus. Leptin has also been shown to reduce neuronal β-secretase activity as well as APOE-dependent Aβ uptake (Fewlass et al., 2004). In the APP/PS1 mouse model of AD, leptin treatment increased proliferation of neuronal precursors in the dentate gyrus subgranular zone (Perez-Gonzalez et al., 2011).
In healthy elderly individuals, higher leptin levels are associated with higher gray matter volumes in the hippocampus (Narita et al., 2009) and less cognitive decline (Holden et al., 2009). Longitudinal studies have shown that both women and men with higher baseline levels of leptin have a decreased risk of incident AD (Lieb et al., 2009). Leptin levels decrease as severity of AD increases, and AD patients typically have lower leptin levels than controls (Bigalke et al., 2011; Warren et al., 2012). This is primarily due to a decrease in BMI over the course of the disease. However, it should be noted that some studies report no difference in leptin levels between AD patients and controls (Theodoropoulou et al., 2012). This may be due to differences in weight loss: one study reported that AD patients who experienced significant weight loss had lower leptin levels than those whose weight remained stable (Power et al., 2001).
5.5 Estrogen and Ghrelin
Ghrelin, another of the adipokines, is the counterpart to leptin. It is the only known appetite-stimulating hormone, and it is predominantly produced by the stomach (Inui et al., 2004; Nakazato et al., 2001). Ghrelin binds to the growth hormone secretagogue receptor (GHSR) in the hypothalamus (De Vriese and Delporte, 2007), where it stimulates the release of growth hormone. There are also ghrelin receptors in the hippocampus (Carlini et al., 2004), and ghrelin has been shown to increase dendritic spine density and promote long-term potentiation (Diano et al., 2006). There is not a clear consensus as to whether women naturally have higher ghrelin levels or if levels are the same between the sexes (Makovey et al., 2007). However, both men and women show an age-related decline in plasma ghrelin levels (Rigamonti et al., 2002) and growth hormone release in response to ghrelin stimulation (Broglio et al., 2003).
Analyses of ovarian hormone regulation of ghrelin indicate that both plasma ghrelin levels and ghrelin mRNA levels in stomach cells increased after ovariectomy, and these effects were reversed with E2 treatment. Additionally, ghrelin has been shown to colocalize with ERα in stomach (Matsubara et al., 2004). This suggests that estrogen is a negative regulator of ghrelin synthesis and thus ghrelin levels would be expected to increase following menopause. However, several studies have shown that HT has no effect on ghrelin levels in postmenopausal women (Lambrinoudaki et al., 2008; Purnell et al., 2003). One study reported increased ghrelin levels in receiving oral HT whereas transdermal HT had no effect (Kellokoski et al., 2005). Another study found that ghrelin levels increased in general over a 1.5 year period; surprisingly, ghrelin levels increased most sharply in those women who initiated and discontinued HT within the 1.5 years of the study (Soni et al., 2011). Conversely, in a study of obese women with metabolic syndrome, HT decreased ghrelin levels (Chu et al., 2006). These findings indicate that HT induces an increase or decrease in ghrelin levels based on metabolic status.
In vitro, ghrelin improves glucose/insulin homeostasis by decreasing insulin resistance, while also decreasing tau phosphorylation via activation of GSK3β (Chen et al., 2010). In vivo, administration of ghrelin agonists has been shown to improve cognition (Atcha et al., 2009) and neurogenesis (Moon et al., 2009). Additionally, ghrelin can protect against synaptic loss and neuronal degeneration induced by Aβ injection into the hippocampus (Moon et al., 2011).
Most studies show that plasma ghrelin levels are the same between AD patients and controls (Proto et al., 2006; Theodoropoulou et al., 2012). However, when challenged with a glucose load, male AD patients had a smaller area-under-the-curve measurement for ghrelin levels (Theodoropoulou et al., 2012). Reduced ghrelin mRNA levels have been observed in the temporal gyrus of patients with AD (Gahete et al., 2010).
5.6 Estrogen and Adiponectin
Adiponectin is the predominant adipokine regulating overall body metabolism and development of the metabolic syndrome (Hanley et al., 2007). It is produced by adipose tissue, and regulates peripheral glucose and insulin levels (Diez and Iglesias, 2003; Dridi and Taouis, 2009). Plasma adiponectin levels are inversely correlated with glucose and insulin levels, and lower adiponectin leads to insulin resistance and dyslipidemia (Cai et al., 2012; Dridi and Taouis, 2009). Further, higher adiponectin levels are associated with lower risk of incident diabetes in prospective studies (Li et al., 2009; Zhu et al., 2010). Adiponectin also appears to play a role in inhibiting inflammatory processes (Hatzis et al., 2013). Adiponectin levels are higher in females than males (Andreasson et al., 2012; Hotta et al., 2000) and increase with age in both sexes (Andreasson et al., 2012; Obata et al., 2012). Unlike leptin and ghrelin - which exert their peripheral effects via the hypothalamus - adiponectin effects appear to be primarily peripherally mediated (Ukkola and Santaniemi, 2002).
Evidence points to ERα as a positive regulator of adiponectin levels in adipose tissue. The balance between ERα and ERβ signaling in adipose tissue changes after menopause, with ERβ becoming the dominant ER (Tomicek et al., 2011). Higher ERβ signaling leads to inhibition of peroxisome proliferator activated receptor gamma (PPARγ), which regulates secretion of adiponectin (Foryst-Ludwig et al., 2008). In vivo studies support this: in rats, OVX was associated with increased ERβ, decreased PPARγ, decreased plasma adiponectin levels and also decreased expression of one adiponectin receptor isoform (Tomicek et al., 2011). Further evidence of positive regulation of adiponectin through ERαcomes from a study using αERKO mice; these animals had lower PPARγ levels and decreased adiponectin compared to WT mice (Ribas et al., 2010). Similarly, in humans, a particular ERα polymorphism that is associated with poorer prognosis after myocardial infarction is also associated with lower serum levels of adiponectin (Yoshihara et al., 2009).
Although there is a slight increase in adiponectin with age, there does not appear to be a significant difference in adiponectin levels associated specifically with menopause (Ahtiainen et al., 2012) or loss of ovarian hormones (Benetti-Pinto et al., 2010). Soni et al found that adiponectin levels increase slightly over 1.5 years in postmenopausal women, but this increase was driven by an increase in adiposity (Soni et al., 2011). As with leptin and ghrelin, and effect of HT on adiponectin is varied, likely because of different HT formulations in different populations. Several studies show increased adiponectin levels in women taking HT (Christodoulakos et al., 2008; Ruszkowska et al., 2013), whereas one study reported decreased adiponectin levels with HT (Im et al., 2006).
A recent study reported that individuals with MCI and AD have higher adiponectin levels both in CSF and plasma (Une et al., 2011). However, other studies have shown no difference in plasma adiponectin levels between AD patients and healthy controls (Bigalke et al., 2011; Warren et al., 2012). The Framingham Heart Study found that a higher baseline adiponectin levels was associated with increased risk of incident AD only in women (van Himbergen et al., 2012). Thus it is unclear if adiponectin itself is associated with AD development, or if it is more closely associated with metabolic disorders - such as obesity (Ukkola and Santaniemi, 2002) or metabolic syndrome (Renaldi et al., 2009) - either of which would increase an individual’s risk for AD.
5.7 Estrogen and Sex Hormone Binding Globulin
Sex hormone-binding globulin (SHBG) is a glycoprotein that binds to hormones as they circulate in the bloodstream. It is produced in the liver, and its production is stimulated by estrogen and testosterone (Plymate et al., 1988). SHBG has different affinities for different hormones; it binds testosterone with a very high affinity, and its affinity is less for estrogen (Wallace et al., 2013). Traditionally it was thought that only unbound (“free”) hormones were able to act on their respective receptors, and through this mechanism SHBG could influence the bioavailability of hormones. However, recent evidence indicates that there is also a G-protein-coupled receptor which binds SHBG on cell membranes (Rosner et al., 2010; Wallace et al., 2013). The SHBG receptor mechanism of action is not yet known; it may allow the bound hormone to have intracellular effects without entering the cell (Rosner et al., 1999), or it may allow the SHBG-hormone complex to enter the cell through endocytosis (Hammes et al., 2005). SHBG binding to its receptor may also prompt expression of estrogen or testosterone receptors on the cell membrane (Wallace et al., 2013). Signaling through the G-protein-coupled receptor promotes intracellular release of cAMP and activation of PKA (Rosner et al., 2010). Interestingly, SHBG bound to estrogen has agonist effects on the intracellular signaling cascade, and SHBG bound to testosterone has antagonist effects (Rosner et al., 2010).
SHBG levels are closely associated with metabolism; in particular, several in vitro and in vivo studies have shown that insulin can inhibit the synthesis of SHBG, decreasing the amount available to bind hormones and increasing hormone bioavailability (Nestler, 1993; Plymate et al., 1988). In humans, there tends to be an inverse relationship between plasma insulin level and SHBG concentration (Akin et al., 2009; Preziosi et al., 1993), which implicates severity of insulin resistance in determining SHBG levels. Insulin resistance and SHBG concentration have been examined in a number of clinical studies, and it has been consistently shown that low SHBG levels correlate with higher HOMA-IR scores (Akin et al., 2007; Akin et al., 2009; Davis et al., 2012b; Kalish et al., 2003; Li et al., 2010; Yasui et al., 2007). This relationship appears independent of BMI (Davis et al., 2012b), but may be mediated by abdominal obesity (Akin et al., 2009). SHBG is also associated with diabetes risk, and higher plasma levels of SHBG have been linked to lower risk of developing T2DM (Ding et al., 2006; Ding et al., 2009; Kalyani et al., 2009).
Women on average have higher SHBG levels than men (Bukowski et al., 2000; Soriguer et al., 2012) (http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/9285). It is unclear whether SHBG levels change with menopause after correcting for changes in insulin levels. Multiple clinical studies have measured plasma SHBG concentrations in pre- and post-menopausal women, and most (Akin et al., 2009; Key et al., 2011; Pasquali et al., 1997) but not all (Burger et al., 2000) have found no effect of menopause status on SHBG levels. The Study of Women’s Health Across the Nation found that overall, there were no significant longitudinal changes in SHBG across the menopause transition, and any small decreases in SHBG levels were driven by changes in adiposity (Wildman et al., 2012). Interestingly, however, some studies have found that the inverse association between HOMA-IR and SHBG levels is only seen in postmenopausal, not premenopausal, women (Akin et al., 2007; Akin et al., 2009; Davis et al., 2012b). After menopause, type of hormone therapy can also affect SHBG levels; based on a number of small clinical studies, oral E2 therapy increases plasma SHBG levels, whereas transdermal therapy has no effect (Selby et al., 1989; Taskinen et al., 1996; Vehkavaara et al., 2000) (reviewed in (Goodman, 2012)).
