A very recent finding with high field MRI is a cellular–compartment specific, and orientation-dependent susceptibility contrast in WM (1, 2), attributed to the myelin sheath that often surrounds axons (see Figure) (2–4). In the major fiber bundles, where myelin appears to be the dominant source of tissue susceptibility shifts (relative to water) (5, 6), distinct R2* values and frequency shifts can be observed for axonal and interstitial water, and water trapped between the layers of the myelin sheath (also called “myelin water”)(2). This compartmental assignment remains tentative but is partly supported by field modeling studies (2–4), based on the geometric arrangement of the myelin sheath and the notion that its susceptibility may be anisotropic (7, 8). If confirmed, this may impact the study of demyelinating pathologies such as multiple sclerosis. More generally, possible compartment and orientation dependent frequency shifts need to be taken into account when quantifying susceptibility with susceptibility mapping techniques.
Figure 1.
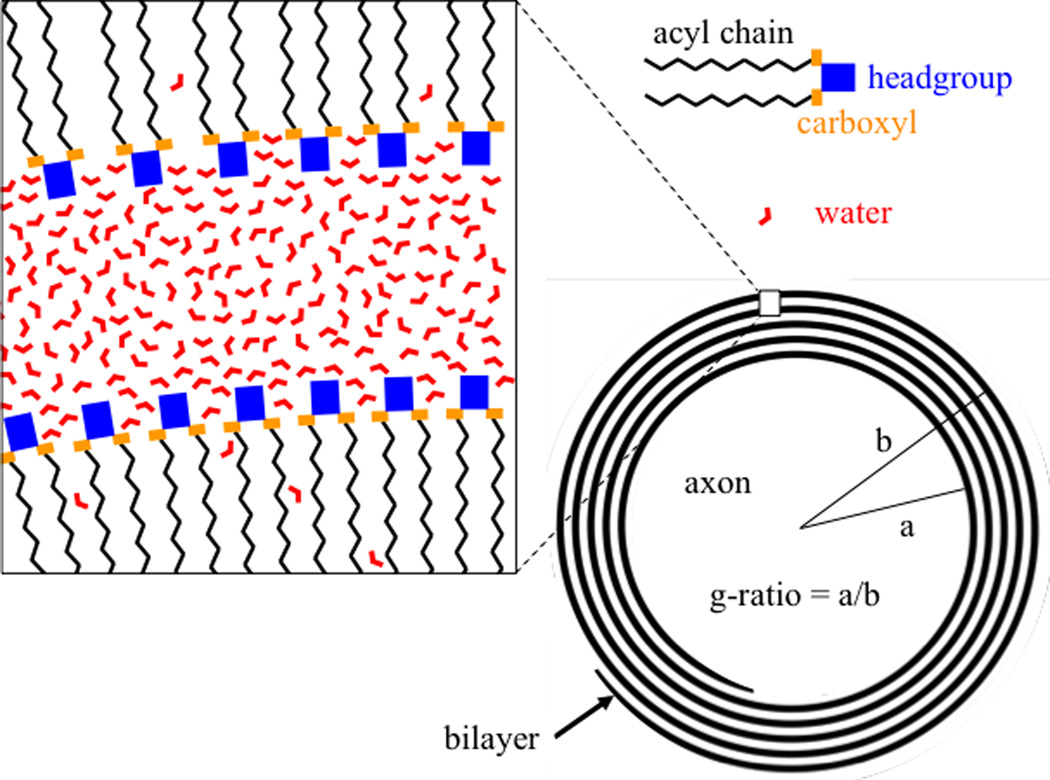
Location of water between myelin layers. Water molecules occupy a predominantly cylindrical annulus between phospholipid bilayers, although some bilayer penetration occurs. Near the headgroups, water experiences reduced mobility and possibly an electrostatic orienting effect. Myelin structure is highly simplified to show only phospholipids, while proteins and other lipids are omitted.
One outstanding issue with the study of frequency shifts in white matter is an apparent discrepancy between theoretical and experimental findings for myelin water (2). Experimental data suggests that the myelin compartment’s mean frequency shift is strongly dependent on orientation and has a sign opposite to that of axonal water (2, 3). For fibers perpendicular to the magnetic field, positive mean frequency shifts in excess of +100 ppb (i.e. ~30 Hz frequency increase at 7T) have been found for myelin water, compared to about −20 ppb for axonal water (2, 3). Theoretical estimates on the other hand only match these experimental data when implicitly assuming that, at the nano-scale, myelin water occupies approximately spherically shaped pockets between the myelin layers (2). In contrast, proper simulation of a more realistic laminar distribution of water between lipid bilayers leads to myelin water frequency shifts that are too small and of the wrong sign (negative rather than positive). For example, a very recent modeling study assuming thin cylindrical myelin water sheaths leads to estimates to about −17 and −45 ppb for myelin and axonal water respectively(4). Here, this discrepancy and potential explanations will be briefly looked at.
