Abstract
Importance
Keratocystic odontogenic tumors of the jaw (KCOTs) affect more than 65% of patients with basal cell nevus syndrome (BCNS). Surgery frequently causes facial disfigurement and is not always curative. Most BCNS-related and some sporadic KCOTs have malignant activation of the Hedgehog (HH) signaling pathway.
Observations
We examined the effect of vismodegib (an oral HH-pathway inhibitor) on KCOT size in BCNS patients enrolled in a clinical trial testing vismodegib for BCC prevention (NCT00957229), using pre and post-treatment MRIs. Four men and 2 women had pretreatment KCOTs, mean longest diameter 2.0cm (range: 0.7–3.3cm), occurring primarily in the mandible. Subjects were treated with vismodegib (150mg/day) for a mean 18 months (SD: 4.8, range: 11–24). Four subjects experienced a size reduction and 2 had no change. Vismodegib reduced the mean longest diameter of KCOTs in all subjects by 1.0cm (95% CI: 0.03, 1.94, p= 0.02) or 50% from baseline. We observed no enlargement of existing KCOTs or new KCOT development.
Conclusions and relevance
Vismodegib shrinks some KCOTs in BCNS patients and may offer an alternative to surgical therapy. These effects were maintained for at least 9 months after drug cessation in 1 patient. Further studies assessing long-term efficacy and optimal maintenance regimens should be performed.
Introduction
Basal cell nevus (Gorlin) syndrome (BCNS) (OMIM#109400) is a rare autosomal dominant disease in which affected individuals can develop a panoply of phenotypic abnormalities, the most prominent of which are basal cell carcinomas (BCCs) of the skin and keratocystic odontogenic tumors of the jaw (KCOTs)1. The incidence of KCOTs varies from 4%–16.5% of oral pathology specimens2; however they affect 65–100% of BCNS patients1. Multiple KCOTs, which typically grow rapidly, are often the earliest presenting feature of BCNS. Surgery is the standard of care but it frequently causes facial disfigurement and often is not curative 2–4. To our knowledge, there is no effective non-surgical therapy for KCOTs.
Patient with BCNS inherit one defective copy of the tumor suppressor gene PATCHED 1 (PTCH1) (OMIM#601309), which encodes a primary inhibitor of the Hedgehog (HH) signaling pathway. Functional loss of the second allele leads to activation of HH pathway signaling5. KCOTs in patients with BCNS and in 30% of sporadic lesions harbor somatically acquired PTCH1 mutations4 and increased HH target gene expression (GLI1)6 _ENREF_7. They have also been seen in heterozygous Ptch1 knockout mice and in K5-Gli2 transgenic mice 6,7. This discovery highlights the possibility that the newly approved targeted HH pathway inhibitor, vismodegib (Erivedge), might be effective for KCOT treatment. We conducted a sub-study in patients enrolled in a recent clinical trial testing vismodegib for BCC prevention8 to determine its effect on KCOT size in patients with BCNS. This study received institutional review board approval by Children’s Hospital Oakland Research Institute, and all participants provided written consent.