Several studies have detected higher SHBG levels in persons with AD relative to healthy controls (Hogervorst et al., 2004; Hoskin et al., 2004; Paoletti et al., 2004). In a recent longitudinal study, elderly men and women who were dementia-free at baseline had a greater risk of incident dementia and AD as their SHBG levels increased (Muller et al., 2010). The increased risk remained significant even after correction for age and BMI; however, it is still unclear whether this increased risk was associated with bioavailable testosterone, or whether it was perhaps associated with another dementia risk factor (such as insulin resistance or diabetes).
6. PET Imaging of Brain Bioenergetic Deficits in Aging and Alzheimer’s Disease
Positron emission tomography (PET) scanning using fluorodeoxyglucose (FDG, a radioisotope of glucose) is a strategy to assess metabolic activity of the brain. The FDG-PET signal is particularly relevant to glucose required for synaptic activity (Landau et al., 2011). In healthy normal individuals, some studies have shown no significant differences in the cerebral metabolic rate of glucose uptake (CMRglu) between the genders (Miura et al., 1990; Tyler et al., 1988), whereas others have shown that females have higher CMRglu rates than males (Andreason et al., 1994; Yoshii et al., 1988). However, none of the studies above corrected for estrogen and progesterone levels, and it has been shown that in premenopausal women there are differences in glucose metabolism based on phase of the menstrual cycle (Reiman et al., 1996a). Thus, it is difficult to discern whether estrogen and progesterone support the same levels of CMRglu as testosterone. In normal aging, CMRglu declines with age, with the most significant declines occurring in the prefrontal cortex (Chetelat et al., 2013; Kalpouzos et al., 2009; Pardo et al., 2007). In the menopausal female brain, this age-related decline in glucose metabolism in prefrontal cortex has been detected, as well as a decline in glucose metabolism in the posterior cingulate that was specific to estrogen deprivation (Rasgon et al., 2005). In a separate study, women had a greater age-related metabolic decline than men (Murphy et al., 1996b).
A reduced rate of brain glucose metabolism is one of the most frequently documented abnormalities in AD. Alzheimer’s patients show a specific pattern of abnormal glucose metabolism in regions of the brain that are most vulnerable to development of pathology, including the temporoparietal cortex, posterior cingulate, hippocampus, and precuneus (Bero et al., 2011; Jagust et al., 2007; Mosconi et al., 2009b; Vaishnavi et al., 2010; Vlassenko et al., 2010). In at-risk populations, a decline in cerebral glucose utilization appears decades prior to the onset of clinical AD (Chen et al., 2011; de Leon et al., 2001; Mosconi et al., 2009b; Reiman et al., 2004) and precedes brain atrophy (De Santi et al., 2001). Decreased glucose uptake correlates with degree of cognitive impairment as measured by the MMSE or the Alzheimer’s Disease Assessment Schedule—Cognition subscale (ADAS-Cog) (Habeck et al., 2012; Minoshima et al., 1997), as well as with lower cerebrospinal fluid (CSF) Aβ42 and higher total tau and phosphotau levels (Petrie et al., 2009).
In persons with mild cognitive impairment, glucose hypometabolism accurately predicts future clinical progression to AD (Chen et al., 2011; Chetelat et al., 2003; Herholz et al., 2011; Landau et al., 2010; Walhovd et al., 2010). The hallmark pattern of hypometabolism in AD can be detected in the aging brain long before the diagnosis of AD (Jagust et al., 2006; Mosconi et al., 2008). In fact, FDG-PET measurements of glucose metabolism are more closely related to changes in cognitive status than CSF or PET measurements of Aβ42 (Jagust et al., 2009).
Consistent with clinical findings, multiple mouse models of AD display a pattern of reduced metabolism in the posterior cingulate/retrosplenial cortex (Nicholson et al., 2010; Valla et al., 2008; Valla et al., 2006b). Specifically, the triple transgenic Alzheimer’s disease (3xTgAD) mouse, which has a measurable decline in mitochondrial enzyme activity at 3 months and develops noticeable plaques by 6 months of age (Yao et al., 2009), has reduced metabolism that is evident as early as 2 months of age (Nicholson et al., 2010). During reproductive senescence, both the normal and 3xTgAD brain show a significant decline in glucose metabolism, which is also evident in the ovariectomized mouse (Ding et al., 2013a; Ding et al., 2013b).
7. Estrogen and Hormone Therapy, the Timing Hypothesis, and Risk of Alzheimer’s Disease
It has been proposed that the loss of estrogen after menopause could significantly contribute to cognitive decline and AD risk in women (Brinton, 2008b). Based on estrogen’s myriad positive effects on mitochondrial energy production and regulation of Aβ accumulation, it seems reasonable to think that estrogen replacement after menopause would have beneficial effects on cognition in general, and might even be able to reduce the risk of AD. Epidemiological studies and clinical trials of hormone therapy and cognition have shown mixed results (Hogervorst et al., 2000; van Amelsvoort et al., 2001). The prevailing data indicate that although HT cannot alter the progression of AD (Asthana et al., 2001; Asthana et al., 1999; Henderson et al., 2000; Mulnard et al., 2000; Wang et al., 2000), it may have the potential to lower the risk of women developing AD in the first place (Wise et al., 2001). Results from several longitudinal studies of HT use in postmenopausal women indicate that long-term HT helps to preserve memory (Jacobs et al., 1998; Maki et al., 2001; Resnick et al., 1997). Most studies also show that HT is associated with better cognitive function in nondemented older women (Duka et al., 2000; Jacobs et al., 1998; Kimura, 1995; Resnick et al., 1997; Robinson et al., 1994; Steffens et al., 1999), although one found no effect of HT on cognitive function (Barrett-Connor and Kritz- Silverstein, 1993). Multiple epidemiological studies - but not all - show that HT reduces the risk of AD, which researchers suggest may be due to delayed disease onset (Henderson et al., 1994; Kawas et al., 1997; Paganini-Hill and Henderson, 1996; Simpkins et al., 1994; Tang et al., 1996; Yaffe et al., 1998; Zandi et al., 2002).
Results from several imaging studies support the idea that postmenopausal HT can modulate brain bioenergetics, likely leading to the maintenance of cognitive function and reduced risk of AD. In the earliest study of brain metabolism in healthy postmenopausal women who were or were not receiving HT, patterns of regional cerebral blood flow (rCBF) were measured during various memory-related tasks (Resnick et al., 1998). Women taking HT showed a different pattern of rCBF than women not taking HT that was particularly evident in brain regions involved in memory systems, and the women taking HT also had superior performance on memory tasks (Resnick et al., 1998). In a follow-up study of the same women two years later, HT users showed increased rCBF over time compared to nonusers in the hippocampus, parahippocampal gyrus, and temporal lobe, regions that are critical for memory formation and are also vulnerable to decreased glucose metabolism in preclinical AD (Maki and Resnick, 2000). As before, the HT users scored higher on a battery of memory tests than nonusers (Maki and Resnick, 2000).
Glucose metabolism in cognitively intact women who were currently taking (ERT+) and had never taken (ERT-) estrogen replacement therapy, as well as women with clinically diagnosed AD, has also been studied using FDG-PET (Eberling et al., 2000). The ERT+ cohort had the highest rates of glucose metabolism, and their metabolic rates were significantly higher than those seen in the women with AD. Women in the ERT-cohort had glucose metabolism rates that were intermediate to the two other groups, but their rates were not significantly different from the women with AD (Eberling et al., 2000). This provides evidence that estrogen depletion can affect glucose metabolism. Similarly, a longitudinal study compared glucose metabolism between cognitively healthy women who did and did not take hormone therapy. At baseline, the two groups of women showed no differences in metabolism; after two years, the women not on hormone therapy had a significant decrease in glucose metabolism in the posterior cingulate cortex - an area of the brain which shows metabolic decline in the earliest stages of AD - whereas the women receiving estrogen therapy did not have a significant metabolic change in this brain region (Rasgon et al., 2005). This preservation of brain metabolism was later shown to be greatest in women taking 17β-estradiol-based hormone therapy formulations (Silverman et al., 2011). Although these studies are observational, they provide evidence that peri- or postmenopausal HT has a positive effect on brain bioenergetic function in women.
A challenge for the field was that results from the Women’s Health Initiative Memory Study (WHIMS) opposed the idea that estrogen was protective against development of AD. In the WHIMS study, both women in the estrogen-alone cohort (Shumaker et al., 2004) and women in the estrogen + progesterone cohort (Shumaker et al., 2003) showed an increased risk of developing AD. However, women in the WHIMS trial were significantly older than women in many of the prior epidemiological studies: on average, they were 65 years old, which is at least a decade past menopause. Additionally, the average BMI of women enrolled in the Women’s Health Initiative study (the population from which the WHIMS women were drawn) was in the overweight range, which likely would have put these women at a higher risk for metabolic diseases such as diabetes, thus increasing their risk for AD (Stefanick et al., 2003). Further - as discussed previously - the unhealthy metabolic status of these women could have potentially attenuated any positive response to hormone therapy.
Thus, a “critical window” theory of hormone replacement has been suggested, based on the concept that estrogen has beneficial effects if taken before or at the time of menopause when neurological health is still intact, but detrimental effects if initiated years after menopause when neurological health may have already begun to decline (Brinton, 2004, 2005). Model systems that have been used to investigate the role of hormone replacement after menopause fall into two distinct classes: preventative interventions in healthy organisms, and “restoration” interventions in organisms with compromised neurological function (Brinton, 2005, 2008a, b). In our laboratory, all systems that have led to the elucidation of neuroprotective effects of estrogen and underlying mechanisms of action have typically used a prevention experimental paradigm (Brinton, 2005, 2008a, b; Yao et al., 2012). Data from other researchers also supports this critical window hypothesis: estrogen replacement in rats shortly after ovariectomy is associated with in improvements in learning, whereas estrogen replacement after a long period of estrogen deprivation is disadvantageous (Daniel et al., 2006; Gibbs, 2000). Further, this prevention paradigm was investigated in women with surgical or pharmacological-induced menopause, where the beneficial effects of HT shortly after menopause paralleled the effects seen in animal model prevention studies (Phillips and Sherwin, 1992; Sherwin, 1988, 2012). An increasing body of clinical literature also supports the concept of a critical window of estrogen benefit, which aligns with the healthy cell bias of estrogen action (Bagger et al., 2005; Berent-Spillson et al., 2010; Brinton, 2008b; Henderson et al., 2005; MacLennan et al., 2006; Maki, 2013; Maki et al., 2011; Shao et al., 2012; Whitmer et al., 2011; Zandi et al., 2002).
