Abstract
The α4 subunit of the GABAA receptor (GABAAR) is highly expressed in the thalamus where receptors containing the α4 and δ subunits are major mediators of tonic inhibition. The α4 subunit also exhibits considerable plasticity in a number of physiological and pathological conditions, raising questions about the expression of remaining GABAAR subunits when the α4 subunit is absent. Immunohistochemical studies of an α4 subunit knockout (KO) mouse revealed a substantial decrease in δ subunit expression in the ventrobasal nucleus of the thalamus as well as other forebrain regions where the α4 subunit is normally expressed. In contrast, several subunits associated primarily with phasic inhibition, including the α1 and γ2 subunits, were moderately increased. Intracellular localization of the δ subunit was also altered. While δ subunit labeling was decreased within the neuropil, some labeling remained in the cell bodies of many neurons in the ventrobasal nucleus. Confocal microscopy demonstrated co-localization of this labeling with an endoplasmic reticulum marker, and electron microscopy demonstrated increased immunogold labeling near the endoplasmic reticulum in the α4 KO mouse. These results emphasize the strong partnership of the δ and α4 subunit in the thalamus and suggest that the α4 subunit of the GABAAR plays a critical role in trafficking of the δ subunit to the neuronal surface. The findings also suggest that previously observed reductions in tonic inhibition in the α4 subunit KO mouse are likely to be related to alterations in δ subunit expression, in addition to loss of the α4 subunit.
Keywords: Immunohistochemistry, Non-synaptic GABA receptors, Plasticity, Receptor trafficking, Tonic inhibition, Ventrobasal nucleus
Introduction
GABAA receptors (GABAARs) that express the α4 subunit have a number of intriguing characteristics that have led to considerable interest in this subunit and its functions. Importantly, GABAARs that contain the α4 and δ subunits, in association with a β subunit, mediate the majority of tonic inhibition in major regions of the forebrain [1] where they are expressed most highly in the thalamus, striatum, molecular layer of the dentate gyrus and outer layers of the cerebral cortex [2, 3]. Consistent with their role in tonic inhibition, the α4 and δ subunits are found primarily at perisynaptic and extrasynaptic locations where they are expected to respond to ambient levels of GABA [4-6]. The α4/β/δ GABAARs are also characterized by their unique pharmacology. While they are unresponsive to classical benzodiazepines, such as flunitrazepam, they are extremely sensitive to neuromodulators such as neurosteroids, ethanol, and general anesthetics such as etomidate [7-9]. Receptors expressing these subunits can thus regulate neuronal activity in response to fluctuations in physiological conditions and may be particularly critical for controlling the excitability of neuronal networks [1, 10, 11].
The α4 subunit of the GABAAR also demonstrates a remarkable degree of plasticity. Marked increases in α4 subunit expression have been observed following withdrawal from or short-term exposure to progesterone [12, 13], following chronic ethanol administration [14-16], and in several models of epilepsy [3, 17-19]. Alpha 4 subunit expression is also increased in several GABAAR subunit knockout (KO) mice, including α1 subunit and α2 subunit-deficient mice [20, 21], as well as in female mice with a specific mutation of the γ2 subunit that decreases its surface expression [22]. The functional significance of such increases in α4 subunit expression remains unclear. Although they are often viewed as compensatory [20, 22], the α4 subunit increases also could lead to changes in receptor properties, including enhanced desensitization that could reduce receptor efficacy in some conditions, such as during prolonged exposure to GABA or during repetitive stimulation [23]. While an increase in expression is the most common alteration in the α4 subunit in several mouse models, a decrease in α4 expression occurs in δ subunit KO mice, and this is likely to be related to the preferential partnership of the α4 and δ subunits [24, 25].
Such plasticity of the α4 subunit has raised questions about the types of subunit changes that might occur following global deletion of the α4 subunit, either because of preferred subunit partnerships or as a compensatory response to loss of the α4 subunit. Specifically, would expression of the δ subunit be altered in the α4 KO mouse, and would changes be limited to GABAAR subunits associated with tonic inhibition or would subunits associated with phasic inhibition also be altered?
This study focused on expression of GABAAR subunits in the ventrobasal (VB) nucleus of the thalamus of the α4 KO mouse, and changes in the regional and cellular localization of remaining GABAAR subunits were studied with immunohistochemical methods. Neurons in the VB nucleus express a well-defined group of GABAAR subunits, including high levels of the α4 subunit, and electrophysiological studies have demonstrated a particularly robust tonic inhibitory current in thalamocortical neurons [26-28]. As expected, a large decrease in tonic inhibition was found in thalamic neurons in the α4 KO mouse [29]. However, it remains unclear whether this decrease in tonic inhibition in the VB nucleus is due solely to absence of the α4 subunits or whether changes in other GABAAR subunits contribute to the functional changes. The current studies revealed decreased expression of the δ subunit of the GABAAR, but with an unexpected retention of δ subunit labeling within the cell bodies of VB neurons. In addition, increased expression of other GABAAR subunits that are normally associated with phasic inhibition were observed in the thalamus. Preliminary reports of some of the findings have been reported previously [30, 31].
Materials and Methods
Animals
Mice that lack the α4 subunit of the GABAAR were produced by targeted disruption of the Gabra4 gene, and their production and characterization have been described previously [29]. The male α4 KO and WT mice used in this study were obtained from heterozygous breeding pairs. All mice were on a mixed C57BL/6J X 129Sv/SvJ genetic background of the F2-F6 generation. All animal use protocols conformed to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of California, Los Angeles, and the University of Pittsburgh.
Immunohistochemistry for Light Microscopy
Tissue preparation
Previously described protocols for tissue preparation were used [3, 25]. Briefly, mice were deeply anesthetized with sodium pentobarbital (90 mg/kg) and perfused through the ascending aorta with 4% paraformaldehyde in 0.12 M phosphate buffer (PB, pH 7.3) (n = 12 WT and 10 α4 KO mice). After perfusion, the brains were maintained in situ at 4°C for 1 h and then removed and postfixed in the same fixative for 1 h. After rinsing, brains were cryoprotected in a 30% sucrose solution, blocked in either the sagittal, horizontal or coronal planes, frozen on dry ice, and sectioned at 30 μm on a cryostat.
Antibodies and immunohistochemical methods
GABAAR subunit-specific antisera that recognize the α1-6, β2-3, γ2 and δ subunits were used in this study. The sources, concentrations and references for specificity of the antibodies are provided in Table I. Prior to immunohistochemistry, free-floating sections were incubated in 1% H2O2 for 30 min and then processed with a water bath heating antigen-retrieval method to reduce endogenous peroxidase-like activity and enhance specific labeling of the receptor subunits [25]. Briefly, the sections were heated to 90°C for 70 min in sodium citrate solution (pH 8.6). After cooling and rinsing in 0.1 M Tris buffered saline (TBS, pH 7.3), sections were processed for immunohistochemistry with standard avidin-biotin-peroxidase methods (Vectastain Elite ABC; Vector Laboratories, Burlingame, CA, USA), as described in detail previously [3, 25]. After immunohistochemical labeling, sections were mounted on slides, dehydrated and cover-slipped. To compare the immunohistochemical labeling for each subunit in WT and α4 KO mice, sections at comparable levels from the two groups of animals were processed identically and in parallel for each step of the immunohistochemical procedures.
