Abstract
Fragile X syndrome (FXS) is caused by mutations in the fragile X mental retardation 1 (FMR1) gene. Most FXS cases occur due to the expansion of the CGG trinucleotide repeats in the 5′ untranslated region (UTR) of FMR1, which leads to hypermethylation and in turn silences the expression of FMRP (fragile X mental retardation protein). Numerous studies have demonstrated that FMRP interacts with both coding and non-coding RNAs and represses protein synthesis at dendritic and synaptic locations. In the absence of FMRP, the basal protein translation is enhanced and not responsive to neuronal stimulation. The altered protein translation may contribute to functional abnormalities in certain aspects of synaptic plasticity and intracellular signaling triggered by Gq-coupled receptors. This review focuses on the current understanding of FMRP function and potential therapeutic strategies that are mainly based on the manipulation of FMRP targets and knowledge gained from FXS pathophysiology.
Keywords: Fragile X syndrome, FMR1, FMRP, Gq-coupled receptors, LTD, LTP, mouse model, RNA-binding protein, translation, therapeutic development
Introduction
FXS is Caused by Mutations in FMR1
Fragile X syndrome (FXS), also referred to as Martin-Bell syndrome or Escalante’s syndrome, is the most common form of inherited intellectual disability (or mental retardation) and autism [1,2]. The incidence of FXS in males is approximately 1 in 2500 to 5000 and in females is 1 in 4000 to 6000 [2]. The cytogenetic discovery of the “fragile site” on the X chromosome in patients [3] and the higher incidence in males strongly suggest FXS as a genetic disease. Positional cloning definitively demonstrates the link between mutations in the fragile X mental retardation 1 (FMR1) gene, whose chromosome locus is at the Xq27.3 fragile site, and FXS [4]. Most FXS patients have a significant expansion of the CGG trinucleotide repeats in the 5′ untranslated region (UTR) of the FMR1 gene. While healthy individuals have 5 to 45 CGG repeats (commonly 29-30 repeats), the affected individuals with full mutation normally have more than 200 repeats [5,6]. A few cases with missense mutations and deletions in the FMR1 gene have also been reported [7-9].
Expansion of the CGG repeats correlates with the significant reduction or lack of expression of the FMR1 gene product FMRP (fragile X mental retardation protein). It has been demonstrated that the altered FMRP expression in FXS patients with >200 CGG repeats may be mediated by different mechanisms. Some studies show that a high number of the CGG repeats may facilitate hypermethylation on the cytosine residues in the proximal regions of FMR1, including the promoter (i.e., about 250 bp to 1 kb upstream of the CGG repeats), leading to transcriptional silencing and consequently lack of protein translation [10,11]. Interestingly, in some alleles with >200 CGG repeats, there is only partial or no increase in methylation [12]. Cells from FXS patients that lack DNA methylation in the FMR1 promoter exhibit normal or even higher levels of FMR1 transcript [13,12]. Nevertheless, the level of FMRP is significantly reduced in FXS samples as compared to the samples from unaffected individuals [12,14], indicating that the expanded CGG repeats in the 5′ UTR may also affect translation efficiency [15]. Other mechanisms posit that full mutations in CGG repeats affect histone modification (including acetylation and methylation) [16,17] and may in turn suppress the activity of the FMR1 promoter.
Animal Models of FXS
The development of valid animal models has been crucial for understanding FXS etiology, the function of FMRP, and has been invaluable in developing potential therapeutics for FXS. The main animal models of FXS have been generated with mouse [18], fruit fly [19,20], and zebrafish [21], in which the genetic ortholog of human FMR1 is deleted. In another mouse model, the wild type Fmr1 allele was mutated to harbor an isoleucine to asparagine mutation (I304N, corresponding to the I367N mutation in a rare FXS patient) [22,7]. It is important to note that the mouse model with an engineered expansion in CGG repeats does not show hypermethylation and lack of FMRP expression [23]. Thus, animal models with perfect construct validity are not available. Stem cells from FXS patients show FMR1 silencing due to DNA hypermethylation upon differentiation [17], and can be used for drug screening and preliminary examination of the gene reactivation therapies [24,25].
Behavioral and physiological examinations have demonstrated that the current animal models show robust if not complete face validity of FXS. Some of the therapeutic strategies, which attenuate certain FXS-related symptoms in the animal models, have now been extended to human clinical trials, indicating reasonable predictive validity. FXS is characterized by mild to severe intellectual disability, susceptibility to seizures, hyperactivity, hypersensitivity to sensory stimuli, and autistic traits such as social anxiety, attention deficit, hand biting or flapping (repetitive behavior), and poor eye contact. Physical manifestations include long facial features with protruding ears, soft skin, connective tissue problems, and large testicles (macroorchidism). Many of these symptoms are recapitulated in the Fmr1 knockout (KO) mouse (Table 1). Fmr1 KO mice show cognitive deficits when examined by Morris water maze ([26,27] but also see [28]), passive avoidance [29-31], contextual fear conditioning ([28] but also see [32]), and object recognition [33,34]. Susceptibility to seizures in Fmr1 KO mice is implicated by wild-running and onset of seizure after receiving a high intensity siren (e.g. 125 dB at 1800-6300 Hz) [35,36]. In addition to audiogenic seizures (AGS), Fmr1 KO mice also show enhanced limbic epileptogenesis and mossy fiber sprouting following a kindling paradigm [37]. Furthermore, electrophysiological studies have identified prolonged epileptiform discharges in the Fmr1 KO hippocampus [38]. Fmr1 KO mice are hyperactive and have more locomotor movement in the open field test [30]. They also show more entries to and spend more time in the center area of the open filed arena [30,39], indicating less anxiety (in contrast to the human FXS phenotype). However, in a modified open field chamber surrounded with mirrored walls, Fmr1 KO mice avoid the center area [40]. Interestingly, independent groups have found that Fmr1 KO mice show more [41], normal [42], or less anxiety [43] in the elevated plus maze test. Hyperarousal and sensorimotor gating phenotypes have been examined by acoustic startle responses and prepulse inhibition (PPI), respectively. While some studies show that low intensity white noise (at 80 dB) elicits higher startle responses but high intensity stimuli (at 120 dB) cause less startle in Fmr1 KO mice [42,44], other studies demonstrate that deletion of Fmr1 gene in mouse causes no change or lower startle in response to different levels of auditory stimuli [45,46]. Reduced PPI (a symptom observed in human FXS patients) [47] is seen in some investigations using Fmr1 KO mice [48,49], while other reports have described increased PPI [35,42,45,47,46]. Autism-related symptoms are also detected in Fmr1 mutant mice [46]. Fmr1 KO mice show less social dominance than wild type animals in the social dominance tube test [40,50]. Fmr1 mutants are less interested in social novelty and social interaction [46,43,51,33]. Defective communication (tested by ultrasonic vocalization)[52] and repetitive behavior (tested by marble burying) [53] are also detected in Fmr1 KO mice [54-56]. FXS model mice harboring the I304N mutation exhibit hyperactivity, decreased acoustic startle response, repetitive behavior, and audiogenic seizures [22]. In addition to the neurological disorders, both Fmr1 KO and I304N mutant mice show enlarged testes (i.e., macroorchidism) [18,22]. Furthermore, increased spine density and immature spines are observed from postmortem FXS samples [57,58], and such cellular abnormalities are detected in different brain regions of Fmr1 KO mice [31,33,59,58] as well as in cultured mutant hippocampal neurons [49].
Table 1.
Human FXS traits that are recapitulated in Fmr1 knockout mice.
| FXS symptoms in human | FXS-related behavior in Fmr1 KO mice |
|---|---|
| Intellectual disability | Defective spatial learning and memory [26,27] Defective passive avoidance memory [30,29] Defective contextual memory [28] Defective recognition memory [33,34] |
| Susceptibility to seizures | Audiogenic seizure [35,36] Enhanced limbic epileptogenesis [37] Prolonged epileptiform discharges [38] |
| Hyperactivity | Increased locomotor movement In open field test [30,43] |
| Hyperarousal | Higher acoustic startle responses to low intensity stimuli [42,44] |
| Abnormal sensorimotor gating (reduced PPI) |
Variable phenotypes in PPI [48,49,35,42,45,47,46] |
| Social anxiety Defective social interaction |
Deficits in social dominance [40,50] Decreased interests in social novelty and social interaction [46,43,51,33] Defective communication (tested by ultrasonic vocalization) [55] |
| Perseveration/repetitive behavior | Increased marble burying [56,22] |
| Macroorchidism | Enlarged testes [18,22] |
| Higher density of immature spine | Higher density of immature spine [31,33,59,58,49] |
In addition to vertebrate models, Drosophila (fruit fly) has been successfully used to study FXS. Flies with mutations in dfmr1, whose gene product shows similar function to that of human FMRP [60], show altered synaptic structure [20], altered social interaction [61], impaired circadian rhythms [61], and defective cognitive function [62]. Additionally, dfmr1 mutants have been used to validate therapeutic efficacy [62], understand signaling dysregulation in FXS, and screen potential pharmacological compounds for FXS therapy [63].
