Abstract
Purpose
Following uniocular anterior chamber (AC) inoculation of HSV-1, the anterior segment of the injected eye becomes inflamed and infected; however, virus does not spread from the anterior segment and infect the retina of the injected eye. The purpose of this study was to identify early infiltrating cells and to determine if infiltrating cells produced interferon γ (IFNγ).
Methods
Euthymic, female, BALB/c mice were injected in one AC with 3 × 104 PFU of HSV-1 (KOS) in a volume of 2µl. Mice from each group were sacrificed at 12, 24, 36, 48, and 72 hrs post injection (p.i.), the eyes were enucleated and frozen sections were stained with antibodies specific for IFNγ, Mac-1 (CD11b), CD49b, F4/80, CD4, CD8 and CD11c. The same antibodies were also used to stain single cell suspensions of ocular cells for flow cytometry.
Results
In the anterior segment of the injected eye, the ciliary body and iris were virus infected and inflamed, and infiltrating cells increased throughout the period of observation. Mac-1+, CD49b+ and F4/80+ cells co-localized with IFNγ in the anterior segment as early as 12hrs p.i., and the percentage of Mac-1+ cells increased in the injected eye beginning at 24hrs p.i. and continued to 72hrs p.i.
Conclusions
Taken together, these results demonstrate that activated microglia are important IFNγ producing cells in the injected eye before day 3 and suggest that IFNγ produced by these cells may be involved in inhibition of anterior to posterior spread of virus in the injected eye.
Keywords: natural killer cells, interferon gamma, immunohistochemistry, flow cytometry, acute retinal necrosis, macrophages, microglia, herpes simplex virus
INTRODUCTION
Acute retinal necrosis (ARN) is a rare but visually devastating disease characterized by circumferential and rapid progression of necrosis, occlusive vasculopathy, vitritis, and inflammation of the anterior segment. In the absence of treatment, ARN usually results in blindness in the affected eye1, 2. In a mouse model of ARN, following inoculation of HSV-1 (KOS) into the AC of one eye of a BALB/c mouse, virus replicates in the anterior segment of the injected eye and then spreads via parasympathetic neurons to the ipsilateral ciliary ganglion at day 2 p.i., the ipsilateral Edinger-Westphal nucleus at day 3 p.i., the ipsilateral suprachiasmatic nucleus (SCN) at day 5 p.i., finally reaching the contralateral optic nerve and retina at day 7 p.i. Despite there being no anatomical barrier between the anterior and posterior segments, virus does not spread directly from the anterior segment to the retina or optic nerve of the injected eye3–6. While the retina of the injected eye is spared in immunocompetent BALB/c mice, thymectomized and T cell depleted BALB/c mice develop bilateral retinitis7. In T cell depleted mice, virus infects both the ipsilateral and contralateral SCN and both optic nerves, but even in the absence of T cells, there is no evidence of direct anterior to posterior spread of virus in the injected eye8–10. In contrast, direct anterior to posterior spread of virus is observed in natural killer (NK) cell depleted BALB/c mice resulting in retinitis in the ipsilateral eye11. These results suggest that NK cells play an important role in protecting the ipsilateral retina by preventing direct anterior to posterior spread of the virus early in the course of HSV-1 infection.
Interferons are important antiviral cytokines involved in activation and differentiation of immune cells via intracellular signaling mechanisms. Interferons initiate production of proteins which inhibit translation and cell growth, induce apoptosis, and promote down regulation of mRNA12, 13. Interferon-gamma (IFNγ) is known as the “immune interferon” because it is specifically produced by cells of the immune system: NK cells, macrophages, neutrophils and T cells14–16, whereas, IFNs α and β are produced by a variety of cell types13, 17. Several studies have demonstrated that IFNγ plays an important role in viral pathogenesis by over expressing IFNγ in the eye and by infection of IFNγ deficient mice. Collectively these studies indicate that IFNγ plays an important role in clearance of viral infection, including HSV-118–22.
Although virus infection is required for the inflammatory response in the injected eye, in order to decipher the pathogenesis of the infection in this eye, the infiltrating cells must be identified, the time of their arrival must be determined, and their capabilities must be understood. Therefore, the overall goal of these studies was to identify the cells which infiltrate the eye after uniocular AC inoculation of HSV-1 and to determine if infiltrating cells produced IFNγ.
MATERIALS AND METHODS
Animals
Adult female BALB/c mice, 6 to 12 weeks old (Taconic, Germantown, NY), were used in all experiments. The mice were housed in accordance with National Institutes of Health guidelines. All study procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia. Mice were maintained on a 12hr light-dark cycle and were given unrestricted access to food and water. Mice were anesthetized with 0.5 to 0.7 ml/kg of a mixture of 42.9 mg/ml ketamine, 8.57 mg/ml xylazine, and 1.43 mg/ml acepromazine before all experimental manipulations. Each group in each experiment had a minimum of 5 mice and experiments were repeated at least once.