Table 1.
Antibodies used in this study
| Target protein | Species | Source/Catalog # | Concentration | References for Specificity |
|---|---|---|---|---|
| α1 | Guinea pig | Dr. J-M Fritschy, Zurich | 1:50,000 | Benke et al. [68] Fritschy and Möhler [69] Kralic et al. [70] |
| α2 | Guinea Pig | Dr. J-M Fritschy, Zurich | 1:10,000 | Fritschy and Möhler [69] |
| α3 | Rabbit | Dr. W. Sieghart, Vienna | 1:1,000 | Pirker et al. [2] |
| α4 | Rabbit | Dr. W. Sieghart, Vienna | 1:2,000 | Bencsits et al. [71] Peng et al. [25] |
| α4 | Rabbit | Chemicon/Millipore AB5457 | 1:1,000 | Tested on α4 KO, Peng and Houser, unpublished data |
| α5 | Guinea pig | Dr. J-M Fritschy, Zurich | 1:3,000 | Fritschy and Möhler [69] Tested on α5 KO, Peng and Houser, unpublished data |
| α6 | Rabbit | Chemicon/Millipore AB5610 | 1:10,000 | — |
| β2 | Rabbit | Chemicon/Millipore AB5561 | 1:1,000 | — |
| β3 | Rabbit | Chemicon/Millipore AB5563 | 1:1,000 | — |
| γ2 | Rabbit | Dr. W. Sieghart. Vienna | 1:2,000 | Tretter et al. [72]; Pirker et al. [2] |
| δ | Rabbit | Dr. W. Sieghart, Vienna | 1:3,000 – 1:4000 | Sperk et al. [73] Peng et al. [25] |
| δ | Rabbit | Dr. W. Sieghart, Vienna | 1:200 (EM) | Tested on δ KO, Peng and Houser, unpublished data |
| KDEL | Mouse | Stressgen SPA-827 | 1:200 | Manufacturer's technical information |
Double-immunofluorescence labeling
To evaluate the intracellular localization of the δ subunit and its potential retention in the endoplasmic reticulum (ER) in the α4 KO mice, double immunofluorescence labeling was used to localize the δ subunit and the C-terminal ER retention signal Lys-Asp-Glu-Leu (KDEL) [32, 33]. After treatment with 1% H2O2 and the antigen retrieval procedure described above, free-floating sections were incubated in 10% normal goat serum in TBS containing 0.3% Triton X-100 for 3 h followed by incubation in a mixture of rabbit anti-δ subunit (1:3,000) and mouse anti-KDEL (1:200) in TBS containing 2% normal goat serum for 3 nights at 4°C. After rinsing in TBS, sections were incubated in a mixture of goat anti-rabbit IgG labeled with Alexa Fluor 555 and goat anti-mouse IgG conjugated to Alexa Fluor 488 (1:500; both from Molecular Probes / Life Technologies, Eugene, OR) for 4 h. To block lipofuscin-like autofluorescence that can occur in large neurons of the thalamus and some other regions of adult mice, sections were treated with a modified autofluorescence blocker, consisting of ammonium acetate buffered copper sulfate (20 mM CuSO4 in 50 mM ammonium acetate buffer, pH 5.0), for 30 min at room temperature [34]. After thorough rinsing in TBS, sections were mounted on slides and coverslipped with the antifade medium Prolong Gold (Molecular Probes).
Data analysis
Single subunit immunolabeling was analyzed with an Axioplan 2 microscope equipped with an AxioCam digital camera system and AxioVision 4.6 software. (Zeiss, Thornwood, NY). To evaluate possible differences in density of GABAAR subunit labeling in the α4 subunit KO mice, three pairs of WT and α4 KO mice of the same age were perfused on the same day. Sections from each animal at comparable levels of the VB nucleus that included the ventral posterior lateral (VPL) thalamic nucleus were processed in the same experimental run with identical conditions for each subunit. Linear black and white digital images of immunolabeling in the VPL, from each side of the brain, were obtained under identical conditions on the same day with stabilized light levels for densitometric analysis (n=3 animals per group; 6 samples per group for each subunit) . The border of the VPL was outlined, and the densities of labeling (grey values) were then analyzed with morphometric AxioVision software (version 4.6; Zeiss). Data were analyzed with Student's t-test, and p < 0.05 was considered statistically significant.
Double-labeled sections were scanned, and digital images were obtained with a LSM 510 META confocal microscope, and confocal images were analyzed with LSM 5 Image Examiner software (Zeiss). Colocalization of the δ subunit with KDEL in thalamic neurons was evaluated qualitatively and compared between WT and α4 KO mice.
Immunogold Labeling for Electron Microscopy
Tissue preparation
Mice were perfused as for light microscopy except that 0.1% glutaraldehyde was added to the 4% paraformaldehyde solution. After perfusion, brains remained in situ for 2 h at 4°C and were then removed from the skull and postfixed for 2 h in the same fixative used for perfusion. Forebrain tissue containing the thalamus was sectioned coronally at 200 μm with a vibratome, and small blocks of tissue were trimmed from the VB nucleus. These specimens were cryoprotected in 5% sucrose and then in 10, 20 and 30% glycerol in 0.12 M PB, pH 7.3, for 2 h each.
Methods for freeze substitution and low-temperature embedding have been described previously [6, 35]. Cryoprotected sections were rapidly plunged into liquid propane cooled by liquid nitrogen to -190°C in a cryofixation unit (EM CPC; Leica, Wien, Austria). Tissues were then transferred to a cryosubstitution unit (EM AFS, Leica) that was programmed for all subsequent steps. Specimens were immersed in 4% uranyl acetate (Electron Microscopy Sciences, Fort Washington, PA) dissolved in anhydrous methanol for 24 h at -90°C, and the temperature was gradually raised to -45°C and held at this temperature for an additional 24 h. Specimens were rinsed in methanol and infiltrated with Lowicryl HM20 resin (Electron Microscopy Sciences) for 48 h at -45°C. The resin was polymerized with ultraviolet light (360 nm) for 24 h at -45°C, and the temperature was then progressively increased until it reached 0°C, where it was maintained for an additional 24 h. Ultrathin sections were cut on a microtome (Reichert-Jung, Vienna, Austria) and picked up on nickel mesh grids that were freshly coated with a Coat-Quick “G” pen (Electron Microscopy Sciences).
Post-embedding immunogold labeling
Methods for immunogold labeling of GABAAR subunits have been described previously [4, 6, 35], and sections from WT and KO animals were processed identically and in parallel. Ultrathin sections were treated with 0.2% sodium hydroxide in distilled water for 5 min and then with 0.01% sodium borohydride in 0.01 M TBS, pH 7.4, for 10 min. After rinsing, ultrathin sections were incubated in 2% human serum albumin (HSA) in TBS containing 0.1% Triton X-100 for 10 min and in the same solution with the addition of 0.05 M glycine for 7 min. Sections were incubated in 2% HSA in TBS for 1.5 h to reduce nonspecific binding and then incubated in primary antisera, rabbit anti-δ subunit (1:200), for 18-24 h at room temperature. After rinsing with 0.05 M Tris-HCl buffer containing polyethylene glycol (50 mg/100 ml), sections were incubated for 2.5 h in the secondary antisera, goat anti-rabbit IgG conjugated to 10 nm colloidal gold particles (GE Healthcare, Piscataway, NJ) diluted 1:20 in 0.05 M Tris-HCl buffer, pH 8.0. Sections were stained with a saturated solution of uranyl acetate for 40 min and lead citrate for 4 min. The sections were studied and photographed with a JEOL 100 CX II electron microscope (Akishima, Japan).
