Abstract
Background
Surfactant protein (SP) D shares target cells with the proinflammatory cytokine TNF-α, an important autocrine stimulator of dendritic cells and macrophages in the airways.
Objective
We sought to study the mechanisms by which TNF-α and SP-D can affect cellular components of the pulmonary innate immune system.
Methods
Cytokine and SP-D protein and mRNA expression was assessed by means of ELISA, Western blotting, and real-time PCR, respectively, by using in vivo models of allergic airway sensitization. Macrophage and dendritic cell phenotypes were analyzed by means of FACS analysis. Maturation of bone marrow–derived dendritic cells was investigated in vitro.
Results
TNF-α, elicited either by allergen exposure or pulmonary overexpression, induced SP-D, IL-13, and mononuclear cell influx in the lung. Recombinant IL-13 by itself was also capable of enhancing SP-D in vivo and in vitro, and the SP-D response to allergen challenge was impaired in IL-13– deficient mice. Allergen-induced increase of SP-D in the airways coincided with resolution of TNF-α release and cell influx. SP-D–deficient mice had constitutively high numbers of alveolar mononuclear cells expressing TNF-α, MHC class II, CD86, and CD11b, characteristics of proinflammatory, myeloid dendritic cells. Recombinant SP-D significantly suppressed all of these molecules in bone marrow–derived dendritic cell cultures.
Conclusions
TNF-α can contribute to enhanced SP-D production in the lung indirectly through inducing IL-13. SP-D, on the other hand, can antagonize the proinflammatory effects of TNF-α on macrophages and dendritic cells, at least partly, by inhibiting production of this cytokine.
Keywords: TNF, SP-D, dendritic cell, mouse model, airway inflammation
Lung collectins (surfactant protein [SP] A and SP-D) and mucosal antigen-presenting cells reside in close proximity in the distal air spaces.1 Collectin/antigen-presenting cell interactions have significant functional consequences in infections and inflammatory diseases2–4 and serve 2 main functions: pathogen clearance and modulation of innate immune cells.5 Compelling evidence was provided to support the importance of SP-A and SP-D in innate immune function by studies on gene-deficient mice. Animals lacking either lung collectins have shown an increased susceptibility to bacterial,6–8 viral,9–11 fungal,12 endotoxin,13 and allergen-induced14,15 lung inflammation. Under normal conditions, however, SP-A−/− mice display no pathologic features in the lung. SP-D−/− mice, on the other hand, show serious alterations, including signs of constitutive alveolar macrophage activation.16–19 Our previous studies in SP-D−/− mice also demonstrated the presence of activated CD3/CD4+ T cells in the airway submucosal tissue with strikingly increased levels of thymus and activation-regulated chemokine, a chemokine responsible for attracting TH2 and dendritic cells.15 These findings suggested that in addition to macrophages, dendritic cells also might be affected by a lack of SP-D and that this lung collectin might have an essential role in maintaining immune homeostasis in the lung under normal, noninflammatory circumstances.
Mature dendritic cells are potent antigen-presenting cells, particularly important in the initiation of naive T cells in the regional lymph nodes.20,21 Under normal conditions, however, dendritic cells reside in the lung tissue in an immature state. These resting cells are scattered throughout in the bronchial and alveolar wall, capturing inhaled pathogens and loading antigenic peptides onto MHC class II molecules butunable to present them to T cells. In response to danger signals, such as pathogens, proinflammatory cytokines, or necrotic cells, dendritic cells start to mature and switch from an antigen-capturing to an antigen-presenting and T cell–stimulating state.21
One of the earliest of the danger signals is the release of TNF-α. This proinflammatory cytokine has autocrine activities on macrophages and dendritic cells and stimulates a wide array of functions, including maturation and activation. Although all mature dendritic cells share a common ability to process and present antigen to naive T cells for the initiation of an immune response, they differ in origin, surface markers, migratory patterns, localization, and cytokine production.22 In murine models of airway inflammation, 2 subsets appeared particularly interesting. Plasmacytoid dendritic cells make up the majority under baseline conditions,23 and these cells were shown to be tolerogenic in allergen-induced inflammation.24–26 On the other hand, myeloid dendritic cells migrate rapidly to the lung during TH2-type inflammation and exert potent TH2 cell activation.24–26 Dendritic cells are generally differentiated by the membrane marker CD11c, although this marker is shared with alveolar macrophages. Myeloid dendritic cells are distinguished by expression of CD11b.
In vitro studies conducted on the direct effects of lung collectins on alveolar macrophages have been somewhat controversial because they found both stimulation27–30 and inhibition27,31–33 of proinflammatory functions, including TNF-α production.34 Only a few studies described the effects of SP-A and SP-D on dendritic cell function.35–37 In these studies the authors found that SP-A inhibited and SP-D augmented the antigen-presenting function of bone marrow–derived dendritic cells.35,37 SP-D, however, inhibited antigen presentation when dendritic cells were isolated from the lung.36 Based on these results, we hypothesized here that TNF-α and SP-D serve as antagonists in the regulation of antigen-presenting cells in the lung.