Report of cases
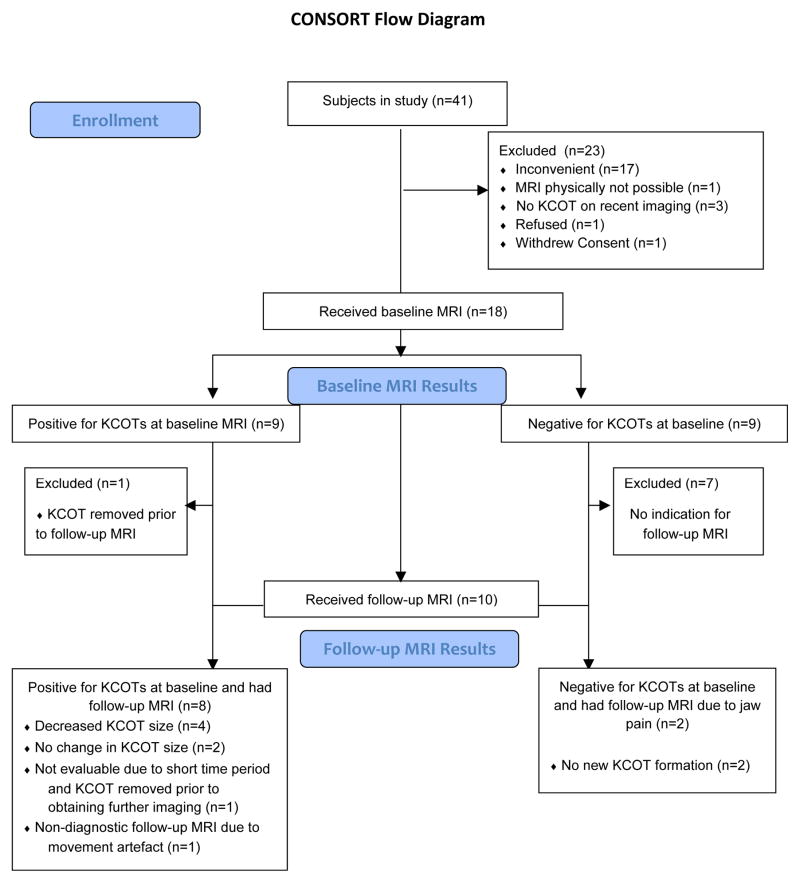
Of 41 BCNS subjects enrolled in a clinical trial (NCT00957229) testing vismodegib 150mg daily for BCC prevention8, 18 participated in the KCOT sub-study and had pre-treatment (baseline) MRIs. We performed post-treatment MRIs in 8 subjects with KCOTs at baseline who had received at least 11 months vismodegib after the pre-treatment MRI (Figure 1).
Figure 1.
CONSORT diagram. A CONSORT flow diagram of patient selection highlighting reasons for exclusion from the case series, as well as, baseline and post-treatment MRI results.
We recorded reasons for not having a MRI, time interval between MRIs, treatment duration and KCOT characteristics. We calculated the percentage reduction in KCOT size (longest diameter in centimeters) from baseline and used the Wilcoxon signed rank test to compare the change in KCOT diameters pre and post-vismodegib.
We excluded subjects without paired MRIs from analysis. We examined face/neck MRI (1.5 or 3.0 T) with coronal, sagittal, and axial T1 fast spin-echo, and multiplanar T2 to demonstrate the intrinsic high T2 signal of KCOTs. A single study radiologist (T.J.) compared all paired MRI images and or/reports.
Results
Of the 41 subjects enrolled in the original clinical trial8 we obtained baseline MRIs in 18, 9 of whom (50%) had KCOTs. Twenty-three subjects refused to participate in the MRI-KCOT study because they had no history of tumors or had negative recent dental imaging, were unable to fit into the MRI scanner, or perceived having an MRI scan as an inconvenience (Figure 1).
We excluded from our analysis, 3 of the 9 subjects with KCOTs diagnosed on baseline MRI, due to tumor removal (N=2) or movement artifact on post-treatment MRI precluding exact measurement (N=1). We performed post-treatment MRIs in 2 subjects with no KCOTs at baseline due to new jaw pain to assess for new growth of KCOTs while receiving vismodegib. Thus a total of 6 subjects with paired KCOT MRIs are included in the final analysis (Table 1, Figure 1).
Table 1.
Percentage change in cyst size from baseline to post-treatment MRI.
| Subject | Age/Sex | KCOT Location | Total months on drug by post- treatment MRI* | Months between MRI’s | Baseline KCOT size (longest diameter, cm) | Post-treatment KCOT size (longest diameter, cm) | % Change in cyst size |
|---|---|---|---|---|---|---|---|
| 1 | 53/F | R maxilla L mandible |
18 | 13 | 3.1 0.7 |
0 0 |
−100 −100 |
| 2 | 59/M | Midline mandible | 24 | 23 | 0.7 | 0.7 | 0 |
| 3 | 51/M | R mandibular ramus | 20# | 23 | 2.3 | 2.3 | 0 |
| 4Ŧ | 48/M | L maxilla L mandible |
11# | 22 | 3.2 0.9 |
0 0.8 |
−100 − 12 |
| 5 | 37/M | L maxilla R mandible |
21 | 19 | 3.3 2.0 |
2.5 1.4 |
−25 −30 |
| 6 | 57/F | Right mandible | 14# | 17 | 1.3 | 1.2 | −8 |
| Mean ±SD | 51±8 | 18.0 ± 4.8 | 19.5 ± 4.0 | 2.0 ± 1.0 | 1.0 ± 0.9 | −50 ± 45 |
Accounting for drug breaks (i.e. a brief period off vismodegib treatment).
