Abstract
This work is to determine whether apolipoprotein E (APOE) genotype modulates the effect of cholinesterase inhibitor (ChEI) treatment on resting state functional connectivity magnetic resonance imaging (rs-fcMRI) in patients with Alzheimer’s disease (AD). We retrospectively studied very mild and mild AD participants who were treated (N=25) or untreated (N=19) with ChEIs with respect to rs-fcMRI measure of 5 resting state networks (RSNs): default mode, dorsal attention (DAN), control (CON), salience (SAL), and sensory-motor. For each network, a composite score was computed as the mean of Pearson’s correlations between pairwise time courses extracted from areas comprising this network. The composite scores were analyzed as a function of ChEI treatment and APOE ε4 allele. Across all participants, significant interactions between ChEI treatment and APOE ε4 allele were observed for all 5 RSNs. Within APOE ε4 carriers, significantly greater composite scores were observed in the DAN, CON and SAL for treated compared to untreated participants. Within APOE ε4 non-carriers, treated and untreated participants did not have significantly different composite scores for all RSNs. These data suggest that APOE genotype affects the response to ChEI using rs-fcMRI. Rs-fcMRI may be useful for assessing the therapeutic effect of medications in AD clinical trials.
Keywords: Alzheimer’s disease (AD), functional magnetic resonance imaging (fMRI), Cholinesterase inhibitor, Apolipoprotein E (APOE), resting state functional connectivity
INTRODUCTION
At autopsy there is a marked loss of cholinergic neurons in the nucleus basalis of Meynert in Alzheimer’s disease (AD) patients 1. Loss of these neurons is the basis for the cholinergic deficit in the cerebral cortex 2,3, and may contribute to clinical symptoms seen in AD 4. Cholinesterase inhibitors (ChEIs) attempt to restore this central cholinergic shortage and may help treat cognition and behavioral changes 5.
Mixed results have been observed concerning the modulation of cognitive response to ChEIs by apolipoprotein E (APOE) genotype in individuals with mild to moderate AD or those with mild cognitive impairment. Early studies showed that APOE ε4 carriers had a worse clinical response to an older ChEI (tacrine) compared to APOE ε4 non-carriers 6,7. Recent studies have suggested that newer ChEIs (donepezil, rivastigmine, and galantamine) may lead to greater cognitive improvements in APOE ε4 carriers than non-carriers 8–11. However, some studies have observed no differences between APOE ε4 carriers and noncarriers in response to treatment with ChEIs 12–14.
Resting state functional connectivity magnetic resonance imaging (rs-fcMRI) non-invasively measures the temporal correlation of spontaneous fluctuations of the blood oxygen level-dependent (BOLD) signal 15. The correlated fluctuations can be observed across spatially distributed regions that recapitulate the topographies of BOLD response induced by performance for various cognitive tasks 16. These rs-fcMRI-observed topographic patterns have been referred to as resting state networks (RSNs). Rs-fcMRI has great promise in assessing the pathophysiology of AD (see reviews by Greicius 17, Broyd et al.18). Our group has recently demonstrated that symptomatic AD participants exhibited rs-fcMRI abnormalities across multiple RSNs that progressively worsen with advancing disease stage 19. However, a limited number of rs-fcMRI studies have investigated the effect of ChEI treatment, with most primarily focused on RSNs involving the hippocampus and cingulate cortex 20,21.
The primary objective of the present work was to retrospectively investigate the effect of ChEI treatment on the integrity of multiple RSNs in patients with very mild and mild AD. In particular, we sought to determine whether APOE genotype would modulate the effect of ChEI treatment on these RSNs.
METHODS
Participants
Participants were community-dwelling volunteers enrolled in studies of aging and memory at the Charles F. and Joanne Knight Alzheimer’s Disease Research Center at Washington University in Saint Louis. Detailed information regarding recruitment has previously been published 22. Inclusion criteria for this study were: 1) a diagnosis of very mild or mild AD dementia, and 2) either not receiving medication for AD or on a stable dose of ChEIs (donepezil, rivastigmine, or galantamine) for at least 15 days, and 3) APOE genotyping.
Individuals were excluded from this study if they had neurological, psychiatric or systemic illness that might impact cognition. This study was approved by the Human Research Protection Office at Washington University in St. Louis and the Institutional Review Board at St. Louis College of Pharmacy. All participants provided written informed consent prior to participating in this study.