Although these animal models do not recapitulate all FXS symptoms and inconsistent phenotypes have been reported, it is evident that the current animal models do show reasonable face validity. The inconsistent behavioral phenotypes observed with Fmr1 KO mice may be due to differences in the experimental protocol, age [64], animal handling (or environmental factors such as different housing facilities and stress [65]), and genetic background [26,46,44,28]. Here, we would like to suggest that these inconsistent observations with animal models might reasonably reflect the fact that human FXS patients do not necessarily display a full spectrum of the symptoms. Importantly, differences in genetic background and environmental factors possibly contribute to the fact that FXS patients do not respond equally to behavioral and medical treatment [66,67].
Role of FMRP in mRNA metabolism
RNA Binding Activity of FMRP
FMRP is expressed in many tissues, but is most abundant in the brain and in the testis. In addition to its expression in the neuronal cell body, FMRP is also detected at dendrites and synapses [68]. Sequence analysis of FMRP reveals several RNA binding domains, which mediate FMRP-RNA interaction [69,70], implicating its function in regulating RNA metabolism. Cellular fractionation experiments demonstrate that FMRP co-sediments with actively translating polyribosomes [71-73], further suggesting its role in regulating mRNA translation.
Among the three canonical RNA binding domains, the two centrally localized hnRNP K-homology KH domains bind to the “kissing complex” tertiary motifs in RNA. The RGG (arginine-glycine-glycine) box is located close to the C terminus and binds to the G-quartet structures in RNA. The I367N missense mutation discovered in a human patient with severe FXS symptoms maps to the RNA binding pocket of the KH2 domain [69]. FMRP with the I367N mutation fails to bind to RNA [69] and polyribosomes [72]. The endogenous I304N-FMRP in mutant mice also does not show robust association with polyribosomes [22]. This suggests that the loss of RNA-binding/translation-regulating function of FMRP may be causal for the phenotypes in FXS.
RNA selection experiments in vitro have revealed that the KH2 domain of FMRP binds to an RNA complex called loop-loop pseudoknot or “kissing complex”. This binding activity is abolished in I304N-FMRP [74]. Further, RNA containing the “kissing complex” but not G-quartet decreases the association of FMRP with polyribosomes [74], suggesting that the “kissing complex” mimics the site that FMRP uses to regulate translation of its target mRNA. However, it is important to note that the kissing complex structure has not been yet convincingly identified in endogenous mRNAs.
The C- terminal RGG box has been found to bind to G-quadruplex RNA secondary structures in vitro [75]. Several FMRP target mRNAs (such as Fmr1, Map1b, and Sema3f) possess the predicted G-quadruplex structures, and in vitro biochemical examinations have confirmed their binding to FMRP [1]. A new structure SoSLIP (Sod1 stem loops) in Sod1 (superoxide dismutase 1) mRNA may also interact with FMRP via the RGG domain [76].
In addition to the RNA binding domains, FMRP possesses two other regions (i.e., nuclear localization signal and nuclear export signal) that enable it to shuttle between the cytoplasm and the nucleus [77]. It is postulated that FMRP may pick up its mRNA targets in the nucleus and transport them to the dendrites, where the local protein synthesis is regulated in an activity-dependent manner. It is shown that some protein-protein interaction domains may also exist to mediate FMRP association with proteins involved in translational regulation [78] and the RNA-induced silencing complex (RISC) [79,80].
RNA Targets of FMRP
It is estimated that FMRP binds to roughly 4% of the mRNAs in the brain [1]. An earlier genome-wide microarray study identified 432 FMRP-interacting mRNAs [81]. A recent study using UV cross-linking to covalently link FMRP to mRNA followed by stringent coimmunoprecipitation and high throughput sequencing identified 842 mRNA targets of FMRP in postnatal 11-25 day mouse brain. Many of the targets are involved in synaptic function, cell signaling, neural development, and autism. Most of the FMRP binding sites are in the coding region but not in the 5′ or 3′ UTR of the mRNA targets and no specific sequence or structural feature has been identified as preferred FMRP-binding motifs [82].
Another recent study used 4-thiouridine (4SU) photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) in HEK293 cells expressing HA-tagged FMRP. Complementary DNA libraries were generated and sequenced. By analyzing the resulting reads, the study identified around 6000 mRNAs that are bound to FMRP. More than 95% of the binding sites were either in the coding region or the 3′ UTR. Unlike the previous studies, this study identified only slightly more binding sites in the coding region than in the 3′ UTR. Further, two RNA-recognition elements (RRE) were identified as ACUK and WGGA (where K is G or U and W is A or U), which were found to occur in more than 50% of the binding sites [83]. Consistent with the study by Darnell et al. [82], many of the identified FMRP targets are also involved in autism spectrum disorder and synaptic signaling. It remains unclear whether these FMRP targets are functionally regulated by FMRP and contribute to cellular and behavioral abnormalities in FXS. Among all the identified FMRP targets, only a handful of them are verified by independent methods and functional studies (see Table 2).
Table 2.
FMRP targets that may be functionally involved in FMRP-regulated translation and FXS-related phenotypes
| Gene symbol |
Protein name |
Validation method |
Changes in FXS | Response to mGluR1/5 stimulation |
Therapeutic effects | Inhibitors |
|---|---|---|---|---|---|---|
| Agap2 | PIKE | HT [82] |
 protein [133] protein [133] |
|||
| App | APP | HT [82,83], CoIP [127] |
 protein [126] protein [126] |
 translation translation[127] |
AGS, hyperactivity, spines, mGluR-LTD [126] |
|
| Arc | Arc | HT [82,83], CoIP [168] |
 protein [168] protein [168] protein [159] protein [159] |
 translation translation[125,100] |
mGluR-LTD [125] | |
| Camk2a | CaMKIIα | HT [82], CoIP [168,149] |
 protein [168,92] protein [168,92] |
 translation translation[89,169,92] |
KN62, KN93 | |
| Cyfip2 | CYFIP2 | HT [82,83] |
 protein [170] protein [170] |
|||
| Dlg4 | PSD-95 | HT [82], miR- 125a [85], G- quadruplex [91] |
 protein [91] protein [91] protein proteindegradation [171]  protein [159] protein [159] |
 translation translation[85,91] |
||
| EF1a | EF1α | CoIP [172] |
 protein [172] protein [172] |
 translation translation[173] |
||
| Fmr1 | FMRP | In vitro [174] |
 or lack of or lack ofexpression |
 translation translationfollowed by degradation [92,98,99,68] |
||
| Gabbr1 | GABA- B1 |
HT [82] | Protein synthesis, AMPAR internalization, abnormal spines, AGS [155,156] |
STX209, baclofen |
||
| Gabrd | GABA-Aδ | In vitro [175] |
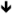 protein [150] protein [150] |
AGS [153], hyperactivity and PPI [154] |
Acamprosate, alphaxalone, gaboxadol, diazepam |
|
| Gria1 | AMPA-1 | HT, Co-IP [149] |
 protein [149,158] protein [149,158]no change [136] |
 translation translation[149] |
||
| Grin1 | NR1 | HT [82], CoIP [158] |
 protein [158] protein [158]no change [136] 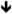 protein [159] protein [159] |
Some clinical efficacy in human patients [160,176] |
Memantine, Acamprosate |
|
| Grin2a | NR2A | miR-125b [84] | no change [136]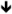 protein [159] protein [159] |
Some clinical efficacy in human patients [160,176] |
Memantine, Acamprosate |
|
| Grin2b | NR2B | HT [82], CoIP [158] |
 protein [158] protein [158]no change [136] 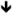 protein [159] protein [159] |
Some clinical efficacy in human patients [160,176] |
Memantine, Acamprosate |
|
| Grm5 | mGluR5 | HT [82] |
 protein [167] protein [167]no change [136] |
mGluR-LTD [106,31], AMPA receptor internalization [101], protein synthesis [106,31,89,90], dendritic spines [31,49,106], visual cortical plasticity [31], AGS [106,31,39], hyperactivity [39], PPI [49,48], social interaction [105], repetitive behavior [54], body weight [31], macroorchidism [106], hypersensitivity [106]. |
MPEP, Fenobam, AFQ056, CTEP |
|
| Gsk3b | GSK3β | HT [82,83] |
 activity [144] activity [144] |