Subjects 3, 4 and 6 took 3 months of drug breaks between MRI’s.
Second post-treatment MRI 9 months off vismodegib: L maxilla KCOT = 0cm, L mandible KCOT = 0.7cm
Study participants included 4 men and 2 women, with a mean age of 51 years (range 37–59) (Table 1). All subjects had prior removal of KCOTs, most having had at least 2 procedures.
In 6 subjects with a total of 9 documented KCOTs we obtained post-treatment MRIs within an average of 19.5 months (SD: 4.0, range 13–23) after baseline imaging. During this interval we treated these subjects with vismodegib for an average of 18.0 months (SD: 4.8, range: 11–24); 3 subjects took drug breaks (i.e. a brief period off vismodegib treatment) (Table 1). The baseline MRI of one of the 6 subjects was performed 5 months after starting vismodegib and two KCOTs were seen. The mean longest diameter of baseline KCOTs was 2.0 cm (range: 0.7–3.3 cm). KCOTs occurred primarily in the mandible (6/9). Of the 6 subjects with tumors, 4 experienced a reduction in KCOT size and 2 experienced no change. Vismodegib reduced the longest diameter of all monitored KCOTs by an average of 1.0cm (95% CI: 0.03, 1.94, p= 0.016), i.e. by 50% from baseline. No enlargement or new KCOTs occurred in any case, including the 2 subjects without KCOTs at baseline. In one subject with100% resolution of one KCOT with vismodegib, there was no recurrence 9 months after drug cessation (Figure 2). All subjects experienced mild side effects (grade 1) of taste loss, muscle cramps and hair loss as previously reported8. Three subjects experienced Grade 2 muscle cramps and GI disturbance necessitating brief drug breaks.
Figure 2.
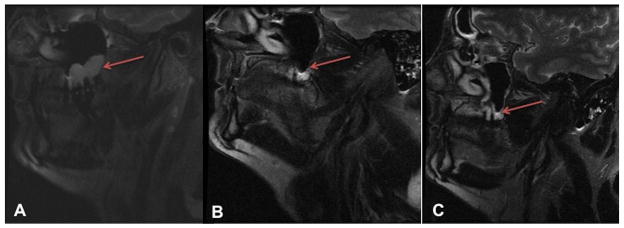
MRI images of subject 4. Serial sagittal T2 weighted MRI images demonstrating the size change in the left maxillary KCOT (red arrows) from A) baseline B) after 11 months of vismodegib and C) 9 months after drug discontinuation.
Discussion
We found that vismodegib can shrink some KCOTs in patients with BCNS, with a mean size reduction of 50% in our study. Two subjects had complete resolution of one or more KCOTs, with no enlargement of existing tumors or development of new lesions in any case. We found no KCOT relapse after 9-months of drug discontinuation in one subject, who also had minimal BCC recurrence at that time.
Vismodegib is an oral HH pathway inhibitor approved for the indefinite treatment of locally advanced/metastatic BCCs. In one case report, Goldberg et al3 incidentally noted resolution of KCOTs in one BCNS patient treated with vismodegib (270mg/day) for 2 years. This dose was used in phase 1-studies before the data demonstrated 150mg/day to be the most efficacious.
