Abstract
Importance
Describing the relationship between the availability of free prescription drug samples and dermatologists’ prescribing patterns on a national scale can help inform policy guidelines on the use of free samples in a physician’s office.
Objective
To investigate the relationships between free drug samples and dermatologists’ local and national prescribing patterns and between the availability of free drug samples and prescription costs.
Design, Setting, and Participants
Cross-sectional study investigating prescribing practices for acne, a common dermatologic condition for which free samples are often available. The settings were, first, the offices of a nationally representative dermatologists from the National Disease and Therapeutic Index (an IMS Health Incorporated database) and, second, an academic medical center clinic without samples. Participants were ambulatory patients who received a prescription from a dermatologist for a primary initial diagnosis of either acne vulgaris or acne rosacea in 2010.
Main Outcome Measures
National trends in dermatologist prescribing patterns, the degree of correlation between the availability of free samples and the prescribing of brand-name medications, and the mean cost of acne medications prescribed per office visit nationally and at an academic medical center without samples.
Results
On a national level, the provision of samples with a prescription by dermatologists has been increasing over time, and this increase directly correlates with the use of the branded generic drugs promoted by these samples. Branded and branded generic drugs comprised most of the prescriptions written nationally (79%), while they represented only 17% at an academic medical center clinic without samples. Because of the increased use of branded and branded generic drugs, the national mean total retail cost of prescriptions at an office visit for acne was conservatively estimated to be 2 times higher (approximately $465 nationally vs $200 at an academic medical center without samples).
Conclusions and relevance
The benefits of free samples in dermatology must be weighed against potential negative impacts on prescribing behavior and prescription costs.
The availability of free drug samples in physicians’ offices has received considerable attention.1–5 A survey conducted in 2003–2004 found that 78% of surveyed physicians had received drug samples; that physicians practicing in solo, 2-person, or group practices had higher odds of receiving samples than those in hospital, health maintenance organization, or university or medical school settings; and that these odds were dependent on medical specialty.1 As physicians continue to receive and provide free samples in clinical practice, it is important to better delineate how physician access to these samples can affect their prescribing behavior.
Advocates and opponents of free drug samples commonly outline several reasons for supporting or discouraging the practice. Samples can be beneficial for patients when used to provide otherwise expensive medications to the uninsured or poor. Samples of alternative medications or formulations can be provided to allow patients to choose a preferred medication, possibly leading to higher adherence.6 In addition, physicians can more easily offer new medications that could have advantages over existing generic alternatives. However, national studies3, 7–9 have repeatedly shown that patients who commonly receive samples are often not those who would financially benefit from their free provision. There are also concerns that samples do not adequately relay consumer medical information to the patient as a pharmacist otherwise would, which could lead to potentially dangerous drug interactions, allergic reactions, or harmful side-effects.10 Samples also add indirectly to the cost of medications, and their aggregate retail value represents approximately $16 billion spent by pharmaceutical companies each year.9
Conflicting evidence exists surrounding the key question of whether the availability of samples alter the prescribing habits of physicians. While some studies2, 11, 12 show that access to samples influences prescribing decisions, other studies13, 14 are less definitive. Surveys demonstrate that physicians do not believe that access to samples influence their behavior, although the availability of drug samples may lead them to prescribe a medication that differs from their preferred drug choice.5, 15, 16 Many of these studies are limited in scope or design by focusing on single-center observations or by relying on physician self-report.
To better understand how physician prescribing behavior may be altered by the provisions of drug samples, we investigated sampling and prescribing patterns specifically in dermatology. 17, 18 Free drug samples provided by pharmaceutical companies are widely available in private, office-based dermatology practices. We investigated prescriptions patterns for patients with acne vulgaris and rosacea for the following reasons: (1) acne is one of the most common indications treated by dermatologists,19 (2) medications for acne are heavily sampled, (3) acne treatment recommendations have not changed considerably in the past decade,20, 21 and (4) multiple bio-equivalent branded, branded generic, and generic medication alternatives exist. Branded generic drugs are specifically defined as products that have novel dosage forms of off-patent products or use a trade name for a molecule that is off-patent.