Clinical assessment
An experienced clinician conducted separate semi-structured interviews with the participant and a collateral source (CS). The clinician then determined whether dementia was present or absent based on the principle of intra-individual cognitive decline relative to previously attained function. The clinician’s judgment was operationalized using the Clinical Dementia Rating (CDR)23, in which CDR 0, 0.5, 1, 2, and 3 corresponded to no dementia (i.e., cognitively normal), very mild, mild, moderate, and severe dementia, respectively. Only CDR 0.5 and CDR 1 participants were included in this study. In addition, CDR-sum of boxes 24 and Mini-Mental State Examination (MMSE) 25 were obtained.
Genotyping
DNA was extracted from peripheral blood samples. Genotyping for APOE was performed using standard procedures previously described 26.
Image acquisition and pre-processing of rs-fcMRI data
MRI data were collected using a Siemens Trio 3.0 Tesla scanner with a twelve-channel head coil. High-resolution structural images were acquired with T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence (echo time [TE] = 16 msec, repetition time [TR] = 2,400 msec, inversion time [TI] = 1,000 msec, flip angle = 8°, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). A two-dimensional spin density/T2-weighted fast spin echo (T2W-FSE) scan was performed (TE = 455 msec, TR = 3,200 msec, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). Two rs-fcMRI scans (164 volumes each) were obtained using a gradient spin-echo sequence (TE = 27 msec, TR = 2.2 sec, 64 × 64 acquisition matrix, flip angle = 90°). Whole-brain coverage was achieved using thirty-six axial slices parallel to the anterior–posterior commissure line with approximately 4.0 mm cubic voxels in each volume. During rs-fcMRI scanning, participants were required to fixate on a visual cross-hair and not fall asleep. All rs-fcMRI data were preprocessed using previously described methods27. Additional details concerning rs-fcMRI data preprocessing and quality assurance are provided in Supplemental Material.
Definition of rs-fcMRI regions of interest
The procedure for generating regions of interest (ROIs) has been previously described19,28. Briefly, rs-fcMRI data acquired from a separate group of 17 healthy young adults were used to generate nodes that comprise 7 RSNs that included the default mode (DMN), dorsal attention (DAN), control (CON), salience (SAL), and auditory, visual, and somatomotor networks. rs-fcMRI data from this young adult group were analyzed using a group-wise spatial independent component analysis (sICA) based upon a published fastICA algorithm 29 implemented in Matlab. This dataset was also analyzed using seed-based correlation mapping, in which initial seed regions were created according to previously published coordinates30. Loci of matching peaks from the ICA and seed based correlation maps were selected as the centers of thirty-six spherical (6 mm radius) ROIs (representing seven RSNs) for the present analysis. Locations of these ROIs were displayed on correlation maps that were created using a representative seed from each RSN using all AD participants (Figure 1 and Supplemental Table S1). The three RSNs corresponding to the auditory, visual, and somatomotor cortices were combined into a single sensory-motor network (SMN).
Figure 1.
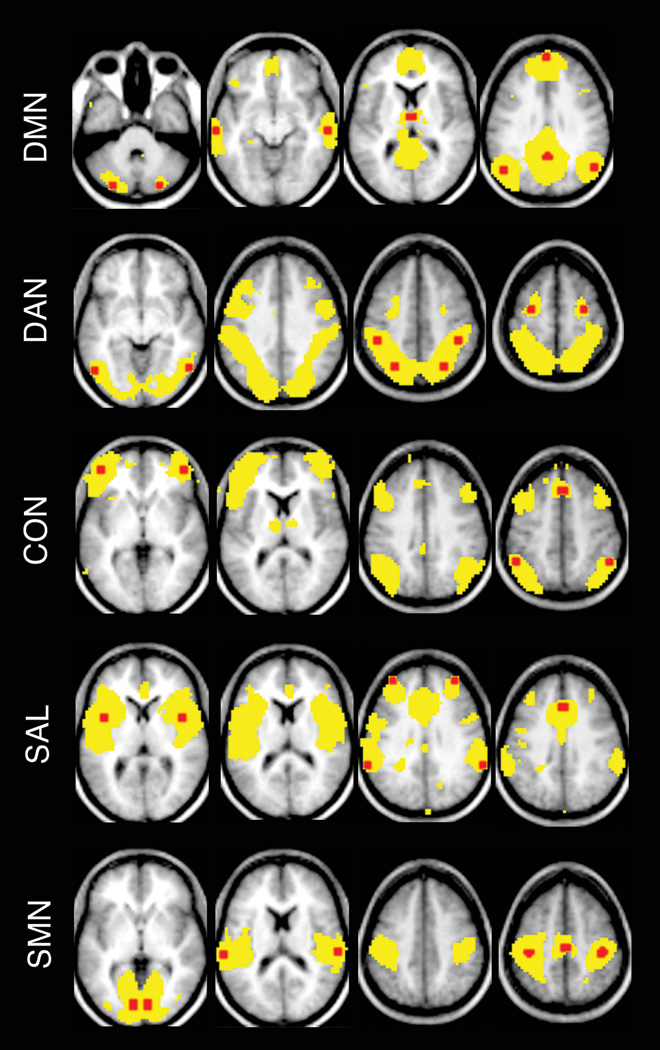
Topographies of resting state networks (RSNs) and locations of seed regions.