Data analysis
After initial studies of the subcellular localization of the δ subunit, immunogold labeling within neuronal cell bodies was analyzed in greater detail. Randomly-selected series of labeled cell body profiles within the VB nucleus were photographed at a primary magnification of 19,000x and a final print magnification of 38,000x. For semi-quantitative analysis, entire regions of cytoplasm within each micrograph, excluding the nucleus, were outlined, and the number of gold particles in each region was counted. The areas of cytoplasm were measured with Image J software (NIH), and the densities of gold particles per μm2 were calculated. Data were obtained from 78 and 48 samples of cytoplasm (essentially equivalent to the number of neurons sampled) in WT and α4 KO specimens respectively (n = 3 mice per group).
In a parallel analysis, the numbers of clusters of gold particles, identified as more than 2 colloidal gold particles in close proximity (less than 20 nm from the nearest gold particle), were counted in the same regions of cytoplasm from WT and α4 KO mice, and the densities per μm2 were determined.
Results
Decreased δ subunit labeling in the α4 KO mouse
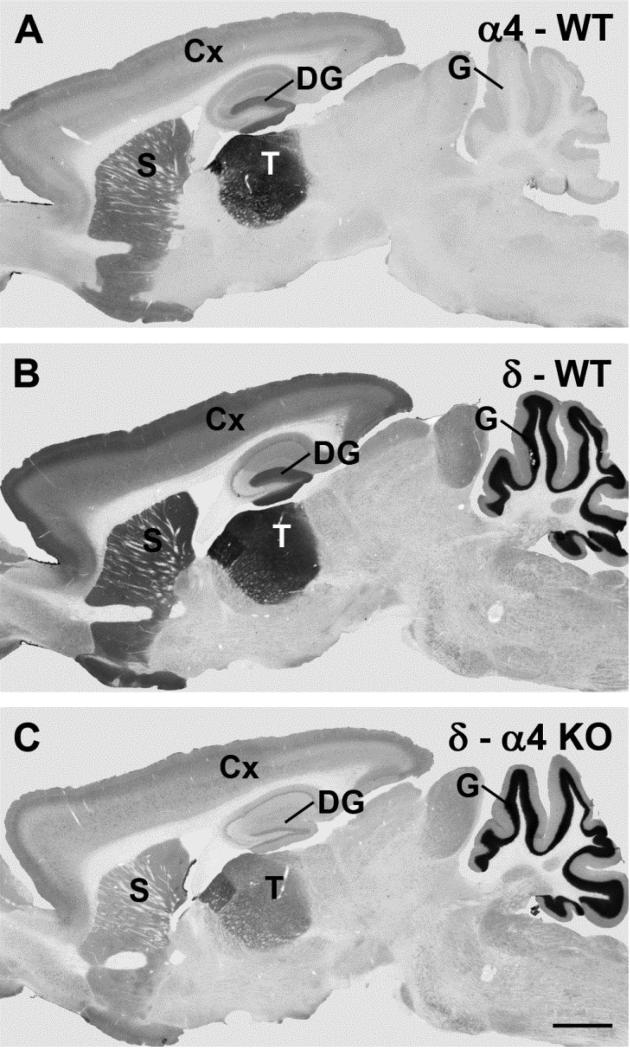
An initial goal of this study was to determine the effects of loss of the α4 subunit (Fig. 1a) on the pattern of δ subunit expression throughout the brain. Alpha 4 subunit expression was first determined in WT mice for comparison with δ subunit labeling in the α4 KO mice. As described previously, α4 subunit expression was primarily confined to the forebrain and was highest in the thalamus, with moderate levels of expression in the striatum, outer layers of the cerebral cortex, and molecular layer of the dentate gyrus (Fig. 1b) [2, 25, 29]. Very little immunohistochemical labeling of the α4 subunit was evident in the cerebellum (Fig. 1b). In WT mice, the pattern of δ subunit labeling (Fig. 1c) was remarkably similar to that of the α4 subunit (Fig. 1b) in the forebrain. However, δ subunit labeling is also present in the granule cell layer of the cerebellum (Fig. 1c), and this distinguished the overall pattern of δ subunit labeling from that of α4 (compare Fig. 1b and 1c).
Fig. 1.
Comparisons of immunolabeling for the α4 and δ subunits of the GABAA receptor (GABAAR) in wild-type (WT) and α4 knockout (KO) mice in sagittal brain sections. a In an α4 KO mice, specific α4 subunit labeling is absent throughout the brain (labeled regions are identified below). b In a WT mouse, the α4 subunit is moderately to strongly labeled in specific forebrain regions, with the highest expression in the thalamus (T), molecular layer of the dentate gyrus (DG), striatum (S) and outer layers of the cerebral cortex (Cx). Virtually no specific α4 subunit labeling is evident in the granule cell layer (G) of the cerebellum. c In a WT mouse, δ subunit labeling closely parallels the pattern of α4 labeling in the forebrain, but is also present at high levels in the granule cell layer of the cerebellum. d In an α4 KO, δ subunit labeling is substantially reduced in all forebrain regions in which the α4 subunit is normally expressed. In contrast, no decrease in δ labeling is evident in the cerebellum which lacks α4 labeling in WT mice (see Panel B). Scale bar = 1 mm for A-D. (Comparisons of α4 subunit labeling in WT and α4 KO mice were originally described in Chandra et al., 2006).
In the α4 KO mouse, the loss of immunohistochemical labeling for the α4 subunit was essentially complete (Fig. 1a) and has been described previously [29]. The δ subunit was also substantially decreased in all forebrain regions in which the α4 subunit is normally localized (Fig. 1d). In contrast, no decrease in δ subunit expression was observed in the cerebellar cortex (Fig. 1d). Thus alterations in δ subunit expression were confined to the specific regions where the α4 subunit is normally present. While regional patterns of labeling were maintained, δ subunit labeling appeared decreased throughout these forebrain regions. The decrease in δ subunit labeling was particularly striking when compared with the maintained levels of labeling in the cerebellum (Fig. 1d). These regional comparisons strongly suggest that the decreases in δ subunit labeling were directly linked to loss of the α4 subunit rather than a more global response to absence of a GABAAR subunit.