METHODS
Animals and sensitization protocols
All experimental animals were housed under specific pathogen-free conditions. Experiments were performed between 8 and 12 weeks of age. BALB/c, C57BL/6, TNF-α–transgenic, and SP-D−/− mice (both on a C57BL/6 background) and IL-4/IL-13 double-knockout and wild-type 129xC57BL/6(F2) mice (provided by the laboratory of Dr Andrew McKenzie, Medical Research Council, Cambridge, United Kingdom38) were used. For the in vitro type II cell culture studies, adult (200 g) Sprague-Dawley rats (Charles River Laboratories, Wilmington, Mass) were used, as previously described.15,39
BALB/c mice were sensitized and challenged with Aspergillus fumigatus, as described previously,38 and studied 0 (not challenged), 1, 6, 12, 24, 48, and 72 hours later. In brief, mice were sensitized intraperitoneally with 20 μg of A fumigatus (Hollister-Stier, Spokane, Wash) together with 20 mg of alum (Imject Alum; Pierce, Rockford, Ill) in 100 μL of PBS at days 0 and 14, followed by intranasal challenge on day 28 with 25 μL of A fumigatus extract in glycerol-PBS. For intranasal treatment, mice were anesthetized by means of isoflurane inhalation, and A fumigatus extract (25 μg) was applied to the left naris.
Anti-TNF-α mAb (a generous gift of Dr Anuk Das, Centocor, King of Prussia, Pa) and isotype control rat IgG1 (0.6 mg in 100 μL of PBS; Sigma, St Louis, Mo) were injected intravenously immediately before allergen challenge. Animals received water and food ad libitum. All experimental procedures were under a protocol approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Bronchoalveolar lavage and cell counts
Bronchoalveolar lavage (BAL) was performed as previously described.38 Briefly, lavage was carried out once with 0.7 mL and twice with 1 mL of sterile PBS. BAL fluid was centrifuged at 4°C for 10 minutes at 400g, and the pellet was resuspended in 1 mL of PBS. Total cell counts were determined from an aliquot of the cell suspension by using a cell counter (Beckman Coulter, Miami, Fla). Differential cell counts were done on cytocentrifuge preparations (Cytospin 3; Thermo Scientific, Waltham, Mass) stained with Kwik Diff (Thermo Scientific), counting 200 to 500 cells from each individual.
Protein assay, cytokine and SP-D ELISA, and SP-D Western blotting
Total protein from cell-free supernatant of the BAL fluids was assessed by using the Bradford Assay (Bio-Rad, Hercules, Calif). IL-13 and TNF-α levels were determined by using commercially available ELISA kits (R&D Systems, Minneapolis, Minn) with detection limits of less than 5 pg/mL. ELISAs were performed according to the manufacturer's instructions. For mouse SP-D ELISAs, we used an in-house anti-SP-D polyclonal antibody and protocol, as previously published.40 Western blotting was performed as previously described.41
Isolation and culture of bone marrow–derived dendritic cells
Bone marrow cells were washed from the femurs and tibias of C57BL/6 or SP-D−/− mice. Single-cell suspensions were pooled from 5 mice, washed in sterile PBS, resuspended in 10% FBS/PBS and biotin-conjugated anti-mouse c-kit antibody (BD PharMingen, San Diego, Calif), and incubated for 45 minutes on ice. Cells were washed, placed in pellets, and resuspended in 500 μL of 10% FBS/PBS and streptavidin-coated microbeads (Miltenyi Biotech, Auburn, Calif). Cells were incubated for 30 to 45 minutes at 4°C under constant rotation. C-kit+ cells were isolated by means of positive selection on a magnetic column. Approximately 0.5 × 106 cells per well were plated in 6-well tissue-culture plates and maintained in Iscove Modified Dulbecco Medium/10% FBS, 1% penicillin/streptomycin, stem cell factor (SCF; 5 ng/mL), GM-CSF (2.5 ng/mL), and IL-4 (2.5 ng/mL). The medium was changed on day 3. On day 4, nonadherent cells were removed into new plates, SCF was replaced by TNF-α (2 ng/mL), and 1 or 5 μg/mL recombinant SP-D was added to some of the cultures. On day 7, nonadherent cells were either harvested for FACS analysis or further cultured in a new plate. FACS analysis of cells was performed on days 0 to 10.
FACS analysis of cells
BAL cells (pooled from 2–3 animals) and bone marrow–derived dendritic cells were resuspended in staining buffer (5 × 105 cells in PBS with 2% FCS and 0.1% sodium azide). Cells were blocked with anti-murine FcIII/II (BD PharMingen) and then incubated with phycoerythrin–conjugated anti-mouse CD11c, phycoerythrin-Cy5–conjugated anti-mouse MHC class II (I-A/I-E), fluorescein isothiocyanate–conjugated anti-mouse CD86, or fluorescein isothiocyanate–conjugated anti-mouse CD11b and their respective isotype controls (all from eBioscience, San Diego, Calif). After cell-surface staining, cells were washed twice with staining buffer and resuspended in Fixation Permeabilization buffer (BD PharMingen) and incubated for 20 minutes at 4°C. Cells were washed with Permeabilization/Wash buffer (BD PharMingen), resuspended in Permeabilization/Wash buffer containing allophycocyanin–conjugated anti-mouse TNF-α antibodies, and incubated for another 30 minutes at 4°C. After intracellular staining, the cells were washed again in Permeabilization/Wash buffer and resuspended in staining buffer. Flow cytometry was performed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, Calif). Data were analyzed by using the Cell Quest software package (BD Biosciences). Thresholds for positive staining were determined from the isotype-matched control samples.
RNA isolation and real-time PCR
Total RNA from lungs was isolated with the Qiagen RNeasy Mini Kit (Qiagen, Valencia, Calif), according to the manufacturer's instructions. RNA from the lung was reverse transcribed with Superscript III reverse transcriptase (Invitrogen, Carlsbad, Calif). Expression differences between groups were analyzed by means of real-time PCR with SYBR Green Jump Start Taq ReadyMix (Sigma). Samples were run in triplicate (n = 3) on an ABI SDS-7500. Results were analyzed by using the relative quantitation (ΔΔCt) method and expressed as fold change (± SEM).