In this study, we assess national temporal trends related to the provision of free drug samples by dermatologists. We use data from a large academic medical center (AMC) without samples to contrast nationally representative data on the prescriptions most commonly written by dermatologists for acne.
Methods
Data Sources
This study was approved by the Stanford Institutional Review Board. The informed consent process was waived to protect the identity of the participants under 45 CFR 164.512(i)(2)(ii)(A), (B), (C). Our analysis of acne treatment examined local data and nationally representative information. Local data for this study were extracted from Stanford University’s Epic electronic database via the Center for Clinical Informatics using the Stanford Translational Research Integrated Database Environment (STRIDE) tool.22
National data for this study was obtained from the National Disease and Therapeutic Index (NDTI).23 The NDTI is a survey of primarily office-based US physicians conducted by IMS Health Incorporated (http://www.imshealth.com), providing nationally representative data on physicians, patients, and treatments.24 Included physicians are selected from the master lists of the American Medical Association and the American Osteopathic Association through random sampling. The geographic and specialty distribution of the selected physicians are designed to mirror national patterns. Each quarter, approximately 3500 physicians are surveyed on 2 consecutive workdays and are asked to detail their clinical encounters with every patient. Physicians self-report patient diagnoses, visit characteristics, patient demographics, and their own demographic information. A unique record is generated for each diagnosis, in which the physician reports all new or continuing medications from the encounter, including prescribed and sampled medications.
Drug prices used in this study were directly quoted from customer service representatives of a major pharmacy in July 2013. The prices apply to a mail-in ordering system for patients without any insurance and do not take into account any manufacturer incentives or pharmacy savings plans. Although these undiscounted prices are likely higher than the average patient’s out-of-pocket costs, they allow for a more direct comparison of prices for the purposes of our analysis.
Patient Selection and Characteristics
Deidentified local patient information on age, sex, race/.ethnicity, and insurance status was provided for all primary, initial diagnoses of acne vulgaris (International Classification of Diseases, Ninth Revision [ICD-9] code 706.1) or rosacea (ICD-9: 695.3) in 2010, the first full year for which complete prescription information was available. Together, these 2 diagnoses comprise what is informally referred to as adult acne and are both investigated to provide a more complete picture of dermatologist prescribing behavior in response to acne. Patients in the local cohort were restricted to those treated by a dermatologist at an academic AMC clinic. Free drug samples have been banned from the AMC clinic location since 2004, and the AMC is used to contrast national prescribing patterns, where samples are ubiquitous. Analyzed prescriptions were restricted to only those written at a patient’s initial encounter to help control for confounding because of acne persistence. The same conditions were used to extract data from the NDTI database.
Classification of Prescriptions
Prescriptions extracted from the NDTI were limited to those written by office-based dermatologists for patients who, on their first visit, received a diagnosis of acne vulgaris or rosacea. “Prescriptions written with a sample” refers to entries in the NDTI in which physician self-reported providing both a prescription for a medication and a sample of the same medication to the patient. Where appropriate, these entries were kept separate from instances in which the physician only administered a prescription to the patient.
Branded and branded generic drugs are analyzed and discussed together for the following 2 main reasons: (1) they are usually priced similarly compared with generic drugs and (2) samples for branded and branded generic drugs are much more prevalent relative to generics. Definitions and examples of drugs in each category are listed in eTable 1 in the Supplement.
Statistical Analysis
Descriptive statistics were used to characterize trends in the local AMC data and the national NDTI data. A Pearson product moment correlation was used to quantify the relationship between sample availability and the proportion of branded generic prescriptions written. The mean estimated cost of acne prescriptions at an initial visit to a dermatologist was calculated using a weighted average of drug prescription frequency and their associated prices (taken from a consistent source), multiplied by the mean number of prescriptions written per office visit. All statistical analyses were performed with available software (SAS, version 9.3; SAS Institute Inc).
Results
National Trends in the Provision of Free Samples by Dermatologists
From the NDTI, we derived temporal trends in the percentage of prescriptions written with a sample in dermatology relative to other medical specialties (Figure 1). The use of free samples in dermatology is comparatively high relative to other medical specialties. For the decade between 2001 and 2010, the proportion of prescriptions written with a sample relative to all prescriptions written increased from 12% to 18% in dermatology, while during the same time period the aggregate proportion for all other specialties decreased from 7% to 4%.23
Figure 1.