AGS, hyperactivity, passive avoidance memory, contextual, and cued fear memory, social preference and social anxiety, defective neurogenesis, spine abnormality [144-146,51,43] |
Lithium, SB216763 |
|
| Hcn1 | HCN1 |
 protein [177] protein [177] |
||||
| Homer 1 | Homer1 | HT [83] | Unchanged [137] | Restored mGluR5 signaling, AGS, anxiety phenotype in open field [137] |
||
| Kcnc1 | Kv3.1; Kv3.2 |
In vitro [75] |
 protein [147] protein [147] |
|||
| Kcnd2 | Kv4.2 | HT [82], in vitro [93] |
 protein [93], protein [93],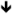 protein [148] protein [148] |
LTP [93] | Heteropodatoxin HpTx2 |
|
| Map1b | MAP1B | HT [82,83] In vitro [88,178] |
 protein protein[168,88,92] |
 translation translation[179,92] |
||
| Mapk1 | MAPK1 or ERK2 |
HT [82,83] |
 phosphorylation phosphorylation[92,139] |
 activation activation[92,89] |
Protein synthesis [89,139], AGS [89,139] |
U0126, SL 327, lovastatin |
| Mmp9 | MMP9 |
 protein [166,41] protein [166,41] |
Spine abnormality, anxiety phenotype in elevate plus maze, AGS, hyperactivity, communication [41,56,164] |
Minocycline | ||
| Mtor | mTOR | HT [82,83] |
 phosphorylation phosphorylation activity [133] activity [133] |
 activation activation[133] |
AGS [89] | Rapamycin |
| Pak1 | PAK1 | HT [82,83] | Spine abnormality, LTP, hyperactivity, repetitive behavior, anxiety, trace fear conditioning, AGS [59,140] |
FRAX486 | ||
| Pik3cb | PI3K P110β catalytic subunit |
HT [83], |
 protein protein activity [133,90] activity [133,90] |
 translation translation activation [90] activation [90] |
Protein synthesis, spine abnormality, AMPA receptor internalization [90] |
LY294002 Wortmannin |
| Pten | PTEN | HT [82,83] |
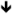 phosphorylation phosphorylation[133] |
|||
| Ptpn5 | STEP | HT [82] |
 protein [50] protein [50] |
 translation translation[180] |
AGS, social interaction, social anxiety [50] |
|
| Rgs5 | RGS5 | In vitro [175] | ||||
| Rps6kb1 | S6K1 | HT [83] |
 phosphorylation phosphorylation[33] |
 activation activation[133] |
Protein synthesis, mGluR-LTD, dendritic spines, novel object recognition, social interaction [33] |
|
| Sapap3/4 | SAPAP3/4 |
 protein [158] protein [158] protein [159] protein [159] |
||||
| Sema3f | SEMA3F | In vitro [181] | ||||
| Shank1 | SHANK1 | HT [82,83], 3’UTR [158] |
 protein [158] protein [158] |
 translation translation[158] |
||
| Shank3 | SHANK3 | HT [82] |
 protein [158] protein [158] |
|||
| Sod1 | SOD1 | In vitro [76] |
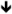 protein [76] protein [76] |
|||
| Tsc2 | TSC2 | HT [82,83] | mGluR-LTD, memory deficit [138] |
HT: high through-put screening; CoIP: coimmunoprecipitation; AGS: audiogenic seizure; PPI: prepulse inhibition;  : increase;
: increase;  : decrease. Therapeutic effects are observed in Fmr1 KO mice when the corresponding FMRP target is genetically reduced or pharmacologically inhibited.
: decrease. Therapeutic effects are observed in Fmr1 KO mice when the corresponding FMRP target is genetically reduced or pharmacologically inhibited.
Interestingly, FMRP also interacts with non-coding microRNAs (miRNAs) [84,79]. Among them, miR-125a and 125b can cooperate with FMRP to regulate the translation of validated FMRP targets PSD-95 and NR2A, respectively [84,85]. Further, overexpression of miR-125b results in longer and thinner spine [84], which is a cellular phenotype of FXS [58]. One functional significance of FMRP-miRNA interaction is that FMRP may regulate translation through coordination with miRNAs that binds to the 3′UTR of FMRP target mRNAs.
Function of FMRP in Regulating mRNA Metabolism
The existence of RNA binding domains in FMRP suggests its function in regulating RNA metabolism. Although some studies have demonstrated that FMRP regulates mRNA transport [86] and stability [87], it is well accepted that FMRP mainly suppresses the translation of its target mRNAs. First, in addition to mRNAs, FMRP also interacts with proteins such as CYFIP1 that regulate translation [78]. Second, FMRP has been found to co-sediment with actively translating polyribosomes particularly in synaptic preparations [71,72]. Many FMRP target mRNAs such as Arc/Arg3.1, CaMKIIα, and PSD-95 localize in dendrites and in dendritic spines. In Fmr1 KO neurons, MAP1B [88] and PSD-95 mRNAs [85] are more enriched in the actively translating polyribosomes rather than in the translationally quiescent messenger ribonucleoprotein (mRNP) complexes. Consequently, the expression of many FMRP targets is up-regulated in the absence of FMRP (see Table 2). It is thus believed that FMRP controls local protein synthesis at synapses by acting as a translational repressor and the loss of such translation control leads to many of the deficits seen in FXS. In a rare case, FMRP binding to Sod1 mRNA positively regulates its translation [76]. It is important to point out that the protein levels of many FMRP targets remain unchanged or even reduced in Fmr1 KO mice (see Table 2). To fully understand how the expression level of FMRP targets is controlled, investigations on compensatory mechanisms and secondary effects are needed.
Considering that FMRP interacts with the translation machinery and some of the FMRP targets (such as S6K1, PI3K, PIKE, and ERK1/2) can indirectly stimulate translation, it is understandable that the basal level of global protein synthesis is increased in Fmr1 KO brain and in cell cultures derived from FXS patients [31,89,90]. In addition to its possible involvement in cap-dependent initiation and elongation steps [82], FMRP may also impede translation through coordination with miRNAs that bind to both FMRP and the 3′UTR of FMRP targets. The interaction between FMRP and components of the RISC (such as AGO1/2 and Dicer) may provide another layer of control on RNA metabolism. For example, the expression of FMRP targets NR2A is regulated through the coordination of FMRP and miR-125, and knockdown of AGO1 increases NR2A expression [84].
As the basal translation is elevated in FXS, activity-dependent up-regulation of translation is dampened in Fmr1 KO neurons. As opposed to wild type neurons, Fmr1 KO neurons do not show increased translation following the activation of mGluR1/5 [91,85,92], NMDAR [93], and upon membrane depolarization [94]. Consequently, certain aspects of mGluR1/5- and NMDAR-dependent synaptic plasticity (such as long-term depression and long-term potentiation) are altered in Fmr1 KO mice [95,96].
Role of FMRP in Regulating Synaptic Function: the mGluR theory and beyond
Exaggerated mGluR1/5-LTD in Fmr1 KO Mice
Synaptic protein synthesis and spine development are altered in FXS, thus it is hypothesized that FMRP regulates synaptic function and plasticity. Huber et al. showed that synaptic long-term depression (LTD) triggered by mGluR1/5 agonist DHPG is enhanced at the CA1 synapses in Fmr1 KO mice [97]. This seminal study builds the foundation of the “mGluR theory”, which highlights that mGluR1/5 signaling is exaggerated in FXS, and explains how FMRP function is connected to mGluR1/5-mediated synaptic responses [95]. Multiple lines of evidence support the functional link between FMRP and mGluR1/5-LTD. First, mGluR1/5-LTD depends on new protein synthesis. The up-regulation of protein translation following mGluR1/5 activation may be related to the dynamic changes in FMRP. The activation of mGluR1/5 triggers rapid increase in FMRP translation [68] but it is followed by FMRP degradation and de-phosphorylation, which may cause un-repression on the translation of certain synaptic molecules [92,98,99]. Indeed, the translation of several FMRP targets is up-regulated following DHPG stimulation (see Table 2). Second, the expression of mGluR1/5-LTD requires AMPA receptor internalization [100]. DHPG-stimulated receptor internalization depends on the translation of certain “LTD” proteins such as Arc and STEP. In the absence of FMRP, the translation of these “LTD” proteins would be less suppressed. The elevated expression of such “LTD” proteins in Fmr1 KO neurons consequently facilitates AMPAR internalization [101] leading to enhanced synaptic depression. It is evident that other mGluR1/5-mediated synaptic functions are also regulated by FMRP. The activation of mGluR1/5 stimulates spine growth [102] and there are more immature spines in FXS neurons [57]. Collectively, mGluR1/5-mediated synaptic function and cellular changes are regulated by FMRP and exaggerated in FXS.