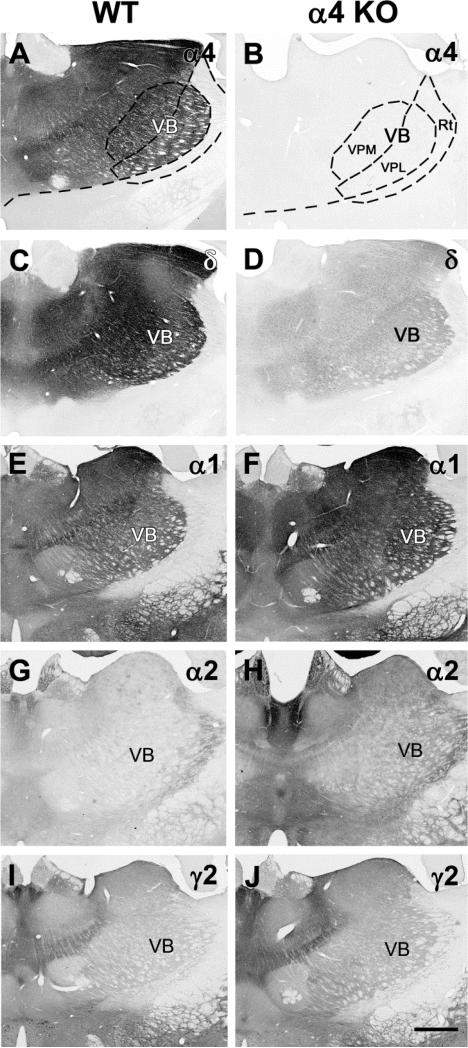
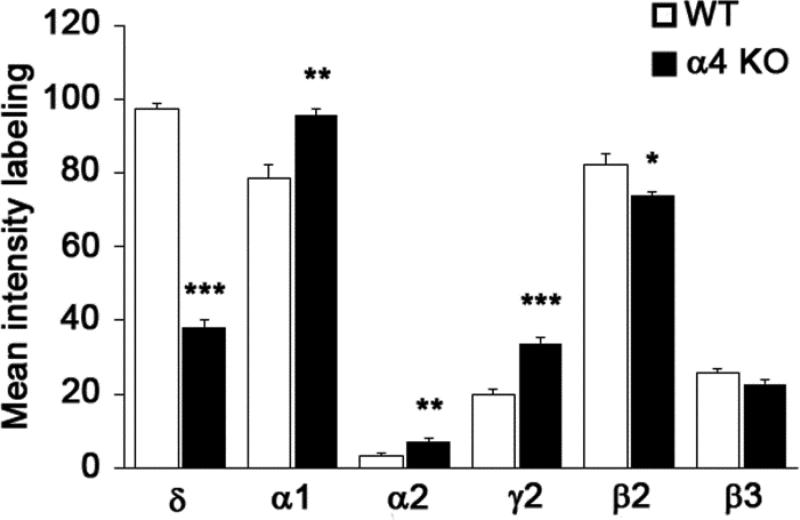
To further characterize the changes in δ subunit expression and compare these with potential changes in other subunits, the analysis focused on alterations in GABAA R subunit expression in the VB nucleus of the thalamus. For consistent sampling, densitometry measurements of GABAAR subunits were made in the VPL nucleus of the VB complex (Fig. 2a,b) at comparable levels of the thalamus (n=3 animals per group; 6 thalamus samples per group for each subunit). The α4 subunit is characteristically high in the VB nucleus in WT mice (Fig. 2a), and immunohistochemical labeling for the α4 subunit was absent in the α4 KO mouse in this region, as well as throughout the thalamus (Fig. 2b). Densitometric analysis of the δ subunit in the VB nucleus of WT and α4 KO mice confirmed a large decrease (60.6%) in δ subunit labeling in the α4 KO mouse (Fig. 2c,d; mean intensity of labeling = 97.23 in WT and 38.28 in KO, p < 0.001, Fig. 3).
Fig. 2.
Comparisons of immunolabeling of GABAAR subunits (α4, δ, α1, α2, γ2) in coronal sections of the thalamus in WT and α4 KO mice. a,b In a WT mouse, strong α4 subunit labeling is present in many thalamic nuclei, including the ventrobasal (VB) complex which includes the ventral posterior medial (VPM) and ventral posterior lateral (VPL) nuclei, but is virtually absent from the nucleus reticularis (Rt). In the α4 KO, no specific labeling is present in the VB nucleus or other thalamic regions where strong α4 subunit labeling is normally present. c,d In a WT mouse, strong δ subunit labeling is evident in the thalamus and closely resembles the pattern of α4 labeling in WT mice. The δ subunit labeling is substantially reduced throughout these regions in the α4 KO. e,f In a WT mouse, α1 labeling is present at moderate levels in much of the thalamus including the VB nuclei and is increased within the same regions in the α4 KO. g,h In a WT mouse, α2 subunit labeling is low throughout much of the thalamus, including the VB nuclei, with the strongest labeling in the Rt and midline nuclei. Labeling is increased slightly throughout the thalamus in the α4 KO. i,j In a WT mouse, γ2 labeling is relatively low in much of the thalamus, including the VB nuclei. Some increases in γ2 labeling are evident in the α4 KO. Scale bar = 500 μm for A-J.
Fig. 3.
Comparisons of the mean intensity of immunolabeling for the major GABAAR subunits in the ventrobasal complex (measurements made in the ventral posterior lateral nucleus) of the thalamus in WT and α4 KO mice (mean ± s.e.m., * p < 0.05; ** p < 0.01; ***p < 0.001).
Alterations in multiple GABAAR subunits in the α4 KO mouse
Labeling of other alpha subunits of the GABAAR was analyzed to determine if there were compensatory changes in this group of subunits in response to loss of the α4 subunit. The α1 subunit was expressed at moderately high levels in the VB nucleus in WT mice, and the labeling increased by 21.7% in the α4 KO mouse (Fig. 2e,f; mean intensity of labeling = 78.62 in WT and 95.71 in KO, p <0.01, Fig. 3). The α2 subunit was expressed at low levels in the VB nucleus of the thalamus, and thus, although densitometry indicated a large percentage increase in this subunit in the α4 KO mouse, the levels of α2 labeling remained comparatively low in the VB nucleus of the α4 KO (Fig. 2g,h; mean intensity of labeling = 3.07 in WT and 7.15 in KO, p <0.01, Fig. 3). Initial studies revealed no obvious changes in the α3, α5 and α6 subunits, and these subunits normally have little or no expression in the VB nucleus. Thus labeling of these subunits was not analyzed further.
The β2 and β3 subunits were also analyzed with densitometry. While labeling of these subunits was variable among animals, a small decrease (10.1%) was observed for the β2 subunit, the major β subunit in the thalamus (mean intensity of labeling = 82.10 in WT and 73.83 in KO; p <0.05, Fig. 3). No significant change in β3 subunit expression was detected (Fig. 3). Finally, the γ2 subunit, which is normally present at relatively low levels in the VB nucleus, showed a significant increase in labeling in the α4 KO mouse, although the levels of labeling remained relatively low in this region (Fig. 2i,j; mean intensity of labeling = 19.87 in WT and 33.84 in KO, p <0.001, Fig. 3). (Baseline levels of γ2 subunit expression in WT mice were higher in other brain regions, including the hippocampus, and increases in γ2 expression were greater in these regions in the α4 KO mouse; Peng and Houser, unpublished findings).
In summary, comparisons among the subunits in the VB nucleus demonstrated the greatest change in the intensity of δ subunit labeling, with a highly significant decrease in the α4 KO mouse (Fig. 2c,d; Fig. 3). In contrast, labeling intensities of the α1, α2 and γ2 subunits were increased (Fig. 2e-j; Fig. 3). Although statistically significant, the increase was relatively small for the α1 subunit, and the labeling intensity remained low for the α2 and γ2 subunits in the VB nucleus of the α4 KO, despite a comparatively large percentage increase (Fig. 3).