Virus
The KOS strain of HSV-1 was used in all experiments. Stock virus was prepared by low multiplicity of infection (0.1 PFU/cell) passage of Vero cells grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 5% fetal bovine serum (FBS) (Hyclone; Logan, UT) and antibiotics. The titer of the virus stock was determined by plaque assay on Vero cells. Aliquots of stock virus were stored at −80°C and a fresh aliquot was thawed and diluted for each experiment.
Anterior chamber (AC) inoculation
Mice were anesthetized and inoculated using the AC route as previously described3. The right eye was proptosed and 3 ×104 plaque-forming units (PFU) of HSV-1 (KOS) in a volume of 2µl were injected into the AC with a 30-gauge needle attached to a 100µl microsyringe (Hamilton, Reno, NV). The inoculum was prepared diluting virus stock in DMEM with antibiotics. Mock injected mice were inoculated in the AC with 2µl of uninfected Vero cell extract diluted in DMEM with antibiotics.
Immuno-electron microscopy (IEM)
Mice were anesthetized and sacrificed by cervical dislocation. Injected eyes were collected at 24, 48 and 72hrs from mock and virus injected mice and fixed in 4% formaldehyde, 0.2% glutaraldehyde in 0.1M NaCacodylate buffer for at least 24hrs at 4°C. The eyes were then dehydrated in a graded alcohol series and embedded in pure resin (LR White, Electron Microscopy Sciences [EMS], Hatfield, PA). Seventy-five nm sections were collected on Pioloform (Ted Pella, Redding, CA) coated, nickel slot grids (EMS). Grids were incubated in blocking solution (1% BSA, 3% normal goat serum [NGS Vector Laboratories, Burlingame, CA], and 0.05% Tween 20 in Tris-buffered saline) for 2hrs, followed by rabbit anti-HSV-1 (Accurate Chemical & Scientific Corporation; Westbury, NY)/anti-rabbit colloidal gold, stained in methanolic uranyl acetate and examined using a Jeol Jem 1230 transmission electron microscope.
Flow cytometry
Single-cell suspensions were prepared from 6 pooled whole injected eyes of normal control, mock injected mice at 24hrs p.i. and from HSV-1 (KOS) infected mice at 24, 48, 72 and 120hrs p.i. The eyes were incubated in 58.5 U/ml of collagenase IV (Sigma-Aldrich, St. Louis, MO) in Hanks Balanced Salt Solution (HBSS, Cellgro Mediatech, Manassas, VA) for 1hr at 37°C, 5% CO2 and pressed through a 70µm nylon cell strainer (BD Falcon, Bedford, MA). Cells were suspended in HBSS and centrifuged at 250 × g at 4°C for 5 min, resuspended in PBS containing 1% FBS, counted and then blocked with 10% mouse serum (Sigma-Aldrich) diluted in staining buffer (1% FBS in PBS) for 15 min at 4°C. Fluorescein (FITC)-anti mouse CD11b (Integrin αM chain, Mac-1 α chain; BD Pharmingen; San Diego, CA), FITC rat IgG2b,κ isotype control (BD Pharmingen), FITC-anti mouse F4/80 (Caltag, Burlingame, CA), Alexa Fluor 488 rat IgG2a isotype control (Caltag), FITC-anti mouse CD4 (L3T4, BD Pharmingen), FITC-anti mouse CD8a (Ly-2, BD Pharmingen), FITC rat IgG2a,κ isotype control (BD Pharmingen), FITC-anti mouse CD49b/Pan-NK cells (BD Pharmingen), FITC rat IgM,κ isotype control (BD Pharmingen), allophycocyanin (APC)-anti mouse CD11c (BD Pharmingen), and APC hamster IgG1,λ isotype control (BD Pharmingen) antibodies were used to identify cells. Flow cytometry of stained cell samples was performed using a FACSCalibur (Becton Dickinson; Franklin Lakes, NJ) and flow cytometry data were collected and analyzed using Cellquest software (Becton Dickinson).
Immunohistochemistry
Mice were deeply anesthetized and perfused transcardially with PBS for approximately 3 min. After perfusion, the injected eye of each mouse was removed, snap-frozen, and embedded in Tissue-Tek O.C.T. Compound (EMS). Eight-to-10µm sections were prepared on positively charged slides (Fisher Scientific, Pittsburgh, PA) using a Microm HM505E Cryostat (EquipNet, Canton, MA). The frozen sections of inoculated eyes were fixed with 4% paraformaldehyde, washed in PBS and blocked with 10% NGS (Vector Laboratories), 1% BSA (Fisher Scientific) and 0.5% Triton X-100 (Sigma-Aldrich) diluted in PBS for 2 hrs. Sections were incubated with anti mouse IFNγ (RMMG-1, PBL Biomedical Laboratories; Piscataway, NJ) washed in PBS, incubated with Texas Red-anti rat (Jackson ImmunoResearch Laboratories; West Grove, PA), washed in PBS, and blocked with 3% rat serum (Sigma-Aldrich) diluted in PBS. Sections were then stained with one of the following antibodies: FITC-anti mouse CD11b (Integrin αM chain, Mac-1 α chain; BD Pharmingen), FITC-anti mouse F4/80 (Caltag Laboratories), FITC-anti mouse CD4 (L3T4, BD Pharmingen), FITC-anti mouse CD8a (Ly-2, BD Pharmingen),or FITC-anti mouse CD49b/Pan-NK cells (BD Pharmingen), washed with PBS, and mounted with VectorShield containing DAPI (Vector Laboratories). Slides were examined using a fluorescence microscope and images were captured using SPOT Advanced (Diagnostic Instruments, Sterling Heights, MI) or AxioVision 4.6 (Carl Zeiss, Germany) computer programs.