Effects of mGluR inhibition on FXS
Based on the mGluR theory, it is postulated that dampening mGluR1/5 activity may be therapeutic for FXS. Indeed, administration of mGluR5 antagonists to animal models of FXS has shown promising therapeutic effects. Specifically, administration of mGluR5 antagonist MPEP attenuates the elevated protein translation [89,90], the enhanced AMPAR internalization [101], the abnormal spine morphology and PPI ([49] but also see [54]), AGS, hyperactivity [39], and repetitive behavior [54] in Fmr1 KO mice. The use of fenobam, a potent negative allosteric modulator of mGluR5, promisingly attenuates spine abnormality [49] and impairments in procedure memory and avoidance discrimination [103]. Genetic approaches have further demonstrated the therapeutic role of mGluR1/5 in FXS. Double mutant mice (heterozygous for mGluR5 and hemizygous for Fmr1) show normal basal translation, spine density, no significant AGS, and normal fear memory extinction. However, macroorchidism is not rescued [31]. Intriguingly, a more recent study found that the mGluR5/Fmr1 double mutants still show AGS, repetitive behavior, and abnormalities in anxiety and memory [45]. Genetic reduction of mGluR1 also fails to correct the major symptoms of FXS [45].
Encouraged by the effects of mGluR5 inhibition, an open label single dose fenobam trial has been performed with 12 adult patients. While there is mild improvement in PPI, no significant effect is observed for CPT (continuous performance test) [104]. Because no adverse reaction was identified, this study warrants further investigation on the therapeutic effect of fenobam. In addition to the available mGluR5 antagonists, new compounds are being developed and tested in clinical trials. These include AFQ056 and CTEP that rescue the enhanced mGluR1/5-LTD, the enhanced protein synthesis, spine morphology, cognitive deficits, hypersensitivity, PPI, and social interaction phenotypes in Fmr1 KO mice [48,105,106]. In a phase II double blind placebo-controlled crossover trial with AFQ056 on 30 adult males for 28 days, administration of AFQ056 resulted in improvement of maladaptive behaviors only in a selective subpopulation of FXS patients with full promoter methylation [67]. Better understanding on the therapeutic value of mGluR5 antagonists may need larger scale trials. Furthermore, the development and clinical trials of other mGluR5 negative allosteric modulators such as STX107 (Seaside therapeutics) and Ro4917523 (Hoffman La-Roche) should aid in understanding the efficacy of mGluR antagonists for the treatment of FXS.
mGluR-Independent Mechanisms
Inhibition of mGluR1/5 rescues some but not all FXS-related symptoms [45] and shows therapeutic effects on a sub-population of FXS patients [67]. This suggests the existence of mGluR-independent mechanisms. Similar to mGluR1/5, activation of another group of Gq-coupled receptors, such as Gq-coupled muscarinic acetylcholine receptors (Gq-mAchR), also triggers AMPAR internalization and translation-dependent LTD. Gq-mAchR-LTD is significantly exaggerated in Fmr1 KO mice [107]. Treating Fmr1 KO mice with an inhibitor of M1 (one subtype of Gq-mAchR) dampens AGS [108]. It remains to be determined whether simultaneous blocking mGluR1/5 and Gq-mAchR offers more robust correction of FXS traits. Moreover, several lines of evidence have demonstrated that alteration of other G-protein coupled receptors is connected to FXS. For example, genetic or pharmacological inhibition of the Gi-coupled muscarinic M4 receptor rescues limited abnormal behaviors in Fmr1 KO mice [109,110]. It was also shown that dopaminergic D1 receptor-mediated AMPAR surface expression and signaling in prefrontal cortex require FMRP, and the D1 receptor agonist SKF81297 attenuates hyperactivity in Fmr1 KO mice [111]. More recently, Costa et al. found a functional cross-talk between 5-HT7 serotonin receptors and mGluR1/5 in Fmr1 KO neurons. Pharmacological activation of 5-HT7 receptor suppresses enhanced mGluR1/5-LTD and AMPAR internalization [112].
In addition to the altered Gq-LTD, abnormal synaptic long-term potentiation (LTP) is also observed in Fmr1 mutants. Studies have shown that FMRP may regulate both the mGluR1/5 and the NMDAR components of LTP. In the wild type visual neocortex, LTP is slightly attenuated by the NMDAR antagonist CPP and severely suppressed by the mGluR1/5 antagonists MPEP and MCPG [113]. The degree of LTP in MPEP-treated slices from wild type mice is similar to that from Fmr1 KO mice without MPEP treatment. These surprising results indicate that the mGluR1/5 function is dampened rather than enhanced in the neocortex of Fmr1 mutants. Further, treating slices from Fmr1 KO mice with MPEP does not correct the LTP deficits. Additionally, Suvrathan et al. found that certain aspects of mGluR1/5-dependent LTP are impaired in the amygdala of Fmr1 KO mice, and application of MPEP fails to correct the impairment [114].
In the hippocampus, LTP induced by the application of glycine depends on both NMDAR and mGluR1/5, and is also impaired in Fmr1 mutants. Interestingly, lower LTP in Fmr1 mutants is slightly dampened by the mGluR1/5 antagonist DL-AP-3 but not by the NMDAR antagonist APV [115]. The involvement of FMRP in regulating NMDAR function has also been suggested by several other studies. Lauterborn et al. reported that LTP induced by weak theta burst stimulation (TBS) at the threshold value (5 burst) is dramatically defective at the CA1 synapses in Fmr1 KO mice [96]. This complements an earlier study documenting that LTP induced by a stronger TBS (10 burst) is normal in the Fmr1 mutant hippocampus [28]. The intensity-dependent LTP phenotypes suggest that the learning and memory deficits in Fmr1 mutants may also depend on the intensity of the behavioral training protocols, and may help explain some inconsistent behavioral results from different labs. Moreover, NMDAR hypofunctions such as lower NMDAR-mediated synaptic transmission and reduced NMDA/AMPA ratio are observed in the dentate gyrus (DG) of Fmr1 mutants [116,117]. Both NMDAR-mediated LTP and LTD at the DG synapses are also impaired in the absence of FMRP [116].
In addition to postsynaptic function, several lines of evidence suggest that FMRP may also regulate presynaptic events. First, FMRP immunoactivity is detected in axons and presynaptic terminals [118]. It is proposed that FMRP may regulate the localization and translation of mRNAs encoding presynaptic proteins [119]. Indeed, proteomic studies have found increases in presynaptic proteins (such as Rab-3A, synapsin-1, and synaptophysin) in Fmr1 KO neurons. Ultrastructural and physiological examinations reveal immature presynaptic terminals and impaired paired pulse facilitation (PPF) at the Schaffer collateral-CA1 synapses of Fmr1 mutants [120]. An independent study failed to observe the PPF deficits, but found that Ca2+ influx in presynaptic neurons and synaptic vesicle recycling are enhanced in Fmr1 KO mutants [121]. Furthermore, presynaptic FMRP may also regulate presynaptic cytoskeleton and the motility of axon growth cone [122]. Interestingly, these altered presynaptic functions in FXS may be mediated through mechanisms independent of translation regulation. A recent study by Deng et al. demonstrates that protein-protein interaction between FMRP and presynaptic BK (the large conductance Ca2+-activated potassium) channels modulates action potential and neurotransmitter release [123]. FMRP also regulates the gating of the Na+-activated potassium channel Slack through direct protein-protein interaction [124].