8. Biomarkers: Systems Biology vs. Individual Markers
As reviewed above, the process of aging in the female involves a shift in bioenergetic systems. Within the brain, this is evidenced by changes in substrate supply, substrate metabolism, and mitochondrial respiration that, when combined, lead to a change from a highly efficient, glucose-driven phenotype to a less efficient, ketone-dependent phenotype (Yao et al., 2011). Peripherally, changes in glucose/insulin homeostasis and adipokine regulation of energy intake and expenditure are coincident with metabolic changes occurring in the brain. Therefore, development of peripheral biomarker profiles, rather than individual markers of components within the system, can serve as a reporter of brain metabolism.
The criteria for a biomarker of AD were proposed in 1998 by the Working Group on Molecular and Biochemical Markers of Alzheimer’s Disease, and have since become standards for the field. The Working Group specified: “the ideal biomarker for AD should detect a fundamental feature of neuropathology and be validated in neuropathologically-confirmed cases; it should have a diagnostic sensitivity >80% for detecting AD and a specificity of >80% for distinguishing other dementias; it should be reliable, reproducible, non-invasive, simple to perform, and inexpensive” (Authors, 1998).
Identification of biomarkers is critical to reliably identify populations at risk for AD, and for development of interventions to prevent, delay, and treat AD. Conceptualization of Alzheimer’s disease is shifting from a disease initiated by a single pathological entity to a failure of multiple interacting systems (Yao et al., 2011). A systems-level perspective predicts that failures at different points within the system can differentially affect future risk of developing AD. This conceptualization predicts that biomarker profiles which define an individual’s risk may shift over time and during the prodromal phase. A further prediction is that multiple therapeutic interventions that target risk phenotypes will be necessary.
Both basic science research and clinical observations suggest that menopausal loss of estrogen plays a significant role in brain aging, and can increase Alzheimer’s disease risk in women (Brinton, 2008b; Paganini-Hill and Henderson, 1994). Evidence indicates that estrogen is a systems-level regulator of brain and whole-body bioenergetics, with a decline in metabolism occurring coincidentally with loss of estrogen at menopause. Of the 5.2 million Americans diagnosed with LOAD, 3.4 million are women and 1.8 million are men (Authors, 2013). Most epidemiological studies indicate higher prevalence and greater lifetime risk of Alzheimer’s disease in women (Authors, 2013; Gao et al., 1998; Seshadri et al., 2006). While prevalence of AD in women is well documented, the incidence rate of AD in women versus men is controversial, with some studies showing higher incidence in women (Fratiglioni et al., 1997; Launer et al., 1999; Ott et al., 1998) and some showing equal incidence between the sexes (Edland et al., 2002; Fillenbaum et al., 1998; Hebert et al., 2001). Upon reviewing the effects of hormone therapy on both brain and whole-body metabolism, it is apparent that while some women may benefit from postmenopausal HT, others may experience detrimental effects. It is also clear that metabolic status can be a key factor in evaluating the risk/benefit profile of hormone therapy.
9. Conclusions
As discussed throughout this review, the decrease in estrogen that women experience during menopause leads to a wide range of changes throughout the metabolic system of the brain and body. Moreover, the tight integration of these systems predicts that alterations within one component of the system will obligate other components to adapt. These adaptations under certain circumstances can compensate for the loss of estrogen regulation. However, such adaptations are highly individualistic. Some women will compensate very well for the rest of their life, whereas women on the other end of the adaptive spectrum will be unable to compensate. Some women will fall in between: they will be able to compensate well, but for a relatively short period of time. We hypothesize that it is women in the latter two categories of compensatory capacity who are at risk for developing late-onset Alzheimer’s disease.
Critical to identifying women at risk for Alzheimer’s is the development of peripheral biomarkers that serve as reporters of the bioenergetic system of the brain. Such a biomarker profile could both identify women at risk and serve as an indicator of therapeutic efficacy. Further, the efficacy of hormone therapy formulations to sustain bioenergetic system function could be assessed using this same biomarker profile.
Highlights.
Estrogen regulates and integrates the metabolic system of the female brain and body.
Estrogen decline coincides with compromised metabolic phenotype of the brain.
Bioenergetic adaptations to estrogen loss could determine Alzheimer’s risk.
Metabolic biomarker profiles could identify at-risk populations.
Biomarker profiles can serve as indicators of therapeutic efficacy.
Acknowledgements
The authors would like to acknowledge Dr. Wendy Mack and Dr. Enrique Cadenas for helpful discussions throughout the process of developing this manuscript which have enriched our understanding of the complexities of human populations and the metabolic system. Funding for development of this review and our research was supported by R01AG032236 (to RDB), P01AG026572 (to RDB and WM), R01AG033288 (to RDB), and F31AG044997 (to JRR).
List of Abbreviations
- 3xTgAD
Triple-transgenic mouse model of Alzheimer’s disease
- αERKO
ERα knockout
- αKGDH
α-ketoglutarate dehydrogenase
- Aβ
Amyloid beta1-42; beta-amyloid
- AD
Alzheimer’s disease
- ArKO
Aromatase knockout
- BMI
Body-mass index
- CEE
Conjugated equine estrogen
- CMRglu
Cerebral metabolic rate of glucose uptake
- COX
Complex IV
- CSF
Cerebrospinal fluid
- DPN
Diarylpropionitrile (an ERβ-selective agonist)
- E1
Estrone
- E2
Estradiol 17β-estradiol
- E3
Estriol
- Estrogen
17b-estradiol unless otherwise specified
- ER
Estrogen receptor
- ERα
Estrogen receptor alpha; also referred to as ESR1
- ERβ
Estrogen receptor beta; also referred to as ESR2
- ERE
Estrogen response element
- FDG
Fluorodeoxyglucose
- HOMA-IR
Homeostatic assessment of insulin resistance
- HT
Hormone therapy
- IDE
Insulin degrading enzyme
- IGF-1
Insulin growth factor-1
- IGF-1R
Insulin growth factor-1 receptor
- IR
Insulin receptor
- MCI
Mild cognitive impairment
- mER
Membrane associated estrogen receptor
- MMSE
Mini-Mental State Exam
- MPA
Medroxyprogesterone acetate
- mtDNA
Mitochondrial DNA
- OVX
Ovariectomized; ovariectomy
- OXPHOS
Oxidative phosphorylation
- PDH
Pyruvate dehydrogenase
- PET
Positron emission tomography
- PPT
Propylpyrazoletriol (an ERα-selective agonist)
- rCBF
Regional cerebral blood flow
- ROS
Reactive oxygen species
- SHBG
Sex hormone-binding globulin
- T2DM
Type II diabetes mellitus
- TCA
Tricarboxylic citric acid
- VaD
Vascular dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya NK, Levin EC, Clifford PM, Han M, Tourtellotte R, Chamberlain D, Pollaro M, Coretti NJ, Kosciuk MC, Nagele EP, et al. Diabetes and Hypercholesterolemia Increase Blood-Brain Barrier Permeability and Brain Amyloid Deposition: Beneficial Effects of the LpPLA2 Inhibitor Darapladib. J Alzheimers Dis. 2013 doi: 10.3233/JAD-122254. [DOI] [PubMed] [Google Scholar]
- Ahima R. Connecting obesity, aging and diabetes. Nature medicine. 2009;15:996–997. doi: 10.1038/nm0909-996. [DOI] [PubMed] [Google Scholar]
- Ahtiainen M, Alen M, Pollanen E, Pulkkinen S, Ronkainen PH, Kujala UM, Kaprio J, Sipila S, Kovanen V. Hormone therapy is associated with better body composition and adipokine/glucose profiles: a study with monozygotic co-twin control design. Menopause. 2012;19:1329–1335. doi: 10.1097/gme.0b013e31825a3344. [DOI] [PubMed] [Google Scholar]
- Akin F, Bastemir M, Alkis E. Effect of insulin sensitivity on SHBG levels in premenopausal versus postmenopausal obese women. Advances in therapy. 2007;24:1210–1220. doi: 10.1007/BF02877767. [DOI] [PubMed] [Google Scholar]
- Akin F, Bastemir M, Alkis E, Kaptanoglu B. SHBG levels correlate with insulin resistance in postmenopausal women. European journal of internal medicine. 2009;20:162–167. doi: 10.1016/j.ejim.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry research. 1994;51:175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Andreasson AN, Unden AL, Elofsson S, Brismar K. Leptin and adiponectin: distribution and associations with cardiovascular risk factors in men and women of the general population. American journal of human biology: the official journal of the Human Biology Council. 2012;24:595–601. doi: 10.1002/ajhb.22279. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Ruiz-Palmero I, Scerbo MJ, Acaz-Fonseca E, Cambiasso MJ, Garcia-Segura LM. Molecular mechanisms involved in the regulation of neuritogenesis by estradiol: Recent advances. The Journal of steroid biochemistry and molecular biology. 2012;131:52–56. doi: 10.1016/j.jsbmb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7:297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Atcha Z, Chen WS, Ong AB, Wong FK, Neo A, Browne ER, Witherington J, Pemberton DJ. Cognitive enhancing effects of ghrelin receptor agonists. Psychopharmacology. 2009;206:415–427. doi: 10.1007/s00213-009-1620-6. [DOI] [PubMed] [Google Scholar]
- Authors. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. Neurobiology of aging. 1998;19:109–116. [PubMed] [Google Scholar]
- Authors Perimenopausal risk factors and future health. Hum Reprod Update. 2011;17:706–717. doi: 10.1093/humupd/dmr020. [DOI] [PubMed] [Google Scholar]
- Authors 2013 Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia. 1980;19:475–481. doi: 10.1007/BF00281829. [DOI] [PubMed] [Google Scholar]
- Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends in neurosciences. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18:1577–1584. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. Jama. 1993;269:2637–2641. [PubMed] [Google Scholar]
- Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr., Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36:627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- Baydas G, Nedzvetskii VS, Nerush PA, Kirichenko SV, Yoldas T. Altered expression of NCAM in hippocampus and cortex may underlie memory and learning deficits in rats with streptozotocin-induced diabetes mellitus. Life Sci. 2003;73:1907–1916. doi: 10.1016/s0024-3205(03)00561-7. [DOI] [PubMed] [Google Scholar]
- Bednarek-Tupikowska G, Filus A, Kuliczkowska-Plaksej J, Tupikowski K, Bohdanowicz-Pawlak A, Milewicz A. Serum leptin concentrations in pre- and postmenopausal women on sex hormone therapy. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2006;22:207–212. doi: 10.1080/09513590600702774. [DOI] [PubMed] [Google Scholar]
- Benedict C, Frey WH, 2nd, Schioth HB, Schultes B, Born J, Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Experimental gerontology. 2011;46:112–115. doi: 10.1016/j.exger.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Benetti-Pinto CL, Castro N, Grassiotto Oda R, Garmes HM. Leptin and adiponectin blood levels in women with premature ovarian failure and age- and weight-matched women with normal menstrual cycles. Menopause. 2010;17:174–177. doi: 10.1097/gme.0b013e3181b00dad. [DOI] [PubMed] [Google Scholar]
- Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69:417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17:692–699. doi: 10.1097/gme.0b013e3181cc49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini E, Maggi A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. Journal of neurochemistry. 1992;58:1923–1929. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Lhotsky A, Wang Y, Dal Forno G, An Y, Metter EJ, Ferrucci L, O’Brien R, Zonderman AB. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. American journal of epidemiology. 2008;168:1179–1189. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigalke B, Schreitmuller B, Sopova K, Paul A, Stransky E, Gawaz M, Stellos K, Laske C. Adipocytokines and CD34 progenitor cells in Alzheimer’s disease. PloS one. 2011;6:e20286. doi: 10.1371/journal.pone.0020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int J Obes Relat Metab Disord. 1996;20:213–219. [PubMed] [Google Scholar]
- Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer’s syndrome. Annals of the New York Academy of Sciences. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Vina J. Mitochondrial oxidant generation is involved in determining why females live longer than males. Frontiers in bioscience: a journal and virtual library. 2007;12:1008–1013. doi: 10.2741/2120. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiology of aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, O’Connor DW, Paykel ES. Vascular risks and incident dementia: results from a cohort study of the very old. Dement Geriatr Cogn Disord. 1998;9:175–180. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology--emerging clinical applications. Nature clinical practice Endocrinology & metabolism. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Reichensperger JD, Brinton RD. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiology of aging. 2006;27:306–317. doi: 10.1016/j.neurobiolaging.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Impact of estrogen therapy on Alzheimer’s disease: a fork in the road? CNS Drugs. 2004;18:405–422. doi: 10.2165/00023210-200418070-00001. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Annals of the New York Academy of Sciences. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Advanced drug delivery reviews. 2008a;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in neurosciences. 2008b;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends in pharmacological sciences. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Neuroendocrinology of Aging. In Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. In: R. K FH, W. K, editors. Saunders Elsevier; Philadelphia: 2010. pp. 163–169. [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu HP. The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiology of aging. 2000;21:475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Brismar T, Maurex L, Cooray G, Juntti-Berggren L, Lindstrom P, Ekberg K, Adner N, Andersson S. Predictors of cognitive impairment in type 1 diabetes. Psychoneuroendocrinology. 2007;32:1041–1051. doi: 10.1016/j.psyneuen.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. The Journal of clinical endocrinology and metabolism. 2003;88:1537–1542. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain research. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, et al. Evidence that oestrogen receptor- alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Bukowski C, Grigg MA, Longcope C. Sex hormone-binding globulin concentration: differences among commercially available methods. Clinical chemistry. 2000;46:1415–1416. [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. The Journal of clinical endocrinology and metabolism. 2000;85:2832–2838. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chen K, Kaszniak AW, Lee W, Alexander GE, Bandy D, Fleisher AS, Caselli RJ, Reiman EM. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology. 2013 doi: 10.1212/WNL.0b013e31828f17de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Honea RA, Vidoni ED, Hutfles L, Brooks WM, Swerdlow RH. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochimica et biophysica acta. 2011 doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF, Besnard AM. Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer’s disease. Metab Brain Dis. 1990;5:179–184. doi: 10.1007/BF00997071. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cai H, Cong WN, Ji S, Rothman S, Maudsley S, Martin B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Current Alzheimer research. 2012;9:5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. The Journal of steroid biochemistry and molecular biology. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiology of aging. 2004;25:105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochemical and biophysical research communications. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- Carr MC. The emergence of the metabolic syndrome with menopause. The Journal of clinical endocrinology and metabolism. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nature medicine. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! American journal of physiology. Endocrinology and metabolism. 2009;297:E836–848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochimica et biophysica acta. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Eshete M, Alworth WL, Yager JD. Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors alpha and beta to human mitochondrial DNA estrogen response elements. Journal of cellular biochemistry. 2004;93:358–373. doi: 10.1002/jcb.20178. [DOI] [PubMed] [Google Scholar]
- Chen K, Ayutyanont N, Langbaum JB, Fleisher AS, Reschke C, Lee W, Liu X, Bandy D, Alexander GE, Thompson PM, et al. Characterizing Alzheimer’s disease using a hypometabolic convergence index. NeuroImage. 2011;56:52–60. doi: 10.1016/j.neuroimage.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cao CP, Li CR, Wang W, Zhang D, Han LL, Zhang XQ, Kim A, Kim S, Liu GL. Ghrelin modulates insulin sensitivity and tau phosphorylation in high glucose-induced hippocampal neurons. Biological & pharmaceutical bulletin. 2010;33:1165–1169. doi: 10.1248/bpb.33.1165. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15:907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy CA. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10236–10241. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, Rutledge S, Mekonnen B, Hauze D, Nagpal S, et al. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–905. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Salmon E, Yakushev I, Bahri MA, Mezenge F, Perrotin A, Bastin C, Manrique A, Scheurich A, et al. Craft S. Cholerton B, Baker LD, editors. Relationships between brain metabolism decrease in normal aging and changes in structural and functional connectivity. NeuroImage. Insulin resistance and pathological brain ageing. Diabet Med. 2013;28:1463–1475. doi: 10.1016/j.neuroimage.2013.03.009. 2011. [DOI] [PubMed] [Google Scholar]
- Chou JL, Shenoy DV, Thomas N, Choudhary PK, Laferla FM, Goodman SR, Breen GA. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. Journal of proteomics. 2011;74:466–479. doi: 10.1016/j.jprot.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Christodoulakos GE, Lambrinoudaki IV, Creatsa MG, Economou EV, Siasou Z, Panoulis CP, Kalligerou I, Papadias C. Circulating levels of atherogenesis-associated adipocytokines and apoptotic markers are differentially influenced by hormone therapy, tibolone and raloxifene in healthy postmenopausal women. Climacteric: the journal of the International Menopause Society. 2008;11:155–165. doi: 10.1080/13697130801954484. [DOI] [PubMed] [Google Scholar]
- Chu MC, Cosper P, Nakhuda GS, Lobo RA. A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertility and sterility. 2006;86:1669–1675. doi: 10.1016/j.fertnstert.2006.04.043. [DOI] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, Craft S. Sex and ApoE Genotype Differences in Treatment Response to Two Doses of Intranasal Insulin in Adults with Mild Cognitive Impairment or Alzheimer’s Disease. J Alzheimers Dis. 2013 doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee, H.G.N. ESR1 estrogen receptor 1 [Homo sapiens (human)] (NCBI) [Google Scholar]
- Committee, H.G.N. ESR2 estrogen receptor 2 (ER beta) [Homo sapiens (human)] [Google Scholar]
- Committee, H.G.N. GPER G protein-coupled estrogen receptor 1 [Homo sapiens (human)] [Google Scholar]
- Cordey M, Gundimeda U, Gopalakrishna R, Pike CJ. Estrogen activates protein kinase C in neurons: role in neuroprotection. Journal of neurochemistry. 2003;84:1340–1348. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001;57:260–264. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiology of aging. 2005;26(Suppl 1):65–69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Current Alzheimer research. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- Curti D, Rognoni F, Gasparini L, Cattaneo A, Paolillo M, Racchi M, Zani L, Bianchetti A, Trabucchi M, Bergamaschi S, et al. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer’s disease (AD) patients. Neurosci Lett. 1997;236:13–16. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- Dallongeville J, Marecaux N, Isorez D, Zylbergberg G, Fruchart JC, Amouyel P. Multiple coronary heart disease risk factors are associated with menopause and influenced by substitutive hormonal therapy in a cohort of French women. Atherosclerosis. 1995;118:123–133. doi: 10.1016/0021-9150(95)05599-r. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P. Understanding weight gain at menopause. Climacteric: the journal of the International Menopause Society. 2012a;15:419–429. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- Davis SR, Robinson PJ, Moufarege A, Bell RJ. The contribution of SHBG to the variation in HOMA-IR is not dependent on endogenous oestrogen or androgen levels in postmenopausal women. Clinical endocrinology. 2012b;77:541–547. doi: 10.1111/j.1365-2265.2011.04301.x. [DOI] [PubMed] [Google Scholar]
- De La Monte SM. Metabolic derangements mediate cognitive impairment and Alzheimer’s disease: role of peripheral insulin-resistance diseases. Panminerva medica. 2012;54:171–178. [PMC free article] [PubMed] [Google Scholar]
- de Lauzon-Guillain B, Fournier A, Fabre A, Simon N, Mesrine S, Boutron-Ruault MC, Balkau B, Clavel-Chapelon F. Menopausal hormone therapy and new-onset diabetes in the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l’Education Nationale (E3N) cohort. Diabetologia. 2009;52:2092–2100. doi: 10.1007/s00125-009-1456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiology of aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- De Vriese C, Delporte C. Influence of ghrelin on food intake and energy homeostasis. Current opinion in clinical nutrition and metabolic care. 2007;10:615–619. doi: 10.1097/MCO.0b013e32829fb37c. [DOI] [PubMed] [Google Scholar]
- Dedeoglu EN, Erenus M, Yoruk P. Effects of hormone therapy and tibolone on body composition and serum leptin levels in postmenopausal women. Fertility and sterility. 2009;91:425–431. doi: 10.1016/j.fertnstert.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Demonacos CV, Karayanni N, Hatzoglou E, Tsiriyiotis C, Spandidos DA, Sekeris CE. Mitochondrial genes as sites of primary action of steroid hormones. Steroids. 1996;61:226–232. doi: 10.1016/0039-128x(96)00019-0. [DOI] [PubMed] [Google Scholar]
- Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- Di Carlo C, Tommaselli GA, Sammartino A, Bifulco G, Nasti A, Nappi C. Serum leptin levels and body composition in postmenopausal women: effects of hormone therapy. Menopause. 2004;11:466–473. doi: 10.1097/01.gme.0000109313.11228.2b. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. American journal of physiology Cell physiology. 2004;286:C655–661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. European journal of endocrinology/European Federation of Endocrine Societies. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- Ding F, Luo J, Yao J, Mao Z, Chen S, Chen K, Reiman EM, Brinton RD. Paper presented at: Society for Neuroscience. New Orleans, LA, USA: 2012. Ovarian hormone loss is associated with reduced brain glucose uptake and a shift to alternative substrates in brain. [Google Scholar]
- Ding F, Yao J, Rettberg JR, Chen S, Brinton RD. Early decline in glucose metabolism, transport and glycolytic capacity are accompanied by a shift to alternative fuel transporters in the aging normal and Alzheimer’s female mouse brain. PloS one. 2013a doi: 10.1371/journal.pone.0079977. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Yao J, Zhao L, Mao Z, Chen S, Brinton RD. Ovariectomy induces a shift in fuel availability and metabolism in the hippocampus of the female transgenic model of familial Alzheimer’s. PloS one. 2013b;8:e59825. doi: 10.1371/journal.pone.0059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi S, Taouis M. Adiponectin and energy homeostasis: consensus and controversy. The Journal of nutritional biochemistry. 2009;20:831–839. doi: 10.1016/j.jnutbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua A, Hennes MI, Hoffmann RG, Maas DL, Krakower GR, Sonnenberg GE, Kissebah AH. Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women. Diabetes. 1996;45:1635–1637. doi: 10.2337/diab.45.11.1635. [DOI] [PubMed] [Google Scholar]