Hematoxylin and Eosin (H and E) staining
The inoculated eye was removed, snap-frozen, and embedded in Tissue-Tek O.C.T. Compound (EMS) as described above. Frozen sections were fixed in cold acetone for 5 min, stained with hematoxylin and eosin (Fisher Scientific), washed, dehydrated in a graded alcohol series, mounted with cytoseal (Richard Allan Scientific, Kalamazoo, MI), and allowed to dry overnight.
RESULTS
HSV-1 in the anterior segment of the injected eye
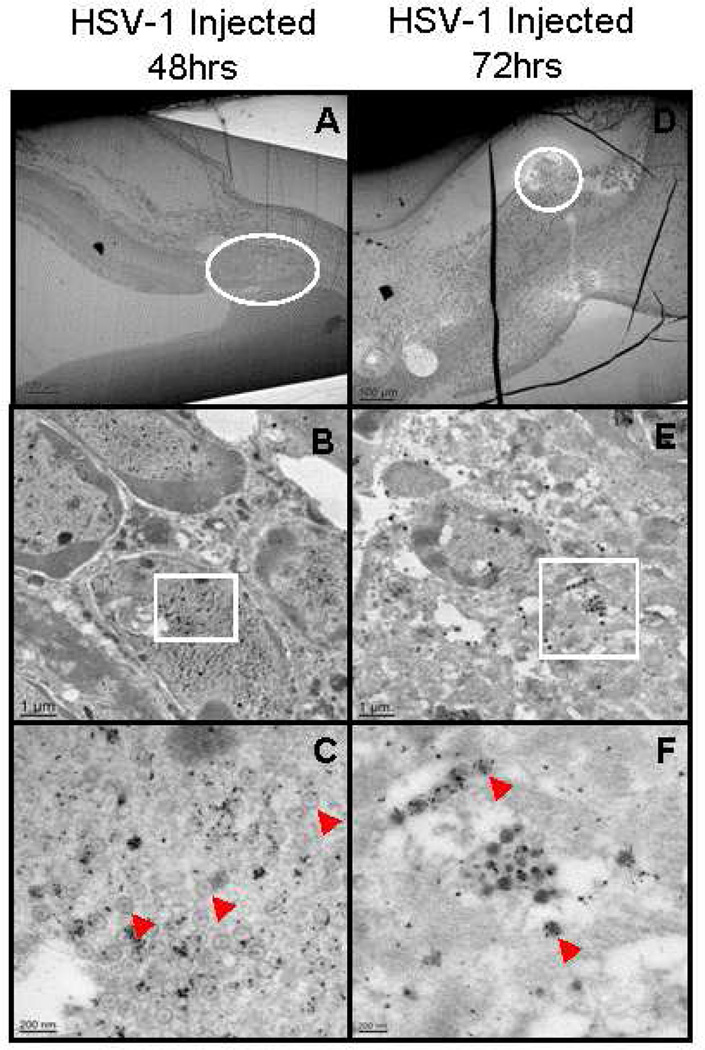
IEM was used to identify virus and viral proteins in cells of the anterior segment early after infection. HSV-1 capsids were not observed in the anterior segment of virus infected mice at 24hrs p.i. (not shown). HSV-1 capsids (Figure1C and 1F, arrowhead) and viral proteins (Figure1C and 1F, gold particles) were present in the nucleus and cytoplasm (Figure 1B, 1C, 1E and 1F) of cells in the anterior segment (Figure1A and 1D) at 48 and 72hrs p.i. HSV-1 capsids were not observed in normal control or mock injected mice (not shown).
Figure 1.
