Abstract
OBJECTIVES
To identify potentially modifiable late-life biological, lifestyle and sociodemographic factors associated with overall and healthy survival to age 85.
DESIGN
Prospective longitudinal cohort study with 21 years of follow-up (1991–2012)
SETTING
The Hawaii Lifespan Study
PARTICIPANTS
1,292 American men of Japanese ancestry (mean age 75.7 years, range 71–82 years) without baseline major clinical morbidity and functional impairments.
MEASUREMENTS
Overall survival and healthy survival (free from six major chronic diseases and without physical or cognitive impairment) to age 85. Factors were measured at late-life baseline examinations (1991–1993).
RESULTS
Of 1,292 participants, 1,000 men (77%) survived to age 85 years (34% healthy) and 309 (24%) survived to age 95 years (<1% healthy). Late-life factors associated with survival and/or healthy survival included biological (body mass index, ankle:brachial index, cognitive score, blood pressure, inflammatory markers); lifestyle (smoking, alcohol use, physical activity), and sociodemographic factors (education, marital status). Cumulative late-life baseline risk factor models demonstrated that age-standardized (at 70 years) probability of survival to age 95 years ranged from 27% (no factors) to 7% (≥5 factors); to age 100 years ranged from 4% (no factors) to 0.1% (≥5 factors). Age-standardized (at 70 years) probability of healthy survival to 90 years ranged from 4% (no factors) to 0.01% (≥ 5 factors). There were nine healthy survivors at age 95 years and one healthy survivor at age 100 years.
CONCLUSION
Several potentially modifiable risk factors in men in late-life (mean age 75.7 years) were associated with markedly increased probability of subsequent healthy survival and longevity.
Keywords: healthy aging, risk factors, longevity, longitudinal cohort study, late-life
INTRODUCTION
Healthy aging is an important goal for older adults, clinicians, and society.1 Identifying potentially modifiable factors to improve the probability of healthy aging may enhance length and quality of life and reduce healthcare costs.2 While several paradigms of healthy aging have been proposed, including “compression of morbidity,”3 “effective aging,”4 and healthspan,5 the paradigm that has most captured the imagination of clinicians and lay public alike is Rowe and Kahn’s concept of “successful aging.”6 It had a transformational effect on the field of gerontology that still reverberates today in the scientific literature and the popular consciousness.7 The Rowe and Kahn model8 set a high bar for “success” in its conceptualization of healthy aging—surviving into old age without major diseases or disability—but one that is consistent with much of the general public’s notion of a “vibrant old age”.
Midlife studies of healthy aging have consistently identified smoking, overweight/obesity, physical activity, alcohol use and marital status as important (and potentially modifiable) for healthy aging.9–11 Guidelines for risk factors in midlife may not be optimal in old age, including those for body mass index (BMI) and body weight,12 cholesterol,13 blood pressure14 and blood sugar.15 Optimal alcohol intake levels for older patients are controversial.16 Smoking may retain relevance into late life and cessation may impact outcomes regardless of how late in life it occurs, but more research is needed.17
Prior studies have examined late-life risk factors associated with survival, summarized in a recent review,18–22 but data are limited on survival free of major clinical diseases, with maintenance of high cognitive and physical function.23,24 Data are particularly limited on late-life factors associated with healthy survival beyond age 85, an increasingly important outcome as nonagenarians and centenarians are the most rapidly growing segment of the oldest-old population.25 Longer survival suggests greater likelihood of spending down retirement savings and facing mounting healthcare and long-term care costs.26 According to retirement planners, married couples have a 25% chance at age 65 that one will survive to 95-plus years.27 Few objective data exist to assess risk for physical and cognitive impairment at these very old ages, with even fewer data on modifying this risk. With more elders surviving into their nineties and beyond, such data are important for financial, healthcare, and long-term care planning.
Previously, we operationalized a phenotype of healthy aging consistent with common paradigms (no major clinical diseases with good physical and cognitive function)9 and found nine common, modifiable midlife risk factors influenced odds for healthy survival up to six fold. Based on this, we hypothesized there are potentially modifiable risk factors present in late life associated with extended healthspan, with some factors different from those in midlife. Therefore, our current study had three principal aims: (1) Identify late-life predictors of healthy survival into very old ages; (2) Compare late-life and midlife predictors, particularly regarding modifiable risk factors; and (3) Assess the odds of achieving further healthy years.
METHODS
Study Population and Procedures
The Honolulu Heart Program (HHP) began in 1965 as a population-based prospective study of cardiovascular diseases with 8,006 American men of Japanese ancestry (AJA) (recruited from World War II service records of 9,877 men with valid contact information), born in 1900–1919, and living on the island of Oahu.28
For the current study, 3,734 subjects (80% of original HHP cohort survivors) were examined at the late-life baseline examination in 1991–1993. We excluded 2,442 men: those with prevalent major chronic diseases, functional impairment or cognitive impairment, in order to focus on development of unhealthy survival among those who were healthy (i.e. true incidence study); and those aged 83 years or older at the late-life baseline exam, to permit at least three years of longitudinal survival follow-up from the late-life baseline. This left an analytic sample of 1,292 men, born in 1909–1919, followed for mortality and healthy survival status up to 21 years (1991–2012).
All examinations were approved by the Institutional Review Board of Kuakini Medical Center. Written informed consent was obtained at each exam.
Risk Factor Measures
The late-life baseline physical examination measured height, weight, grip strength, timed 10-foot walk, seated blood pressure, forced expiratory volume in the first second (FEV1), ankle-brachial index (ABI), cognitive function (Cognitive Abilities Screening Instrument, CASI),29 and depressive symptoms (Center for Epidemiologic Studies Depression 11-Item Scale, CESD-11).30 Fasting blood samples provided total cholesterol, triglycerides, high-density lipoprotein (HDL), fibrinogen, white blood cell count (WBC), hemoglobin, insulin, and glucose levels. Current and past smoking history, alcohol consumption, physical activity, social and demographic characteristics were collected by self-report.