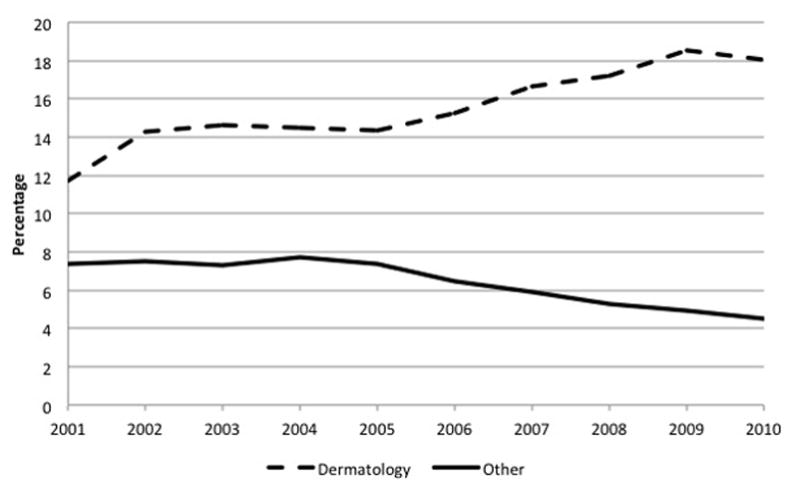
Trend in the percentage of prescriptions written with a sample by dermatologists compared to physicians in other medical specialties on a nationally projected basis.
“Other” specialties correspond to allergy, cardiology, surgery, endocrinology, family practice, general practice, gastroenterology, geriatrics, hematology, internal medicine, nephrology, neurology, obstetrics/gynecology, oncology, ophthalmology, pediatrics, psychiatry, pulmonary diseases, rheumatology, and urology. (Source: IMS National Disease and Therapeutic Index™ January, 2001 – December, 2010, IMS Health Incorporated. All Rights Reserved.)
The percentage of prescriptions written with a sample by dermatologists has increased even more dramatically, from 10% in 2001 to 25% in 2010, for acne vulgaris and rosacea specifically. 23 In direct, positive correlation (r=0.92) to this finding is the increase in the percentage of branded generics prescribed by dermatologists relative to branded and generic drugs for the indication (Figure 2). Because the free samples being marketed are often for branded generic drugs, it makes intuitive sense that these 2 are correlated. In contrast, the percentage of generic medications prescribed has remained flat and in absolute numbers has decreased in the same time period.
Figure 2.
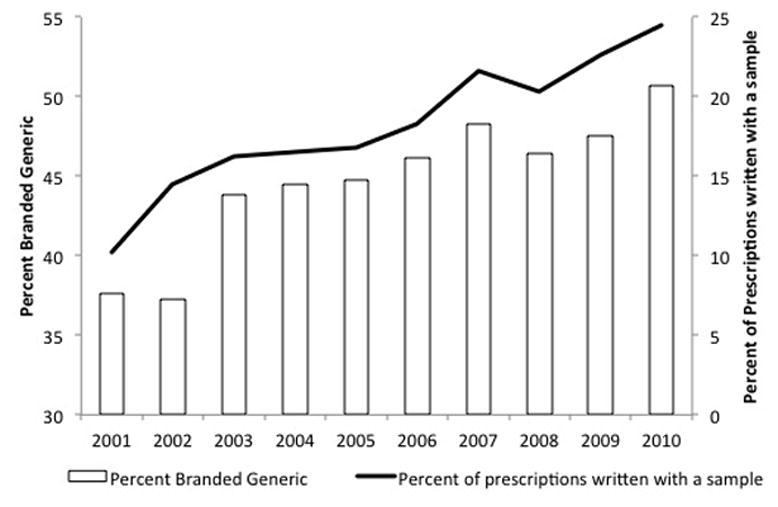
The percent of prescriptions written with a sample and the percent of branded generic drugs as prescribed by office-based dermatologists for patients with acne vulgaris and acne rosacea on a nationally projected basis.
(Source: IMS National Disease and Therapeutic Index™ January, 2001 – December, 2010, IMS Health Incorporated. All Rights Reserved.)