Using a representative seed for each RSN, correlation maps were created for all AD participants. Group average maps thresholded at z(r)>0.1 are shown using yellow. All a priori seed regions (6-mm spheres) (red) from a RSN are overlaid on the group average map. Atlas coordinates for each seed region are provided in Supplemental Table S1. In particular, the posterior cingulate cortex was used as seed region for default mode network (DMN); the left posterior intraparietal sulcus for dorsal attention network (DAN); the left anterior prefrontal cortex for control network (CON), the left insular cortex for salience network (SAL), and the left primary visual cortex, the left primary auditory cortex and the left motor cortex for the sensory-motor network (SMN).
Subject-level ROI-based measure of functional connectivity
The mean time course, extracted from the preprocessed rs-fcMRI data, was obtained from each ROI. Pearson’s correlation coefficients were computed between pairwise ROI time courses across all ROIs within a given RSN. The correlation coefficients were converted to z values using Fisher's transformation. For each RSN, correlation coefficients [z(r)] across all ROI pairs included in this network were averaged to form a composite score. The composite score has been shown to correlate with disease severity of AD 19. This approach to statistical inference achieves data reduction and reduces the impact of sampling error across node pairs. Additional analysis concerning the influence of negative correlation on composite score is presented in Supplemental Figure S1.
Statistical analysis
For each RSN, subject-level composite scores were analyzed as a function of ChEI (treated vs. untreated) and APOE genotype (presence vs. absence of at least one ε4 allele) by an analysis of variance (ANOVA). The main effects of ChEI and APOE genotype and their interaction were tested for each RSN. If a significant interaction was observed, the effect of ChEI was tested separately in APOE ε4 carriers and non-carriers, and the effect of APOE genotype was assessed separately in treated and untreated participants. The five RSNs were analyzed separately (using 5 ANOVA models) with a statistical threshold for significance of p < 0.05, uncorrected for multiple comparisons (SPSS 19.0 Chicago, IL).
RESULTS
Demographic information for the entire cohort is provided in Table 1. The demographic variables were not significantly different between the very mild and mild AD participants treated and untreated with ChEIs (all p ≥ 0.25). Within the ChEIs-treated participants (N=25), 21 received donepezil, 3 were prescribed galantamine and 1 was taking rivastigmine. For ChEIs-treated patients, the duration between initiation of treatment and acquisition of MRI ranged from 4 to 78 months with a median of 18 months.
Table 1.
Participants demographics
| Participants | Untreated | Treated | p value |
|---|---|---|---|
| N = 44 | N = 19 | N = 25 | |
| Mean Age (SD), year | 75.8 (6.9) | 76.2 (5.1) | 0.81 |
| Age range, year | 64-88 | 65-84 | |
| Sex, %Male | 42.1 | 48.0 | 0.77 |
| Mean Education (SD), year | 14.7 (2.9) | 14.7 (2.8) | 1.00 |
| Mean MMSE (SD) | 25.5 (3.7) | 26.5 (3.1) | 0.33 |
| CDR (No. of 0.5/1) | 16/3 | 21/4 | 1.00 |
| Mean CDR sum of boxes (SD) | 2.3 (1.6) | 2.8 (1.5) | 0.25 |
| APOE genotype, % ε4+ | 57.9 | 64.0 | 0.76 |
SD: standard deviation, MMSE: mini-mental state examination, for which the range of scores is from 30 (“best”) to 0 (“worst”), CDR: Clinical Dementia Rating, for which CDR 0.5 and CDR 1 indicate very mild and mild AD respectively, the CDR sum of boxes (the sum of individual CDR domain scores) range from 0 to 18, with lower scores indicating better performance. APOE: Apolipoprotein E
Effects of ChEI and APOE genotype on RSN composite scores for the entire cohort
For each RSN, an ANOVA model assessed the effects of ChEI, APOE genotype and their possible interaction on RSN composite scores of this RSN. The interaction between ChEI and APOE genotype was significant for each of the 5 RSNs (DMN: F = 4.903, p = 0.033; DAN: F = 5.022, p = 0.031; CON: F = 8.924, p = 0.005; SAL: F = 6.638, p = 0.014 and SMN: F = 4.523, p = 0.040). Across the 5 RSNs, neither the effects of ChEI nor APOE genotype were significant for any of the RSN composite scores (ChEI effect: all p ≥ 0.477; APOE ε4 effect: all p ≥ 0.144).