Continuous variables were dichotomized (high/low) using conventional cutpoints or median values. Published risk cutpoints from national expert panels or mortality and morbidity studies included waist-hip ratio (>0.99),31 FEV1 (<2.1L),32 diastolic blood pressure (DBP) (>90mmHg),33 ABI (<0.9),34 city blocks walked per day (<12),35 triglycerides (≥150mg/dl),33 HDL (<40mg/dl),33 fasting glucose (≥126mg/dl),36 fasting insulin (≥20μU/ml),37 fibrinogen (>3.51g/L),38 WBC (>6000 cells/mm3),39 CASI scores (intermediate scores 74–81.9 versus high scores ≥82)29 and depressive symptoms (CESD-11 score ≥9).30
Factors without published cutpoints were dichotomized by median levels, including handgrip strength (≤33kg), gait speed (≤0.75m/s), and physical activity index (PAI) (≤30.4). Several variables had U-shaped relationships with survival and were divided based on mortality distribution and previous literature: BMI (≥25kg/m2, 19 to <25kg/m2, and <19kg/m2);40 SBP (<120mmHg, 120–160mmHg, and >160mmHg);33 and hemoglobin(<13g/dl, 13–15g/dl, and >15g/dl).41
Additional variable definitions included: education (≥12 versus <12 years of education),9 self-rated health (fair/poor versus good/excellent),42 smoking (never, past and current), and alcohol intake (standardized into ounces of alcohol per month: never drinker = 0 oz/month; mild intake >0 to ≤15 oz/month, totaling ≤1 drink/day; and moderate to heavy alcohol consumption >15 oz/month, totaling >1 drink/day).43
Outcome Measures
Chronic disease and survival data were obtained through comprehensive surveillance of hospital discharges, death certificates, autopsies and repeat examinations44 through 2012.
To identify factors associated with non-survival and unhealthy survival, subjects were assigned to one of three survival phenotypes: non-survivors, unhealthy survivors, and healthy survivors. Non-survivors were men who died prior to age 85 years (mean age at death = 81.3, range = 73.9–84.9). Unhealthy survivors were classified according to a phenotype that operationalized Rowe and Kahn’s popular criteria:9 men who survived to age 85 years with one or more of six major, age-related chronic diseases (coronary heart disease (CHD), stroke, cancer (excluding non-melanoma skin cancer), chronic obstructive pulmonary disease (COPD), Parkinson’s disease (PD) and treated diabetes mellitus), physical impairment and/or cognitive impairment. Healthy survivors were men who survived to age 85 free of these chronic diseases, physical and cognitive impairment.9
Physical impairment was defined as difficulty walking ½ mile.9 Cognitive impairment was defined as CASI score <74.45 Chronic disease, physical impairment and cognitive impairment were determined at each examination from 1991–1993 through 2012.
Statistical Analysis
In order to examine the relationship between risk factors at late-life baseline (age mid-70’s) with healthy, unhealthy and non-survival, three analyses were performed.
First, general linear models (GLM) compared mean late-life baseline (herein referred to as “baseline”) risk factors by survival phenotypes, adjusting for age at baseline. This provided a raw estimate of the differences between risk factors across survival phenotypes.
Second, separate logistic regression models examined associations between baseline risk factors and likelihood of: (1) non-survival versus overall survival, and (2) unhealthy survival versus healthy survival. Due to the large number of variables considered in the analyses, stepwise logistic regression was used to select the subset of variables for the final model, by including only variables with p values smaller than the preselected significance level (p<0.1). Each variable meeting the preselected significance level was added individually until no additional variables met this preselected significance level. Sensitivity analyses were performed including all participants up through age 84 years at baseline and excluding the first 3 years of follow up, and the results did not change significantly (data not shown). All p values were 2-tailed. P values ≤0.05 were considered significant in regression analyses.
Third, separate survival curves examined age-standardized (at age 70) years of overall survival and healthy survival by number of risk factors present using follow-up data through 2012. As in our previous study of mid-life risk factors,9 the objective was to estimate the probability of overall or healthy survival based on total number of risk factors, using an easily-understood risk score based on simply adding the number of risk factors. Cumulative effects of multiple risk factors were assessed using a survival risk score. The risk factors used in the survival risk score were selected from the variables significant in the stepwise logistic regression models of non-survival vs. survival, while the risk factors used in the healthy survival risk score were selected from the variables significant in the stepwise logistic regression models of unhealthy vs. healthy survival. Dummy variables were created to correspond to number of risk factors in the survival risk score (0–4 and ≥5) and the healthy survival risk score. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Of the 1,292 men included in this study, 292 (23%) died before age 85 years (non-survivors); 556 (43%) survived to 85 years with disease or disability (unhealthy survivors); and 444 (34%) survived to age 85 years free of major chronic disease, physical disability and cognitive impairment (healthy survivors). Of this study sample, 983 men (76.1 %) died before 95 years, 300 men (23.2 %) were unhealthy survivors, and only 9 (<1%) were healthy survivors at age 95 years.
Table 1 displays age-adjusted baseline late-life characteristics of the three survival phenotypes for age 85: healthy survivors, unhealthy survivors, and non-survivors. Sociodemographically, fewer healthy survivors were unmarried. Biologically, healthy survivors had faster 10-foot walk time, greater handgrip strength and lower DBP than unhealthy survivors and non-survivors. Although all men were cognitively intact at baseline (CASI score ≥74), healthy survivors had higher CASI scores than the other groups. Fewer healthy survivors had depressive symptoms and fair/poor self-rated health. Healthy survivors also had lower fasting glucose, fasting insulin, fibrinogen and WBC. Regarding health habits, healthy survivors smoked less or were nonsmokers, drank less alcohol, had more physical activity and walked more.
Table 1.