Data analysis
We used Prism4 software (GraphPad, Inc, San Diego, Calif), Student t tests for pairwise comparison, and 2-way ANOVA for analysis of time courses and dose responses. Data are expressed as means ± SEM. A P value of less than .05 was considered statistically significant.
RESULTS
Expression of TNF-α in the airways was associated with enhanced production of SP-D
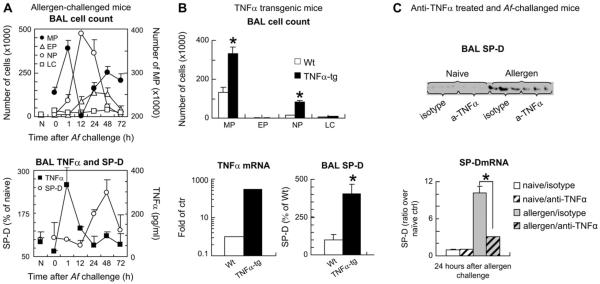
To study whether a regulatory link exists between TNF-α and SP-D, we first investigated the temporal relationship between expression of these molecules using our previously described model of single-allergen challenge of sensitized mice.15,41,42 To dissect the pathologic events characterizing the onset and resolution of the inflammatory airway response, separate groups of animals were investigated 1, 12, 24, 48, and 72 hours after allergen challenge, and sensitized but nonchallenged (0 hour) and naive mice were used as control animals. There was a significant influx of mononuclear cells into the airways very early, 1 hour after allergen challenge of sensitized mice. This influx paralleled the peak of TNF-α release into the airways. Macrophage numbers showed a second peak 48 hours later; however, it coincided with the height of SP-D expression and dampened levels of TNF-α. Generally, increasing expression of SP-D was inversely associated with the inflammatory changes (Fig 1, A), suggesting that TNF-α is part of the initiation phase and SP-D is part of the resolution phase of the airway inflammatory response.
FIG 1.
Expression of TNF-α in the airways was associated with increased numbers of macrophages, and it preceded enhanced production of SP-D. A, BALB/c mice were sensitized and challenged with Aspergillus fumigatus (Af) and studied before (0) and after challenge. B, Constitutive TNF-α expression was targeted into lung type II alveolar epithelial cells by using an SP-C promoter construct. C, Mice were injected with anti-TNF-α or isotype control antibodies (0.5 mg) immediately before allergen challenge and were studied 24 hours later. Total and differential cell counts were assessed, and TNF-α and SP-D protein release was analyzed by means of ELISA (Fig 1, A and B) and Western blotting (Fig 1, C; 3 representative bands of n 5 6 in each group). TNF-α (Fig 1, B) and SP-D (Fig 1, C) mRNA expression in the lung tissue was studied by means of real-time PCR. Results are presented as means ± SEMs (n ≥ 6). *P < .05. N, Naive mice; Wt, wild-type; TNFα-tg, TNF-α–transgenic mice; MP, macrophage; EP, eosinophil; NP, neutrophil; LC, Lymphocyte.
To study whether TNF-α without allergen challenge can contribute to upregulation of SP-D production, we used a model of TNF-α–transgenic mice. These mice specifically overexpress TNF-α in alveolar type II cells under the control of the human SP-C promoter.43–45 As shown in Fig 1, B, the expression of the gene encoding TNF-α was almost 300-fold higher in TNF-α–transgenic mice compared with that seen in wild-type control animals. Although the total protein content in the BAL fluid of these mice was not significantly different (not shown), there was a 4-fold increase in SP-D levels in TNF-α–transgenic mice compared with that seen in wild-type control animals, suggesting that TNF-α might play a regulatory role in increased SP-D production. To test this hypothesis, we treated the mice immediately before allergen challenge with a single intravenous injection of anti-TNF-α antibody (0.5 mg). Blocking TNF-α resulted in a significant inhibition of SP-D protein in the BAL fluid and mRNA in the lung tissue, indicating that inhibition of SP-D protein release into the BAL fluid (Fig 1, C, top panel) was commensurate with inhibition of SP-D mRNA production isolated from the lung tissue of the mice (Fig 1, C, bottom panel).
To investigate whether TNF-α can directly regulate SP-D synthesis, we used an in vitro type II alveolar epithelial cell culture system, as described previously.39 Addition of TNF-α to these cells up to 100 ng, however, did not alter SP-D levels recovered from the culture (data not shown).
IL-13 directly stimulated enhanced production of SP-D in the airways
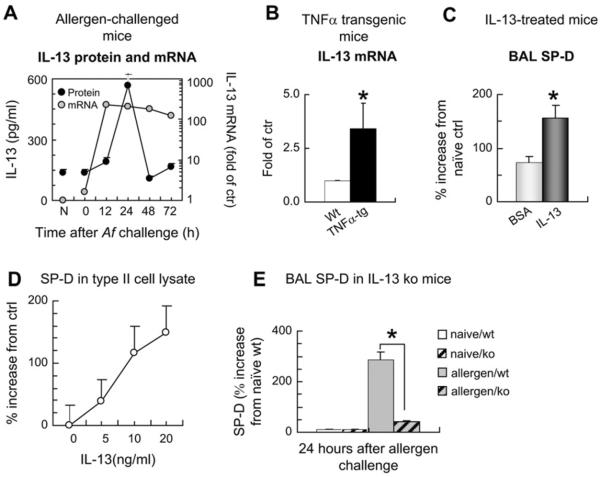
High TNF-α levels both in the allergen challenge model and the TNF-α overexpression model were associated with IL-13 mRNA activation (Fig 2, A and B) and IL-13 protein release (Fig 2, A). Intratracheal treatment of the mice with recombinant IL-13 significantly enhanced SP-D levels in the BAL fluid (Fig 2, C), indicating that TNF-α–associated IL-13 can be responsible for enhanced SP-D production in the distal airways. To confirm that the changes in the BAL fluid represent what happens at the tissue level, we showed in type II alveolar epithelial cell cultures that addition of IL-13 directly enhanced SP-D protein levels in a dose-dependent manner in the cell lysates (Fig 2, D). Furthermore, mice deficient in IL-13 were significantly impaired in their SP-D responses after sensitization and challenge with allergen (Fig 2, E).