It is unclear why vismodegib was effective in some, but not all of our subjects. The three patients with multiple KCOTs in our study appeared to have had the best response, regardless of size, months of treatment or drug holidays. This may reflect the increased proliferative activity associated with multiple tumors, a finding that has been associated with protein truncating PTCH1 mutations in both sporadic and syndrome-related KCOTs9. Another hypothesis is that higher doses of vismodegib are required to induce shrinkage. Indeed, the dose used in our study was almost half of that used in Goldberg et al’s case report3 and, cyclopamine (another HH pathway inhibitor) has been found to significantly arrest the growth of KCOT cells in a dose-dependent manner in vitro10. However, since all our patients received the same FDA-approved dose of vismodegib, we were not able to assess any dose-dependent response.
In one subject, there was no KCOT recurrence 9 months after drug cessation. In fact, there was further size reduction, albeit minimal, of the mandibular tumor. Interestingly, this subject had recurrence of only 1 BCC in the same time period, a much lower recurrence rate than previously reported8. The incidence of KCOT recurrence in reported series varies from 0%- 62%, with a higher recurrence rate in BCNS-related tumors (over 80%) as compared to sporadic tumors11. This variability could relate to the diverse nature of the cases reported, the different treatment protocols used and variations in post-treatment monitoring. The management of KCOTs remains controversial. Conservative therapy, such as simple enucleation or marsupialization has a recurrence rate of 50% compared to a lower rate following aggressive complete resection12. Recurrence, which typically develops within 5 years of treatment, is thought to occur due to incomplete removal of the original cysts, the presence of microscopic satellite cysts or new cyst development in adjacent areas13. Relapse has been reported even after 41 years14, necessitating long-term follow-up, particularly as these tumors have locally aggressive behavior and, rarely, malignant potential (primary intraosseous squamous cell carcinoma)4.
Limitations of this study include possible selection bias as only a subset of subjects elected to undergo imaging, selecting for patients with recent/symptomatic KCOTs. Most of the 41 clinical trial subjects reported previous surgical treatment for KCOTs. We studied only patients with BCNS, thus limiting the generalizability of the results to syndrome-related KCOTs. However, since 30% of sporadic KCOTs harbor PTCH1 mutation4, vismodegib could be effective for a significant percentage of sporadic tumors. In addition, analysis of KCOT tissue to assess for PTCH1 mutations could explain some of the variability in response. Strengths include the availability of pre and post-treatment MRI images for KCOT size analysis, as well as the largest number to date of patients with KCOTs treated with vismodegib. We chose to image with MRI instead of dental radiographs in order to improve KCOT measurement and to avoid exposure to ionizing radiation, which can induce BCCs in BCNS patients15.
In conclusion, our findings confirm functional involvement of the HH pathway in KCOTs and illustrate the usefulness of treatment with molecularly targeted drugs like vismodegib. This is particularly important for patients with multiple KCOTs, who can be left with facial disfigurement and speech impediment following multiple surgical procedures 3. Vismodegib offers a non-surgical treatment option, potentially revolutionizing management of KCOTs in BCNS. It is difficult to predict which patients develop KCOTs due to variable expression in BCNS; however, MRI may be used for screening during the initial diagnostic work-up and for those with jaw symptoms. Further studies into optimal maintenance regimes of vismodegib for KCOT treatment are required. An ongoing clinical trial (NCT01556009) assessing its use as maintenance therapy for BCCs may yield more information.
Acknowledgments
Funding: Supported by Genentech, a Clinical and Translational Science Award from the National Institutes of Health (UL1RR02413), grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1K23AR056736 to Dr. Tang and 5P30AR044535-11 to Dr. Bickers) and the National Cancer Institute (R01CA109584 to Dr. Epstein), a Damon Runyon Cancer Research Foundation Clinical Investigator Award (CI-54-11 to Dr. Tang) and funding from the Swim Across America Foundation and the Michael J, Rainen Family Foundation.
Footnotes
Financial disclosures
Relationships relevant to this manuscript: None reported.
All other relationships: JYT and EE are consultants for Genentech. EE is also consultant for Novartis and owns stock options in Curis and Infinity. The authors disclose no other potential conflicts of interest.