Baseline late-life characteristics by survival (to age 85 years) phenotype, standardized to age 76.
| Baseline Characteristic | Overall Sample n=1292 | Healthy Survival n=444 (34%) | Unhealthy Survival n=556 (43%) | Nonsurvivaln=292 (23%) | p valuea |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age at Baseline, mean (±SD), yb | 75.7 ± 2.8 | 77.0±3.1 | 75.2±2.5 | 74.9±2.4 | <.001 |
| Years of Education | 10.9 ± 3.1 | 11.3±3.2 | 10.6±3.0 | 10.6±2.9 | 0.004 |
| Unmarried, (%) | 16.4 ± 37.0 | 12.7±35.1 | 14.9±34.7 | 24.9±42.2 | <.001 |
| Anthropometric and physiologic | |||||
| Body Mass Index, kg/m2 | 23.7 ± 2.9 | 23.4±2.8 | 24.0±2.7 | 23.4±3.1 | 0.970 |
| Waist/hip ratio | 0.9 ± 0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.022 |
| FEV1, L | 2.2 ± 0.5 | 2.2±0.4 | 2.2±0.4 | 2.1±0.5 | <.001 |
| 10-foot walk time, seconds | 3.6 ± 1.0 | 3.5±0.9 | 3.6±1.0 | 3.8±1.1 | <.001 |
| Grip Strength, kg | 32.4 ± 5.8 | 33.0±5.2 | 32.5±5.6 | 31.4±5.9 | <.001 |
| Seated systolic blood pressure, mmHg | 148.9 ± 21.7 | 147.0±20.0 | 149.5±21.4 | 150.5±24.3 | 0.044 |
| Seated diastolic blood pressure mmHg | 81.6 ± 10.4 | 80.7±9.4 | 81.7±10.2 | 82.8±11.9 | 0.010 |
| Hypertension, (%)c | 73.0 ± 44.4 | 72.2±44.4 | 73.7±44.2 | 72.8±45.0 | 0.877 |
| CASId score | 88.3 ± 5.9 | 90.0±5.3 | 87.3±6.0 | 87.4±5.7 | <.001 |
| Ankle-brachial index | 1.1 ± 0.1 | 1.1±0.1 | 1.1±0.1 | 1.0±0.1 | <.001 |
| CESD-11 scoree | 3.4 ± 3.3 | 2.9±3.0 | 3.5±3.5 | 3.9±3.4 | <.001 |
| Fair or Poor self-rated health (%) | 23.6 ± 42.5 | 18.5±39.8 | 24.5±42.6 | 30.1±45.6 | <.001 |
| Hematologic and biochemical | |||||
| Total Cholesterol (mg/dl) | 194.8 ± 31.1 | 194.8±32.0 | 195.6±30.1 | 193.0±31.6 | 0.465 |
| Triglycerides (mg/dl) | 148.8 ± 93.3 | 143.3±80.1 | 152.6±89.1 | 150.1±116.7 | 0.360 |
| HDL Cholesterol (mg/dl) | 51.7 ± 12.8 | 52.7±12.7 | 50.6±12.1 | 52.3±13.9 | 0.708 |
| Fasting glucose (mg/dl) | 106.8 ± 15.7 | 104.8±11.4 | 107.7±16.9 | 108.0±18.4 | 0.011 |
| Fasting insulin (μU/ml) | 13.9 ± 8.1 | 12.8±7.0 | 14.2±7.4 | 15.0±10.3 | <.001 |
| Fibrinogen (mg/dl) | 299.3 ± 57.2 | 288.4±50.3 | 301.8±58.0 | 311.4±62.3 | <.001 |
| WBC (1,000 cells/mm3) | 6.1 ± 2.0 | 5.7±2.6 | 6.1±1.6 | 6.5±1.8 | <.001 |
| Hemoglobin (g/dl) | 15.1 ± 1.2 | 15.1±1.1 | 15.2±1.1 | 15.1±1.4 | 0.423 |
| Health habits | |||||
| Current smoker (%) | 7.3 ± 26.1 | 3.8±19.3 | 7.0±25.5 | 13.5±34.2 | <.001 |
| Past smoker (%) | 54.9 ± 49.8 | 53.8±50.0 | 53.1±49.7 | 60.1±49.0 | 0.112 |
| Never smoked (%) | 37.8 ± 48.5 | 42.4±49.6 | 39.9±48.7 | 26.4±43.8 | <.001 |
| Smoking, pack-years | 23.2 ± 30.7 | 17.6±25.5 | 23.1±31.0 | 32.6±35.3 | <.001 |
| Alcohol consumption (oz/mo) | 19.0 ± 41.4 | 15.2±26.3 | 17.4±42.2 | 28.0±55.3 | <.001 |
| Physical Activity Indexf | 31.5 ± 4.6 | 31.9±4.7 | 31.6±4.6 | 30.9±4.4 | 0.005 |
| City blocks walked per day | 16.2 ± 17.8 | 17.8±17.6 | 16.1±17.5 | 14.1±18.5 | 0.010 |
p value for trend test
Data are expressed as means (±SD) unless otherwise indicated. Age range 71–82 years at baseline; men aged 83 years and older at baseline were excluded.
Hypertension was defined as blood pressure of 140/90 or higher or antihypertensive medication use.
CASI = Cognitive Abilities Screening Instrument, score range 0–100
CES-D 11 (Centers for Epidemiologic Studies - Depression 11-item scale), scores ≥9 meet criteria for presence of depressive symptoms.
Metabolic work performed in a typical 24-hour day, measured at late-life baseline.
Table 2 shows age-adjusted odds ratios on initial logistic regression analyses for factors that were significantly (p<0.05) associated with either non-survival versus survival or unhealthy versus healthy survival. Sociodemographically, unmarried men were less likely to survive and of those who survived; less education was associated with increased odds of poor health. Biologically, U-shaped mortality relationships necessitated three-level variables for BMI, SBP, and hemoglobin. Lower BMI, SBP and hemoglobin were associated with non-survival, while higher BMI and SBP were associated with unhealthy survival. Consistently high ORs for both non-survival and unhealthy survival were associated with slower 10-foot walk times, higher SBP and DBP, low-normal cognitive function on CASI exam, lower ABI, fair/poor self-rated health, high fasting insulin, high fibrinogen and high-normal WBC (≥6000 cells/mm3). Regarding health habits, poor outcomes were observed for current smoking and walking fewer blocks per day.