Effects of Manipulating FMRP Targets on FXS
As the basal level of translation is elevated in Fmr1 KO neurons, it is generally accepted that FMRP suppresses the translation of its target mRNAs. Thus, it is postulated that the elevated expression of certain “key” FMRP targets may be causal for FXS, and dampening such “key” targets may be therapeutic. However, this therapeutic approach is still in its infancy. Only a handful of studies show that suppressing FMRP targets attenuates cellular abnormalities [125,93] and certain (but not all) behavioral phenotypes of FXS [50,41,126]. Most of the verified FMRP targets are involved in synaptic plasticity, neurotransmission, and neuronal signaling. Some targets have a connection to neurological and psychiatric disorders. The therapeutic value of FMRP targets is investigated using a combination of genetic and pharmacological approaches.
Arc (activity-regulated cytoskeleton-associated protein), an FMRP target, is associated with the synaptic cytoskeleton network and regulates AMPA receptor (e.g. GluR1) trafficking [100,125]. Its involvement in FXS is implicated by the observation that the basal level of Arc expression is elevated in Fmr1 KO neurons [98]. Further, Arc translation is rapidly stimulated by the activation of mGluR1/5 and is required for mGluR1/5-LTD [100,125]. Genetic deletion of Arc in wild type and Fmr1 KO mice results in no significant mGluR1/5-LTD [125]. Another FMRP target, APP (amyloid beta precursor protein) [127] is also a structural protein that regulates synaptic function [128] as well as neurodegeneration. While overexpression of APP in Fmr1 KO mice increases seizure susceptibility [129], reduction of APP in Fmr1 KO mice rescues multiple FXS symptoms including AGS, higher density of immature spines, and the enhanced mGluR1/5-LTD [126]. These studies identify a link between FXS and Alzheimer’s disease (AD) [130], and suggest that therapies developed for AD to reduce APP level may be used to treat FXS. Interestingly, Arc expression level is also significantly higher in AD patients, and genetic deletion of Arc reduces amyloid-beta level in a mouse model of AD [131]. The expression level of another FMRP target, STEP (striatal-enriched tyrosine phosphatase), is elevated in Fmr1 KO neurons as well as in AD patients. Strikingly, genetic reduction of STEP rescues certain FXS phenotypes [50] and also reverses cognitive impairment in AD mice [132].
A number of FMRP targets are functionally involved in the PI3K (phosphoinositide 3-kinase) and MAPK (mitogen-activated protein kinase) pathways (see Table 2), which positively regulate ribosomal function and translation in an activity-dependent manner. It is also important to note that the activity of both PI3K and MAPK is required for LTP and LTD. In Fmr1 KO neurons, PI3K activity and the expression of its catalytic subunit p110 are elevated [90,133]. Inhibition of PI3K by LY294002 suppresses the cellular phenotypes of FXS including exaggerated basal translation, GluR1 internalization, and spine density [90]. Increased expression of an up-stream activator of PI3K (i.e. PIKE or PI3K enhancer) and increased activity of a down-stream effector of PI3K (i.e. mTOR or mammalian target of rapamycin) are observed in the hippocampus of Fmr1 KO mice. Furthermore, activity of an up-stream suppressor of PI3K (i.e. PTEN or phosphatase and tensin homolog) is decreased in the hippocampus of Fmr1 KO mice [133]. However, inhibition of mTOR with rapamycin only suppresses mGluR1/5-LTD in wild type [134] but not Fmr1 KO mice [133]. Treating Fmr1 KO mice with rapamycin does not suppress the elevated basal translation, but does dampen AGS [89]. Homer1, another FMRP target, is an adaptor protein that connects mGluR1/5 and the PI3K signaling cascade [135]. Although Homer1 mRNA interacts with FMRP, its expression is unchanged in Fmr1 KO neurons [136]. Intriguingly, mGluR5 associates less with the long Homer isoforms but more with the short Homer1a in Fmr1 KO mice [136,137]. Genetic deletion of Homer1a in Fmr1 KO mouse decreases AGS and center occupancy in the open field test without affecting the enhanced mGluR1/5-LTD and basal translation [137]. As mentioned earlier, some FMRP targets are related to autism. The mRNA of TSC2, which regulates mTOR activity, binds to FMRP. Genetic mutation of Tsc2 is linked to TSC (tuberous sclerosis complex) disease that displays symptoms of autism and mental retardation. Tsc2 heterozygous KO mice show reduced mGluR1/5-LTD and protein synthesis. Genetic reduction of Tsc2 in Fmr1 KO mice normalizes mGluR1/5-LTD and memory deficits to the wild type level [138].
ERK1/2 (extracellular signal-regulated kinase 1/2), a component of the MAPK pathway, is an FMRP target. The activity rather than the expression level of ERK1/2 is elevated in Fmr1 KO mice. Pharmacological inhibition of ERK1/2 reduces AGS, elevated protein translation, and prolonged epileptiform discharges in Fmr1 KO mice [38,89]. Osterweil et al. reported similar therapeutic effects in Fmr1 KO mouse by using lovastatin, (a clinically approved cholesterol-lowering drug) to suppress ERK1/2 activity [139]. Several molecules up- and down-stream of ERK1/2 are also FMRP targets. PAK1 (p21-activated kinase 1) is a positive regulator of ERK1/2 and plays an important role in spine morphology. In addition to its mRNA, PAK1 protein also interacts with FMRP. Expression of dominant negative PAK1 in Fmr1 KO mice rescues the higher spine density, cortical LTP deficits, hyperactivity, repetitive behavior, and impairment in trace fear conditioning [59]. Additionally, significant therapeutic effects can also be observed in Fmr1 KO mice using a potent PAK inhibitor [140].
Although studies on the therapeutic function of ERK1/2 and PI3K have shown some degree of controversy [90,89], the two signaling pathways may converge and co-regulate the activity of some FMRP targets. S6K1 (ribosomal protein S6 kinase 1) is involved in ribosome biogenesis and regulates protein translation; its activity can be up-regulated by ERK1/2- and PI3K-mediated phosphorylation at different residues [141]. Although the expression level of S6K1 is normal in Fmr1 KO neurons, phospho-S6K1 is elevated [133]. Genetic deletion of S6K1 in Fmr1 KO mice rescues the enhanced mGluR1/5-LTD, abnormal spine morphology, and deficits in recognition memory and social interaction. However, some FXS-related phenotypes such as hyperactivity and repetitive behavior are not corrected [33]. GSK3 is another FMRP target whose activity can be down-regulated by PI3K- [142] and ERK1/2-mediated phosphorylation [143]. Although PI3K and ERK1/2 activity are elevated in Fmr1 KO mice, the phosphorylation of both GSK3 and GSK3 is decreased in FXS [144]. Thus GSK3 activity is abnormally higher in Fmr1 KO neurons. Treating Fmr1 KO mice with GSK3 inhibitors lithium and SB-216763 attenuates AGS, hyperactivity, defective cognitive function (such as passive avoidance memory, contextual, and cued fear memory), deficits in social interaction (such as social preference and social anxiety), defective neurogenesis, and abnormal spine morphology in cortical and newborn hippocampal neurons [144-146,51,43]. An open label study with 15 FXS males treated with lithium showed that lithium improves social and maladaptive behavior as well as auditory memory. However, 7 individuals had side effects of polydipsia and polyuria [66].
Another group of FMRP targets includes voltage-gated potassium channels (Kv3.1 and Kv4.2) [75,147,148,93], ligand-gated channels (such as GABA-A, AMPA, and NMDA receptors) [82,84,149], and G protein-coupled GABA-B receptors [82]. Kv4.2 plays important roles in regulating excitability, seizures, and plasticity. Lee et al. found that the Kv4.2 mRNA is localized in the dendrites, and its 3′ UTR binds FMRP and is required for FMRP-dependent translation suppression [93]. Functionally, inhibiting Kv4.2 rescues the defective LTP in Fmr1 KO mice [93]. Intriguingly, Gross et al. found that FMRP facilitates the translation of Kv4.2, whose expression is decreased in Fmr1 KO mouse brain [148]. A couple of studies show evidence that different subunits of GABA-A receptors [150-152] are down-regulated in Fmr1 KO mice. It is not surprising that GABA-A receptor agonists and positive allosteric modulators (such as diazepam and ganaxolone), which are clinically used as anticonvulsants, attenuate AGS [153]. Another GABA system modulator, gaboxadol, reduces hyperactivity and PPI without affecting acoustic startle and cued memory in Fmr1 KO mice [154]. Although GABA-B receptor mRNA is identified as an FMRP target by high-throughput screening [82], its expression level in FXS is not known. However, its functional relevance is demonstrated by the fact that GABA-B agonists baclofen and arbaclofen (STX209) rescue AGS in Fmr1 KO mice [155,156]. Further, arbaclofen treatment reduces the enhanced basal translation, AMPA receptor internalization, and spine density in Fmr1 KO mice [156]. A Phase 2 randomized, double blind, and placebo controlled crossover trial with STX209 in 55 males and 8 females showed that STX209 treatment improves social behavior in all patients [157]. It has been demonstrated that the expression level of NMDAR subunits (i.e. NR1 and NR2B) is increased in the neocortex and hippocampus of Fmr1 KO mice ([158], but also see [136] and [159]). FMRP also suppresses the translation of NR2A [84]. Thus, there is possibly a combination of excessive NMDAR function and deficient GABA function (but also see [116]). Based on this hypothesis, acamprosate, which acts as an NMDAR antagonist and GABA-A agonist, was used in an open-label clinical study. Young FXS patients receiving acamprosate show significant improvement in social behavior and reduction in hyperactivity [160].