Investigating the actual medications that were provided as samples and prescribed by dermatologists in offices nationwide supports the observed trends. The top 5 medications prescribed overall and with samples by office-based dermatologists for initial encounters of patients with acne on a national level for 3 separate years in the past decade are listed in Table 1. The composition of each list is markedly different between years, indicating that the medication preferences of dermatologists shift over time. However, when comparing within-year patterns, the most common medications prescribed overall and the medications prescribed with a sample are similar. In 2005, for example, the top 4 medications prescribed with a sample were also the top 4 medications for which a prescription was written, both with and without an accompanying sample. This implies that most of the commonly prescribed drugs for acne were available and dispensed as samples in the office and that these frequently prescribed medications were preferred in years when samples for them were available.
Table 1.
Top five medications prescribed overall and with samples by office-based dermatologists for initial patient encounters of acne on a nationally projected basis for three different years
| Top Five Medications Prescribed Overall | Top Five Medications Prescribed with a Free Sample | |
|---|---|---|
| 2010 | Epiduo® | Epiduo® |
| Doxycycline Hyclate | Metrogel® | |
| Metrogel® | Solodyn® | |
| Solodyn® | Ziana® | |
| Differin® | Oracea® | |
| 2005 | Differin® | Differin® |
| Benzaclin® | Duac® | |
| Duac® | Benzaclin® | |
| Retin-A Micro® | Retin-A Micro® | |
| Doxycycline Hyclate | Metrogel® | |
| 2001 | Differin® | Differin® |
| Tetracycline HCl | Retin-A Micro® | |
| Cleocin T® | Tazorac® | |
| Benzamycin® | Metrolotion® | |
| Retin-A Micro® | Triaz® |
(Source: IMS National Disease and Therapeutic Index™ January, 2001 – December, 2010, IMS Health Incorporated. All Rights Reserved.)
National versus AMC Prescription Patterns
Nationally representative data from the NDTI database was compared were compared with local data from an AMC. The patient characteristics at each site are listed in eTable 2 in the Supplement. In general, the AMC cohort was older, had fewer patients of white race/ethnicity, and had a higher percentage of patients covered by public insurance (defined as Medicare or Medicaid).
The 10 most commonly prescribed medications by dermatologists at the first diagnosis of acne in 2010 are listed in Table 2. Of the most commonly prescribed medications nationally, 9 out of 10 are classified as branded or branded generic medications, and samples are commonly given with all of them, ranging from 33% to 62% of the time. In fact, 12 out of the 15 most commonly sampled medications were also within the top 15 most prescribed overall to patients (data not shown). Of 9 branded or branded generic medications, 8 have less expensive commercially available generic equivalents.
Table 2.
Ten most common medications and their associated generic equivalents, as prescribed by Dermatologists nationally and at an academic medical center at the first diagnosis of acne in 2010
| % Rx given with sample | National Estimate | Generic Equivalent | Academic Medical Center | Generic Equivalent |
|---|---|---|---|---|
| 51% | Epiduo® | (adapalene and benzoyl peroxide) | Tretinoin | -- |
| 4% | Doxycycline Hyclate | -- | Doxycycline Hyclate | -- |
| 48% | Metrogel® | (metronidazole) | Benzoyl Peroxide | -- |
| 45% | Solodyn® | (minocycline HCl) | Clindamycin Phosphate | -- |
| 42% | Differin® | (adapalene) | Adapalene | -- |
| 49% | Finacea® | -- | Triamcinolone Acetonide | -- |
| 55% | Ziana® | (clindamycin phosphate and tretinoin) | Metronidazole | -- |
| 47% | Duac® | (clindamycin and benzoyl peroxide) | Metrocream® | (metronidazole) |
| 33% | Benzaclin® | (clindamycin and benzoyl peroxide) | Cephalexin | -- |
| 62% | Oracea® | (doxycycline) | Minocycline HCl | -- |
(Source: STRIDE and IMS National Disease and Therapeutic Index™ January, 2010 – December, 2010, IMS Health Incorporated. All Rights Reserved.)
Only available as Avage or Tazorac, but prescribed as such.