Effects of ChEI on RSN composite scores in APOE ε4 carriers
Demographic variables were not significantly different between ChEI-treated (N=16) and untreated (N=11) APOE ε4 carriers (all p ≥ 0.34) (Supplemental Table S2A). In general, across all 5 RSNs, the treated APOE ε4 carriers exhibited greater RSN composite scores than those APOE ε4 carriers who remained untreated. However, statistically significant increases were only observed in the DAN, CON and SAL (all p < 0.05) (Figure 1).
Effects of ChEI on RSN composite scores in APOE ε4 noncarriers
Demographic variables were also similar between the treated (N=9) and untreated (N=8) APOE ε4 non-carriers (all p ≥ 0.20) (Supplemental Table S2B). On average, treated APOE ε4 non-carriers had lower RSN composite scores than untreated APOE ε4 non-carrier individuals. However, group differences were not statistically significant (all p ≥ 0.114) (Figure 1).
We examined the effect of APOE genotype on RSN composite scores in treated and untreated AD participants separately. Significant differences were found for several RSNs between APOE ε4 non-carriers and carriers in either treated or untreated groups (Supplemental Materials).
DISCUSSION
The present work demonstrates that among individuals with very mild and mild AD, APOE ε4 carriers and non-carriers were affected differentially by ChEI treatment with respect to rs-fcMRI measures of RSN integrity. Specifically, within APOE ε4 carriers, functional connectivity was increased in all 5 RSNs in treated compared to untreated individuals, with statistically significant increases seen in the DAN, CON and SAL. In contrast, within APOE ε4 non-carriers, functional connectivity was decreased across the 5 RSNs in treated compared to untreated individuals, but none of these effects were statistically significant.
We observed that ChEI treatment is associated with significant changes in functional connectivity that occur only in APOE ε4 carriers. In the largest randomized control trial evaluating the effect of a ChEI (donepezil) in persons with mild cognitive impairment, treatment significantly delayed the progression to AD over 24 months, but only within APOE ε4 carriers 8. A sub-study derived from this trial has subsequently shown that patients treated with this ChEI had a trend toward lower rates of hippocampal atrophy compared to individuals receiving placebo, but this effect was only seen in APOE ε4 carriers 31. Our findings are consistent with these previous studies 8–11,31 suggesting that the presence of APOE ε4 allele may modulate the response to ChEIs. However, other studies 12–14 have reported that APOE ε4 allele does not affect the response to ChEI treatment. These conflicting results are difficult to reconcile and may reflect differences in sample size, outcome measures, follow-up periods, and ChEI pharmacodynamics. Although further studies are needed to elucidate the biological underpinnings for drug-genotype interactions, the present results suggest that genotyping for APOE may be beneficial for determining therapeutic strategies for very mild and mild AD individuals.
The topographic distribution of the RSNs significantly affected by ChEI treatment is consistent with prior reports. Previous studies 32,33 have compared the BOLD response to various cognitive paradigms before and after ChEI (donepezil) treatment. These studies have reported that treatment is associated with increased activation in the lateral prefrontal areas 32,33. In a positron emission tomography study comparing acetylcholinesterase activity before and after ChEI (donepezil) therapy, the greatest inhibition of acetylcholinesterase activity was observed in the anterior cingulate cortex 34. Since the lateral prefrontal cortex and the anterior cingulate comprise principle nodes of the CON and SAL respectively, the available data suggest that the enhancement of prefrontal activity may be involved by the mechanism of action for donepezil treatment.