Minocycline, an FDA-approved broad-spectrum tetracycline antibiotic, shows some promising therapeutic effects in human FXS patients. In a pilot open label study on 50 FXS individuals, minocycline treatment improved cognition, language and behavior [161]. In another open label study, 20 FXS individuals treated with minocycline showed improvement in irritability and other global behavior tests [162]. A double blind, placebo-controlled, crossover trial with minocycline treatment in FXS children resulted in improvements in anxiety and mood-related behavior [163]. Treating Fmr1 KO neurons and mice with minocycline corrects abnormal spine morphology, anxiety phenotype, defective ultrasonic vocalization, AGS, and hyperactivity [41,56,164]. Possible mechanisms include minocycline-mediated inhibition of ribosome function [165] or MMP9 (matrix metallopeptidase 9), which is an FMRP target whose expression level is elevated in FXS [166,41].
Connecting the mGluR Theory and FMRP Targets
The mGluR theory and FMRP-mediated translation have been fundamental to the development of therapeutic approaches for FXS. It is not clear how the dys-regulated translation of FMRP targets contributes to elevated mGluR1/5 signaling in FXS. A recent study reported that the expression of mGluR5 itself, whose mRNA is identified as an FMRP target through high through-put screening [82], is slightly but significantly elevated in the prefrontal cortex of FXS patients [167]. However, another study has shown that the expression level of mGluR5 is not changed in the forebrain and cerebellum of Fmr1 KO mice [136]. Although, as a major downstream effector of mGluR1/5 signaling, ERK1/2 shows higher activity in Fmr1 KO neurons, its total expression level is not changed [139]. Thus, the changes in ERK1/2 may represent an outcome of the elevated mGluR1/5 signaling rather than having a causal role on the enhanced mGluR5 function. Investigation on the molecules in the PI3K cascade has found that the expression levels of both PIKE and the p110 subunit of PI3K are elevated in the absence of FMRP [90,133]. Considering that PI3K activation can be triggered by mGluR1/5 stimulation, the enhanced expression of these FMRP-targets may contribute to the elevated basal mGluR1/5 activity in FXS.
Future Directions
Since the positional cloning of the FMR1 gene, there have been tremendous advances in understanding the function of FMRP, which have led to rational designs of therapeutic approaches. While new agents are being examined in animal models and clinical trials, successful repurposing the available drugs such as memantine, acamprosate, minocycline, fenobam, baclofen, lithium, and lovastatin could benefit FXS patients without involving the lengthy drug development process. Because FMRP regulates many aspects of neuronal function, simultaneous manipulation of multiple FMRP targets and/or signaling pathways should also deserve significant consideration.
Although the prevailing theory posits that FMRP suppresses translation and mGluR1/5 signaling, significant involvement of FMRP in up-regulating protein synthesis, mGluR1/5-independent synaptic function, and protein-protein interaction have also been identified. It remains to be determined how these new functions of FMRP are relevant to FXS etiology.
Acknowledgements
This work was supported by research grants from the National Institute of Health (MH093445) and FRAXA Research Foundation. I (Hongbing Wang) would like to pay special thanks to Dr. Richard Olsen who provided valuable advice and guidance and shared his enthusiasm in science during my graduate study.
References
- 1.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- 2.Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012;122(12):4314–4322. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krawczun MS, Jenkins EC, Brown WT. Analysis of the fragile-X chromosome: localization and detection of the fragile site in high resolution preparations. Hum Genet. 1985;69(3):209–211. doi: 10.1007/BF00293026. [DOI] [PubMed] [Google Scholar]
- 4.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 5.Peprah E. Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet. 2012;76(2):178–191. doi: 10.1111/j.1469-1809.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, Hirsch B, Jacky P, McDowell GA, Popovich B, Watson M, Wolff DJ. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3(3):200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJI. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3(1):31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- 8.Coffee B, Ikeda M, Budimirovic DB, Hjelm LN, Kaufmann WE, Warren ST. Mosaic FMR1 deletion causes fragile X syndrome and can lead to molecular misdiagnosis: a case report and review of the literature. Am J Med Genet A. 2008;146A(10):1358–1367. doi: 10.1002/ajmg.a.32261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins SC, Bray SM, Suhl JA, Cutler DJ, Coffee B, Zwick ME, Warren ST. Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males. Am J Med Genet A. 2010;152A(10):2512–2520. doi: 10.1002/ajmg.a.33626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 11.Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 12.Pietrobono R, Tabolacci E, Zalfa F, Zito I, Terracciano A, Moscato U, Bagni C, Oostra B, Chiurazzi P, Neri G. Molecular dissection of the events leading to inactivation of the FMR1 gene. Hum Mol Genet. 2005;14(2):267–277. doi: 10.1093/hmg/ddi024. [DOI] [PubMed] [Google Scholar]
- 13.Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet. 2000;94(3):232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Tabolacci E, Moscato U, Zalfa F, Bagni C, Chiurazzi P, Neri G. Epigenetic analysis reveals a euchromatic configuration in the FMR1 unmethylated full mutations. Eur J Hum Genet. 2008;16(12):1487–1498. doi: 10.1038/ejhg.2008.130. [DOI] [PubMed] [Google Scholar]
- 15.Dobson T, Kube E, Timmerman S, Krushel LA. Identifying intrinsic and extrinsic determinants that regulate internal initiation of translation mediated by the FMR1 5− leader. BMC Mol Biol. 2008;9:89. doi: 10.1186/1471-2199-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffee B, Zhang F, Ceman S, Warren ST, Reines D. Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile x syndrome. Am J Hum Genet. 2002;71(4):923–932. doi: 10.1086/342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiges R, Urbach A, Malcov M, Frumkin T, Schwartz T, Amit A, Yaron Y, Eden A, Yanuka O, Benvenisty N, Ben-Yosef D. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell. 2007;1(5):568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78(1):23–33. [PubMed] [Google Scholar]
- 19.Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34(6):961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107(5):591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 21.den Broeder MJ, van der Linde H, Brouwer JR, Oostra BA, Willemsen R, Ketting RF. Generation and characterization of FMR1 knockout zebrafish. PLoS One. 2009;4(11):e7910. doi: 10.1371/journal.pone.0007910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zang JB, Nosyreva ED, Spencer CM, Volk LJ, Musunuru K, Zhong R, Stone EF, Yuva-Paylor LA, Huber KM, Paylor R, Darnell JC, Darnell RB. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genet. 2009;5(12):e1000758. doi: 10.1371/journal.pgen.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, Van der Linde HC, Nelson DL, Oostra BA, Willemsen R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res. 2007;313(2):244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiurazzi P, Pomponi MG, Willemsen R, Oostra BA, Neri G. In vitro reactivation of the FMR1 gene involved in fragile X syndrome. Hum Mol Genet. 1998;7(1):109–113. doi: 10.1093/hmg/7.1.109. [DOI] [PubMed] [Google Scholar]
- 25.Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of the FMR1 gene. Hum Mol Genet. 1999;8(12):2317–2323. doi: 10.1093/hmg/8.12.2317. [DOI] [PubMed] [Google Scholar]
- 26.Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9(6):562–574. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 27.Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100(2):423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- 28.Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94(1):185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 29.Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135(3):999–1009. doi: 10.1016/j.neuroscience.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 30.Qin M, Kang J, Smith CB. Increased rates of cerebral glucose metabolism in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(24):15758–15763. doi: 10.1073/pnas.242377399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Dam D, D’Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, Oostra BA, Kooy RF, De Deyn PP. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res. 2000;117(1-2):127–136. doi: 10.1016/s0166-4328(00)00296-5. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron. 2012;76(2):325–337. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ventura R, Pascucci T, Catania MV, Musumeci SA, Puglisi-Allegra S. Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: involvement of dopamine in the medial prefrontal cortex. Behav Pharmacol. 2004;15(5-6):433–442. doi: 10.1097/00008877-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103(4):1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 36.Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41(1):19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 37.Qiu LF, Lu TJ, Hu XL, Yi YH, Liao WP, Xiong ZQ. Limbic epileptogenesis in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19(7):1504–1514. doi: 10.1093/cercor/bhn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25(35):8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49(7):1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4(7):420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 41.Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46(2):94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res. 2002;927(1):8–17. doi: 10.1016/s0006-8993(01)03309-1. [DOI] [PubMed] [Google Scholar]
- 43.Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2011;14(5):618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Errijgers V, Fransen E, D’Hooge R, De Deyn PP, Kooy RF. Effect of genetic background on acoustic startle response in fragile X knockout mice. Genet Res (Camb) 2008;90(4):341–345. doi: 10.1017/S0016672308009415. [DOI] [PubMed] [Google Scholar]