First, it should be noted that there appear to be a couple of flaws in the most recent study (4) that directly affect its frequency estimates. One is an apparently incorrect evaluation of its equation [39] for the estimate of myelin water frequency shift. Substituting the parameter values suggested by the authors leads to a myelin water frequency shift of −17 ppb (quoted above) rather than the value of −47 ppb reported in (4) (this problem, pointed out to the authors on July 26 2003 via a private communication, appears to have been corrected in online versions of (4)downloaded after August 3). A secondary issue is the authors’ use of an unrealistic myelin g-ratio (see Figure) of 0.5, lower than the range of around 0.55–0.9 widely reported for CNS fibers (e.g. (9–11)). For example, using a g-ratio of 0.7 would results in lower estimates of −10 ppb and −23 ppb for myelin and axonal frequency shifts respectively. While this axonal shift is consistent with measurements and earlier modeling work, the myelin shift clearly is not (−10 versus +100 ppb). Thus, despite the apparently more accurate assumptions for the geometry of the myelin water environment, this model has less explanatory power than rival models (2, 3) that are able to accurately match the measurement data with realistic myelin susceptibility values. Importantly, it should be realized that by not specifically defining the water accessible space within the myelin sheath, these rival methods use the local magnetization of myelin to calculate the field experienced by a water proton (12), which is equivalent to implicitly assuming the myelin water to be restricted to spherical pockets.
How to explain the surprising finding that spherical rather than cylindrical shapes appear more appropriate to model the field experienced by myelin water? The answer to this question will likely require more modeling and experimental studies. Below, some potential explanations will be highlighted, including: (i) the water protons generating the NMR signal are not where it is assumed they are, (ii) the anisotropy is not where it is assumed it is; (iii) the are errors in calculating the magnetic field or its sampling by water protons.
Although a hollow cylinder may seem an appropriate shape to model the water-accessible space between the lipid bilayers of myelin, it may not accurately represent the actual water distribution. For example, the bilayer itself can have a water content of up to about 20% (13), partly within the about 0.5 nm deep pockets between the headgroups of the phospholipid molecules (see Figure), and partly deeper between the phospholipids (13). In addition, the 3–4 nm thick space between bilayers is not contiguously occupied by water molecules but contains proteolipid protein and myelin basic protein as well (13, 14). A further complication is that lipid bilayers are not rigid planes but rather have considerable flexibility leading to a temporally varying structure (15). Nevertheless, these apparent shortcomings of the model appear to be relatively minor and too insignificant to substantially alter the mean field experienced by the water protons.
Another model assumption is the notion that myelin’s anisotropic susceptibility entirely originates from the hydrocarbon chains of its phospholipid molecules (16). Since this part of the myelin sheath is hardly accessible by water, approximation of the water environment by cylindrical spaces appears appropriate. However, it has been suggested that some of the anisotropy may arise from phospholipid headgroups (17), which are hydrophilic and more readily accessible by water. For example, the molar anisotropy associated of carboxyl, which is part of the headgroup (See Figure), is about a quarter of that of the lipid acyl chain (16). Any field effect experienced by these protons is effectively shared with water protons further away from bilayer-water interface through diffusion, which is thought to be fast on the T2 time scale (see e.g. (18)). The fact that these water spaces do not have the clear cylindrical geometry assumed by the model, may have contributed to the discrepant findings. Nevertheless, it remains unclear if the headgroups are sufficiently oriented to substantially contribute to the observed anisotropy.
Lastly, field calculations based on the Fourier method (2), and analytical approaches (3, 4) are macroscopic continuum approaches, i.e. they do not explicitly calculate the magnetic dipole fields of individual water molecules, but rather use the Lorentz-sphere construct to approximate their effect (12, 19, 20). This construct assumes that, due to extensive averaging of many dipoles within the sphere, their contribution to the local field cancels out and can be neglected. Calculations taking into account the effect of discrete dipoles suggest that this assumption holds for spheres that enclose as few as a few hundred dipoles (19). Imperfect cancellation may occur for protons in the water accessible space between the phospholipid headgroups at the myelin-water interface surface, whose size is only several times the dimension of the water molecule. However, when considering that the measured water frequency results from extensive spatial (over all water accessible space between the sheaths) and motional averaging, the effect of discrete dipoles can likely be safely neglected.
Various other explanations are possible including rather exotic ones. For example, although there is increasing evidence for an anisotropic susceptibility of white matter (2, 3, 7, 8, 21), this may not directly originate from the lipid bilayer itself. In fact, it is possible that some of the water in the hydration layers of myelin may have a net orientation preference (see Figure) (22), which combined with the anisotropic susceptibility of the water molecule (23)may contribute to the anisotropic magnetic properties of white matter. Further studies will be required to investigate this, and other possibilities mention above.