Electron micrographs showing virus capsids (arrows) and virus proteins (identified by colloidal gold particles) in the cytoplasm and nucleus of cells in the anterior segment of the injected eye at 48 and 72hrs p.i. B and E, enlargement of area circled in A and D. C and F enlargement of boxed area in B and E. Original magnification: A and D – 50×; B and E – 5,000×; C – 25,000×, F – 20,000×
Infiltrating cells in the injected eye
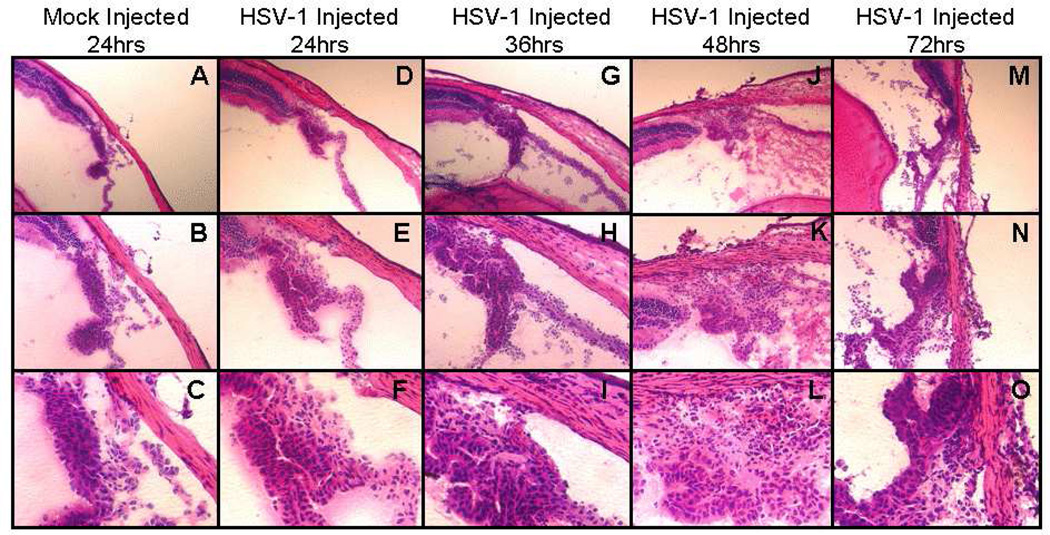
H and E staining was used to examine inflammation and cell infiltration in the anterior segment of the injected eye after HSV-1 infection. H and E staining showed structural disorganization and infiltrating cells in the anterior segment beginning at 36hrs p.i. and continuing through 72hrs p.i. (Figure 2H, 2K and 2N). Cellular debris from necrotic and/or apoptotic cells was observed in the anterior and posterior chambers of virus infected mice beginning at 36hrs p.i. (Figure 2G, 2J, and 2M). Moreover, cells with multilobed nuclei, characteristic of neutrophils, were observed beginning 48hrs after infection in the ciliary body, iris (Figure2L and 2O) and cornea (not shown).
Figure 2.
Photomicrographs of hematoxylin and eosin staining of the ciliary body in the injected eye of mock injected mice 24hrs p.i. and of virus injected mice 48, 72 and 120hrs infected mice (original magnification: A, D, G, J, M – 100×; B, E, H, K, N – 200×; C, F, I, L,O – 400×).
Flow cytometry was used to quantify cell types activated and/or infiltrating the injected eye after AC injection of HSV-1 (KOS). As shown in Table 1, the number of cells in the eyes of mock inoculated mice was only slightly increased (0.56%) compared with normal, non-injected control eyes. In HSV-1 infected mice, the total number of cells per virus injected eye increased by 29% at 24hrs p.i., 22% at 48hrs p.i., and 44% at 72hrs p.i. At 120hrs p.i., the number of cells had decreased slightly to 39%.
Table 1.
Number of cells per eye
| Normal Control |
Mock Injected 24hrs |
HSV-1Injected 24hrs |
HSV-1 Injected 48hrs |
HSV-1 Injected 72hrs |
HSV-1 Injected 120hrs |
|---|---|---|---|---|---|
| 5.21 ×106 | 5.24×106 (0.56%)* | 7.34×106 (29%) | 6.64×106 (22%) | 9.23×106 (44%) | 8.60×106 (39%) |
% increase compared with normal control, average of two independent experiments
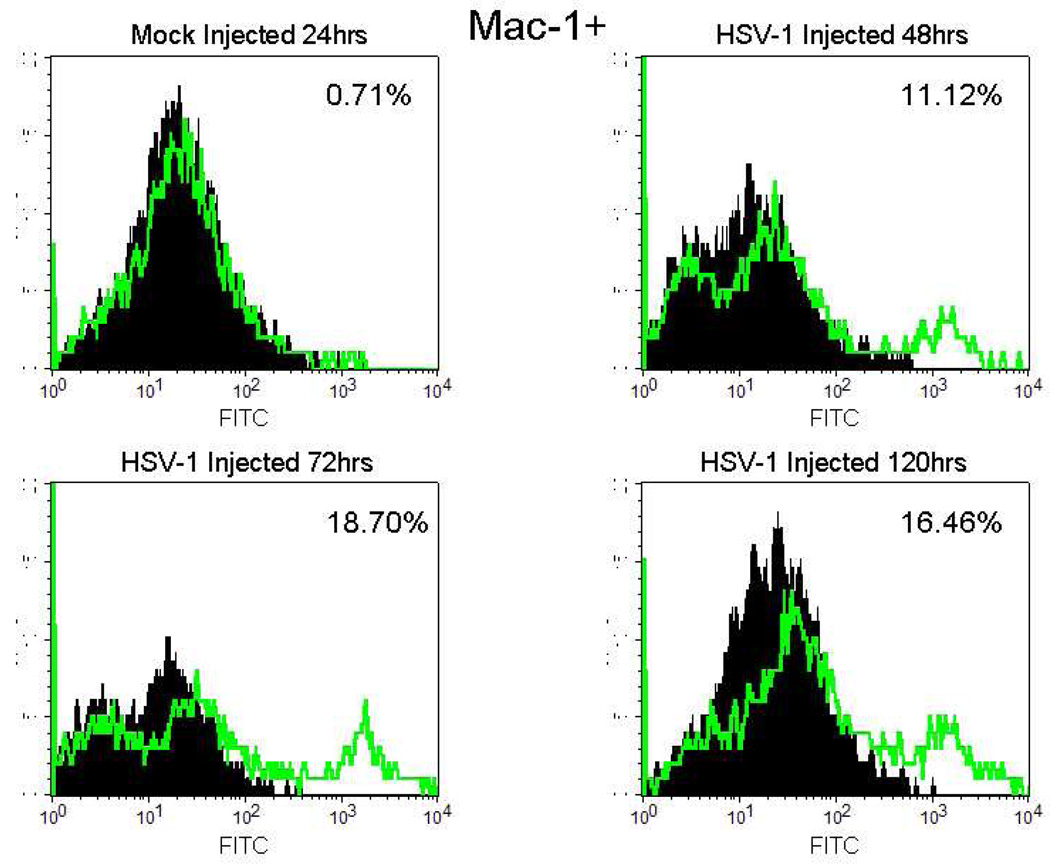