Dermatologists nationally and at the AMC prescribed very different medications for patients with acne vulgaris and rosacea in 2010 (Table 2). Only 1 of the 10 most commonly prescribed medications at the AMC was also commonly prescribed on a national level. Expanding the comparison to the top 20 most frequently prescribed medications still only resulted in a 35% overlap (7 of 20 medications) between the AMC and the national estimates.
The prescription variations between the AMC and national offices can be explained by examining the very different proportions of branded, branded generic, and generic medications that were prescribed in 2010. Nationally, while patients more frequently received prescriptions for branded or branded generic medications, patients at the AMC were overwhelmingly prescribed generic medications. For all commonly prescribed medications, defined as drugs prescribed 3 or more times in 2010, at the AMC, 17% (230 of 1364) of prescriptions were for branded or branded generic drugs and 83% (1134 of 1364) of prescriptions were for generic drugs (Figure 3). This is in stark contrast to medications prescribed by office-based dermatologists on a national level for patients manifesting acne for the first time, where 79% of prescriptions were branded or branded generic and 21% were generic.
Figure 3.
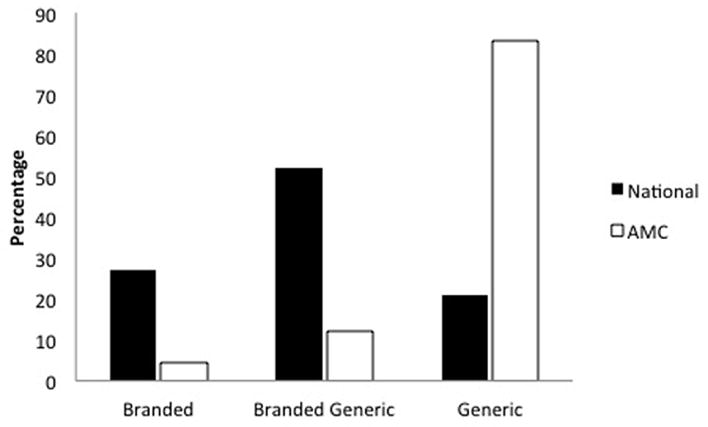
Percentage of all branded, branded generic, and generic medications for acne vulgaris and rosacea prescribed during a patient’s initial visit in 2010 at an academic medical center (AMC) and on a nationally projected basis.
Analyses are restricted to prescriptions written more than 3 times in 2010 at the AMC. (Source: STRIDE and IMS National Disease and Therapeutic Index™ January, 2010 – December, 2010, IMS Health Incorporated. All Rights Reserved.)
Costs Associated with Acne Prescriptions
Because the percentage of branded, branded generic, and generic medications prescribed on a national level contrast starkly with those written at the AMC and because branded and branded generic drugs are more expensive than generics, there will also be cost differences between the 2 groups. The mean retail cost of medications prescribed for acne was much higher nationally compared with the AMC. Using the top 20 most prescribed medications, which is approximately 63% of all prescriptions written in each database, and accounting for the difference in number of prescriptions written per visit at each site, the mean estimated costs of medications per patient visit nationally was $465 using estimates from the NDTI, and $200 for AMC (eTable 3 in the Supplement). In other words, the national mean retail cost of the prescriptions received at an office visit for acne is conservatively 2 times higher compared to the AMC, where samples were unavailable.
Discussion
The receipt of free samples by physicians is prevalent and controversial. The purported beneficial and harmful effects of the provision of free drug samples and the degree to which their availability can influence physician prescribing behavior are uncertain. In this study, we used local and national data to highlight how the provision of samples is associated with the prescribing behavior of dermatologists and how the availability of samples correlates with the prescription of more expensive branded generic drugs over less expensive generic alternatives. Our analysis also suggests that longitudinal prescribing preferences are at least in part related to what is contemporaneously available as free samples.