Table 2.
Age-Adjusted ORs of Selected Baseline Risk Factors by Survival Status at 85 Years.
| Baseline Risk Factor | Nonsurvival vs. Survival (n= 292 vs. 1000) | Unhealthy vs. Healthy Survival (n=556 vs. 444) | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Sociodemographic | ||||
| Low Education (<12 y) | 1.03 (0.79–1.34) | 0.838 | 1.69 (1.29–2.20) | <.001 |
| Unmarried in late-life | 2.11 (1.51–2.94) | <.001 | 1.16 (0.80–1.68) | 0.446 |
| Anthropometric and physiologic | ||||
| BMI <19 | 2.79 (1.59–4.87) | <.001 | 0.64 (0.31–1.31) | 0.219 |
| BMI 19 to <25 | 1.00 (reference) | -- | 1.00 (reference) | -- |
| BMI ≥25 | 1.02 (0.76–1.36) | 0.912 | 1.69 (1.26–2.28) | <.001 |
| High Waist/hip ratio (>0.99) | 1.20 (0.83–1.73) | 0.326 | 1.49 (1.01–2.21) | 0.044 |
| Lower FEV1 (<2.1) | 1.67 (1.26–2.21) | <.001 | 1.12 (0.85–1.48) | 0.430 |
| Slower walk speed (≤0.75 m/s)g | 1.40 (1.07–1.83) | 0.013 | 1.27 (0.97–1.66) | 0.080 |
| Lower Grip Strength (<33kg) | 1.29 (0.99–1.70) | 0.063 | 1.08 (0.83–1.42) | 0.558 |
| SBP <120 mmHg | 1.68 (1.01–2.78) | 0.044 | 1.02 (0.59–1.78) | 0.944 |
| SBP 120–160 mmHg | 1.00 (reference) | -- | 1.00 (reference) | -- |
| SBP >160 mmHg | 1.38 (1.02–1.85) | 0.036 | 1.57 (1.16–2.13) | 0.004 |
| High Seated DBP (>90mmHg) | 1.50 (1.08–2.08) | 0.015 | 1.86 (1.29–2.69) | 0.001 |
| Hypertension | 0.98 (0.73–1.32) | 0.909 | 1.13 (0.84–1.51) | 0.427 |
| Intermediate CASI score (74–81.9) | 1.39 (0.97–1.98) | 0.070 | 2.75 (1.87–4.05) | <.001 |
| Low ABI (<0.9) | 2.12 (1.30–3.45) | 0.003 | 2.31 (1.26–4.25) | 0.007 |
| High CESD-11 score (≥9) | 1.50 (0.93–2.44) | 0.097 | 1.54 (0.90–2.63) | 0.116 |
| Fair or Poor self-rated health | 1.54 (1.13–2.09) | 0.007 | 1.41 (1.02–1.96) | 0.038 |
| Hematologic and biochemical | ||||
| High Triglycerides (≥150mg/dl) | 0.90 (0.68–1.18) | 0.439 | 1.16 (0.89–1.53) | 0.275 |
| Low HDL Cholesterol (<40mg/dl) | 1.06 (0.75–1.52) | 0.730 | 1.37 (0.95–1.96) | 0.092 |
| High glucose (≥126mg/dl) | 1.30 (0.79–2.13) | 0.301 | 2.49 (1.38–4.46) | 0.002 |
| High insulin (≥ 20μU/ml) | 1.34 (0.95–1.88) | 0.094 | 1.54 (1.06–2.25) | 0.023 |
| High Fibrinogen (>351 mg/dl) | 2.00 (1.43–2.80) | <.001 | 2.09 (1.38–3.17) | <.001 |
| High WBC ( >6000 cells/m3) | 1.69 (1.29–2.22) | <.001 | 1.56 (1.18–2.05) | 0.002 |
| Hemoglobin <13 g/dl | 2.25 (1.18–4.32) | 0.014 | 0.85 (0.40–1.81) | 0.680 |
| Hemoglobin 13–15g/dl | 1.00 (reference) | -- | 1.00 (reference) | -- |
| Hemoglobin >15g/dl | 1.19 (0.89–1.58) | 0.240 | 1.21 (0.92–1.59) | 0.173 |
| Health habits | ||||
| Never smoker | 1.00 (reference) | -- | 1.00 (reference) | -- |
| Past smoker | 1.76 (1.29–2.39) | <.001 | 1.03 (0.78–1.35) | 0.835 |
| Current smoker | 3.75 (2.29–6.12) | <.001 | 1.99 (1.06–3.75) | 0.033 |
| Never Alcohol use | 0.80 (0.57–1.12) | 0.191 | 1.10 (0.81–1.50) | 0.545 |
| Low Alcohol use (1–15 oz/mo) | 1.00 (reference) | -- | 1.00 (reference) | -- |
| Higher Alcohol use (>15 oz/mo) | 1.54 (1.10–2.15) | 0.011 | 1.04 (0.73–1.47) | 0.830 |
| Physical Activity Index ≤30.4 | 1.42 (1.08–1.86) | 0.012 | 0.98 (0.75–1.28) | 0.876 |
| Fewer blocks walked/day (<12) | 1.45 (1.10–1.91) | 0.008 | 1.25 (0.95–1.64) | 0.106 |
Walk speed determined on timed 10-foot (3m) walk at usual pace.
Table 3 shows variables significant on age-adjusted logistic regression analyses that were included in stepwise logistic regression analyses. Three variables, blood pressure, high fibrinogen, and high white blood count were significantly or borderline associated with increased likelihood of both non-survival and unhealthy survival. Several variables selected for the non-survival outcome analyses differed from the unhealthy survival outcome analyses. Low BMI was associated with non-survival versus survival, while high BMI was associated with unhealthy versus healthy survival. Unmarried status, current and past smoking, high alcohol intake and low physical activity index were associated with non-survival versus survival, but were not significant for the unhealthy survival outcome. Lower education, low-normal CASI and low ankle-brachial index were associated with unhealthy versus healthy survival, but were not significant in the non-survival outcome model.