Author Contributions: Dr(s) Ally, Tang, Joseph, Mackay-Wiggan, Bickers and Epstein had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Tang and Epstein. Acquisition of data: Ally, Tang, Joseph, Thompson, Lindgren, Raphael, Ulerio, Chanana, Mackay-Wiggan, Bickers and Epstein. Analysis and interpretation of data: Ally, Tang, Joseph and Epstein. Drafting of the manuscript: Ally, Tang and Epstein. Critical revision of the manuscript for important intellectual content: Ally, Tang, Joseph, Mackay-Wiggan, Bickers and Epstein. Statistical analysis: Ally and Tang. Obtained funding: Tang, Bickers and Epstein. Administrative, technical, or material support: Ally, Tang and Epstein. Study supervision: Tang and Epstein.
References
- 1.Gorlin RJ. Nevoid basal-cell carcinoma syndrome. Medicine. 1987 Mar;66(2):98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ozkan A, Bayar GR, Altug HA, Sencimen M, Senel B. Management of keratocystic odontogenic tumour with marsupialisation, enucleation and Carnoy’s solution application: a case report. Oral health and dental management. 2012 Jun;11(2):69–73. [PubMed] [Google Scholar]
- 3.Goldberg LH, Landau JM, Moody MN, Kazakevich N, Holzer AM, Myers A. Resolution of odontogenic keratocysts of the jaw in basal cell nevus syndrome with GDC-0449. Archives of dermatology. 2011 Jul;147(7):839–841. doi: 10.1001/archdermatol.2011.50. [DOI] [PubMed] [Google Scholar]
- 4.Li TJ. The odontogenic keratocyst: a cyst, or a cystic neoplasm? Journal of dental research. 2011 Feb;90(2):133–142. doi: 10.1177/0022034510379016. [DOI] [PubMed] [Google Scholar]
- 5.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nature reviews. Cancer. 2008 Oct;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grachtchouk M, Liu J, Wang A, et al. Odontogenic keratocysts arise from quiescent epithelial rests and are associated with deregulated hedgehog signaling in mice and humans. The American journal of pathology. 2006 Sep;169(3):806–814. doi: 10.2353/ajpath.2006.060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimi K, Ohki K, Kumamoto H, et al. Immunohistochemical and genetic analysis of mandibular cysts in heterozygous ptc knockout mice. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2003 Feb;32(2):108–113. doi: 10.1034/j.1600-0714.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 8.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. The New England journal of medicine. 2012 Jun 7;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan S, Li TJ. PTCH1 mutations in odontogenic keratocysts: are they related to epithelial cell proliferation? Oral oncology. 2009 Oct;45(10):861–865. doi: 10.1016/j.oraloncology.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Ren C, Amm HM, DeVilliers P, et al. Targeting the sonic hedgehog pathway in keratocystic odontogenic tumor. The Journal of biological chemistry. 2012 Aug 3;287(32):27117–27125. doi: 10.1074/jbc.M112.367680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madras J, Lapointe H. Keratocystic odontogenic tumour: reclassification of the odontogenic keratocyst from cyst to tumour. Journal (Canadian Dental Association) 2008 Mar;74(2):165–165h. [PubMed] [Google Scholar]
- 12.Kaczmarzyk T, Mojsa I, Stypulkowska J. A systematic review of the recurrence rate for keratocystic odontogenic tumour in relation to treatment modalities. International journal of oral and maxillofacial surgery. 2012 Jun;41(6):756–767. doi: 10.1016/j.ijom.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Morgan TA, Burton CC, Qian F. A retrospective review of treatment of the odontogenic keratocyst. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2005 May;63(5):635–639. doi: 10.1016/j.joms.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Yagyuu T, Kirita T, Sasahira T, Moriwaka Y, Yamamoto K, Kuniyasu H. Recurrence of keratocystic odontogenic tumor: clinicopathological features and immunohistochemical study of the Hedgehog signaling pathway. Pathobiology: journal of immunopathology, molecular and cellular biology. 2008;75(3):171–176. doi: 10.1159/000124977. [DOI] [PubMed] [Google Scholar]
- 15.Epstein E., Jr Genetic determinants of basal cell carcinoma risk. Medical and pediatric oncology. 2001 May;36(5):555–558. doi: 10.1002/mpo.1129. [DOI] [PubMed] [Google Scholar]