We show that nationally representative data are incongruent with locally observed prescription patterns in the absence of samples. This is important in part to demonstrate the broad effect that sample provision can have on prescribing patterns, as well as to emphasize the large cost implications that are a result of this modified behavior. Specifically, the increased prescribing of branded generics, as shown in Figure 3, increases overall costs to the health care system. Reiffen and Ward25 demonstrate that the introduction of a branded generic drug, which is essentially a generic version of a manufacturer’s currently branded drug introduced before patent expiration, can result in higher drug prices in the long run. They show that generic prices are pushed higher with the entry of branded generic medications into the market, which in turn implies that branded generics, as a strategy for drug manufacturers, can be advantageous by increasing the firm’s profits. Previous research has shown that physicians in general are not aware of the costs of drugs they are prescribing26. This takes on significance relative to the estimates by Payette and Grant-Kels, 27 which show that an average cost savings of $60 per prescription could be achieved by switching from a brand name to a generic dermatologic medication. With rising retail costs of prescription drugs28, it will be ever more important for physicians to be cognizant of how pharmaceutical marketing practices can affect their habits and potentially inflate the costs of prescribed drugs.
On a national level, the percentage of prescriptions written with a free sample by dermatologists for patients with acne has increased over time. As dermatologists increasingly utilize samples in their practice, the proportion of branded generic medications prescribed also increases over time at a similar rate. By virtue of what is made available to dermatologists as samples, most sampled medications are categorized as branded generic medications. The observed national trend whereby an increase in sample use has coincided with the increased prescription of branded generic medications suggests that dermatologists are increasingly likely to prescribe drug samples that are made available. Furthermore, dermatologists’ preferences for acne medications changes over time, but largely coincides with what medications are available as samples in that time period. In other words, dermatologists are using more samples, are prescribing more branded generic drugs as sample use increases, and are mirroring the specific medications they are prescribing to what is distributed to them by pharmaceutical representatives.
Our study has several limitations. The causal nature of the relationship between the availability of free samples and a preference for more expensive branded medications may be uncertain. The observed differences in prescribing habits may be attributed to other forms of pharmaceutical marketing that were not adequately captured in our study, such as the number of visits by or gifts from pharmaceutical representatives. The use of co-pay discount cards, which can also influence prescribing patterns, was not captured in this study and is an area that should be explored in future investigations. The observed differences in prescribing patterns could also be a reflection of the culture and the preferences of the dermatologists at the AMC, independent of the presence of drug samples. Specifically, broad differences in ethical norms at AMCs and private dermatology practices with regard to interactions with pharmaceutical companies could be contributing to these observations. There were also differences in patient demographics between those patients at the AMC and on a national level, but we could not perform a multivariate regression analysis to account for such factors because of the nature of the data in the NDTI. Given these limitations, the AMC data primarily serve to contrast the national data and offer an alternative scenario to national data that otherwise suggests a larger, systemic problem. The retail value of the medications assessed in this study does not reflect the value of the prescriptions that were actually filled and is a proxy for the actual cost to the health care system. Patients are likely to get branded and branded generic drugs at a much less expensive out-of-pocket price than listed, but this does not change the fact that some entity in the health care system is shouldering the burden of these costs. Also, although organizations such as The Joint Commission29 act to regulate drug sampling nationally, variation may exist between practices at AMCs. For example, while the AMC in this study does not have any samples, others may have free samples for over-the-counter medications, which may affect prescription patterns. From a care delivery standpoint, patient expectations and satisfaction were not documented between the 2 sites. Cost aside, the patient’s perception of the quality of care may be higher because of the availability of samples. If so, practicing physicians must weigh this fact when considering the benefits and drawbacks of drug sampling.
Conclusions
While there are many benefits and drawbacks of providing free drug samples, minimizing their use has been advocated by professional organizations and physician practices. Dermatologists, and physicians more generally, should be aware of how the availability of free samples influences physician prescribing behavior and increases health care expenses. The negative consequences of drug sampling affect clinical practice on a national level, and policies should be in place to properly mitigate their inappropriate influence on prescribing patterns.