Table 3.
Stepwise Logistic Regression Model of Risk of Death (Nonsurvival) or Unhealthy Survival (Usual Survival) at Age 85 Years
| Risk Factor | Nonsurvival vs. Survival (n=292 vs. 1000)a | Unhealthy vs. Healthy Survival (n=556 vs. 444) | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Sociodemographic | ||||
| Low Education (<12 y) | Not included | 1.45 (1.08–1.95) | .014 | |
| Unmarried at exam 4 | 1.51 (1.01–2.26) | .043 | Not included | |
| Anthropometric and physiologic | ||||
| Low BMI (<19kg/m2 vs. ≥19) | 2.25 (1.12–4.51) | .023 | Not included | |
| High BMI (≥25 kg/m2 vs.<25) | Not included | 1.65 (1.20–2.27) | .002 | |
| High Waist/hip ratio (>0.99) | Not included | Not selected | ||
| Lower FEV1 (<2.1) | 1.32 (0.97–1.80) | .076 | Not included | |
| Lower Grip Strength (<33kg) | Not selected | Not included | ||
| Slow walk speed (≤0.75 m/s) | Not selected | Not selected | ||
| SBP <120 mmHg | Not selected | Not included | ||
| SBP 120–160 mmHg | 1.00 (reference) | Not included | ||
| SBP >160 mmHg | Not selected | Not included | ||
| SBP >160 mmHg vs. ≤ 160 mmHg | Not included | 1.49 (1.07–2.07) | .019 | |
| High DBP (>90mmHg) | 1.53 (1.06–2.21) | .024 | Not selected | |
| Intermediate CASI score (74–81.9) | Not selected | 2.48 (1.59–3.85) | <.001 | |
| Low ABI (<0.9) | Not selected | 2.12 (1.08–4.16) | .029 | |
| Depressive Symptoms (CESD≥9) | Not selected | Not included | ||
| Fair or Poor self-rated health | Not selected | 1.36 (0.95–1.93) | .091 | |
| Hematologic and biochemical | ||||
| Low HDL Cholesterol (<40mg/dl) | Not included | Not selected | ||
| High Glucose (≥126mg/dl) | Not included | Not selected | ||
| High insulin (≥ 20μU/ml) | Not selected | Not selected | ||
| High Fibrinogen (>351 mg/dl) | 1.59 (1.09–2.32) | .017 | 1.98 (1.26–3.10) | .003 |
| High WBC ( >6000 cells/m3) | 1.30 (0.95–1.77) | .098 | 1.39 (1.03–1.87) | .031 |
| Hemoglobin (<13g/dl vs ≥13) | Not selected | Not included | ||
| Health habits | ||||
| Past smoker vs. never | 1.46 (1.04–2.04) | .030 | Not included | |
| Current smoker vs. never | 2.32 (1.30–4.15) | .005 | Not selected | |
| Alcohol >15 oz/month vs. ≤15 oz/mo | 1.52 (1.10–2.09) | .011 | Not included | |
| Low Physical Activity Index ≤30.4 | 1.41 (1.04–1.91) | .025 | Not included | |
| Fewer blocks walked/day (<12) | 1.32 (0.98–1.79) | .068 | Not selected | |
N= sample size for final logistic regression models (final model n=1138 for mortality model; n=914 for healthy survival model due to missing data). Variables not selected by the nonsurvival versus survival model included: grip strength, slow walk speed, systolic blood pressure, low-normal cognitive function (CASI) score, low ABI, depressive symptoms, fair or poor self-rated health, elevated fasting insulin level and low hemoglobin. Variables not selected by the unhealthy survival versus healthy survival model included: high waist-hip ratio, slow walk speed, higher diastolic blood pressure, current smoking, city blocks walked, low HDL cholesterol, high glucose level and elevated fasting insulin level. Age was forced into the models (data not shown). Not included indicates not selected for model due to nonsignificance (p>.05) on univariate analyses.
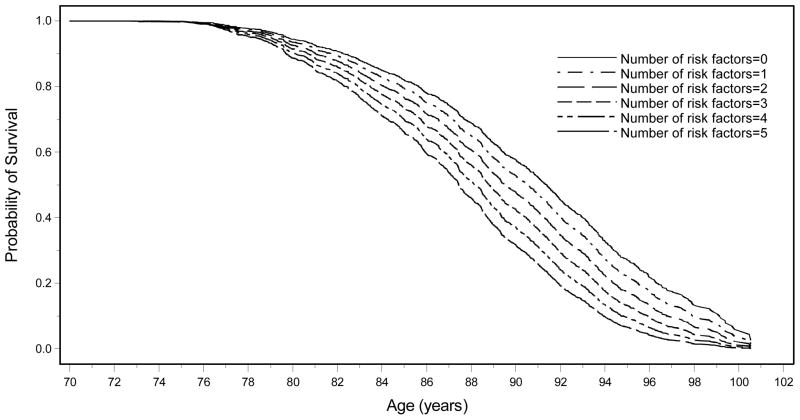
Figure 1 displays the years of further survival by number of risk factors present, age-standardized at 70 years. There is clear separation in years of survival based on number of late-life risk factors present, with survival up to and beyond age 100 years more common among men with no risk factors. Among men with zero risk factors at late-life baseline (standardized to age 70 years), 58% survived to age 90, compared to 32% of men with 5 or more risk factors. Among men with zero late-life risk factors, 27% survived to age 95 years, compared to 7% of men with 5 or more risk factors. Among men with zero late-life risk factors, 4% survived to age 100 years, compared to 0.1% of men who had 5 or more risk factors.
Figure 1. Age-standardized (at 70) probability of survival by number of risk factors present at baseline.