Detection of cellular markers was used to quantify specific immune cell types: Mac-1 (activated microglia), F4/80 (systemic macrophages), CD4 (T helper cells) CD8 (cytotoxic T cells), CD49b (pan NK cells), and CD11c (plasmacytoid dendritic cells). As shown in Table 2, the percent of Mac-1+ cells in the eye of normal control mice and of mock injected mice was low (0.80% and 0.71% respectively). In the injected eye of HSV-1 infected mice, the percent of Mac-1+ cells had increased to 3.59% at 24hrs p.i. and remained elevated at 20hrs p.i. (16.46%) (Table 2 and Figure 3). No F4/80+ cells were observed in normal eyes or mock injected eyes while a small percentage of the cells were F4/80+ at 72hrs p.i. and 120hrs p.i. (0.61% and 1.87% respectively) (Table 2). The percentage of CD49b+ cells was increased above mock-injected eyes only at 120hrs p.i. (3.4%) (Table 2). There were no differences in the percentage of CD4+, CD8+ or CD11c+ cells between normal control or mock injected mice and virus injected mice at any time point (Table 2). In these studies, it would have been ideal to use flow cytometry to quantify the cell types producing IFNγ by double staining for cell type and IFNγ. However, we were unable to double stain cell suspensions for cellular markers and IFNγ perhaps because of the extensive manipulation of the cells required to double stain cells for flow cytometry.
Table 2.
Types of infiltrating cells after uniocular anterior chamber inoculation of HSV-1.
| Group | Mac1+ (%) | F4/80+ (%) | CD4+ (%) | CD8+ (%) | CD49b+ (%) | CD11c+ (%) |
|---|---|---|---|---|---|---|
| Normal Control | 0.80* | 0 | 0.83 | 0 | 0 | 0 |
| Mock Injected 24hrs | 0.71 | 0 | 0 | 0.32 | 0.87 | 0 |
| HSV-1 Injected 24hrs | 3.59 | 0 | 0 | 0.04 | 0.62 | 0.04 |
| HSV-1 Injected 48hrs | 11.12 | 0 | 0.23 | 0.41 | 0.85 | 0 |
| HSV-1 Injected 72hrs | 18.70 | 0.61 | 0 | 0 | 0.75 | 0 |
| HSV-1 Injected 120hrs | 16.46 | 1.87 | 0.94 | 0.61 | 3.4 | 0 |
Percentages are % of isotype matched control staining subtracted from % of cell marker staining. Data are representative of two independent experiments
Figure 3.
Flow cytometric histograms of infiltrating cells isolated from mock injected mouse eyes at 24hrs p.i. and HSV-1 (KOS) injected eyes at 48, 72 and 120hrs p.i. Cells were stained with FITC-conjugated anti mouse Mac-1 (green line) or FITC-conjugated Rat IgG2b,κ (solid black). Results were analyzed by flow cytometry. Percentages are % of isotype matched control staining subtracted from % of cell marker staining. Data are representative of two independent experiments.