Acknowledgments
Funding/Support: Dr. Stafford’s contribution to this study was supported in part by a mid-career mentoring award from the National Heart, Lung and Blood Institute (K24-HL086703).
| Funding/Sponsor was involved? | ||
|---|---|---|
| Design and conduct of the study | Yes ___ | No _X__ |
| Collection, management, analysis, and interpretation of data | Yes ___ | No _X__ |
| Preparation, review, or approval of the manuscript | Yes ___ | No _X__ |
| Decision to submit the manuscript for publication | Yes ___ | No _X__ |
Role of the Sponsors: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): National Disease and Therapeutic Index™ (1997–2009), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
We are indebted to David Peng, MD and Jean Tang, MD for their critical review of the manuscript. Additionally, the authors would like to thank Raymond R. Balise, PhD for his valuable guidance, as well as the Stanford Center for Clinical Informatics and the STRIDE project, which was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR025744. This work was previously published as an abstract and presented as a poster at both the 72nd annual Society for Investigative Dermatology meeting (May 2012) and at the 38th annual Society for Pediatric Dermatology meeting (July 2012).
Footnotes
Financial Disclosure: M.P. Hurley and A.T. Lane have no conflicts of interest to report. R.S. Stafford reports past expert testimony for Mylan Pharmaceuticals, a manufacturer of generic medications regarding patterns of doxycycline use in the treatment of rosacea.
Author Contributions: Mr. Michael Hurley and Dr. Alfred Lane had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Hurley, Lane, Stafford. Analysis and interpretation of data: Hurley, Stafford, Lane. Drafting of the manuscript: Hurley. Critical revision of the manuscript for important intellectual content: Hurley, Stafford, Lane. Statistical analysis: Hurley. Obtained funding: NA Administrative, technical, or material support: NA Study supervision: Lane
References
- 1.Campbell EG, Gruen RL, Mountford J, Miller LG, Cleary PD, Blumenthal D. A national survey of physician-industry relationships. N Engl J Med. 2007 Apr 26;356(17):1742–1750. doi: 10.1056/NEJMsa064508. [DOI] [PubMed] [Google Scholar]
- 2.Adair RF, Holmgren LR. Do drug samples influence resident prescribing behavior? A randomized trial. Am J Med. 2005 Aug;118(8):881–884. doi: 10.1016/j.amjmed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Martin J, Vittinghoff E, Guglielmo BJ, Udkow T, MacDougall C. National estimates and predictors of prescription medication sample use in the United States, 1999–2005. Journal of the American Pharmacists Association. 2010;50(6):677–685. doi: 10.1331/JAPhA.2010.09086. [DOI] [PubMed] [Google Scholar]
- 4.Hall KB, Tett SE, Nissen LM. Perceptions of the influence of prescription medicine samples on prescribing by family physicians. Med Care. 2006 Apr;44(4):383–387. doi: 10.1097/01.mlr.0000204017.71426.53. [DOI] [PubMed] [Google Scholar]
- 5.Chew LD, O’Young TS, Hazlet TK, Bradley KA, Maynard C, Lessler DS. A physician survey of the effect of drug sample availability on physicians’ behavior. J Gen Intern Med. 2000 Jul;15(7):478–483. doi: 10.1046/j.1525-1497.2000.08014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastiaens L, Chowdhury S, Gitelman L. Medication samples and drug compliance. Psychiatr Serv. 2000 Jun;51(6):819. doi: 10.1176/appi.ps.51.6.819. [DOI] [PubMed] [Google Scholar]
- 7.Cutrona SL, Woolhandler S, Lasser KE, Bor DH, McCormick D, Himmelstein DU. Characteristics of recipients of free prescription drug samples: a nationally representative analysis. Am J Public Health. 2008 Feb;98(2):284–289. doi: 10.2105/AJPH.2007.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander GC, Zhang J, Basu A. Characteristics of patients receiving pharmaceutical samples and association between sample receipt and out-of-pocket prescription costs. Med Care. 2008 Apr;46(4):394–402. doi: 10.1097/MLR.0b013e3181618ee0. [DOI] [PubMed] [Google Scholar]