The probabilities of survival are estimated assuming all 6 groups start at age 70 in the graph. All participants were Japanese American men followed from late-life baseline (1991–1993) to 2012. Survival risk score indicates the number of risk factors (unmarried, low body mass index <19 kg/m2, low forced expiratory volume in 1 minute (FEV1 <2.1), high diastolic blood pressure >90mmHg, past smoker, current smoker, alcohol use >15 oz/month, low physical activity index, fewer than 12 city blocks walked/day, higher fibrinogen >351 mg/dl, higher white blood count >6000 cells/m3.
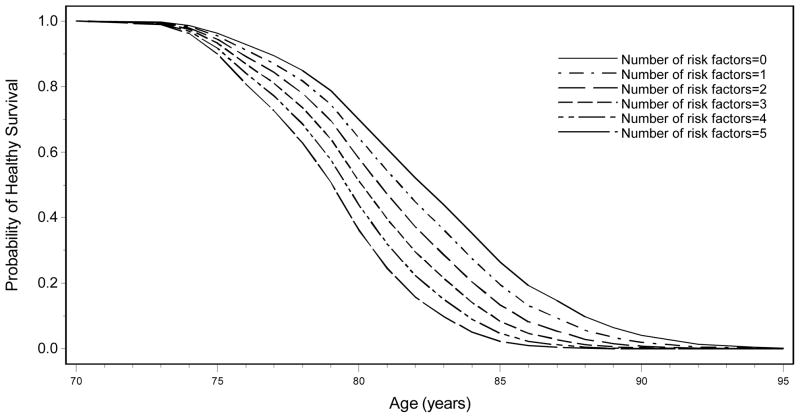
Figure 2 displays the years of healthy survival by number of risk factors present, age-standardized at 70 years. There is clear separation in years of healthy survival based on number of late-life risk factors present. Healthy survival to age 85 years was 13-fold more common among men with no risk factors at late-life baseline (standardized to age 70 years, 26%) compared to men with 5 or more risk factors (2%). Among men with zero risk factors at late-life baseline, 4% remained healthy at age 90 years, compared to 0.01% of men with 5 or more risk factors at late-life baseline. Only nine men met operationalized Rowe and Kahn9 healthy survival criteria at age 95 (0.2%) and only one man (0.002%) met healthy survival criteria at age 100.
Figure 2. Age-standardized (at 70) probability of healthy survival (free of chronic disease, cognitive impairment and disability) by number of risk factors present at baseline.
The probabilities of healthy survival are estimated assuming all 6 groups start at age 70 in the graph. All participants were Japanese American men followed from late-life baseline (1991–1993) to 2012. Among those alive at age 85, exceptional survival was defined as absence of 6 major chronic diseases and absence of physical and cognitive disability. Survival risk score indicates number of risk factors (education < 12years, body mass index ≥25 kg/m2, systolic blood pressure >160, CASI score 74–81.9, ankle-brachial index <0.9, poor self-rated health, high fibrinogen >351 mg/dl, higher white blood count >6000 cells/m3).
DISCUSSION
Aging seniors, healthcare providers, and policy makers share a common stake in optimizing odds for good health at older ages. Therefore, we need evidence-based information on potentially modifiable risk factors. While only 29.7% of 65–74 year olds report a disability, by age 75-plus, 49.6% report one or more disabilities.46 Although definitive evidence requires interventional studies, this prospective, observational cohort study identified several potentially modifiable late-life factors associated with subsequent healthy survival, suggesting that even in old age, it may not be too late to impact future health.
The findings that emerged may share some similarities but also have important differences with prior studies of midlife. Hypertension in both midlife and late life was a risk factor for non-survival and unhealthy survival. However, the adverse effects of high triglycerides and high glucose, while robust in midlife,9 are not as apparent in late life. Higher BMI in midlife9 was associated with both non-survival and unhealthy survival, while in late life, low BMI was associated with non-survival and higher BMI was associated with unhealthy survival. Current smoking had an important role in both midlife9 and late life. Thus, the current study provides additional evidence for clinicians to continue to encourage smoking cessation, regardless of age. While increased physical activity in midlife did not appear protective,9 low physical activity in late life was associated with increased mortality. In midlife,9 drinking more than three alcoholic drinks per day was associated with both non-survival and unhealthy survival, while in late-life drinking more than one drink per day (15 ounces/month) was associated with non-survival, suggesting lower alcohol limits in late life.
Interestingly, inflammatory factors, including fibrinogen and WBC count, appear to have increasingly important roles in late life. Elevated levels of each predicted increased mortality risk, possibly reflecting dysregulation of the immune system and the inflammatory state that accompanies aging.47 Subclinical diseases in late life, including subclinical cardiovascular disease (lower ABI) and early cognitive impairment (lower CASI score), were associated with higher risk for unhealthy survival, supporting a role for continued risk factor modification and research on more effective means of secondary prevention. Our findings are supported by one other study, the Cardiovascular Health Study (CHS), which assessed white men and women aged 65-plus years who maintained cognitive function, ADL independence (rather than ability to walk ½ mile) and avoided major chronic diseases with an average eight years of follow-up.24 Factors associated with healthy survival included younger age, regular physical activity, lower rates of subclinical cardiovascular disease and related risk factors (ABI, carotid intimal thickness, EKG abnormality, diabetes, smoking, and C-reactive protein).
Most importantly, we found a clear dose-response relation between number of risk factors and age-standardized probability of overall and healthy survival, with a 13-fold increased probability of living another 10 years in good health and up to 40-fold increased probability of living another 25 years, by avoiding particular late-life risk factors. Healthy survival to age 85 years was 13-fold more common among men with no risk factors at late-life baseline (standardized to age 70 years, 26%) compared to men with 5 or more risk factors (2%), and overall survival to age 100 years was 40-fold more common among men with zero late-life risk factors (4%) compared to 0.1% of men who had 5 or more risk factors.