FIG 2.
Enhanced SP-D expression in the airways is directly stimulated by IL-13. A, BALB/c mice were sensitized and challenged with Aspergillus fumigatus (Af) and studied at the indicated time points. B, Constitutive TNF-α expression was targeted into lung type II alveolar epithelial cells by using an SP-C promoter construct. C, Recombinant mouse IL-13 (1.5 μg) or BSA was administered intratracheally to sensitized mice, and BAL fluid SP-D levels were studied 24 hours later. D, Isolated rat type II alveolar epithelial cells were cultured with IL-13 for 4 days. E, IL-13–deficient mice were sensitized and challenged with the allergen and studied 24 hours later. IL-13 protein in BAL fluid (Fig 2, A) was assessed by means of ELISA. IL-13 mRNA (Fig 2, A and B) in the lung was studied by means of real-time PCR. SP-D protein content was analyzed by using Western blotting (Fig 2, C–E). Results are presented as means ± SEMs (n ≥ 6). *P < .05. N, Naive mice; Wt, wild-type, TNFα-tg, TNF-α–transgenic mice.
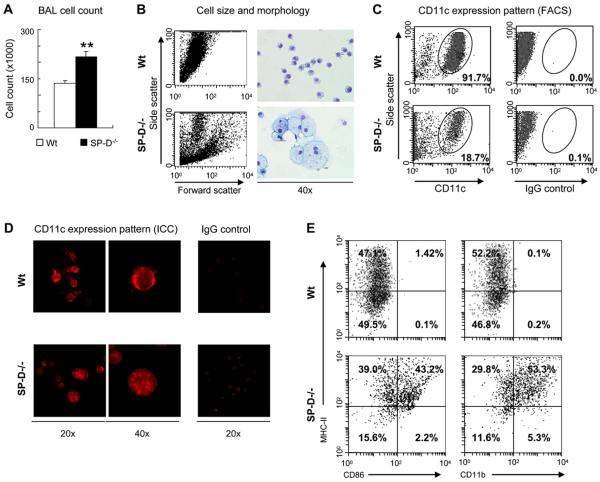
Lack of SP-D in SP-D−/− mice resulted in abnormal count, morphology, and activation of CD11c-expressing cells in the BAL fluid
In contrast to the inhibitory effects of high SP-D concentrations on macrophage numbers, in mice lacking SP-D, there was a constitutively increased number of these cells in the BAL fluid (P < .0001; Fig 3, A). To further evaluate the effects of the presence and absence of SP-D on antigen-presenting cells in vivo, we analyzed BAL fluid cells for their morphology and expression of CD11c, a marker for both macrophages and dendritic cells. Remarkable differences in size and granularity can be seen between SP-D−/− and wild-type mice by means of FACS analysis on side and forward scatter (Fig 3, B, left panels). In addition, cytospin preparations of BAL fluid cells showed abnormally enlarged foamy macrophages in SP-D−/− mice (Fig 3, B, right lower panels).
FIG 3.
Lack of SP-D in gene-deficient (SP-D−/−) mice resulted in abnormal count, morphology, and activation of CD11c-expressing cells in the BAL fluid. A, Total counts of BAL fluid cells from wild-type (Wt) and SP-D−/− mice (mean ± SEM of n 5 8). B, Morphologic differences between Wild-type and SP-D−/− BAL fluid samples by means of FACS analysis (left panels) and light microscopic analysis of cytospin preparations (right panels). C and D, Expression of the dendritic cell and macrophage marker CD11c on wild-type and SP-D−/− BAL fluid cells, as assessed by labeling with phycoerythrin-conjugated anti-mouse mAb by means of FACS analysis (Fig 3, C) and immunocytochemistry (Fig 3, D). E, CD11c+ cells were gated in the BAL fluid of SP-D−/− and wild-type mice and analyzed for expression of MHC class II, CD86, and CD11b by using mAbs. Fig 3, B through E: Representative samples of 3 independent experiments. Isotype controls were also used for MHC class II, CD86, and CD11b antibodies (data not shown).
In contrast to our expectations, the numbers of CD11c+ cells appeared much less abundant in BAL fluid (Fig 3, C) and in whole lung-digests (data not shown) obtained from SP-D−/− mice when compared with numbers seen in wild-type animals by means of FACS analysis. To test whether CD11c was diminished in SPD−/− mice because of possible internalization of this membrane molecule, we also performed immunochemical labeling. Indeed, CD11c was characteristically membrane localized in wild-type BAL fluid cells, whereas it appeared inside the cytoplasm in most of the BAL fluid cells in SP-D−/− mice (Fig 3, D).