- Dubuc GR, Phinney SD, Stern JS, Havel PJ. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism: clinical and experimental. 1998;47:429–434. doi: 10.1016/s0026-0495(98)90055-5. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women. Neurology. 2000;55:875–877. doi: 10.1212/wnl.55.6.875. [DOI] [PubMed] [Google Scholar]
- Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester. Minn. Arch Neurol. 2002;59:1589–1593. doi: 10.1001/archneur.59.10.1589. [DOI] [PubMed] [Google Scholar]
- El Seifi S, Green IC, Perrin D. Insulin release and steroid-hormone binding in isolated islets of langerhans in the rat: effects of ovariectomy. The Journal of endocrinology. 1981;90:59–67. doi: 10.1677/joe.0.0900059. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Dawoodi S, Raju M, Thumma A, Hynan LS, Maasumi SH, Reisch JS, O’Bryant S, Jenkins M, Barber R, et al. Androgen receptor gene and sex-specific Alzheimer’s disease. Neurobiology of aging. 2013 doi: 10.1016/j.neurobiolaging.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Heyman A, Huber MS, Woodbury MA, Leiss J, Schmader KE, Bohannon A, Trapp-Moen B. The prevalence and 3-year incidence of dementia in older Black and White community residents. J Clin Epidemiol. 1998;51:587–595. doi: 10.1016/s0895-4356(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr., Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS genetics. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48:132–138. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- Fusco R, Galgani M, Procaccini C, Franco R, Pirozzi G, Fucci L, Laccetti P, Matarese G. Cellular and molecular crosstalk between leptin receptor and estrogen receptor-{alpha} in breast cancer: molecular basis for a novel therapeutic setting. Endocrine-related cancer. 2010;17:373–382. doi: 10.1677/ERC-09-0340. [DOI] [PubMed] [Google Scholar]
- Gahete MD, Rubio A, Cordoba-Chacon J, Gracia-Navarro F, Kineman RD, Avila J, Luque RM, Castano JP. Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer’s disease patients. J Alzheimers Dis. 2010;22:819–828. doi: 10.3233/JAD-2010-100873. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain research Brain research reviews. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Progress in brain research. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Cardona-Gomez GP, Chowen JA, Azcoitia I. Insulin-like growth factor-I receptors and estrogen receptors interact in the promotion of neuronal survival and neuroprotection. Journal of neurocytology. 2000;29:425–437. doi: 10.1023/a:1007125626308. [DOI] [PubMed] [Google Scholar]
- Garris DR, Williams SK, Coleman DL, Morgan CR. Glucose utilization by the mouse brain: influence of age and diabetes. Brain research. 1984;317:141–146. doi: 10.1016/0165-3806(84)90091-9. [DOI] [PubMed] [Google Scholar]
- GeneCards. Estrogen Receptor 1, C.H.G. Center, editor. Weizmann Institute of Science; Rehovot, Israel: [Google Scholar]
- GeneCards Summary for estrogen receptor 1 (ESR1); GeneCards. Estrogen Receptor 2, C.H.G. Center, editor. Weizmann Institute of Science; Rehovot, Israel: GeneCards Summary for estrogen receptor 2 (ESR2) [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Haroutunian V, Zhang H, Park LC, Shi Q, Lesser M, Mohs RC, Sheu RK, Blass JP. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000a;48:297–303. [PubMed] [Google Scholar]
- Gibson GE, Park LC, Sheu KF, Blass JP, Calingasan NY. The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem Int. 2000b;36:97–112. doi: 10.1016/s0197-0186(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20(Suppl 2):S591–607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Zhang H, Sheu KF, Bogdanovich N, Lindsay JG, Lannfelt L, Vestling M, Cowburn RF. Alpha-ketoglutarate dehydrogenase in Alzheimer brains bearing the APP670/671 mutation. Ann Neurol. 1998;44:676–681. doi: 10.1002/ana.410440414. [DOI] [PubMed] [Google Scholar]
- Goodman MP. Are all estrogens created equal? A review of oral vs. transdermal therapy. J Womens Health (Larchmt) 2012;21:161–169. doi: 10.1089/jwh.2011.2839. [DOI] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes & development. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Goran MI, Smith A, Kent E. Leptin in postmenopausal women: influence of hormone therapy, insulin, and fat distribution. The Journal of clinical endocrinology and metabolism. 2000;85:1770–1775. doi: 10.1210/jcem.85.5.6602. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Hamzelou A, Johnston JM, Smith MA, Ashford JW, Tezapsidis N. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and beta-amyloid in neurons. Biochemical and biophysical research communications. 2011;414:170–174. doi: 10.1016/j.bbrc.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009a;455:191–194. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochemical and biophysical research communications. 2009b;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, Ashford JW, Smith MA, Tezapsidis N. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochemical and biophysical research communications. 2008;376:536–541. doi: 10.1016/j.bbrc.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, E.C.W. Hormones and cardiovascular health in women. Hum Reprod Update. 2006;12:483–497. doi: 10.1093/humupd/dml028. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism: clinical and experimental. 1991;40:1323–1326. doi: 10.1016/0026-0495(91)90037-w. [DOI] [PubMed] [Google Scholar]
- Habeck C, Risacher S, Lee GJ, Glymour MM, Mormino E, Mukherjee S, Kim S, Nho K, DeCarli C, Saykin AJ, et al. Relationship between baseline brain metabolism measured using [(1)(8)F]FDG PET and memory and executive function in prodromal and early Alzheimer’s disease. Brain imaging and behavior. 2012;6:568–583. doi: 10.1007/s11682-012-9208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm S, Martinez-Rodriguez G, Rodriguez L, Prat F, Mylonas CC, Carrillo M, Zanuy S. Cloning, characterisation, and expression of three oestrogen receptors (ERalpha, ERbeta1 and ERbeta2) in the European sea bass, Dicentrarchus labrax. Molecular and cellular endocrinology. 2004;223:63–75. doi: 10.1016/j.mce.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Bowden D, Wagenknecht LE, Balasubramanyam A, Langfeld C, Saad MF, Rotter JI, Guo X, Chen YD, Bryer-Ash M, et al. Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic Hispanics and African-Americans. The Journal of clinical endocrinology and metabolism. 2007;92:2665–2671. doi: 10.1210/jc.2006-2614. [DOI] [PubMed] [Google Scholar]
- Hatzis G, Deftereos S, Tousoulis D, Bouras G, Giannopoulos G, Anatoliotakis N, Tsounis D, Stefanadis C. Adiponectin: merely a bystander or the missing link to cardiovascular disease? Current topics in medicinal chemistry. 2013 doi: 10.2174/1568026611313020005. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Hawkins MB, Thomas P. The unusual binding properties of the third distinct teleost estrogen receptor subtype ERbetaa are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinology. 2004;145:2968–2977. doi: 10.1210/en.2003-0806. [DOI] [PubMed] [Google Scholar]
- Hawkins MB, Thornton JW, Crews D, Skipper JK, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10751–10756. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhang SS, Meng Q, Li S, Zhu HH, Raquil MA, Alderson N, Zhang H, Wu J, Rui L, et al. Shp2 controls female body weight and energy balance by integrating leptin and estrogen signals. Molecular and cellular biology. 2012;32:1867–1878. doi: 10.1128/MCB.06712-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? American journal of epidemiology. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- Herholz K, Westwood S, Haense C, Dunn G. Evaluation of a calibrated (18)F-FDG PET score as a biomarker for progression in Alzheimer disease and mild cognitive impairment. J Nucl Med. 2011;52:1218–1226. doi: 10.2967/jnumed.111.090902. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Experimental gerontology. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiology of aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Munch G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacology & therapeutics. 2007;113:154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, Park YK, Lee KW, Kim SH. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause. 2007;14:835–840. doi: 10.1097/GME.0b013e31802cddca. [DOI] [PubMed] [Google Scholar]
- Hoskin EK, Tang MX, Manly JJ, Mayeux R. Elevated sex-hormone binding globulin in elderly women with Alzheimer’s disease. Neurobiology of aging. 2004;25:141–147. doi: 10.1016/s0197-4580(03)00046-0. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Im JA, Lee JW, Lee HR, Lee DC. Plasma adiponectin levels in postmenopausal women with or without long-term hormone therapy. Maturitas. 2006;54:65–71. doi: 10.1016/j.maturitas.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:439–456. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. The Journal of clinical endocrinology and metabolism. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149:3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. Journal of neuroendocrinology. 2012;24:236–248. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiology of aging. 2007;28:1670–1681. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Tang MX, Stern Y, Sano M, Marder K, Bell KL, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59:673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69:871–877. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, Murphy MP, Beckett TL, Finch CE, Brinton RD, et al. 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153:5467–5479. doi: 10.1210/en.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD. Role of body fat distribution and the metabolic complications of obesity. The Journal of clinical endocrinology and metabolism. 2008;93:S57–63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI. Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. The Journal of clinical endocrinology and metabolism. 2003;88:1646–1652. doi: 10.1210/jc.2002-021375. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, Gapstur SM, Golden SH. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. The Journal of clinical endocrinology and metabolism. 2009;94:4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Kellokoski E, Poykko SM, Karjalainen AH, Ukkola O, Heikkinen J, Kesaniemi YA, Horkko S. Estrogen replacement therapy increases plasma ghrelin levels. The Journal of clinical endocrinology and metabolism. 2005;90:2954–2963. doi: 10.1210/jc.2004-2016. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Bienias JL, Shah A, Meeke KA, Schneider JA, Soriano E, Bennett DA. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer’s disease: relationship to Mini-Mental State Examination scores. Current Alzheimer research. 2008;5:45–51. doi: 10.2174/156720508783884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, Rollison DE, Dorgan JF, Brinton LA, Overvad K, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. British journal of cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Horm Behav. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. Brain cytochrome oxidase in Alzheimer’s disease. Journal of neurochemistry. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Kostanyan A, Nazaryan K. Rat brain glycolysis regulation by estradiol-17 beta. Biochimica et biophysica acta. 1992;1133:301–306. doi: 10.1016/0167-4889(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukatay V, Agar A, Gumuslu S, Yargicoglu P. Effect of sulfur dioxide on active and passive avoidance in experimental diabetes mellitus: relation to oxidant stress and antioxidant enzymes. Int J Neurosci. 2007;117:1091–1107. doi: 10.1080/00207450600934531. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. Bmj. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrinoudaki IV, Christodoulakos GE, Economou EV, Vlachou SA, Panoulis CP, Alexandrou AP, Kouskouni EE, Creatsas GC. Circulating leptin and ghrelin are differentially influenced by estrogen/progestin therapy and raloxifene. Maturitas. 2008;59:62–71. doi: 10.1016/j.maturitas.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiology of aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR, Jr., et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. American journal of epidemiology. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- Li C, Ford ES, Li B, Giles WH, Liu S. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33:1618–1624. doi: 10.2337/dc09-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. Jama. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer’s disease: an epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Cheng D, Tang MX, Schupf N, Mayeux R. Central Obesity in the Elderly is Related to Late-onset Alzheimer Disease. Alzheimer Dis Assoc Disord. 2011a doi: 10.1097/WAD.0b013e318222f0d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007a;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007b;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Small S, Biessels GJ. Should we target insulin resistance to prevent dementia due to Alzheimer disease? Arch Neurol. 2011b;68:17–18. doi: 10.1001/archneurol.2010.339. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. American journal of epidemiology. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res. 2003;74:512–523. doi: 10.1002/jnr.10791. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Lynch NA, Ryan AS, Berman DM, Sorkin JD, Nicklas BJ. Comparison of VO2max and disease risk factors between perimenopausal and postmenopausal women. Menopause. 2002;9:456–462. doi: 10.1097/00042192-200211000-00012. [DOI] [PubMed] [Google Scholar]
- Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. The Journal of endocrinology. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Dennerstein L, Clark M, Guthrie J, LaMontagne P, Fornelli D, Little D, Henderson VW, Resnick SM. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain research. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiology of aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158:227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone - an opposite-sex twin study. Clinical endocrinology. 2007;66:530–537. doi: 10.1111/j.1365-2265.2007.02768.x. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique C, Lastra G, Habibi J, Mugerfeld I, Garro M, Sowers JR. Loss of Estrogen Receptor alpha Signaling Leads to Insulin Resistance and Obesity in Young and Adult Female Mice. Cardiorenal medicine. 2012;2:200–210. doi: 10.1159/000339563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Rimm EB, Colditz GA, Willett WC, Nathan DM, Arky RA, Rosner B, Hennekens CH, Speizer FE, Stampfer MJ. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Annals of epidemiology. 1992;2:665–673. doi: 10.1016/1047-2797(92)90011-e. [DOI] [PubMed] [Google Scholar]
- Mao Z, Zhao L, Yao J, Ding F, Cadenas E, Brinton RD. Sex-dependent bioenergetic and metabolic gene expression in the hippocampus: Female brain ages differently from male brain Paper presented at: Society for Neuroscience; New Orleans, LA, USA. 2012. [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- Maruszak A, Zekanowski C. Mitochondrial dysfunction and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:320–330. doi: 10.1016/j.pnpbp.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Marwarha G, Dasari B, Prasanthi JR, Schommer J, Ghribi O. Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimers Dis. 2010;19:1007–1019. doi: 10.3233/JAD-2010-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiacoma F, Lindsay JG, Bettendorff L, Rice J, Kish SJ. Brain protein and alpha-ketoglutarate dehydrogenase complex activity in Alzheimer’s disease. Ann Neurol. 1996;39:592–598. doi: 10.1002/ana.410390508. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo F, Bergeron C, Kish SJ. Brain alpha-ketoglutarate dehydrogenase complex activity in Alzheimer’s disease. Journal of neurochemistry. 1993;61:2007–2014. doi: 10.1111/j.1471-4159.1993.tb07436.x. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides. 2004;25:289–297. doi: 10.1016/j.peptides.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiology of aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasce LP, White GR, Harrison CJ, Boyle JM. Localization of the estrogen receptor locus (ESR) to chromosome 6q25.1 by FISH and a simple post-FISH banding technique. Genomics. 1993;17:263–265. doi: 10.1006/geno.1993.1320. [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Frontiers in neuroendocrinology. 2006;27:391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. Journal of medicinal chemistry. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Frontiers in neuroendocrinology. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. The Journal of comparative neurology. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology. 2008;149:3306–3312. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. The Journal of comparative neurology. 2001;429:355–371. [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. The Journal of comparative neurology. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura SA, Schapiro MB, Grady CL, Kumar A, Salerno JA, Kozachuk WE, Wagner E, Rapoport SI, Horwitz B. Effect of gender on glucose utilization rates in healthy humans: a positron emission tomography study. J Neurosci Res. 1990;27:500–504. doi: 10.1002/jnr.490270410. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell death and differentiation. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- Moon M, Choi JG, Nam DW, Hong HS, Choi YJ, Oh MS, Mook-Jung I. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-beta1-42 oligomer-injected mice. J Alzheimers Dis. 2011;23:147–159. doi: 10.3233/JAD-2010-101263. [DOI] [PubMed] [Google Scholar]
- Moon M, Kim S, Hwang L, Park S. Ghrelin regulates hippocampal neurogenesis in adult mice. Endocrine journal. 2009;56:525–531. doi: 10.1507/endocrj.k09e-089. [DOI] [PubMed] [Google Scholar]
- Moor AN, Gottipati S, Mallet RT, Sun J, Giblin FJ, Roque R, Cammarata PR. A putative mitochondrial mechanism for antioxidative cytoprotection by 17beta-estradiol. Experimental eye research. 2004;78:933–944. doi: 10.1016/j.exer.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis. 2006;9:101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. Journal of the neurological sciences. 2007;257:206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochimica et biophysica acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced Mitochondria Cytochrome Oxidase Activity in Adult Children of Mothers with Alzheimer’s Disease. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110866. E, L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiology of aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009a;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009b;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, Tsui W, Ginestroni A, Bessi V, Fayyazz M, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- Mott NN, Pak TR. Characterisation of human oestrogen receptor beta (ERbeta) splice variants in neuronal cells. Journal of neuroendocrinology. 2012;24:1311–1321. doi: 10.1111/j.1365-2826.2012.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Sex hormone binding globulin and incident Alzheimer’s disease in elderly men and women. Neurobiology of aging. 2010;31:1758–1765. doi: 10.1016/j.neurobiolaging.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. Jama. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1996a;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996b;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. Journal of neurochemistry. 1994;63:2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Hormone and metabolic research = Hormon-und Stoffwechselforschung = Hormones et metabolisme. 2002;34:758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Fuentes E, Soria B, Ripoll C. Estrogen and xenoestrogen actions on endocrine pancreas: from ion channel modulation to activation of nuclear function. Steroids. 2004;69:531–536. doi: 10.1016/j.steroids.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Narita K, Kosaka H, Okazawa H, Murata T, Wada Y. Relationship between plasma leptin level and brain structure in elderly: a voxel-based morphometric study. Biological psychiatry. 2009;65:992–994. doi: 10.1016/j.biopsych.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Nejat EJ, Chervenak JL. The continuum of ovarian aging and clinicopathologies associated with the menopausal transition. Maturitas. 2010;66:187–190. doi: 10.1016/j.maturitas.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Nestler JE. Sex hormone-binding globulin: a marker for hyperinsulinemia and/or insulin resistance? The Journal of clinical endocrinology and metabolism. 1993;76:273–274. doi: 10.1210/jcem.76.2.8432767. [DOI] [PubMed] [Google Scholar]
- Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer’s disease highlights common regional vulnerability across AD mouse models. Brain research. 2010;1347:179–185. doi: 10.1016/j.brainres.2010.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Current drug targets CNS and neurological disorders. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC neuroscience. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Brinton RD. Estradiol in vivo regulation of brain mitochondrial proteome. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Flight IH, Rees MC. Oestrogen and progestogen hormone replacement therapy for peri-menopausal and post-menopausal women: weight and body fat distribution. Cochrane Database Syst Rev. 2000:CD001018. doi: 10.1002/14651858.CD001018. [DOI] [PubMed] [Google Scholar]
- Northam EA, Rankins D, Cameron FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol. 2006;2:78–86. doi: 10.1038/ncpneuro0097. [DOI] [PubMed] [Google Scholar]
- Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta neuropathologica. 2009;118:151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, et al. Oxidative damage is the earliest event in Alzheimer disease. Journal of neuropathology and experimental neurology. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- O’Sullivan AJ, Crampton LJ, Freund J, Ho KK. The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest. 1998;102:1035–1040. doi: 10.1172/JCI2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y, Yamada Y, Takahi Y, Baden MY, Saisho K, Tamba S, Yamamoto K, Umeda M, Furubayashi A, Matsuzawa Y. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clinical endocrinology. 2012 doi: 10.1111/cen.12041. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. The Journal of clinical endocrinology and metabolism. 2000a;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000b;95:333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam Study. American journal of epidemiology. 1998;147:574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. American journal of epidemiology. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- Pallottini V, Bulzomi P, Galluzzo P, Martini C, Marino M. Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infectious disorders drug targets. 2008;8:52–60. doi: 10.2174/187152608784139631. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Congia S, Lello S, Tedde D, Orru M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62:301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage. 2007;35:1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev. 2001;25:311–323. doi: 10.1016/s0149-7634(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr., Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr., Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994a;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr., Parks J, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer’s disease brain. Neurology. 1994b;44:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Bertazzo D, Casimirri F, Pascal G, Tortelli O, Labate AM, Virgilio-Menopause-Health Group Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Metabolism: clinical and experimental. 1997;46:5–9. doi: 10.1016/s0026-0495(97)90159-1. [DOI] [PubMed] [Google Scholar]
- Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. International journal of clinical practice. 2010;64:1808–1812. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SB, Hansen PS, Lund S, Andersen PH, Odgaard A, Richelsen B. Identification of oestrogen receptors and oestrogen receptor mRNA in human adipose tissue. European journal of clinical investigation. 1996;26:262–269. doi: 10.1046/j.1365-2362.1996.145278.x. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. The Journal of clinical endocrinology and metabolism. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- Pentti K, Tuppurainen MT, Honkanen R, Sandini L, Kroger H, Alhava E, Saarikoski S. Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. European journal of endocrinology/European Federation of Endocrine Societies. 2009;160:979–983. doi: 10.1530/EJE-09-0151. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;24(Suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH. Coenzyme A-acetylating enzymes in Alzheimer’s disease: possible cholinergic ‘compartment’ of pyruvate dehydrogenase. Neurosci Lett. 1980;18:105–110. doi: 10.1016/0304-3940(80)90220-7. [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl 2):97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. Journal of neurochemistry. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- Pimenta F, Maroco JO, Ramos C, Leal I. Predictors of weight variation and weight gain in peri- and post-menopausal women. Journal of health psychology. 2013 doi: 10.1177/1359105313483153. [DOI] [PubMed] [Google Scholar]
- Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. The Journal of clinical endocrinology and metabolism. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med. 1995;123:673–675. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Pouliot MC, Despres JP, Nadeau A, Moorjani S, Prud’Homme D, Lupien PJ, Tremblay A, Bouchard C. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2001;12:167–170. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosi P, Barrett-Connor E, Papoz L, Roger M, Saint-Paul M, Nahoul K, Simon D. Interrelation between plasma sex hormone-binding globulin and plasma insulin in healthy adult women: the telecom study. The Journal of clinical endocrinology and metabolism. 1993;76:283–287. doi: 10.1210/jcem.76.2.8432770. [DOI] [PubMed] [Google Scholar]
- Proto C, Romualdi D, Cento RM, Spada RS, Di Mento G, Ferri R, Lanzone A. Plasma levels of neuropeptides in Alzheimer’s disease. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2006;22:213–218. doi: 10.1080/09513590500519385. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Weigle DS, Breen P, Cummings DE. Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. The Journal of clinical endocrinology and metabolism. 2003;88:5747–5752. doi: 10.1210/jc.2003-030513. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiology of aging. 2011;32:1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang SC, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer’s disease. Age Ageing. 1994;23:396–399. doi: 10.1093/ageing/23.5.396. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. Journal of biomedicine & biotechnology. 2006;2006:31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends in molecular medicine. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Armstrong SM, Matt KS, Mattox JH. The application of positron emission tomography to the study of the normal menstrual cycle. Hum Reprod. 1996a;11:2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996b;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaldi O, Pramono B, Sinorita H, Purnomo LB, Asdie RH, Asdie AH. Hypoadiponectinemia: a risk factor for metabolic syndrome. Acta medica Indonesiana. 2009;41:20–24. [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Revilla R, Revilla M, Villa LF, Cortes J, Arribas I, Rico H. Changes in body composition in women treated with gonadotropin-releasing hormone agonists. Maturitas. 1998;31:63–68. doi: 10.1016/s0378-5122(98)00080-2. [DOI] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. American journal of physiology Endocrinology and metabolism. 2010;298:E304–319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti AE, Pincelli AI, Corra B, Viarengo R, Bonomo SM, Galimberti D, Scacchi M, Scarpini E, Cavagnini F, Muller EE. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. The Journal of endocrinology. 2002;175:R1–5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- Robinson D, Friedman L, Marcus R, Tinklenberg J, Yesavage J. Estrogen replacement therapy and memory in older women. Journal of the American Geriatrics Society. 1994;42:919–922. doi: 10.1111/j.1532-5415.1994.tb06580.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. The Journal of clinical endocrinology and metabolism. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Molecular and cellular endocrinology. 2010;316:79–85. doi: 10.1016/j.mce.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. The Journal of steroid biochemistry and molecular biology. 1999;69:481–485. doi: 10.1016/s0960-0760(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Ruszkowska B, Sokup A, Kulwas A, Kwapisz J, Goralczyk K, Socha MW, Rhone P, Rosc D. Adiponectin and endothelial markers in post-menopausal women taking oral or transdermal hormone therapy. Acta obstetricia et gynecologica Scandinavica. 2013 doi: 10.1111/aogs.12136. [DOI] [PubMed] [Google Scholar]
- Ryan J, Carriere I, Carcaillon L, Dartigues JF, Auriacombe S, Rouaud O, Berr C, Ritchie K, Scarabin PY, Ancelin ML. Estrogen receptor polymorphisms and incident dementia: The prospective 3C study. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013 doi: 10.1016/j.jalz.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. The Journal of clinical endocrinology and metabolism. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- Sabo-Attwood T, Kroll KJ, Denslow ND. Differential expression of largemouth bass (Micropterus salmoides) estrogen receptor isotypes alpha, beta, and gamma by estradiol. Molecular and cellular endocrinology. 2004;218:107–118. doi: 10.1016/j.mce.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. Journal of neurochemistry. 2011;118:460–474. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes, obesity & metabolism. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. The Journal of clinical endocrinology and metabolism. 2010;95:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz CM, Hanaire H, Vellas BJ, Sinclair AJ, Andrieu S. Diabetes mellitus as a modulator of functional impairment and decline in Alzheimer’s disease. The Real.FR cohort. Diabet Med. 2011 doi: 10.1111/j.1464-5491.2011.03445.x. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Craft S, Brooks SJ, Frey WH, 2nd, Benedict C. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Molecular neurobiology. 2012;46:4–10. doi: 10.1007/s12035-011-8229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby P, McGarrigle HH, Peacock M. Comparison of the effects of oral and transdermal oestradiol administration on oestrogen metabolism, protein synthesis, gonadotrophin release, bone turnover and climacteric symptoms in postmenopausal women. Clinical endocrinology. 1989;30:241–249. doi: 10.1111/j.1365-2265.1989.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke; a journal of cerebral circulation. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- Shadoan MK, Anthony MS, Rankin SE, Clarkson TB, Wagner JD. Effects of tibolone and conjugated equine estrogens with or without medroxyprogesterone acetate on body composition and fasting carbohydrate measures in surgically postmenopausal monkeys. Metabolism: clinical and experimental. 2003;52:1085–1091. doi: 10.1016/s0026-0495(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Shadoan MK, Kavanagh K, Zhang L, Anthony MS, Wagner JD. Addition of medroxyprogesterone acetate to conjugated equine estrogens results in insulin resistance in adipose tissue. Metabolism: clinical and experimental. 2007;56:830–837. doi: 10.1016/j.metabol.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Shao H, Breitner JC, Whitmer RA, Wang J, Hayden K, Wengreen H, Corcoran C, Tschanz J, Norton M, Munger R, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79:1846–1852. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behavioral neuroscience. 2012;126:123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu KF, Kim YT, Blass JP, Weksler ME. An immunochemical study of the pyruvate dehydrogenase deficit in Alzheimer’s disease brain. Ann Neurol. 1985;17:444–449. doi: 10.1002/ana.410170505. [DOI] [PubMed] [Google Scholar]
- Shi J, Simpkins JW. 17 beta-Estradiol modulation of glucose transporter 1 expression in blood-brain barrier. The American journal of physiology. 1997;272:E1016–1022. doi: 10.1152/ajpendo.1997.272.6.E1016. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ. Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta) Experimental neurology. 2004;190:468–477. doi: 10.1016/j.expneurol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of comparative neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. The Journal of comparative neurology. 2001;436:64–81. [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Current Alzheimer research. 2011;8:563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Geist CL, Kenna HA, Williams K, Wroolie T, Powers B, Brooks J, Rasgon NL. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36:502–513. doi: 10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer’s disease. Neurobiology of aging. 1994;15(Suppl 2):S195–197. doi: 10.1016/0197-4580(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria--physiological and pathological implications. Molecular and cellular endocrinology. 2008;290:51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. Journal of neurochemistry. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr., Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Colacicco AM, D’Introno A, Capurso C, Torres F, Grigoletto F, Maggi S, Del Parigi A, Reiman EM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- Soni AC, Conroy MB, Mackey RH, Kuller LH. Ghrelin, leptin, adiponectin, and insulin levels and concurrent and future weight change in overweight, postmenopausal women. Menopause. 2011;18:296–301. doi: 10.1097/gme.0b013e3181f2e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13:72–78. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- Soriguer F, Rubio-Martin E, Fernandez D, Valdes S, Garcia-Escobar E, Martin-Nunez GM, Esteva I, Martin-Almaraz MC, Rojo-Martinez G. Testosterone, SHBG and risk of type 2 diabetes in the second evaluation of the Pizarra cohort study. European journal of clinical investigation. 2012;42:79–85. doi: 10.1111/j.1365-2362.2011.02559.x. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8:402–407. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Frontiers in neuroendocrinology. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. Journal of medicinal chemistry. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Annals of epidemiology. 2003;13:S78–86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Norton MC, Plassman BL, Tschanz JT, Wyse BW, Welsh-Bohmer KA, Anthony JC, Breitner JC. Enhanced cognitive performance with estrogen use in nondemented community-dwelling older women. Journal of the American Geriatrics Society. 1999;47:1171–1175. doi: 10.1111/j.1532-5415.1999.tb05195.x. [DOI] [PubMed] [Google Scholar]
- Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Molecular pharmacology. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Stolk RP, Breteler MM, Ott A, Pols HA, Lamberts SW, Grobbee DE, Hofman A. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997;20:792–795. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- Stoltzner SE, Berchtold NC, Cotman CW, Pike CJ. Estrogen regulates bcl-x expression in rat hippocampus. Neuroreport. 2001;12:2797–2800. doi: 10.1097/00001756-200109170-00009. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka K, Nakano M, Totsune-Nakano H, Minakami H, Tero-Kubota S, Ikegami Y. Mechanism of O2-generation in reduction and oxidation cycle of ubiquinones in a model of mitochondrial electron transport systems. Biochimica et biophysica acta. 1988;936:377–385. doi: 10.1016/0005-2728(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67:77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85:3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009;17:737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochimica et biophysica acta. 2011 doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Experimental neurology. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer’s disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr., Davis RE, Parker WD., Jr. Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Tang M, Subbiah MT. Estrogens protect against hydrogen peroxide and arachidonic acid induced DNA damage. Biochimica et biophysica acta. 1996;1299:155–159. doi: 10.1016/0005-2760(95)00227-8. [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Taskinen MR, Puolakka J, Pyorala T, Luotola H, Bjaorn M, Kaarianen J, Lahdenpera S, Ehnholm C. Hormone replacement therapy lowers plasma Lp(a) concentrations. Comparison of cyclic transdermal and continuous estrogen-progestin regimens. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:1215–1221. doi: 10.1161/01.atv.16.10.1215. [DOI] [PubMed] [Google Scholar]