- 9.Tjia J, Briesacher BA, Soumerai SB, et al. Medicare beneficiaries and free prescription drug samples: a national survey. J Gen Intern Med. 2008 Jun;23(6):709–714. doi: 10.1007/s11606-008-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace LS, Keenum AJ, Roskos SE, Blake GH, Colwell ST, Weiss BD. Suitability and readability of consumer medical information accompanying prescription medication samples. Patient Educ Couns. 2008 Mar;70(3):420–425. doi: 10.1016/j.pec.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Miller DP, Mansfield RJ, Woods JB, Wofford JL, Moran WP. The impact of drug samples on prescribing to the uninsured. South Med J. 2008 Sep;101(9):888–893. doi: 10.1097/SMJ.0b013e3181814d52. [DOI] [PubMed] [Google Scholar]
- 12.Boltri JM, Gordon ER, Vogel RL. Effect of antihypertensive samples on physician prescribing patterns. Fam Med. 2002 Nov-Dec;34(10):729–731. [PubMed] [Google Scholar]
- 13.Hartung DM, Evans D, Haxby DG, Kraemer DF, Andeen G, Fagnan LJ. Effect of Drug Sample Removal on Prescribing in a Family Practice Clinic. Ann Fam Med. 2010 Sep 1;8(5):402–409. doi: 10.1370/afm.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groves KE, Sketris I, Tett SE. Prescription drug samples--does this marketing strategy counteract policies for quality use of medicines? J Clin Pharm Ther. 2003 Aug;28(4):259–271. doi: 10.1046/j.1365-2710.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 15.Pinckney RG, Helminski AS, Kennedy AG, Maclean CD, Hurowitz L, Cote E. The effect of medication samples on self-reported prescribing practices: a statewide, cross-sectional survey. J Gen Intern Med. 2011 Jan;26(1):40–44. doi: 10.1007/s11606-010-1483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan MA, Dana J, Loewenstein G, Zinberg S, Schulkin J. Interactions of doctors with the pharmaceutical industry. J Med Ethics. 2006 Oct;32(10):559–563. doi: 10.1136/jme.2005.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alikhan A, Sockolov M, Brodell RT, Feldman SR. Drug samples in dermatology: Special considerations and recommendations for the future. Journal of the American Academy of Dermatology. 2010;62(6):1053–1061. doi: 10.1016/j.jaad.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 18.Sams WM, Freedberg IM. The dermatology-industry interface: defining the boundaries. J Am Acad Dermatol. 2000 Sep;43(3):550–554. doi: 10.1067/mjd.2000.107502. [DOI] [PubMed] [Google Scholar]
- 19.Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004 Mar;9(2):126–130. doi: 10.1046/j.1087-0024.2003.09108.x. [DOI] [PubMed] [Google Scholar]
- 20.Haider A, Shaw JC. Treatment of acne vulgaris. JAMA. 2004 Aug 11;292(6):726–735. doi: 10.1001/jama.292.6.726. [DOI] [PubMed] [Google Scholar]
- 21.Seidler EM, Kimball AB. Meta-analysis comparing efficacy of benzoyl peroxide, clindamycin, benzoyl peroxide with salicylic acid, and combination benzoyl peroxide/clindamycin in acne. J Am Acad Dermatol. 2010 Jul;63(1):52–62. doi: 10.1016/j.jaad.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 22.Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE--An integrated standards-based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391–395. [PMC free article] [PubMed] [Google Scholar]
- 23.National Disease and Therapeutic Index. Plymouth Meeting. Pennsylvania: IMS Health; 2011. [Google Scholar]
- 24.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003 Jan 1;41(1):56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 25.Payette M, Grant-Kels JM. Generic drugs in dermatology: Part II. J Am Acad Dermatol. 2012 Mar;66(3):353 e351–353 e315. doi: 10.1016/j.jaad.2011.11.945. [DOI] [PubMed] [Google Scholar]
- 26.Reiffen D, Ward MR. ‘Branded Generics’ As A Strategy To Limit Cannibalization of Pharmaceutical Markets. 2005 May; http://www.ftc.gov/be/healthcare/wp/12_Reiffen_BrandedGenericsAsAStrategy.pdf.
- 27.Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007 Sep;4(9):e283. doi: 10.1371/journal.pmed.0040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prices for Brand Name Drugs Increasing at Record Rates. Washington, DC: AARP; Aug, 2010. http://assets.aarp.org/www.aarp.org_/articles/health/rx_watchdog_08-10.pdf. [Google Scholar]
- 29.Commission TJ. Medication Management. Sample Medications. Nov 24; http://www.jointcommission.org/mobile/standards_information/jcfaqdetails.aspx?StandardsFAQId=277&StandardsFAQChapterId=76.