We further analyzed these cells because of their greater number, abnormal appearance, and constitutively internalized CD11c suggesting activation. When we gated on the remaining CD11c+ cells of SP-D−/− BAL fluid (or in whole lung digest), we observed an increased expression of MHC class II, CD86 (a costimulatory molecule), and CD11b (a marker of myeloid dendritic cells; Fig 3, E). Thus in addition to abnormal macrophages, we detected the presence of activated myeloid dendritic cells in the BAL fluid of SP-D−/− mice.
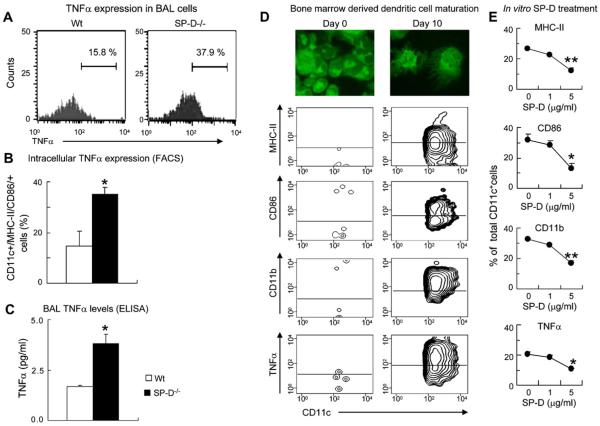
Lack of SP-D enhanced and presence of SP-D suppressed TNF-α expression in CD11c+ cells
To further investigate the effect of a lack of SP-D on alveolar antigen-presenting cell function, we assessed the levels of intracellular (Fig 4, A and B) and soluble (Fig 4, C) TNF-α in SP-D−/− mice. Because these were naive animals on a C57BL/6 background, the levels of TNF-α released in the BAL fluid were low. Nevertheless, there were significantly increased amounts of TNF-α in SP-D−/− mice compared with that seen in control animals (P < .05; Fig 4, C).
FIG 4.
Lack of SP-D enhanced and presence of SP-D suppressed TNF-α expression in CD11c+ cells. A, Representative FACS histograms of intracellular TNF-α expression in CD11c/MHC class II/CD86–positive BAL fluid cells. B, Quantitative analysis of TNF-α in wild-type (Wt) and SP-D−/− BAL fluid cells. C, Cell-free BAL supernatant was analyzed for TNF-α in naive wild-type and SP-D−/− mice (mean ± SEM of n = 6). *P < .05. D and E, Bone marrow–derived c-kit+ cells were cultured as described. Fig 4, D: Cells were analyzed for morphologic characteristics by using carboxyfluorescein succinimidyl ester labeling of cytospin preparations (top panels) and FACS on day 0 (left panels) and day 10 (right panels). MHC class II, CD86, CD11b, and TNF-α expression was assessed on CD11c-gated cells. Fig 4, E: Recombinant mouse SP-D was added on day 4 of the culture. CD11c+ cells were analyzed for MHC class II, CD86, CD11b, and TNF-α expression on day 10 (mean ± SEM of n = 6). *P < .05, **P < .01.
The source of TNF-α in vivo was assessed by means of intracellular labeling of cells obtained from BAL fluid, as well as digested whole-lung samples, from the mice by using flow cytometry. These results show that CD11c+ cells that were also positive for expression of MHC class II and CD86 in the BAL fluid of SP-D−/− mice expressed more than twice the amount of TNF-α when compared with that seen in wild-type mice (P < .05; Fig 4, A and B). We repeated these studies with cells from whole-lung digests and found very similar data (data not shown). Although activated macrophages can contribute to the increased TNF-α levels, expression of MHC class II and CD86 on CD11c+ cells suggests that dendritic cells also participate in TNF-α production. Indeed, simultaneous expression of CD11b (Fig 3, E) or TNF-α (Fig 4, A and B) on CD11c/MHC class II/CD86-positive BAL fluid cells of SP-D−/− mice indicates the presence of activated myeloid dendritic cells that are likely good producers of this proinflammatory cytokine. Because myeloid dendritic cell maturation is an important process in eliciting airway inflammatory changes, we investigated the effects of SP-D on maturation of bone marrow–derived dendritic cells in vitro. C-kit+ cells were isolated from bone marrow cell suspensions and cultured in the presence of SCF, GM-CSF, and IL-4 until day 4. Nonadherent cells then were further cultured without SCF in the presence of SP-D until day 10. Although most c-kit+ cells were negative for the expression of CD11c, MHC class II, CD86, CD11b, and TNF-α on day 4 (Fig 4, D, left panel), by day 10, approximately 90% of the nonadherent cells were morphologically mature dendritic cells with numerous long extensions (Fig 4, D, top right panel). Most of these cells were positive for CD11c and expressed high levels of MHC class II and the costimulatory molecule CD86, as well as CD11b, integrin marker of myeloid dendritic cells. Moreover, most dendritic cells were positive for TNF-α expression (Fig 4, D, bottom right panel).
Fig 4, E, shows that SP-D significantly inhibited MHC class II (P < .0001), CD86 (P < .05), and CD11b (P < .0001) expression. Additionally, there was a significant reduction in the production of TNF-α by dendritic cells in the presence of SP-D, as measured by means of intracellular TNF-α staining (P <.05). These results confirmed that SP-D has a suppressive effect on the maturation of myeloid dendritic cells and suggested that this action might occur at least in part by inhibiting autocrine production of TNF-α.