The effect of ChEIs (in particular donepezil) on rs-fcMRI has been most recently studied using voxelwise whole brain analyses 20,21. Goveas and colleagues observed that administration of donepezil increased functional correlations between the hippocampus and multiple cortical or subcortical regions 20. Another study reports that donepezil treatment is associated with an enhanced functional connectivity between cingulate cortex and other brain areas 21. We demonstrated that ChEI treatment was associated with significant increases in functional correlations within the DAN, CON and SAL. While further work incorporating distinct analytic strategies is needed, the available data may collectively suggest that rs-fcMRI is useful for detecting the therapeutic effect in AD clinical trials.
The present work has several limitations. The present study used global signal regression as a preprocessing technique35. This technique provides a simple way to remove noise correlations associated with variations in heart rate and breathing36, however, it may mathematically induces negative correlations in seed-based analysis37,38. We defined the ROIs only from regions that showed positive correlations with a priori seeds. We computed a RSN composite score from only ROIs belonging to a particular network. These procedures maximally mitigate the confounding impacts of global signal regression on the present results. Further studies are needed to confirm our findings using alternative methods correcting for variations in heart rate and breathing39,40. Our study was limited by both cross-sectional design and small sample size. We were unable to collect data before and after treatment to determine whether the clinical response was consistent with rs-fcMRI observations (i.e., treatment-associated symptomatic improvement occurs in APOE ε4 carriers but not in APOE ε4 non-carriers). In addition, the present statistical results fail to survive stringent Bonferroni correction due to our rather limited sample size. A prospective, placebo-controlled trail with a larger sample size is needed to confirm observed effects.
Supplementary Material
Figure 2.
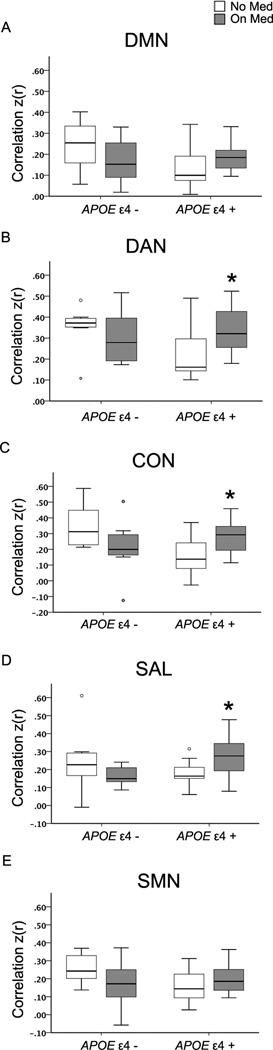
The effect of cholinesterase inhibitor (ChEI) treatment on composite score resting state functional connectivity in apolipoprotein E (APOE) ε4 allele carriers and noncarriers with very mild and mild Alzheimer’s disease.
Boxes plot with network composite scores with whiskers extending to 1.5 interquartile range.
*: p < 0.05. The circles represent outliers.
On Med refers to participants receiving ChEI treatment, No Med refers to participants not received treatment for AD. See Figure 1 for additional abbreviations.
Acknowledgements
This work was supported from support from the National Institute of Mental Health (NIMH) (K23MH081786) (BMA), National Institute of Nursing Research (NINR) (R01NR012907 and R01NR012657) (BMA), Alzheimer’s Association (BA and LW), The St. Louis College of Pharmacy through the Office for Research on Aging (ORA) (20-122-3000-1336), P01AG03991 and P50 AG05681 (JCM), and The American Roentgen Ray Society Foundation (TB).
We thank the Knight ADRC’s Clinical Core for participant assessments (participants were enrolled under NIA grants P01AG026276, P01AG03991, and P50AG05681) and Genetics Core for Apolipoprotein E genotyping.