IFNγ production and immune cells
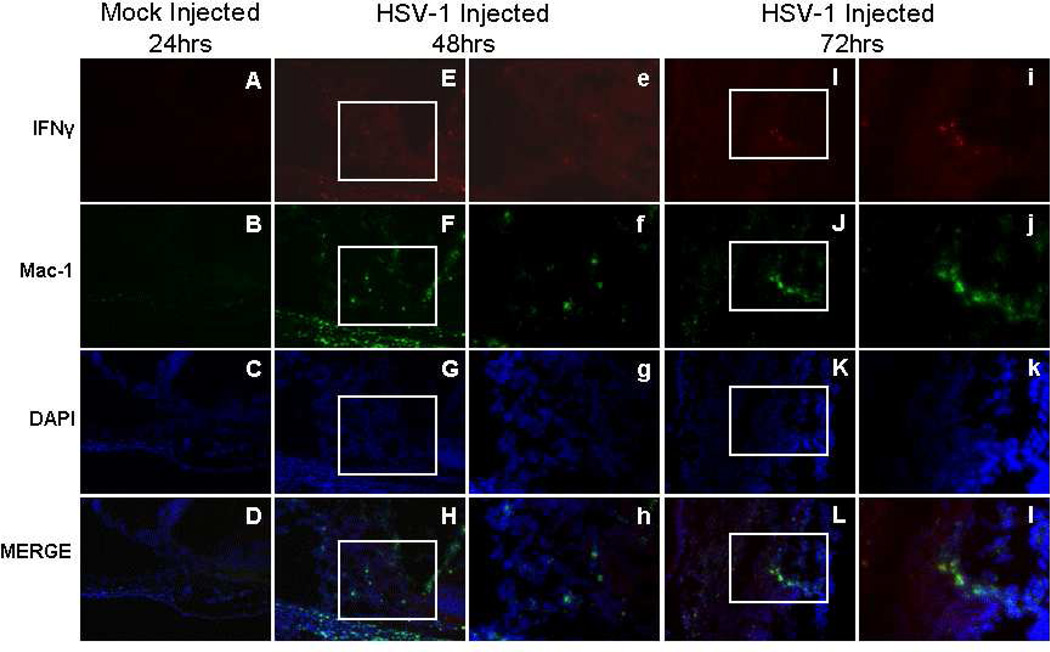
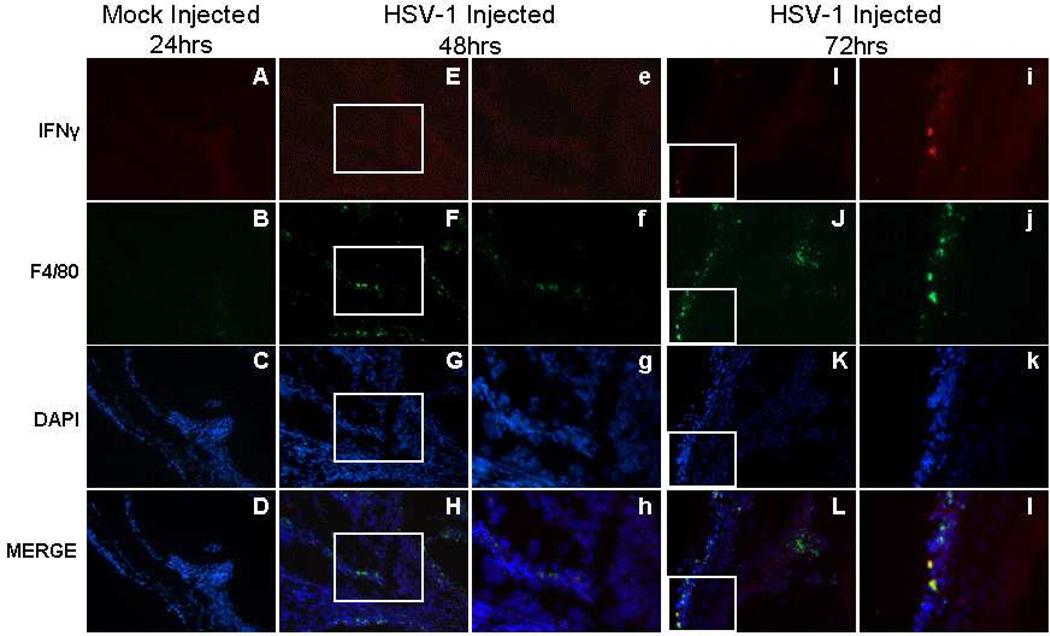
Immunofluorescence was used to identify cells secreting IFNγ and to determine their location in the injected eye following uniocular AC inoculation of HSV-1 (KOS). Activated microglia (Mac-1+), systemic macrophages (F4/80+) and NK cells (CD49b+) were observed in the anterior segment of the injected eye as early as 12hrs p.i. (not shown) and continuing to 72hrs p.i. (Figures 4, 5, and 6). An occasional Mac-1+, F4/80+, or CD49b+ cell was observed in normal control (not shown) or mock injected animals. Single stained IFNγ+ cells or cells double stained for IFNγ and Mac-1, IFNγ and F4/80, or IFNγ and CD49b were rarely observed in control animals. Beginning at 12hrs p.i., Mac-1+IFNγ+ cells were observed in the limbus of the anterior segment of the injected eye in 56% of the mice. As shown in Figure 4, some but not all, Mac-1+ cells were also IFNγ+ and IFNγ+Mac-1+ cells were observed in the limbus, ciliary body, iris and cornea at 48 and 72hrs p.i. (Table 3; Figure 4).
Figure 4.
Photomicrographs of the ciliary body of the injected eye showing the location of IFNγ+ and Mac-1+ cells in mock injected animals 24hrs p.i. and in virus injected animals 48 and 72hrs p.i., A–L (200×); e–l (400×).
Figure 5.
Photomicrographs of the ciliary body of the injected eye showing the location of IFNγ+ and F4/80+ cells in mock injected animals 24hrs p.i. and in virus injected animals 48 and 72hrs p.i., A–L (200×); e–l (400×).