Altered subcellular localization of the δ subunit in the α4 KO mouse
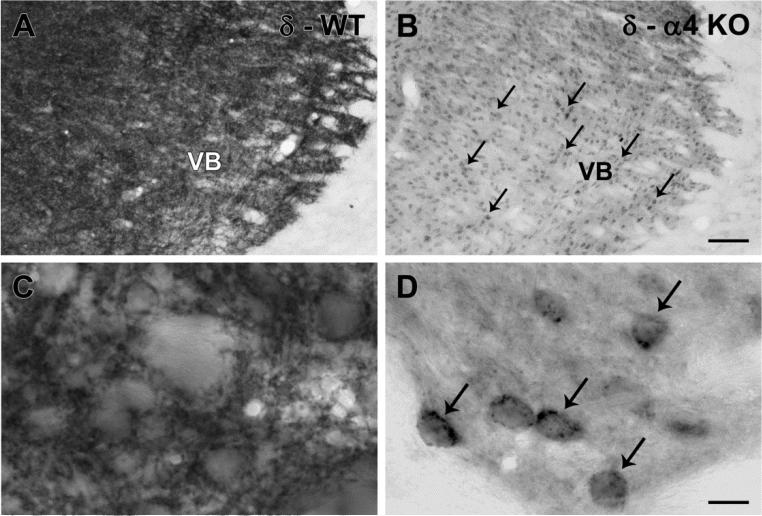
The patterns of δ subunit labeling were then studied in greater detail. In the VB nucleus of WT mice, δ subunit labeling was prominent throughout the region, reflecting abundant labeling of dendrites in the neuropil surrounding neuronal cell bodies (Fig. 4a,c). In contrast, in the α4 KO mouse, labeling within the neuropil was substantially decreased, but intracellular labeling was evident in many cell bodies (Fig. 4b,d). Such labeling suggested that δ subunits were retained within the cytoplasm and could be sequestered in organelles such as the endoplasmic reticulum.
Fig. 4.
δ subunit labeling in the ventrobasal (VB) nucleus of the thalamus in WT and α4 KO mice. a In a WT mouse, δ subunit labeling is abundant throughout the VB nucleus, reflecting substantial labeling of dendritic processes within the neuropil. b In an α4 KO mouse, labeling within the neuropil is reduced, but some labeling remains concentrated in cell bodies (examples at arrows) throughout the VB nucleus. c,d At higher magnification of the regions, a rich network of δ subunit-labeled processes in the WT mouse (c) contrasts with the comparatively light labeling in the neuropil but distinct labeling of cell bodies (examples at arrows) in the α4 KO mouse (d). Scale bars = 100 μm for A and B; 10 μm for C and D.
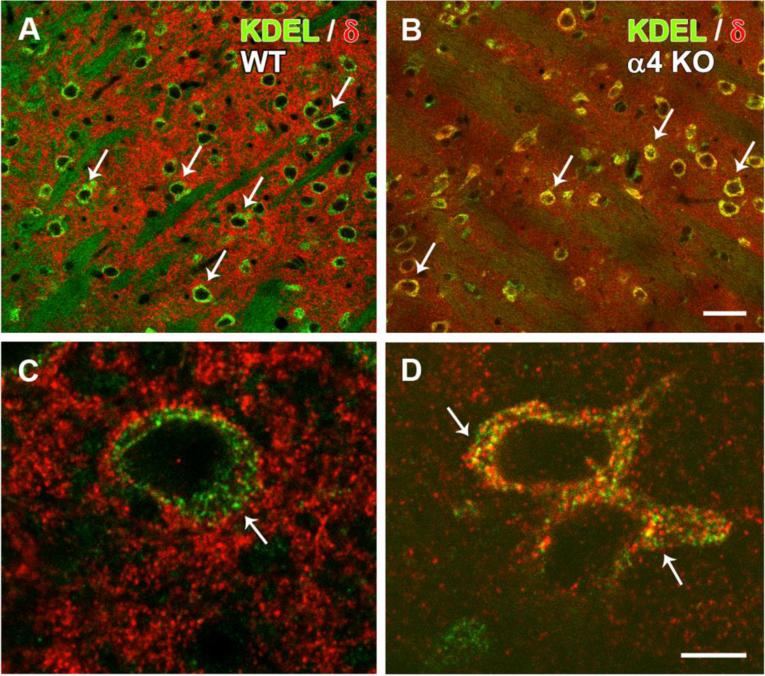
To evaluate this possibility, double labeling for the δ subunit and the ER retention motif KDEL was used to compare the localization patterns in WT and α4 KO mice. Little co-localization of the δ subunit and KDEL was evident in thalamic neurons of WT mice (Fig. 5a,c). While KDEL was localized primarily to the cell bodies, strong δ subunit labeling was found in dendritic processes within the neuropil, with comparatively little labeling in neuronal cell bodies (Fig. 5a,c). In contrast, in the α4 KO mouse, δ subunit labeling was reduced in the neuropil, but was evident in cell bodies (Fig. 5b,d). Distinct punctate labeling of the δ subunit was present within the cytoplasm, and double labeling for the δ subunit and KDEL was evident (Fig. 5b,d). These findings suggest that, while the overall level of δ subunit labeling was reduced in the VB nucleus of the α4 KO mouse, an alteration in the cellular localization of the δ subunit also occurs, with apparent retention of the δ subunit within the ER and associated organelles.
Fig. 5.
Double-labeling of the δ subunit (red) and KDEL (green), a marker of endoplasmic reticulum (ER), in confocal images of the ventrobasal (VB) nucleus in WT and α4 KO mice. a,b In a WT mouse, δ subunit labeling is distributed throughout the neuropil, whereas many neuronal cell bodies (examples at arrows) are labeled primarily for KDEL. In contrast, in an α4 KO mouse, δ subunit labeling within the neuropil is less distinct, and δ localization within neuronal cell bodies is increased, as indicated by yellow labeling of numerous neuronal somata (examples at arrows). c,d At higher magnification in a WT mouse, KDEL labeling is confined primarily to the cytoplasm of the cell bodies (arrow), consistent with ER localization, and δ subunit labeling is evident primarily in the surrounding neuropil. In contrast, in the α4 KO, δ subunit labeling is most highly concentrated in the neuronal cytoplasm (arrows) where colocalization with KDEL is evident (yellow labeling). Scale bars = 25 μm for A and B; 5 μm for C and D.
Ultrastructural evidence for altered subcellular labeling of the δ subunit in the α4 KO mouse
Postembedding immunogold labeling was used to compare the subcellular localization of the δ subunit in the VB nucleus of WT and α4 KO mice. In WT mice, immunogold labeling for the δ subunit was found on dendrites at both perisynaptic and extrasynaptic locations (Fig. 6a,b). Perisynaptic localization was defined as within 30 nm of either end of the postsynaptic thickening [35], while labeling on or very near the plasma membrane at further distances from the synapse was considered extrasynaptic. Localization of the δ subunit directly at the synaptic contact was rarely observed. This pattern of δ subunit labeling in the VB nucleus closely resembled the predominantly perisynaptic and extrasynaptic localization of the δ subunit in the cerebellum and dentate gyrus in normal C57BL/6 mice [4, 35, 36].