- Theodoropoulou A, Metallinos IC, Psyrogiannis A, Vagenakis GA, Kyriazopoulou V. Ghrelin and leptin secretion in patients with moderate Alzheimer’s disease. The journal of nutrition, health & aging. 2012;16:472–477. doi: 10.1007/s12603-012-0058-4. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Kuhad A, Bishnoi M, Chopra K. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharmacol Biochem Behav. 2009;93:183–189. doi: 10.1016/j.pbb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Tomicek NJ, Lancaster TS, Korzick DH. Increased estrogen receptor beta in adipose tissue is associated with increased intracellular and reduced circulating adiponectin protein levels in aged female rats. Gender medicine. 2011;8:325–333. doi: 10.1016/j.genm.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;24:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- Tyler JL, Strother SC, Zatorre RJ, Alivisatos B, Worsley KJ, Diksic M, Yamamoto YL. Stability of regional cerebral glucose metabolism in the normal brain measured by positron emission tomography. J Nucl Med. 1988;29:631–642. [PubMed] [Google Scholar]
- Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med (Berl) 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- Une K, Takei YA, Tomita N, Asamura T, Ohrui T, Furukawa K, Arai H. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2011;18:1006–1009. doi: 10.1111/j.1468-1331.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- Vagelatos NT, Eslick GD. Type 2 Diabetes as a Risk Factor for Alzheimer’s Disease: The Confounders, Interactions, and Neuropathology Associated With This Relationship. Epidemiologic reviews. 2013 doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Gonzalez-Lima F, Reiman EM. FDG autoradiography reveals developmental and pathological effects of mutant amyloid in PDAPP transgenic mice. Int J Dev Neurosci. 2008;26:253–258. doi: 10.1016/j.ijdevneu.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006a;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Schneider L, Reiman EM. Age- and transgene-related changes in regional cerebral metabolism in PSAPP mice. Brain research. 2006b;1116:194–200. doi: 10.1016/j.brainres.2006.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Yaari R, Wolf AB, Kusne Y, Beach TG, Roher AE, Corneveaux JJ, Huentelman MJ, Caselli RJ, Reiman EM. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsvoort T, Compton J, Murphy D. In vivo assessment of the effects of estrogen on human brain. Trends in endocrinology and metabolism: TEM. 2001;12:273–276. doi: 10.1016/s1043-2760(01)00422-2. [DOI] [PubMed] [Google Scholar]
- van Himbergen TM, Beiser AS, Ai M, Seshadri S, Otokozawa S, Au R, Thongtang N, Wolf PA, Schaefer EJ. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Gibbs EM, Simpson IA. Glucose utilization and glucose transporter proteins GLUT-1 and GLUT-3 in brains of diabetic (db/db) mice. The American journal of physiology. 1997;272:E267–274. doi: 10.1152/ajpendo.1997.272.2.E267. [DOI] [PubMed] [Google Scholar]
- Vehkavaara S, Hakala-Ala-Pietila T, Virkamaki A, Bergholm R, Ehnholm C, Hovatta O, Taskinen MR, Yki-Jarvinen H. Differential effects of oral and transdermal estrogen replacement therapy on endothelial function in postmenopausal women. Circulation. 2000;102:2687–2693. doi: 10.1161/01.cir.102.22.2687. [DOI] [PubMed] [Google Scholar]
- Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males: control of longevity by sex hormones. Science of aging knowledge environment: SAGE KE. 2005;2005:pe17. doi: 10.1126/sageke.2005.23.pe17. [DOI] [PubMed] [Google Scholar]
- Vina J, Sastre J, Pallardo FV, Gambini J, Borras C. Role of mitochondrial oxidative stress to explain the different longevity between genders: protective effect of estrogens. Free radical research. 2006;40:1359–1365. doi: 10.1080/10715760600952851. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK. Large-scale chemical dissection of mitochondrial function. Nature biotechnology. 2008;26:343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brewer J, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jr., Jennings RG, Karow D, Dale AM. Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. AJNR American journal of neuroradiology. 2010;31:347–354. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clinical endocrinology. 2013;78:321–329. doi: 10.1111/cen.12086. [DOI] [PubMed] [Google Scholar]
- Walton C, Godsland IF, Proudler AJ, Wynn V, Stevenson JC. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. European journal of clinical investigation. 1993;23:466–473. doi: 10.1111/j.1365-2362.1993.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. Journal of neurochemistry. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Wang JM, Hou X, Adeosun S, Hill R, Henry S, Paul I, Irwin RW, Ou XM, Bigler S, Stockmeier C, et al. A dominant negative ERbeta splice variant determines the effectiveness of early or late estrogen therapy after ovariectomy in rats. PloS one. 2012;7:e33493. doi: 10.1371/journal.pone.0033493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- Warren MW, Hynan LS, Weiner MF. Lipids and adipokines as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2012;29:151–157. doi: 10.3233/JAD-2012-111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain research. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain research. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Wildman RP, Tepper PG, Crawford S, Finkelstein JS, Sutton-Tyrrell K, Thurston RC, Santoro N, Sternfeld B, Greendale GA. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women’s Health Across the Nation. The Journal of clinical endocrinology and metabolism. 2012;97:E1695–1704. doi: 10.1210/jc.2012-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Plassman BL, Burke J, Benjamin S. Preventing Alzheimer’s disease and cognitive decline. Evidence report/technology assessment. 2010:1–727. [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Jeffery RW, Burton LR, Thorson C, Kuller LH, Folsom AR. Change in waist-hip ratio with weight loss and its association with change in cardiovascular risk factors. Am J Clin Nutr. 1992;55:1086–1092. doi: 10.1093/ajcn/55.6.1086. [DOI] [PubMed] [Google Scholar]
- Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Frontiers in neuroendocrinology. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES. Perimenopause: an update. The Nursing clinics of North America. 2004;39:117–129. doi: 10.1016/j.cnur.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17 Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Wynn V, Adams PW, Godsland I, Melrose J, Niththyananthan R, Oakley NW, Seed M. Comparison of effects of different combined oral-contraceptive formulations on carbohydrate and lipid metabolism. Lancet. 1979;1:1045–1049. doi: 10.1016/s0140-6736(79)92949-0. [DOI] [PubMed] [Google Scholar]
- Wynn V, Doar JW. Some effects of oral contraceptives on carbohydrate metabolism. Lancet. 1966;2:715–719. doi: 10.1016/s0140-6736(66)92978-3. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56:211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiology of aging. 2009;30:607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. Jama. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Yager JD, Chen JQ. Mitochondrial estrogen receptors--new insights into specific functions. Trends in endocrinology and metabolism: TEM. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Douchi T, Yamamoto S, Oki T, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstetrics and gynecology. 2001;97:338–342. doi: 10.1016/s0029-7844(00)01181-9. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr., Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, et al. Mitochondrial localization of estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiology of aging. 2012;33:1507–1521. doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Petanceska SS, Montine TJ, Holtzman DM, Schmidt SD, Parker CA, Callahan MJ, Lipinski WJ, Bisgaier CL, Turner BA, et al. Aging, gender and APOE isotype modulate metabolism of Alzheimer’s Abeta peptides and F-isoprostanes in the absence of detectable amyloid deposits. Journal of neurochemistry. 2004;90:1011–1018. doi: 10.1111/j.1471-4159.2004.02532.x. [DOI] [PubMed] [Google Scholar]
- Yao J, Rettberg JR, Klosinski LP, Cadenas E, Brinton RD. Shift in brain metabolism in late onset Alzheimer’s disease: implications for biomarkers and therapeutic interventions. Molecular aspects of medicine. 2011;32:247–257. doi: 10.1016/j.mam.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Zhao L, Mao Z, Chen S, Wong KC, To J, Brinton RD. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor beta-selective phytoSERM treatments. Brain research. 2013 doi: 10.1016/j.brainres.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui T, Tomita J, Miyatani Y, Yamada M, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R, Ishii Y, et al. Associations of adiponectin with sex hormone-binding globulin levels in aging male and female populations. Clinica chimica acta; international journal of clinical chemistry. 2007;386:69–75. doi: 10.1016/j.cca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Yates CM, Butterworth J, Tennant MC, Gordon A. Enzyme activities in relation to pH and lactate in postmortem brain in Alzheimer-type and other dementias. Journal of neurochemistry. 1990;55:1624–1630. doi: 10.1111/j.1471-4159.1990.tb04948.x. [DOI] [PubMed] [Google Scholar]
- Ye L, Wang F, Yang RH. Diabetes impairs learning performance and affects the mitochondrial function of hippocampal pyramidal neurons. Brain research. 2011;1411:57–64. doi: 10.1016/j.brainres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Yoshihara R, Utsunomiya K, Gojo A, Ishizawa S, Kanazawa Y, Matoba K, Taniguchi K, Yokota T, Kurata H, Yokoyama J, et al. Association of polymorphism of estrogen receptor-alpha gene with circulating levels of adiponectin in postmenopausal women with type 2 diabetes. Journal of atherosclerosis and thrombosis. 2009;16:250–255. doi: 10.5551/jat.e471. [DOI] [PubMed] [Google Scholar]
- Yoshii F, Barker WW, Chang JY, Loewenstein D, Apicella A, Smith D, Boothe T, Ginsberg MD, Pascal S, Duara R. Sensitivity of cerebral glucose metabolism to age, gender, brain volume, brain atrophy, and cerebrovascular risk factors. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1988;8:654–661. doi: 10.1038/jcbfm.1988.112. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Armellini F, Milani MP, De Marchi M, Todesco T, Robbi R, Bergamo-Andreis IA, Bosello O. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. Int J Obes Relat Metab Disord. 1992;16:495–504. [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. Jama. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Schneider LS, Brinton RD. Estrogen receptor beta-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause. 2011a;18:1131–1142. doi: 10.1097/gme.0b013e3182175b66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Morgan TE, Mao Z, Lin S, Cadenas E, Finch CE, Pike CJ, Mack WJ, Brinton RD. Continuous versus cyclic progesterone exposure differentially regulates hippocampal gene expression and functional profiles. PloS one. 2012;7:e31267. doi: 10.1371/journal.pone.0031267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17 beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiology of aging. 2011b;32:1949–1963. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- Zhao X, MacBride MM, Peterson BR, Pfaff DW, Vasudevan N. Calcium flux in neuroblastoma cells is a coupling mechanism between non-genomic and genomic modes of estrogens. Neuroendocrinology. 2005;81:174–182. doi: 10.1159/000087000. [DOI] [PubMed] [Google Scholar]
- Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424–434. doi: 10.2337/db11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Pankow JS, Ballantyne CM, Couper D, Hoogeveen RC, Pereira M, Duncan BB, Schmidt MI. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. The Journal of clinical endocrinology and metabolism. 2010;95:5097–5104. doi: 10.1210/jc.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]