DISCUSSION
Generation of the pleiotropic cytokine TNF-α induces development of inflammatory responses characterizing many respiratory diseases, including asthma, chronic obstructive pulmonary disease, and acute lung injury.46 We have previously shown that the lung collectin SP-D has immunosuppressive activities15 and that it protects against inflammatory changes of the lung.40 In this study we investigated the regulatory link between these innate immune molecules. Our data demonstrate an early increase of TNF-α levels in response to allergen challenge in sensitized mice and late increases in SP-D associated with resolution of the inflammatory changes. Lack of SP-D in the BAL fluid of gene-deficient naive mice resulted in increased numbers of activated macrophages and myeloid dendritic cells that constitutively expressed TNF-α. On the other hand, when added exogenously, SP-D inhibited maturation and TNF-α production of myeloid (bone marrow–derived) dendritic cells in vitro. Because chronic inflammation in TNF-α–transgenic mice was not affected by high SP-D levels, we propose that this lung collectin exerts its immunosuppressive activities in the airways, at least partly, by suppressing TNF-α.
Our findings are in accordance with studies that showed that TNF-α upregulation is part of the early pathologic changes occurring in asthmatic subjects and in murine models of asthma.47,48 Because TNF-α expression preceded increases in SP-D levels during the allergic airway response, we tested whether this cytokine can contribute to upregulation of SP-D production without allergic sensitization. Interestingly, mice specifically overexpressing TNF-α in alveolar type II cells43–45 had increased SP-D levels. In contrast, treatment of mice with an anti-TNF-α antibody significantly inhibited SP-D expression. Recombinant TNF-α by itself, however, did not increase SP-D production in epithelial cell cultures.39 Therefore it is possible that instead of direct gene induction, TNF-α affects SP-D production indirectly. At least in part, the extensive cell damage of airway and alveolar epithelial cells that was previously described by Miyazaki et al43 could account for increased SP-D expression in TNF-α–overexpressing mice. However, such tissue changes were found in 6-month-old mice and were not present in the animals used in our experiments at the age of 8 weeks. On the other hand, high TNF-α levels both in the allergen challenge model and the TNF-α overexpression model were associated with IL-13 mRNA activation and IL-13 protein release. Because IL-13 has been shown to induce SP-D production in vitro and in vivo,15 the increased SP-D expression might be a result of the upregulation of IL-13. Indeed, intratracheal treatment of mice and isolated alveolar epithelial cells with recombinant IL-13 significantly enhanced SP-D levels in the BAL fluid and in the cell lysates, respectively. Furthermore, IL-13–deficient mice were not able to mount an SP-D response after allergen challenge. Thus TNF-α–associated IL-13 might be responsible for enhanced SP-D production in the distal airways.
There is a growing body of evidence that TNF-α and SP-D share cellular targets in modulating the innate immune system and contribute on multiple levels to the pathogenesis of acute and chronic airway inflammation. Early recruitment of antigen-presenting cells into the airways, an important element of the initial inflammatory response,24,49 paralleled release of TNF-α, with a peak 1 hour after allergen challenge of sensitized mice. On the other hand, appearance of high levels of SP-D 48 hours later coincided with a second wave of mononuclear cell influx but no TNF-α release. We speculate that a TNF-α–induced upregulation of SP-D in allergic inflammation or in TNF-α–overexpressing mice might be a mechanism of protection that enables the restoration of immunologic homeostasis at the conclusion of the inflammatory response.4 This hypothesis is supported by our data on SP-D–deficient mice. Absence of SP-D in these animals had severe effects on the resident antigen-presenting cell population in the lung. FACS analysis of the BAL fluid cells showed that levels of CD11c, a cell-surface marker for dendritic cells and macrophages, was significantly decreased, but it appeared intracellularly in a granular pattern, suggesting internalization and possible association with lysosomes. Several studies have shown that SP-D enhances phagocytosis of inhaled pathogens10,50 and that SP-D–deficient mice have reduced clearance of pathogen-derived DNA fragments.51 This was initially attributed to the opsonizing function of SP-D. However, it was recently suggested that SP-D also regulates surface expression of CD11c,52 which is an important β2-integrin mediator of phagocytosis of microbes53 and apoptotic cells.54 Similarly to our findings, Senft et al52 observed that cell-surface expression of CD11c was drastically reduced and appeared in the lysosomes in the BAL fluid cells of SP-D–deficient mice. Reduced surface expression of CD11c and an abrogated phagocytic activity might contribute to increased production of TNF-α.55 Indeed, by means of FACS analysis, we found significantly more TNF-α+ cells in the BAL fluid of SP-D–deficient mice than in wild-type mice.
The released TNF-α detected in the BAL fluid in our asthma model and in the SP-D–deficient mice is most likely antigen-presenting cell derived. Therefore SP-D–mediated inhibition of TNF-α production in these cells can serve as a potent mechanism by which suppression of antigen-presenting cell function occurs. Because myeloid dendritic cell maturation is an important process in airway inflammation,24 we investigated the effects of SP-D on maturation of bone marrow–derived dendritic cells in vitro. SP-D significantly inhibited MHC class II, CD86, and CD11b expression and abolished production of TNF-α in these cells. In the in vivo situation, this can have 2 main implications. First, immature dendritic cells are efficient in removal of inhaled antigenic material. Second, immature dendritic cells are not able to initiate a local T-cell response (rather they induce tolerance). Thus high levels of SP-D can confer protection from chronic inflammation in the airways and lung parenchyma by inhibiting local maturation of the highly proinflammatory dendritic cell population.
Taken together, our study indicates that TNF-α initiates not only proinflammatory changes during the airway response but also anti-inflammatory mechanisms, including enhanced production of SP-D at the later stages. We propose that such an increase in SP-D is necessary to counterbalance the action of TNF-α and that the antagonistic effects of these innate immune regulators might be manifested through antigen-presenting cell function in the airways.
Clinical implications.