- 45.Thomas AM, Bui N, Graham D, Perkins JR, Yuva-Paylor LA, Paylor R. Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome. Behav Brain Res. 2011;223(2):310–321. doi: 10.1016/j.bbr.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietropaolo S, Guilleminot A, Martin B, D’Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One. 2011;6(2):e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9(4):417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 48.Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buijsen RA, Pop AS, Gomezmancilla B, Nelson DL, Willemsen R, Gasparini F, Oostra BA. AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis. 2011;42(3):311–317. doi: 10.1016/j.nbd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 49.de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31(1):127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012;11(5):586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One. 2010;5(3):e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wohr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 53.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl) 2012;219(1):47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- 55.Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile X syndrome reveals limited, call type specific deficits. PLoS One. 2012;7(9):e44816. doi: 10.1371/journal.pone.0044816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 2012;1439:7–14. doi: 10.1016/j.brainres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 57.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98(2):161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 58.Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26(27):7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104(27):11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20(22):8536–8547. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34(6):973–984. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 62.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45(5):753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 63.Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4(4):256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 64.Yun SW, Platholi J, Flaherty MS, Fu W, Kottmann AH, Toth M. Fmrp is required for the establishment of the startle response during the critical period of auditory development. Brain Res. 2006;1110(1):159–165. doi: 10.1016/j.brainres.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 65.Qin M, Xia Z, Huang T, Smith CB. Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience. 2011;194:282–290. doi: 10.1016/j.neuroscience.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29(4):293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 67.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F, Hadjikhani N, Martinet D, Meyer J, Beckmann JS, Delange K, Brun A, Bussy G, Gasparini F, Hilse T, Floesser A, Branson J, Bilbe G, Johns D, Gomez-Mancilla B. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3(64):64ra61. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 68.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94(10):5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siomi H, Choi M, Siomi MC, Nussbaum RL, Dreyfuss G. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994;77(1):33–39. doi: 10.1016/0092-8674(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 70.Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74(2):291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- 71.Corbin F, Bouillon M, Fortin A, Morin S, Rousseau F, Khandjian EW. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum Mol Genet. 1997;6(9):1465–1472. doi: 10.1093/hmg/6.9.1465. [DOI] [PubMed] [Google Scholar]
- 72.Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997;1(1):109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- 73.Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17(5):1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005;19(8):903–918. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107(4):489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 76.Bechara EG, Didiot MC, Melko M, Davidovic L, Bensaid M, Martin P, Castets M, Pognonec P, Khandjian EW, Moine H, Bardoni B. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 2009;7(1):e16. doi: 10.1371/journal.pbio.1000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996;5(8):1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- 78.Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134(6):1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 79.Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7(2):113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- 80.Plante I, Davidovic L, Ouellet DL, Gobeil LA, Tremblay S, Khandjian EW, Provost P. Dicer-derived microRNAs are utilized by the fragile X mental retardation protein for assembly on target RNAs. J Biomed Biotechnol. 2006;2006(4):64347. doi: 10.1155/JBB/2006/64347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107(4):477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 82.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ascano M, Jr., Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, Williams Z, Ohler U, Tuschl T. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492(7429):382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65(3):373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 2011;42(5):673–688. doi: 10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14(6):926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, Bagni C. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10(5):578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101(42):15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30(46):15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30(32):10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100(24):14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51(4):441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72(4):630–642. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, Base CK, Greenough WT. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101(50):17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27(40):10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niere F, Wilkerson JR, Huber KM. Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression. J Neurosci. 2012;32(17):5924–5936. doi: 10.1523/JNEUROSCI.4650-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nalavadi VC, Muddashetty RS, Gross C, Bassell GJ. Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J Neurosci. 2012;32(8):2582–2587. doi: 10.1523/JNEUROSCI.5057-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104(39):15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99(3):1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Veloz MF, Buijsen R, Willemsen R, Cupido A, Bosman LW, Koekkoek SK, Potters JW, Oostra BA, De Zeeuw CI. The effect of an mGluR5 inhibitor on procedural memory and avoidance discrimination impairments in Fmr1 KO mice. Genes Brain Behav. 2012;11(3):325–331. doi: 10.1111/j.1601-183X.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46(4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gantois I, Pop AS, de Esch CE, Buijsen RA, Pooters T, Gomez-Mancilla B, Gasparini F, Oostra BA, D’Hooge R, Willemsen R. Chronic administration of AFQ056/Mavoglurant restores social behaviour in Fmr1 knockout mice. Behav Brain Res. 2013;239:72–79. doi: 10.1016/j.bbr.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 106.Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27(43):11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Carpenter RL, Paylor R. Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile X syndrome. Psychopharmacology (Berl) 2011;217(1):143–151. doi: 10.1007/s00213-011-2276-6. [DOI] [PubMed] [Google Scholar]
- 109.Veeraragavan S, Graham D, Bui N, Yuva-Paylor LA, Wess J, Paylor R. Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS) Behav Brain Res. 2012;228(1):1–8. doi: 10.1016/j.bbr.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behav Neurosci. 2011;125(5):783–790. doi: 10.1037/a0025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, Zhao MG, Zhuo M. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59(4):634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 112.Costa L, Spatuzza M, D’Antoni S, Bonaccorso CM, Trovato C, Musumeci SA, Leopoldo M, Lacivita E, Catania MV, Ciranna L. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry. 2012;72(11):924–933. doi: 10.1016/j.biopsych.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 113.Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci U S A. 2007;104(7):2454–2459. doi: 10.1073/pnas.0610875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107(25):11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shang Y, Wang H, Mercaldo V, Li X, Chen T, Zhuo M. Fragile X mental retardation protein is required for chemically-induced long-term potentiation of the hippocampus in adult mice. J Neurochem. 2009;111(3):635–646. doi: 10.1111/j.1471-4159.2009.06314.x. [DOI] [PubMed] [Google Scholar]
- 116.Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2012;22(2):241–254. doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- 117.Yun SH, Trommer BL. Fragile X mice: reduced long-term potentiation and NMethyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. J Neurosci Res. 2011;89(2):176–182. doi: 10.1002/jnr.22546. [DOI] [PubMed] [Google Scholar]