The strengths of the current study are several. One, it is the only known study to operationalize the Rowe and Kahn paradigm of healthy aging from late life, reflecting both age-associated diseases and disabilities, with follow-up into the nonagenarian and centenarian years. Two, in addition to the long 21-year follow-up, this study also provides a comprehensive examination of 26 risk factors, some not previously studied in late-life healthy aging. This longitudinal study design, in which men entered the study in midlife in 1965 and were all born in 1909–1919, minimized bias due to age at entry and birth cohort. While not an interventional study and thus causation cannot be established, several interesting findings emerged that may have implications for patient care, research, and public health.
There are several limitations to this study. The study population was comprised of Japanese-American men, and generalizability to other populations may be limited due to genetic, sociocultural, cohort or other effects. Our sample likely had greater longevity than other populations, as 50% of our cohort who were healthy at late-life baseline and 25% of our original 1965 cohort recruited in midlife (45–68 years old) survived to age 90. Additionally, these findings may not be generalizable to women, who were not included in this cohort.
Several implications of these data for aging seniors are worth noting. While both survival and healthy survival appear markedly enhanced for those who avoid late-life risk factors, the limits of aging “successfully” by Rowe and Kahn criteria were clearly apparent. By the nonagenarian years, even for those who avoided all major risk factors, only 4% were still “healthy” at age ninety and only one person remained healthy until age one hundred. While the Rowe and Kahn criteria set a high bar for health, so do many aging baby boomers. Nevertheless, despite frequent stories in the media about extraordinarily healthy and active centenarians, the probability of most us becoming centenarians is low48 and the likelihood of being healthy by common standards is even lower.49 In the current study, even if you were a healthy septuagenarian and avoided major risk factors, the probability of healthy survival to age one hundred was only 0.002% (1/444).
On an encouraging note, for those of us who wish to maximize our longevity, perhaps in somewhat less robust health, there may be much we can do. For healthy, active, septuagenarian men with no major risk factors, a substantial number in the current study lived into their 90s and 100s (58% to age 90 years, 27% to age 95 years, and 4% to age 100 years). While the current study did not assess women, they generally outnumber men 4:1 by the centenarian years50 so there is reason to believe their odds for longevity are even better. Healthy septuagenarians who avoid common late-life risk factors would be well advised to plan ahead to mitigate financial, health and long-term care challenges, which are likely to accompany such longevity.
CONCLUSION
Even in late life, we found risk and protective factors, some modifiable, associated with likelihood of overall and healthy survival. Future research is needed to determine whether modification of these risk factors in late life will enhance overall and healthy survival. This study suggests that there may be much we can do to improve our probability of healthy aging and longevity—even at older ages.
Acknowledgments
The authors gratefully acknowledge the participants of the Honolulu Heart Program/Honolulu-Asia Aging Study/Hawaii Lifespan Study, for their 48 years of commitment to this research.
Funding sources: This research was supported by: The John A. Hartford Center of Excellence in Geriatrics, Department of Geriatric Medicine, John A. Burns School of Medicine, University of Hawaii; Kuakini Medical Center; and the US National Institutes of Health (contract N01-AG-4-2149, Grants 5 U01 AG019349-05, 5R01AG027060-06 (Kuakini Hawaii Lifespan Study), 5R01AG038707-02 (Kuakini Hawaii Healthspan Study) and 1R13AG041931 from the National Institute on Aging and contract N01-HC-05102 from the National Heart, Lung, and Blood Institute).
Footnotes
Related paper presentations: Portions of this study were presented at the Annual Meeting of the American Geriatrics Society in May 2008 and at the Gerontological Society of America Annual Meeting in November 2010.
Author Contributions:
Study concept, design and acquisition of data: Bradley J. Willcox, Randi Chen, Kamal Masaki, Qimei H, J. David Curb, John Grove, Timothy Donlon
Analysis and interpretation of data: Christina L. Bell, Randi Chen, Kamal Masaki, Priscilla Yee, Qimei He, John Grove, D. Craig Willcox, Leonard W. Poon, Bradley J. Willcox
Preparation of manuscript: Christina L. Bell, Kamal Masaki, Randi Chen, Qimei He, Bradley J. Willcox
Critical revision of manuscript: Christina L. Bell, Randi Chen, Kamal Masaki, Priscilla Yee, Qimei He, John Grove, Timothy Donlon, J. David Curb, D. Craig Willcox, Leonard W. Poon, Bradley J. Willcox
Conflict of Interest
Drs. Bell, Masaki, and Curb, Willcox (DCW, BJW) and Poon received grant funding from NIH.
Sponsor’s Role: The investigators retained full independence in the conduct of this research and the funding organizations had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.National Institute on Aging. Living Long and Well in the 21st Century - Strategic Directions for Research on Aging. National Institute on Aging. 2007 [Google Scholar]
- 2.Olshansky SJ, Perry D, Miller RA, et al. Pursuing the longevity dividend: Scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- 3.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 4.Curb JD, Guralnik JM, LaCroix AZ, et al. Effective aging. Meeting the challenge of growing older. J Am Geriatr Soc. 1990;38:827–828. doi: 10.1111/j.1532-5415.1990.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen SL, Sebastiani P, Dworkis DA, et al. Health span approximates life span among many supercentenarians: Compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 7.Poon LW, Fry C, Kahana E, et al. [Accessed January 27, 2013.];Healthy successful aging: a public health mandate. www.publichealth.uga.edu/geron/research/healthy-successful-aging-public-health-mandate.