Negative feedback regulation between TNF-α and SP-D is important for facilitating resolution of acute inflammatory changes in the respiratory tract and preventing the development of chronic conditions, such as asthma.
Acknowledgments
Supported by grant R01-AI055593-01 and Centocor.
We thank Dr Yi Zhang for expert guidance on the dendritic cell culture studies. Dr Andrew McKenzie (Medical Research Council, Cambridge, UK), Dr K. Fan Chung, and Dr Puneeta Nath (National Heart and Lung Institute, Imperial College, London, United Kingdom) are gratefully acknowledged for the IL-4/IL-13 double-knockout mice and for providing the allergen-sensitized/challenged and control BAL samples obtained from these animals.
Abbreviations used
- BAL
Bronchoalveolar lavage
- SCF
Stem cell factor
- SP
Surfactant protein
Footnotes
Disclosure of potential conflict of interest: A. M. Das owns stock in, has patent licensing arrangements with, and is employed by Centocor. A. Haczku has received research support from Centocor. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Crouch EC. Surfactant protein-D and pulmonary host defense. Respir Res. 2000;1:93–108. doi: 10.1186/rr19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JY, Reid KB. The immunoregulatory roles of lung surfactant collectins SP-A, and SP-D, in allergen-induced airway inflammation. Immunobiology. 2007;212:417–25. doi: 10.1016/j.imbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–9. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 4.Haczku A. Role and regulation of lung collectins in allergic airway sensitization. Pharmacol Ther. 2006;110:14–34. doi: 10.1016/j.pharmthera.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–7. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3:161–6. doi: 10.1016/s1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- 7.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, et al. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165:3934–40. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- 8.Korfhagen TR. Surfactant protein A (SP-A)-mediated bacterial clearance: SP-A and cystic fibrosis. Am J Respir Cell Mol Biol. 2001;25:668–72. doi: 10.1165/ajrcmb.25.6.f221. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Siddiqui J, Hendry M, Akiyama J, Edmondson J, Brown C, et al. Surfactant protein-A–deficient mice display an exaggerated early inflammatory response to a beta-resistant strain of influenza A virus. Am J Respir Cell Mol Biol. 2002;26:277–82. doi: 10.1165/ajrcmb.26.3.4584. [DOI] [PubMed] [Google Scholar]
- 10.LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, et al. Surfactant protein-D enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31:193–9. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 11.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol. 2001;167:5868–73. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 12.Atochina EN, Beck JM, Preston AM, Haczku A, Tomer Y, Scanlon ST, et al. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect Immun. 2004;72:6002–11. doi: 10.1128/IAI.72.10.6002-6011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borron P, McIntosh JC, Korfhagen TR, Whitsett JA, Taylor J, Wright JR. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L840–7. doi: 10.1152/ajplung.2000.278.4.L840. [DOI] [PubMed] [Google Scholar]
- 14.Schaub B, Westlake RM, He H, Arestides R, Haley KJ, Campo M, et al. Surfactant protein D deficiency influences allergic immune responses. Clin Exp Allergy. 2004;34:1819–26. doi: 10.1111/j.1365-2222.2004.02068.x. [DOI] [PubMed] [Google Scholar]
- 15.Haczku A, Cao Y, Vass G, Kierstein S, Nath P, Atochina-Vasserman EN, et al. IL-4 and IL-13 form a negative feedback circuit with surfactant protein-D in the allergic airway response. J Immunol. 2006;176:3557–65. doi: 10.4049/jimmunol.176.6.3557. [DOI] [PubMed] [Google Scholar]
- 16.Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci U S A. 1998;95:11869–74. doi: 10.1073/pnas.95.20.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawgood S, Ochs M, Jung A, Akiyama J, Allen L, Brown C, et al. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1002–10. doi: 10.1152/ajplung.00118.2002. [DOI] [PubMed] [Google Scholar]
- 18.Hawgood S, Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol. 2001;63:495–519. doi: 10.1146/annurev.physiol.63.1.495. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M, Whitsett JA. Alveolar macrophages and emphysema in surfactant protein-D-deficient mice. Respirology. 2006;11(Suppl):S37–40. doi: 10.1111/j.1440-1843.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 21.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–50. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, et al. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–48. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 24.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–9. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohl J, Wills-Karp M. Complement regulates inhalation tolerance at the dendritic cell/T cell interface. Mol Immunol. 2007;44:44–56. doi: 10.1016/j.molimm.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Wills-Karp M. Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc. 2007;4:247–51. doi: 10.1513/pats.200704-046AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano H, Sohma H, Muta T, Nomura S, Voelker DR, Kuroki Y. Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J Immunol. 1999;163:387–95. [PubMed] [Google Scholar]
- 28.Weikert LF, Lopez JP, Abdolrasulnia R, Chroneos ZC, Shepherd VL. Surfactant protein A enhances mycobacterial killing by rat macrophages through a nitric oxide-dependent pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L216–23. doi: 10.1152/ajplung.2000.279.2.L216. [DOI] [PubMed] [Google Scholar]
- 29.Barr FE, Pedigo H, Johnson TR, Shepherd VL. Surfactant protein-A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. Am J Respir Cell Mol Biol. 2000;23:586–92. doi: 10.1165/ajrcmb.23.5.3771. [DOI] [PubMed] [Google Scholar]