Fig. 6.
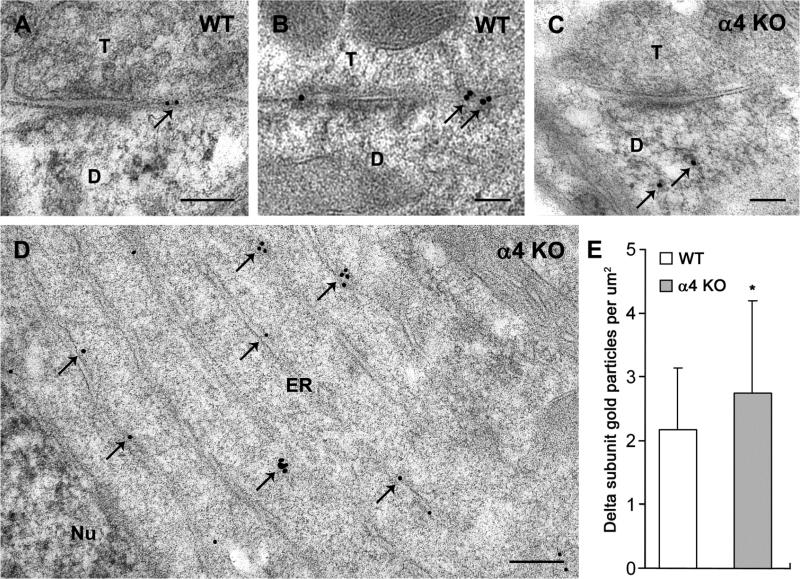
Comparison of immunogold labeling of the δ subunit in electron micrographs of the ventrobasal (VB) nucleus in WT and α4 KO mice. a,b In WT mice, δ subunit labeling is located predominantly at perisynaptic and extrasynaptic locations (arrows) near synaptic contacts between axon terminals (T) and postsynaptic dendrites (D). c In an α4 KO mouse, labeling along the plasma membranes near synaptic contacts appears reduced, and immunogold particles are evident within the cytoplasm (arrows). d In an α4 KO mouse, immunogold labeling for the δ subunit (arrows) is present within the cytoplasm of the cell body, and clusters of immunogold particles are evident near stacks of endoplasmic reticulum (ER), adjacent to the neuronal nucleus (Nu). e Comparisons of immunogold labeling in neuronal cell bodies of the VB nucleus indicate a higher concentration of immunogold particles in the cytoplasm in α4 KO mice than in WT mice (mean ± s.e.m., p < 0.05). Scale bars = 0.1 μm for A-C; 0.2 μm for D.
In contrast, in the α4 KO mice, labeling along the plasma membrane of VB neurons was limited. However, immunogold labeling was observed within the cytoplasm, occasionally near synaptic contacts (Fig. 6c), but more frequently within the cell bodies (Fig. 6d). The intracellular labeling included clusters of gold particles near segments of ER (Fig. 6d), consistent with an increased concentration of the δ subunit at this location in the α4 KO mouse. Semi-quantitative analysis of immunogold particles in WT and α4 KO mice demonstrated a 26.7% increase in immunogold labeling of the δ subunit within the cytoplasm of the cell body in the α4 KO (2.17 gold particles per um2 in WT and 2.75 gold particles per um2 in the α4 KO, p < 0.05; Fig. 6e). In addition, the density of clusters of immunogold particles was greater in the cell body cytoplasm in the α4 KO mouse than in WT (0.19 clusters per μm2 in WT and 0.33 clusters per μm2 in the α4 KO; p < 0.01).
Discussion
A major finding of this study was the marked decrease in δ subunit expression in the α4 KO mouse in regions where the α4 subunit would normally be expressed. In addition, in the VB nucleus of the thalamus, the cellular localization of remaining δ subunit labeling was altered. While a large decrease in dendritic labeling was evident in the neuropil, δ subunit labeling was evident in many cell bodies. Such intracellular labeling could represent retention of δ subunits within the cytoplasm and lack of incorporation into functional receptors that could reach the cell surface. Thus δ subunit function is likely to be severely impaired, and this could contribute significantly to the deficits that have been observed in α4 KO mice.
Potential contributions of decreased δ subunit expression to functional deficits in α4 KO mice
The large decrease in GABAAR-mediated tonic inhibition in the thalamus of the α4 KO mouse could be related not only to the absence of the α4 subunit [29] but also to the decrease in δ subunit expression. Likewise, the lack of responses to 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol (THIP; gaboxadol) in the thalamus [29], as well as other brain regions of the α4 KO mouse [37], is consistent with a decrease in the δ subunit, as THIP is recognized as a GABAAR agonist that at low concentrations preferentially activates δ subunit-containing GABAARs [38, 39]. Indeed, the marked decrease in tonic inhibition and lack of responsiveness to THIP in the VB nucleus in δ subunit KO mice [38, 40] appear very similar to the changes reported in this region in the α4 KO mouse [29]. Other functional changes in the α4 KO mouse, including the decreased response to isoflurane [41, 42], could also result from the combined effects of loss of the α4 subunit and substantial decreases in δ subunit-containing GABAARs.
Separating the effects of the α4 and δ subunits in the thalamus are particularly difficult because the majority of GABAARs responsible for tonic inhibition in this region contain both subunits [43]. Furthermore, these subunits are altered in parallel in the corresponding KO mice; the α4 subunit is decreased in the δ subunit KO mouse [25], and the δ subunit is decreased in the hippocampus of the α4 KO mouse [37, 44-46] as well as in the thalamus (present study). Thus, combined loss or decrease of the two subunits in the thalamus and dentate gyrus could contribute to the increased seizure susceptibility in both α4 and δ subunit KO mice [8, 47, 48]. These findings are also consistent with marked decreases in high affinity muscimol binding in both the α4 and δ KO mice, and essentially all such binding is lost in the double α4/δ KO mouse [49]. Together these results emphasize the normally strong partnership of the δ and α4 subunits of the GABAAR.
Similar decreases in δ subunit labeling in α4 and α6 KO mice
In the α4 KO mouse, δ subunit labeling of dendrites in the VB nucleus was strongly decreased, and this resembled the virtual loss of δ subunit labeling in the cerebellum in the α6 KO mouse [50]. The α4 and α6 subunits are homologous subunits, with α4 preferentially expressed in the forebrain and α6 expressed primarily in granule cells of the cerebellum [51, 52]. Thus similar changes in δ subunit expression might be expected in the α4 and α6 KO mice. Due to nearly complete loss of specific δ subunit labeling, the α6 KO mouse has been considered essentially a double knockout of α6 and δ subunits in the cerebellum [50], and GABAAR-mediated tonic inhibition in cerebellar granule cells was also severely reduced [53]. Thus the δ subunit depletion and the decreases in GABAAR-mediated tonic inhibition in the cerebellum of the α6 KO and the thalamus of the α4 KO mice appear very similar. Interestingly, the loss of such tonic inhibition in the α6 subunit-deficient mouse led to a form of homeostatic plasticity in which neuronal excitability is regulated by a voltage-independent potassium conductance [54]. Whether similar adaptive changes occur in the α4 KO mouse is not known, but the previous findings emphasize the fundamental importance of tonic inhibition.