REFERENCES
- 1.van Gelderen P, de Zwart JA, Lee J, Sati P, Reich DS, Duyn JH. Nonexponential T2* decay in white matter. Magn Reson Med. 2012;67(1):110–117. doi: 10.1002/mrm.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sati P, van Gelderen P, Silva AC, Reich DS, Merkle H, de Zwart JA, Duyn JH. Micro-compartment specific T2() relaxation in the brain. Neuroimage. 2013;77:268–278. doi: 10.1016/j.neuroimage.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wharton S, Bowtell R. Fiber orientation-dependent white matter contrast in gradient echo MRI. Proc Natl Acad SciUSA. 2012;109(45):18559–18564. doi: 10.1073/pnas.1211075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukstanskii AL, Yablonskiy DA. On the role of neuronal magnetic susceptibility and structure symmetry on gradient echo MR signal formation. Magn Reson Med. 2013 doi: 10.1002/mrm.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Li W, Johnson GA, Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage. 2011;56(3):930–938. doi: 10.1016/j.neuroimage.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Shmueli K, Kang BT, Yao B, Fukunaga M, van Gelderen P, Palumbo S, Bosetti F, Silva AC, Duyn JH. The contribution of myelin to magnetic susceptibility-weighted contrasts in high-field MRI of the brain. Neuroimage. 2012;59(4):3967–3975. doi: 10.1016/j.neuroimage.2011.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Wu B, Avram AV, Liu C. Magnetic susceptibility anisotropy of human brain in vivo and its molecular underpinnings. Neuroimage. 2012;59(3):2088–2097. doi: 10.1016/j.neuroimage.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Shmueli K, Fukunaga M, van Gelderen P, Merkle H, Silva AC, Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci USA. 2010;107(11):5130–5135. doi: 10.1073/pnas.0910222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatzopoulou E, Miguez A, Savvaki M, Levasseur G, Muzerelle A, Muriel MP, Goureau O, Watanabe K, Goutebroze L, Gaspar P, et al. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J Nerosc. 2008;28(30):7624–7636. doi: 10.1523/JNEUROSCI.1103-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy J, Ellis EA, Hope GM, Emerson S. Maintenance of myelinated fibre g ratio in acute experimental allergic encephalomyelitis. Brain. 1991;114(Pt 1A):281–294. [PubMed] [Google Scholar]
- 11.Hildebrand C, Hahn R. Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. J Neurol Sci. 1978;38(3):421–434. doi: 10.1016/0022-510x(78)90147-8. [DOI] [PubMed] [Google Scholar]
- 12.Chu SC, Xu Y, Balschi JA, Springer CS., Jr Bulk magnetic susceptibility shifts in NMR studies of compartmentalized samples: use of paramagnetic reagents. Magn Reson Med. 1990;13(2):239–262. doi: 10.1002/mrm.1910130207. [DOI] [PubMed] [Google Scholar]
- 13.Vandenheuvel FA. Structural Studies of Biological Membranes: The Structure of Myelin. Ann NY Acad Sci. 1965;122:57–76. doi: 10.1111/j.1749-6632.1965.tb20192.x. [DOI] [PubMed] [Google Scholar]
- 14.De Felici M, Felici R, Ferrero C, Tartari A, Gambaccini M, Finet S. Structural characterization of the human cerebral myelin sheath by small angle x-ray scattering. Phys Med Biol. 2008;53(20):5675–5688. doi: 10.1088/0031-9155/53/20/007. [DOI] [PubMed] [Google Scholar]
- 15.Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Quarterly Reviews of Biophysics. 1975;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- 16.Lonsdale K. Diamagnetic anisotropy of organic molecules. Proc Royal Soc Lond Series A-Mathematical and Physical Sciences. 1939;171(A947):0541–0568. [Google Scholar]
- 17.Sakurai I, Kawamura Y, Ikegami A, Iwayanagi S. Magneto-orientation of lecithin crystals. Proc Natl Acad Sci USA. 1980;77(12):7232–7236. doi: 10.1073/pnas.77.12.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen FY, Peters GH, Taub H, Miskowiec A. Diffusion of water and selected atoms in DMPC lipid bilayer membranes. J Chem Phys. 2012;137(20):204910. doi: 10.1063/1.4767568. [DOI] [PubMed] [Google Scholar]
- 19.Aravamudhan S. Magnetized materials: contributions inside Lorentz ellipsoids. Indian J Phys and Proc Indian Assoc Cultivation of Science. 2005;79(9):985–989. [Google Scholar]
- 20.Jackson JD. Classical Electrodynamics. John Wiley and Sons Limited; 1962. [Google Scholar]
- 21.van Gelderen P, Mandelkow H, de Zwart JA, Duyn JH. The anisotropy of myelin magnetic susceptibility; Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City USA: 2013. p. 708. [Google Scholar]
- 22.Chapman G, McLauchlan KA. Oriented Water in Sciatic Nerve of Rabbit. Nature. 1967;215(5099):391. doi: 10.1038/215391a0. [DOI] [PubMed] [Google Scholar]
- 23.Taft H, Dailey BP. Magnetic Susceptibility of H2O Molecule. J Chem Phys. 1969;51(3):1002. [Google Scholar]