Drs. Wang, Roe, Ances as well as Mr. Day, Mr. Brier, and Mr. Thomas report no disclosures. Dr. Benzinger consults for Biomedical Systems, Inc. and ICON Medical Imaging and receives research support from Avid Radiopharmaceuticals. Dr. Morris is currently participating in clinical trials of anti-dementia drugs sponsored by Janssen Alzheimer Immunotherapy, Eli Lilly, and Pfizer. He reports consulting for AstraZeneca, Bristol-Myers Squibb, Eisai, Elan/Janssen Alzheimer Immunotherapy Program, Genentech, Eli Lilly, Merck, Novartis, Otsuka Pharmaceuticals, Pfizer/Wyeth, and Schering Plough.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 2.Bowen DM, Smith CB, White P, et al. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976 Sep;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 3.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 4.Perry EK, Blessed G, Tomlinson BE, et al. Neurochemical activities in human temporal lobe related to aging and Alzheimer-type changes. Neurobiology of aging. 1981 Winter;2(4):251–256. doi: 10.1016/0197-4580(81)90032-4. [DOI] [PubMed] [Google Scholar]
- 5.Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56(9):1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 6.Poirier J, Delisle MC, Quirion R, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farlow MR, Lahiri DK, Poirier J, et al. Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer's disease. Neurology. 1998 Mar;50(3):669–677. doi: 10.1212/wnl.50.3.669. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005 Jun 9;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 9.Patterson CE, Todd SA, Passmore AP. Effect of apolipoprotein E and butyrylcholinesterase genotypes on cognitive response to cholinesterase inhibitor treatment at different stages of Alzheimer's disease. The pharmacogenomics journal. 2011 Dec;11(6):444–450. doi: 10.1038/tpj.2010.61. [DOI] [PubMed] [Google Scholar]
- 10.Bizzarro A, Marra C, Acciarri A, et al. Apolipoprotein E epsilon4 allele differentiates the clinical response to donepezil in Alzheimer's disease. Dementia and geriatric cognitive disorders. 2005;20(4):254–261. doi: 10.1159/000087371. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Kim SY, Na HR, et al. Effect of ApoE genotype on response to donepezil in patients with Alzheimer's disease. Dementia and geriatric cognitive disorders. 2008;25(5):445–450. doi: 10.1159/000124752. [DOI] [PubMed] [Google Scholar]
- 12.Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001 Aug 14;57(3):489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 13.Aerssens J, Raeymaekers P, Lilienfeld S, et al. APOE genotype: no influence on galantamine treatment efficacy nor on rate of decline in Alzheimer's disease. Dementia and geriatric cognitive disorders. 2001 Mar-Apr;12(2):69–77. doi: 10.1159/000051238. [DOI] [PubMed] [Google Scholar]
- 14.Rigaud AS, Traykov L, Latour F, et al. Presence or absence of at least one epsilon 4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer's disease. Pharmacogenetics. 2002 Jul;12(5):415–420. doi: 10.1097/00008571-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology. 2008 Aug;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 18.Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience and biobehavioral reviews. 2009 Mar;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Brier MR, Thomas JB, Snyder AZ, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci. 2012 Jun 27;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goveas JS, Xie C, Ward BD, et al. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer's disease patients treated with donepezil assessed by resting-state fMRI. Journal of magnetic resonance imaging : JMRI. 2011 Oct;34(4):764–773. doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Antuono PG, Xie C, et al. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer's disease after 12-week donepezil treatment. NeuroImage. 2012 Apr 2;60(2):1083–1091. doi: 10.1016/j.neuroimage.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998 Mar;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 24.Berg L, Miller JP, Baty J, et al. Mild senile dementia of the Alzheimer type. 4. Evaluation of intervention. Ann Neurol. 1992 Mar;31(3):242–249. doi: 10.1002/ana.410310303. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol. 2003 Aug;54(2):163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 27.Shulman GL, Pope DL, Astafiev SV, et al. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010 Mar 10;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon BJ, Raichle ME, Snyder AZ, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Network. 1999;10(10):626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010 Jan;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 31.Jack CR, Jr, Petersen RC, Grundman M, et al. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiology of aging. 2008 Sep;29(9):1285–1295. doi: 10.1016/j.neurobiolaging.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004 Jul;127(Pt 7):1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 33.Petrella JR, Prince SE, Krishnan S, et al. Effects of donepezil on cortical activation in mild cognitive impairment: a pilot double-blind placebo-controlled trial using functional MR imaging. AJNR. American journal of neuroradiology. 2009 Feb;30(2):411–416. doi: 10.3174/ajnr.A1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohnen NI, Kaufer DI, Hendrickson R, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 2005 Mar;76(3):315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox MD, Zhang D, Snyder AZ, et al. The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology. 2009 Jun;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dijk KR, Hedden T, Venkataraman A, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010 Jan;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy K, Birn RM, Handwerker DA, et al. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009 Feb 1;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissenbacher A, Kasess C, Gerstl F, et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009 Oct 1;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 39.van Buuren M, Gladwin TE, Zandbelt BB, et al. Cardiorespiratory effects on default-mode network activity as measured with fMRI. Human brain mapping. 2009 Sep;30(9):3031–3042. doi: 10.1002/hbm.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009 Oct 1;47(4):1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