Unique patterns of δ subunit labeling in VB neurons in the α4 KO mouse
Despite clear similarities, some differences in δ subunit labeling in the α4 and α6 KO mice were noted. In the thalamus of the α4 KO mouse, persistent labeling in many cell bodies in the VB nucleus contrasted with the virtual loss of immunohistochemical labeling of the δ subunit in the cerebellum of the α6 KO mouse. This loss of δ subunit labeling in the cerebellum was considered to result from loss of the normal partnership with the α6 subunit and resultant rapid degradation of the δ subunit [50]. However, in cell bodies of the VB nucleus in the α4 KO mouse, some δ subunit labeling was retained and colocalization with KDEL, a commonly used marker of the ER, has led to our interpretation that the labeling represents retention of the δ subunit in the ER and associated organelles within cell bodies.
Reasons for these different patterns of δ subunit labeling are not known, but they could reflect regional or cell-specific differences in GABAAR subunit processing and assembly. Basic differences in the size of the neurons could play a role, with limited accumulation occurring in the cell bodies of smaller neurons, such as the cerebellar granule cells. A lack of labeling of the somata of dentate granule cells in the α4 KO mouse [44] is consistent with this suggestion. In contrast, the larger size of the thalamocortical neurons in the VB nucleus and the high expression of the δ subunit in this region could lead to accumulation of the δ subunit in the cytoplasm prior to degradation. Nevertheless, in both the α6 and α4 subunit KO mice, the functional outcome would be very similar due to a large loss of δ subunit expression on the cell surface and thus a similarly large loss of functional δ subunit-containing receptors.
Interestingly, we did not detect such sequestration of the α4 subunit in cell bodies of VB neurons in the δ subunit KO mouse [25] . Although the α4 subunit was decreased in the δ subunit KO mouse, the decreased expression appeared to occur diffusely throughout the neuron, and a concentration of α4 labeling in the neuronal cell bodies was not observed in the thalamus.
The different intracellular localization of the remaining subunits in α4 and δ KO mice is likely to be related to the partnership rules among α4, δ and γ2 subunits. In the δ KO mice, an alternate partnership of the remaining α4 subunit with the γ2 subunit has been proposed [24, 25, 55], and previous studies have suggested that the δ and γ2 subunits may compete for partnership with the α4 subunit during development. Thus, in the δ subunit KO mouse, the α4 subunit may associate with increased γ2 subunits to form functional receptors with an apparently normal cellular distribution [25]. In contrast, the δ and γ2 subunit are considered mutually exclusive [56-58], and thus are unlikely to assemble in the same receptor in response to loss of the α4 subunit. As a result, the δ subunit may be retained within the ER and subsequently degraded [50], as primarily fully-assembled GABAARs are trafficked to the cell surface [33, 59].
In a previous study of the α4 KO mouse, a marked decrease in surface localization of the δ subunit was also observed in hippocampal pyramidal cells, and the α4 KO mice were considered to be functional knock-downs of the δ-containing receptors [37]. In CA1 pyramidal cells, the decrease in δ subunit surface labeling led to an increase in the intracellular to surface ratio for this subunit, and these findings also support a role for the α4 subunit in trafficking of the δ subunit to the plasma membrane. However, the patterns of immunolabeling differed from those in the current study as the levels of intracellular δ subunit labeling were unchanged in the hippocampal neurons [37], perhaps again suggesting regional differences in GABAAR subunit processing and assembly.
Potential importance of the α4 subunit for trafficking of GABAARs involved in tonic inhibition
The current findings strongly suggest that, in thalamocortical neurons, the α4 subunit is critical for trafficking δ subunit-containing receptors to the cell surface. These in vivo findings closely parallel those in transfected mouse fibroblasts in which epitope-tagged δ subunits remained within the cytoplasm until the α4 and β3 subunits were introduced [60]. Functional receptors containing the δ subunit were then expressed on the cell surface. The current findings further emphasize the importance of appropriate subunit partners for trafficking of the δ subunit to the cell surface and suggest that the α4 subunit plays a critical role in such surface expression and related tonic inhibition.
The functional consequences of several epilepsy-related mutant GABAAR subunits have been linked to ER retention and abnormal processing and trafficking of these subunits [61-63]. Thus multiple mechanisms, including altered subunit partnership, can lead to subunit accumulation within the cytoplasm. Such alterations may not only limit surface expression of GABAARs but also could impair protein synthesis and general neuronal function [64]. While most previous studies have emphasized changes in subunits involved in phasic (synaptic) inhibition, such alterations could have major consequences for GABAARs mediating tonic inhibition.
In addition to the well recognized partnership of the α4 and δ subunits, the δ subunit can form functional GABAARs with the α1 subunit in some cell types and brain regions, including several cell lines and interneurons of the hippocampal formation [44, 65]. Whether such partnerships are formed in the VB nucleus of the α4 subunit KO mouse is not known. The extent of such assembly is clearly insufficient to rescue the tonic inhibition.
Possible compensation by GABAAR subunits associated with phasic inhibition in the α4 KO mouse
While the δ subunit was substantially decreased in the α4 KO mouse, an increase in other subunits was noted, including α1, α2 and γ2 subunits. Interestingly, all of these subunits are localized predominantly at synaptic sites and mediate phasic inhibition. The mechanisms responsible for such increases in synaptic subunits following loss of a predominantly extrasynaptic GABAAR subunit remain uncertain. One possibility is that the increased expression occurs in response to loss of a limited amount of α4 subunit expression directly at the synapse. In instances where the α4 subunit associates with the γ2 subunit, a synaptic localization might be expected [45] (although nonsynaptic localization can also occur [35]). Thus following loss of synaptically localized α4 subunits, the γ2 subunit could associate with increased α1 or α2 subunits and maintain or enhance phasic inhibition. Currently, little change in phasic inhibition has been identified in the VB nucleus in the α4 KO mice [29]. However, some compensatory changes in synaptic GABAAR subunits occur in dentate gyrus granule cells of α4 KO mice [45], and increased modulation of these responses by zolpidem suggests the possible involvement of α1 or α2 subunits in these responses [46].
Alternatively, increases in GABAAR subunits that are associated with phasic inhibition could be occurring as a homeostatic response to the marked decrease in tonic inhibition. Such reciprocal changes in subunits involved in phasic and tonic inhibition have been observed in several mouse lines with genetically-altered GABAAR subunits [20, 22, 25]. In these same mouse models, subunits that are partners in either tonic or phasic inhibition tend to vary in parallel, and similar coordinated changes in α4 and δ subunits have been described in some normal physiological conditions, such as puberty [66].
In contrast, in several pathological conditions and models, the rules for partnership and such homeostatic responses appear to be broken, and alternative, perhaps less effective, subunit changes occur. As discussed previously, a marked increase in α4 subunit expression is observed in a number of conditions, including withdrawal from progesterone [12], chronic administration of ethanol [15, 67], and multiple models of epilepsy [3, 17, 18]. However, in these models, the increase in α4 subunit expression is frequently accompanied by a decrease in δ subunit labeling [3, 16, 17, 19], rather than a parallel increase in the two subunit partners. The lack of parallel changes and resulting altered assembly of GABAARs could lead to suboptimal function and altered responses to modulators of tonic inhibition. This contrast between changes in GABAAR KO mice and those in conditions such as epilepsy may signal both compensatory and pathological forms of GABAAR subunit plasticity.