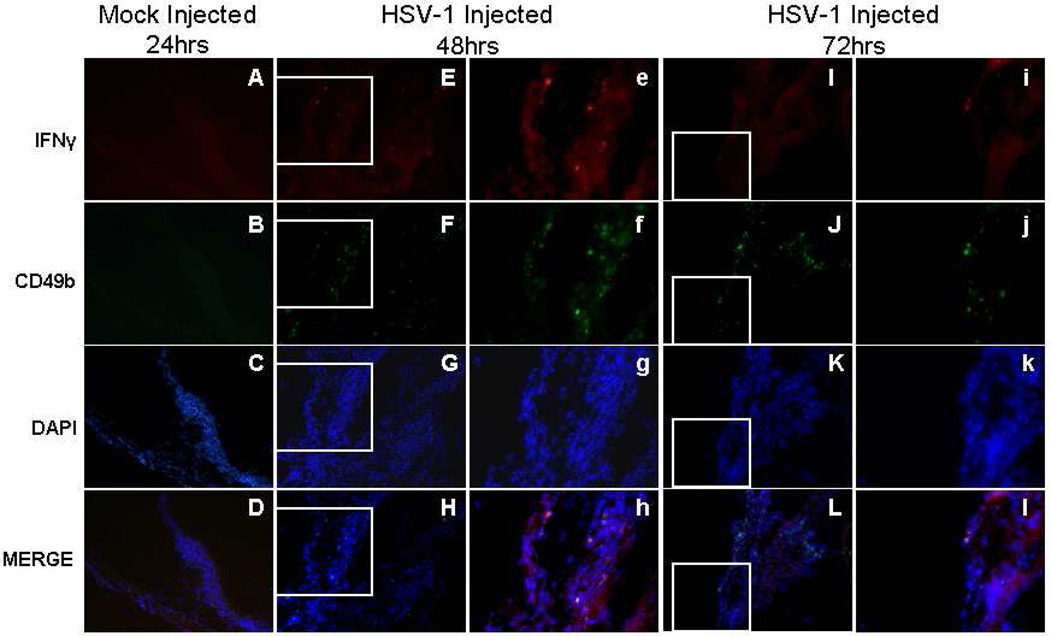
Figure 6.
Photomicrographs of the ciliary body of the injected eye showing the location of IFNγ+ and CD49b+ cells in mock injected animals 24hrs p.i. and in virus injected animals 48 and 72hrs p.i., A-L (200×); e–l (400×).
Table 3.
IFNγ positive microglia, macrophages, and NK cells in the anterior segment
| Group | IFNγ+Mac-1+ | IFNγ+F4/80+ | IFNγ+CD49b+ |
|---|---|---|---|
| Normal Control | 0/3*(0%) | 0/3(0%) | 0/3(0%) |
| Mock Injected 24hrs | 0/4(0%) | 0/3(0%) | 0/4(0%) |
| HSV-1 Injected 12hrs | 5/9(56%) | 3/9(33%) | 2/9(22%) |
| HSV-1 Injected 24hrs | 10/11(91%) | 9/11(82%) | 4/11(36%) |
| HSV-1 Injected 36hrs | 8/9(89%) | 8/9(89%) | 5/9(56%) |
| HSV-1 Injected 48hrs | 9/9(100%) | 9/9(100%) | 9/9(100%) |
| HSV-1 Injected 72hrs | 7/7(100%) | 6/7(86%) | 6/7(86%) |
Number of mice with IFNγ+ and Mac-1+ cells, IFNγ+ and F4/80+ cells, and IFNγ+ and CD49b+ cells in the anterior segment/total (% positive)
F4/80+ cells expressing IFNγ were observed beginning at 12hrs p.i. in and around the limbus of 33% of the mice (Table 3). As shown in Figure 5, some but not all F4/80+ cells, were also IFNγ+, and F4/80+IFNγ+ cells were observed in the limbus, ciliary body, iris and cornea of most of the mice beginning at 24hrs and continuing to 72hrs p.i. (Table 3; Figure 5).
CD49b+ cells expressing IFNγ were observed beginning at 12hrs p.i. in the limbus of the anterior segment in 22% of mice (Table 3), and as shown in Figure 6, some but not all, CD49b+ cells were also IFNγ+. CD49b and IFNγ double positive cells were observed through 72hrs p.i. in the limbus, ciliary body, iris and cornea (Table 3; Figure 6).
Both CD8+ and CD4+ cells expressing IFNγ were observed at 24hrs p.i. in the limbus of the anterior segment (not shown). Some, but not all, CD4+ or CD8+ cells were also IFNγ+, and CD4+IFNγ+ cells as well as CD8+IFNγ+ cells were observed through 72hrs p.i. (not shown).
DISCUSSION
Polymorphonuclear leukocytes (PMNs) and NK cells have been implicated in protecting the ipsilateral retina from direct anterior to posterior spread of HSV-1 (KOS) following uniocular AC injection11, 23 but the mechanisms by which these immune cells control virus spread in the injected eye have not been elucidated. IFNγ is an antiviral cytokine produced by cells of the innate immune response (neutrophils, macrophages, microglia, and NK cells)14–16, 24, 25. Since the innate immune response is initiated between 0–96 hours after infection and HSV-1 (KOS) titers in the inoculated eye peak around day 2 or day 3 p.i.26, it is reasonable to expect that IFNγ producing cells arrive and/or are activated on or before day 3 p.i.