- 118.Christie SB, Akins MR, Schwob JE, Fallon JR. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 2009;29(5):1514–1524. doi: 10.1523/JNEUROSCI.3937-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akins MR, Berk-Rauch HE, Fallon JR. Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits. 2009;3:17. doi: 10.3389/neuro.04.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klemmer P, Meredith RM, Holmgren CD, Klychnikov OI, Stahl-Zeng J, Loos M, van der Schors RC, Wortel J, de Wit H, Spijker S, Rotaru DC, Mansvelder HD, Smit AB, Li KW. Proteomics, ultrastructure, and physiology of hippocampal synapses in a fragile X syndrome mouse model reveal presynaptic phenotype. J Biol Chem. 2011;286(29):25495–25504. doi: 10.1074/jbc.M110.210260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deng PY, Sojka D, Klyachko VA. Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci. 2011;31(30):10971–10982. doi: 10.1523/JNEUROSCI.2021-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32(1-2):37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 123.Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77(4):696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13(7):819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Westmark CJ, Westmark PR, O’Riordan KJ, Ray BC, Hervey CM, Salamat MS, Abozeid SH, Stein KM, Stodola LA, Tranfaglia M, Burger C, Berry-Kravis EM, Malter JS. Reversal of fragile X phenotypes by manipulation of AbetaPP/Abeta levels in Fmr1KO mice. PLoS One. 2011;6(10):e26549. doi: 10.1371/journal.pone.0026549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5(3):e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korte M, Herrmann U, Zhang X, Draguhn A. The role of APP and APLP for synaptic transmission, plasticity, and network function: lessons from genetic mouse models. Exp Brain Res. 2012;217(3-4):435–440. doi: 10.1007/s00221-011-2894-6. [DOI] [PubMed] [Google Scholar]
- 129.Westmark CJ, Westmark PR, Beard AM, Hildebrandt SM, Malter JS. Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. Int J Clin Exp Pathol. 2008;1(2):157–168. [PMC free article] [PubMed] [Google Scholar]
- 130.Malter JS, Ray BC, Westmark PR, Westmark CJ. Fragile X Syndrome and Alzheimer’s Disease: Another story about APP and beta-amyloid. Curr Alzheimer Res. 2010;7(3):200–206. doi: 10.2174/156720510791050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu J, Petralia RS, Kurushima H, Patel H, Jung MY, Volk L, Chowdhury S, Shepherd JD, Dehoff M, Li Y, Kuhl D, Huganir RL, Price DL, Scannevin R, Troncoso JC, Wong PC, Worley PF. Arc/Arg3.1 regulates an endosomal pathway essential for activity-dependent beta-amyloid generation. Cell. 2011;147(3):615–628. doi: 10.1016/j.cell.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107(44):19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30(2):694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24(28):6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6(11):1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- 136.Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25(39):8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, Hu JH, Worley PF, Gibson JR, Huber KM. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15(3):431–440. S431. doi: 10.1038/nn.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480(7375):63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Osterweil EK, Chuang SC, Chubykin AA, Sidorov M, Bianchi R, Wong RK, Bear MF. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77(2):243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko HY, Lin GG, Govindarajan A, Choi SY, Tonegawa S. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci U S A. 2013;110(14):5671–5676. doi: 10.1073/pnas.1219383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou X, Lin DS, Zheng F, Sutton MA, Wang H. Intracellular calcium and calmodulin link brain-derived neurotrophic factor to p70S6 kinase phosphorylation and dendritic protein synthesis. J Neurosci Res. 2010;88(7):1420–1432. doi: 10.1002/jnr.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156(6):885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal. 2008;20(1):206–218. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56(2):463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79(4):632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo W, Murthy AC, Zhang L, Johnson EB, Schaller EG, Allan AM, Zhao X. Inhibition of GSK3beta improves hippocampus-dependent learning and rescues neurogenesis in a mouse model of fragile X syndrome. Hum Mol Genet. 2012;21(3):681–691. doi: 10.1093/hmg/ddr501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010;30(31):10263–10271. doi: 10.1523/JNEUROSCI.1125-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31(15):5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27(20):5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19(7):1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121(1):238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 152.El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377(3):141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- 153.Heulens I, D’Hulst C, Van Dam D, De Deyn PP, Kooy RF. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav Brain Res. 2012;229(1):244–249. doi: 10.1016/j.bbr.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 154.Olmos-Serrano JL, Corbin JG, Burns MP. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci. 2011;33(5):395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pacey LK, Heximer SP, Hampson DR. Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol. 2009;76(1):18–24. doi: 10.1124/mol.109.056127. [DOI] [PubMed] [Google Scholar]
- 156.Henderson C, Wijetunge L, Kinoshita MN, Shumway M, Hammond RS, Postma FR, Brynczka C, Rush R, Thomas A, Paylor R, Warren ST, Vanderklish PW, Kind PC, Carpenter RL, Bear MF, Healy AM. Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABA(B) receptors with arbaclofen. Sci Transl Med. 2012;4(152):152ra128. doi: 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV, Gonzalez-Heydrich J, Wang PP, Carpenter RL, Bear MF, Hagerman RJ. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med. 2012;4(152):152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- 158.Schutt J, Falley K, Richter D, Kreienkamp HJ, Kindler S. Fragile X mental retardation protein regulates the levels of scaffold proteins and glutamate receptors in postsynaptic densities. J Biol Chem. 2009;284(38):25479–25487. doi: 10.1074/jbc.M109.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2011;108(6):2587–2592. doi: 10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Erickson CA, Wink LK, Ray B, Early MC, Stiegelmeyer E, Mathieu-Frasier L, Patrick V, Lahiri DK, McDougle CJ. Impact of acamprosate on behavior and brain-derived neurotrophic factor: an open-label study in youth with fragile X syndrome. Psychopharmacology (Berl) 2013;228(1):75–84. doi: 10.1007/s00213-013-3022-z. [DOI] [PubMed] [Google Scholar]
- 161.Utari A, Chonchaiya W, Rivera SM, Schneider A, Hagerman RJ, Faradz SM, Ethell IM, Nguyen DV. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil. 2010;115(5):433–443. doi: 10.1352/1944-7558-115.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM, Ethell DW. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. 2010;10:91. doi: 10.1186/1471-2377-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leigh MJ, Nguyen DV, Mu Y, Winarni TI, Schneider A, Chechi T, Polussa J, Doucet P, Tassone F, Rivera SM, Hessl D, Hagerman RJ. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J Dev Behav Pediatr. 2013;34(3):147–155. doi: 10.1097/DBP.0b013e318287cd17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE, Razak KA, Ethell DW, Ethell IM. Long-lasting effects of minocycline on behavior in young but not adult Fragile X mice. Neuroscience. 2013;246:186–198. doi: 10.1016/j.neuroscience.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci. 2013 doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, Durbin-Johnson B, Hagerman RJ, Tassone F. High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am J Med Genet A. 2013 doi: 10.1002/ajmg.a.36023. [DOI] [PubMed] [Google Scholar]
- 167.Lohith TG, Osterweil EK, Fujita M, Jenko KJ, Bear MF, Innis RB. Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile X syndrome? Mol Autism. 2013;4(1):15. doi: 10.1186/2040-2392-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112(3):317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 169.Kao DI, Aldridge GM, Weiler IJ, Greenough WT. Altered mRNA transport, docking, and protein translation in neurons lacking fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2010;107(35):15601–15606. doi: 10.1073/pnas.1010564107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hoeffer CA, Sanchez E, Hagerman RJ, Mu Y, Nguyen DV, Wong H, Whelan AM, Zukin RS, Klann E, Tassone F. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 2012;11(3):332–341. doi: 10.1111/j.1601-183X.2012.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tsai NP, Wilkerson JR, Guo W, Maksimova MA, DeMartino GN, Cowan CW, Huber KM. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151(7):1581–1594. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sung YJ, Dolzhanskaya N, Nolin SL, Brown T, Currie JR, Denman RB. The fragile X mental retardation protein FMRP binds elongation factor 1A mRNA and negatively regulates its translation in vivo. J Biol Chem. 2003;278(18):15669–15678. doi: 10.1074/jbc.M211117200. [DOI] [PubMed] [Google Scholar]
- 173.Huang F, Chotiner JK, Steward O. The mRNA for elongation factor 1alpha is localized in dendrites and translated in response to treatments that induce long-term depression. J Neurosci. 2005;25(31):7199–7209. doi: 10.1523/JNEUROSCI.1779-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. Embo J. 2001;20(17):4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37(3):417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 176.Erickson CA, Mullett JE, McDougle CJ. Open-label memantine in fragile X syndrome. J Autism Dev Disord. 2009;39(12):1629–1635. doi: 10.1007/s10803-009-0807-3. [DOI] [PubMed] [Google Scholar]
- 177.Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(−/y) mouse model of fragile X syndrome. Cell Rep. 2012;1(3):225–233. doi: 10.1016/j.celrep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Menon L, Mader SA, Mihailescu MR. Fragile X mental retardation protein interactions with the microtubule associated protein 1B RNA. Rna. 2008;14(8):1644–1655. doi: 10.1261/rna.1100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen YL, Shen CK. Modulation of mGluR-dependent MAP1B translation and AMPA receptor endocytosis by microRNA miR-146a-5p. J Neurosci. 2013;33(21):9013–9020. doi: 10.1523/JNEUROSCI.5210-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Menon L, Mihailescu MR. Interactions of the G quartet forming semaphorin 3F RNA with the RGG box domain of the fragile X protein family. Nucleic Acids Res. 2007;35(16):5379–5392. doi: 10.1093/nar/gkm581. [DOI] [PMC free article] [PubMed] [Google Scholar]