Conclusions
In mice with knockout of a specific GABAAR subunit, the resulting receptor changes are seldom limited to the subunit that is deleted [20, 25], and the resulting changes provide new insights into the rules for GABAAR assembly. The current studies of the α4 KO mice emphasize the preferential partnership of two major subunits that mediate tonic inhibition in the thalamus, with a decrease in δ subunit expression occurring in parallel with the absence of the α4 subunit. The study also demonstrates the importance of the α4 subunit for trafficking δ subunit-containing receptors to the cell surface in vivo. Translating these findings to the development of new treatment approaches for enhancing tonic inhibition in conditions with increased network excitability is a challenging opportunity.
Acknowledgments
We thank Drs. Jean-Marc Fritschy and Werner Sieghart for generously sharing their GABAAR subunit-specific antisera that have made these studies possible. We also thank Christine Huang and Yliana Cetina for outstanding assistance with tissue processing and figure preparation. This work was supported by National Institutes of Health Grants NS075245 (CRH), AA14022 and AA13004 (GEH), AA007680 (RWO), and Veterans Affairs Medical Research Funds (CRH).
Footnotes
Conflict of interest The authors report no conflict of interest concerning the materials and methods used in this study or the findings specified in the paper.
Contributor Information
Zechun Peng, Department of Neurobiology David Geffen School of Medicine at the University of California, Los Angeles Los Angeles, California.
Nianhui Zhang, Department of Neurobiology David Geffen School of Medicine at the University of California, Los Angeles Los Angeles, California.
Dave Chandra, Department of Anesthesiology and Pharmacology & Chemical Biology University of Pittsburgh Pittsburgh, Pennsylvania.
Gregg E. Homanics, Department of Anesthesiology and Pharmacology & Chemical Biology University of Pittsburgh Pittsburgh, Pennsylvania
Richard W. Olsen, Department of Molecular and Medical Pharmacology David Geffen School of Medicine at the University of California, Los Angeles Los Angeles, California
Carolyn R. Houser, Department of Neurobiology David Geffen School of Medicine at the University of California, Los Angeles Los Angeles, California Research Service VA Greater Los Angeles Healthcare System Los Angeles, California.
References
- 1.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 2.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 3.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen RW, Sieghart W, International Union of Pharmacology LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- 11.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 13.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of γ-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- 15.Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor α4 subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- 16.Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzer C, Tsunashima K, Wanzenböck C, Fuchs K, Sieghart W, Sperk G. GABAA receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- 18.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 19.Rajasekaran K, Joshi S, Sun C, Mtchedlishvilli Z, Kapur J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- 21.Panzanelli P, Gunn BG, Schlatter MC, Benke D, Tyagarajan SK, Scheiffele P, Belelli D, Lambert JJ, Rudolph U, Fritschy JM. Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J Physiol. 2011;589:4959–4980. doi: 10.1113/jphysiol.2011.216028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretschmannova K, Hines RM, Revilla-Sanchez R, Terunuma M, Tretter V, Jurd R, Kelz MB, Moss SJ, Davies PA. Enhanced tonic inhibition influences the hypnotic and amnestic actions of the intravenous anesthetics etomidate and propofol. J Neurosci. 2013;33:7264–7273. doi: 10.1523/JNEUROSCI.5475-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Lüddens H. Altered receptor subtypes in the forebrain of GABAA receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- 25.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 26.Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 29.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Z, Chandra D, Homanics GE, Olsen RW, Houser CR. 2007 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. Multiple changes in GABAA receptor subunit localization in the thalamus of an α4 subunit-deficient mouse. Program No. 350.1. Online. [Google Scholar]
- 31.Zhang N, Chandra D, Homanics GE, Houser CR. 2007 Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. Ultrastructural localization of the δ subunit of the GABAA receptor in the mouse thalamus and its alterations in α4 subunit-deficient mice. Program No. 350.2. Online. [Google Scholar]
- 32.Pelham HR. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- 33.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric γ-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 34.Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- 35.Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the γ-aminobutyric acid receptor subunit α4 reduces functional δ-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, Balfour DJ, Lambert JJ, Belelli D. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3- ol: a selective role for δ-GABAA receptors. Eur J Neurosci. 2009;29:1177–1187. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- 41.Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;324:1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- 42.Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI, Fanselow MS, Homanics GE, Sonner JM. Gamma-aminobutyric acid type A receptor alpha 4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2009;109:1816–1822. doi: 10.1213/ANE.0b013e3181bf6ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 44.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 45.Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 46.Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I. Subunit compensation and plasticity of synaptic GABAA receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci. 2011;5:110, 1–8. doi: 10.3389/fnins.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 48.Chandra D, Werner DF, Liang J, Suryanarayanan A, Harrison NL, Spigelman I, Olsen RW, Homanics GE. Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol Clin Exp Res. 2008;32:10–18. doi: 10.1111/j.1530-0277.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halonen LM, Sinkkonen ST, Chandra D, Homanics GE, Korpi ER. Brain regional distribution of GABAA receptors exhibiting atypical GABA agonism: roles of receptor subunits. Neurochem Int. 2009;55:389–396. doi: 10.1016/j.neuint.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJH, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benke D, Michel C, Mohler H. GABAA receptors containing the α4-subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- 53.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 54.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tretter V, Hauer B, Nusser Z, Mihalek RM, Höger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA δ subunit gene leads to an up-regulation of γ2 subunit- containing receptors in cerebellar granule cells. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- 56.Shivers BD, Killisch I, Sprengel R, Sontheimer H, Köhler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 57.Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of δ-subunit containing GABAA receptors from rat brain. Eur J Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 58.Araujo F, Ruano D, Vitorica J. Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 59.Kittler JT, McAinsh K, Moss SJ. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol. 2002;26:251–268. doi: 10.1385/MN:26:2-3:251. [DOI] [PubMed] [Google Scholar]
- 60.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL. δ subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of α4β2δ GABAA receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang JQ, Shen W, Macdonald RL. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci. 2009;29:2845–2856. doi: 10.1523/JNEUROSCI.4772-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macdonald RL, Kang JQ. mRNA surveillance and endoplasmic reticulum quality control processes alter biogenesis of mutant GABAA receptor subunits associated with genetic epilepsies. Epilepsia 53 Suppl. 2012;9:59–70. doi: 10.1111/epi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang JQ, Shen W, Macdonald RL. Trafficking-deficient mutant GABRG2 subunit amount may modify epilepsy phenotype. Ann Neurol. 2013;74:547–559. doi: 10.1002/ana.23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matthews DB, Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- 68.Benke D, Mertens S, Möhler H. Ubiquitous presence of GABAA-receptors containing the α1 subunit in rat brain demonstrated by immunoprecipitation and immunohistochemistry. Mol Neuropharmacol. 1991;1:103–110. [Google Scholar]
- 69.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 70.Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABAA receptor alpha1 subunit knockout mice. J Pharm Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- 71.Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native γ-aminobutyric acidA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- 72.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sperk G, Schwarzer C, Tsunashima K, Fuchs J, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]