- 30.Kremlev SG, Phelps DS. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am J Physiol Lung Cell Mol Physiol. 1997;272:L1070–7. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh JC, Mervin-Blake S, Conner E, Wright JR. Surfactant protein A protects growing cells and reduces TNF-alpha activity from LPS-stimulated macrophages. Am J Physiol Lung Cell Mol Physiol. 1996;271:L310–9. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- 32.Arias-Diaz J, Garcia-Verdugo I, Casals C, Sanchez-Rico N, Vara E, Balibrea JL. Effect of surfactant protein A (SP-A) on the production of cytokines by human pulmonary macrophages. Shock. 2000;14:300–6. doi: 10.1097/00024382-200014030-00010. [DOI] [PubMed] [Google Scholar]
- 33.Chabot S, Salez L, McCormack FX, Touqui L, Chignard M. Surfactant protein A inhibits lipopolysaccharide-induced invivo production of interleukin-10 by mononuclear phagocytes during lung inflammation. Am J Respir Cell Mol Biol. 2003;28:347–53. doi: 10.1165/rcmb.4883. [DOI] [PubMed] [Google Scholar]
- 34.Lekkala M, LeVine AM, Linke MJ, Crouch EC, Linders B, Brummer E, et al. Effect of lung surfactant collectins on bronchoalveolar macrophage interaction with Blastomyces dermatitidis: inhibition of tumor necrosis factor alpha production by surfactant protein D. Infect Immun. 2006;74:4549–56. doi: 10.1128/IAI.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinker KG, Martin E, Borron P, Mostaghel E, Doyle C, Harding CV, et al. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1453–63. doi: 10.1152/ajplung.2001.281.6.L1453. [DOI] [PubMed] [Google Scholar]
- 36.Hansen S, Lo B, Evans K, Neophytou P, Holmskov U, Wright JR. Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens. Am J Respir Cell Mol Biol. 2007;36:94–102. doi: 10.1165/rcmb.2006-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L232–41. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 38.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin IL-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–72. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y, Tao JQ, Bates SR, Beers MF, Haczku A. IL-4 induces production of the lung collectin surfactant protein-D. J Allergy Clin Immunol. 2004;113:439–44. doi: 10.1016/j.jaci.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 40.Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, et al. Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D. Respir Res. 2006;7:85. doi: 10.1186/1465-9921-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haczku A, Atochina EN, Tomer Y, Cao Y, Campbell C, Scanlon ST, et al. The late asthmatic response is linked with increased surface tension and reduced surfactant protein B in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L755–65. doi: 10.1152/ajplung.00062.2002. [DOI] [PubMed] [Google Scholar]
- 42.Scanlon ST, Milovanova T, Kierstein S, Cao Y, Atochina EN, Tomer Y, et al. Surfactant protein-A inhibits Aspergillus fumigatus-induced allergic T-cell responses. Respir Res. 2005;6:97. doi: 10.1186/1465-9921-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–9. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita M, Shannon JM, Ouchi H, Voelker DR, Nakanishi Y, Mason RJ. Serum surfactant protein D is increased in acute and chronic inflammation in mice. Cytokine. 2005;31:25–33. doi: 10.1016/j.cyto.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Kumarathasan P, Blais E, Goegan P, Yagminas A, Guenette J, Adamson IY, et al. 90-day repeated inhalation exposure of surfactant Protein-C/tumor necrosis factor-alpha, (SP-C/TNF-alpha) transgenic mice to air pollutants. Int J Toxicol. 2005;24:59–67. doi: 10.1080/10915810590921379. [DOI] [PubMed] [Google Scholar]
- 46.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFalpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004;4:1817–28. doi: 10.1016/j.intimp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 48.Nakae S, Lunderius C, Ho LH, Schafer B, Tsai M, Galli SJ. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. J Allergy Clin Immunol. 2007;119:680–6. doi: 10.1016/j.jaci.2006.11.701. [DOI] [PubMed] [Google Scholar]
- 49.Vermaelen KY, Cataldo D, Tournoy K, Maes T, Dhulst A, Louis R, et al. Matrix metalloproteinase-9-mediated dendritic cell recruitment into the airways is a critical step in a mouse model of asthma. J Immunol. 2003;171:1016–22. doi: 10.4049/jimmunol.171.2.1016. [DOI] [PubMed] [Google Scholar]
- 50.Hartshorn KL, White MR, Crouch EC. Contributions of the N- and C-terminal domains of surfactant protein d to the binding, aggregation, and phagocytic uptake of bacteria. Infect Immun. 2002;70:6129–39. doi: 10.1128/IAI.70.11.6129-6139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palaniyar N, Clark H, Nadesalingam J, Shih MJ, Hawgood S, Reid KB. Innate immune collectin surfactant protein D enhances the clearance of DNA by macrophages and minimizes anti-DNA antibody generation. J Immunol. 2005;174:7352–8. doi: 10.4049/jimmunol.174.11.7352. [DOI] [PubMed] [Google Scholar]
- 52.Senft AP, Korfhagen TR, Whitsett JA, LeVine AM. Surfactant protein D regulates the cell surface expression of alveolar macrophage beta(2)-integrins. Am J Physiol Lung Cell Mol Physiol. 2007;292:L469–75. doi: 10.1152/ajplung.00297.2006. [DOI] [PubMed] [Google Scholar]
- 53.Antal JM, Cunningham JV, Goodrum KJ. Opsonin-independent phagocytosis of group B streptococci: role of complement receptor type three. Infect Immun. 1992;60:1114–21. doi: 10.1128/iai.60.3.1114-1121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu B, Jennings JH, Sonstein J, Floros J, Todt JC, Polak T, et al. Resident murine alveolar and peritoneal macrophages differ in adhesion of apoptotic thymocytes. Am J Respir Cell Mol Biol. 2004;30:687–93. doi: 10.1165/rcmb.2003-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]