- 8.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 9.Willcox BJ, He Q, Chen R, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- 10.Terry DF, Pencina MJ, Vasan RS, et al. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J Am Geriatr Soc. 2005;53:1944–1950. doi: 10.1111/j.1532-5415.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 11.Britton A, Shipley M, Singh-Manoux A, et al. Successful aging: The contribution of early-life and midlife risk factors. J Am Geriatr Soc. 2008;56:1098–1105. doi: 10.1111/j.1532-5415.2008.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens J, Cai J, Pamuk ER, et al. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 13.Iribarren C, Reed DM, Burchfiel CM, et al. Serum total cholesterol and mortality. Confounding factors and risk modification in Japanese-American men. JAMA. 1995;273:1926–1932. [PubMed] [Google Scholar]
- 14.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Boscardin WJ, Stijacic Cenzer I, et al. The risks and benefits of implementing glycemic control guidelines in frail older adults with diabetes mellitus. J Am Geriatr Soc. 2011;59:666–672. doi: 10.1111/j.1532-5415.2011.03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang I, Guralnik J, Wallace RB, et al. What level of alcohol consumption is hazardous for older people? Functioning and mortality in U.S. and English national cohorts. J Am Geriatr Soc. 2007;55:49–57. doi: 10.1111/j.1532-5415.2006.01007.x. [DOI] [PubMed] [Google Scholar]
- 17.LaCroix AZ, Lang J, Scherr P, et al. Smoking and mortality among older men and women in three communities. N Engl J Med. 1991;324:1619–1625. doi: 10.1056/NEJM199106063242303. [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Murabito JM. The Epidemiology of Longevity and Exceptional Survival. Epidemiologic reviews. 2013 doi: 10.1093/epirev/mxs013. [epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta A, Henley W, Lang I, et al. Predictors of extraordinary survival in the Iowa established populations for epidemiologic study of the elderly: cohort follow-up to “extinction”. J Am Geriatr Soc. 2011;59:963–971. doi: 10.1111/j.1532-5415.2011.03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yates LB, Djousse L, Kurth T, et al. Exceptional longevity in men: modifiable factors associated with survival and function to age 90 years. Arch Intern Med. 2008;168:284–290. doi: 10.1001/archinternmed.2007.77. [DOI] [PubMed] [Google Scholar]
- 21.Stessman J, Hammerman-Rozenberg R, Cohen A, et al. Physical activity, function, and longevity among the very old. Arch Intern Med. 2009;169:1476–1483. doi: 10.1001/archinternmed.2009.248. [DOI] [PubMed] [Google Scholar]
- 22.Rajpathak SN, Liu Y, Ben-David O, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–1512. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort--the cardiovascular health study all stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman AB, Arnold AM, Naydeck BL, et al. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 25.Vaupel JW. The remarkable improvements in survival at older ages. Philos Trans R Soc Lond B Biol Sci. 1997;352:1799–1804. doi: 10.1098/rstb.1997.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spillman BC, Lubitz J. The effect of longevity on spending for acute and long-term care. N Engl J Med. 2000;342:1409–1415. doi: 10.1056/NEJM200005113421906. [DOI] [PubMed] [Google Scholar]
- 27.Maximizing Your Workforce: Employees Over 50 in Today’s Global Economy. Wharton University of Pennsylvania/AARP; Nov 10, 2004. [Google Scholar]
- 28.Kagan A, editor. The Honolulu Heart Program: An Epidemiologic Study of Coronary Heart Disease and Stroke. Amsterdam: Harwood Academic Press; 1996. [Google Scholar]
- 29.Launer LJ, Masaki K, Petrovitch H, et al. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 30.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 31.Price GM, Uauy R, Breeze E, et al. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 32.Sharp DS, Enright PL, Chiu D, et al. Reference values for pulmonary function tests of Japanese-American men aged 71 to 90 years. Am J Respir Crit Care Med. 1996;153:805–811. doi: 10.1164/ajrccm.153.2.8564136. [DOI] [PubMed] [Google Scholar]
- 33.The Seventh Report of the Joint National Committee on Prevention. Detection, Evaluation, and Treatment of High Blood Pressure. [Accessed September 10, 2010];National High Blood Pressure Education Program. 2004 at http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.htm. [PubMed]
- 34.Heald CL, Fowkes FG, Murray GD, et al. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189:61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Abbott RD, White LR, Ross GW, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 36.Standards of medical care in diabetes--2010. Diabetes Care. 33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burchfiel CM, Curb JD, Arakaki R, et al. Cardiovascular risk factors and hyperinsulinemia in elderly men: The Honolulu Heart Program. Ann Epidemiol. 1996;6:490–497. doi: 10.1016/s1047-2797(96)00103-2. [DOI] [PubMed] [Google Scholar]
- 38.Yano K, Grove JS, Chen R, et al. Plasma fibrinogen as a predictor of total and cause-specific mortality in elderly Japanese-American men. Arterioscler Thromb Vasc Biol. 2001;21:1065–1070. doi: 10.1161/01.atv.21.6.1065. [DOI] [PubMed] [Google Scholar]
- 39.Ruggiero C, Metter EJ, Cherubini A, et al. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007;49:1841–1850. doi: 10.1016/j.jacc.2007.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Expert Committee. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 41.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: The Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 42.Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. Am J Public Health. 1990;80:446–452. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galanis DJ, Joseph C, Masaki KH, et al. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: The Honolulu-Asia Aging Study. Am J Public Health. 2000;90:1254–1259. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbott RD, Curb JD, Rodriguez BL, et al. Age-related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol. 2003;56:479–486. doi: 10.1016/s0895-4356(02)00611-x. [DOI] [PubMed] [Google Scholar]
- 45.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA. 1996;276:955–960. [PubMed] [Google Scholar]
- 46. [Accessed August 8, 2011.];American Community Survey. 2007 http://factfinder.census.gov.
- 47.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Carnes BA, Olshansky SJ, Hayflick L. Can human biology allow most of us to become centenarians? J Gerontol A Biol Sci Med Sci. 2013;68:136–142. doi: 10.1093/gerona/gls142. [DOI] [PubMed] [Google Scholar]
- 49.Andersen-Ranberg K, Schroll M, Jeune B. Healthy centenarians do not exist, but autonomous centenarians do: A population-based study of morbidity among Danish centenarians. J Am Geriatr Soc. 2001;49:900–908. doi: 10.1046/j.1532-5415.2001.49180.x. [DOI] [PubMed] [Google Scholar]
- 50.Willcox BJ, Willcox DC, Ferrucci L. Secrets of healthy aging and longevity from exceptional survivors around the globe: Lessons from octogenarians to supercentenarians. J Gerontol A Biol Sci Med Sci. 2008;63:1181–1185. doi: 10.1093/gerona/63.11.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]