Uniocular AC inoculation of HSV-1 (KOS) results in acute inflammation of the anterior segment, involving the cornea, iris and ciliary body, with cell infiltration and loss of structural integrity beginning 24hrs p.i.3. Early in infection, virus is present in the ciliary body and iris of the injected eye4. Virus infection of the anterior segment was confirmed in this study by IEM, and viral antigen positive cells and viral capsids were observed in the nucleus and cytoplasm of cells in the ciliary body and iris of the injected eye at 48 and 72hrs p.i. While signs of virus infection and virus have been observed in the anterior segment as early as 24hrs p.i.3, 4, no capsids were seen by EM at this time, a finding which is probably not surprising due to the time required for a cycle of virus replication, 18–20hrs in cell culture and somewhat longer in vivo27.
The retina develops from the diencephalon of the neural tube and is considered part of the CNS28. Microglia are the resident macrophages of the CNS; these cells have been previously observed in the AC of the eye after HSV-1 infection and have also been implicated in IFNγ production25, 29. In this study, IFNγ producing microglia were observed in the anterior segment of the injected eye beginning at 12hrs p.i. and extending to at least 72hrs p.i. These results suggest that microglia migrate to the anterior segment of the injected eye where they proliferate and contribute to early protection of the ipsilateral retina (on or before day 3 p.i.) by production of IFNγ. This idea is not without precedent, since microglia migrate in the retina, proliferate, become more phagocytic and produce cytokines in response to retinal degeneration30, 31.
The Mac-1 antigen, also known as the complement receptor (CR3), is expressed on subtypes of neutrophils, macrophages, myeloid dendritic cells, NK cells, microglia and B cells32–35. Since neutrophils have been observed in the HSV-1 injected ipsilateral eye prior to day 323, Mac-1+ cells may represent a subpopulation of neutrophils.
NK cells are major producers of IFNγ during the innate immune response and they have been shown to play a role in protecting mice from HSV-1 infections11, 36, 37. Although CD49b+ staining (and co-localization with IFNγ+ cells) was observed from 24 to 72hrs p.i., the largest number of CD49b+ NK cells was observed at 120hrs p.i. in virus injected mice. In NK depleted mice, evidence of HSV-1 infection of the retina in the injected eye is observed by day 511. The results presented in this manuscript suggest that NK cells contribute to protection of the ipsilateral retina beginning around day 4 p.i. Although the reason for the several days delay in recruitment of these cells is not known, one possible explanation is that the delay in recruitment of NK cells to this eye reflects the time until the blood ocular barrier has been compromised which, in turn, allows NK cells to enter the eye.
Macrophages are important antigen presenting cells in the innate immune response and previous results suggest macrophages are a source of IFNγ in the uninoculated contralateral eye of BALB/c mice on and after day 9 p.i.14. Other studies have shown that cultured macrophages from BALB/c mice produce IFNγ after stimulation with IL-12 and IL-1815. In the studies presented herein, F4/80+ staining (and co-localization with IFNγ+ cells) was observed from 24 to 72 hrs p.i. However, in the lfow cytometry studies, F4/80+ systemic macrophages were not detected in the virus injected eye until 72hrs p.i. and even at 120hrs p.i. only 1.87% of the infiltrating cells were F4/80+. F4/80+ macrophages are resident in the AC of normal mice38 and are important in anterior chamber associated immune deviation (ACAID), in which delayed type immunity is suppressed and pathogens are eliminated in the absence of inflammation by excluding effector immune cells39. There are two possible reasons why only a few F4/80+ cells were identified in the injected eye early after HSV-1 infection of the AC: 1) phagocytic macrophage recruitment and entry to the infected eye is prevented by the blood ocular barrier or 2) antigen presenting resident macrophages migrate from the eye to initiate ACAID39.
There were no differences in numbers of CD4+, CD8+ and CD11c+ infiltrating cells between normal control mice and virus infected mice at any time point which suggests that these cells do not contribute to prevention of anterior to posterior virus spread in the injected eye. T cells (CD4+ or CD8+) produce IFNγ, but significant numbers of these cells would be expected to be present later (i.e. after 96hrs p.i.) as part of the adaptive immune response. Plasmacytoid dendritic cells (CD11c+) produce large quantities of IFNα and β in response to virus infection17, 40 and although these cells are resident in the iris and ciliary body of mice38, we were unable to quantify CD11c+ cells in the infected eye perhaps because they are immature sentinel antigen capturing dendritic cells that stain weakly for CD11c and only after stimulation by virus do they mature into CD11c+ migratory cells that are not found in the eye38.
Taken together, the results from these studies suggest microglia are important resident immune cells and involved in early sparing of the ipsilateral retina (before day 3) after AC inoculation of BALB/c mice with HSV-1 (KOS). There was an increase in infiltrating cells in the AC of the injected eye and virus infected cells were observed at the junction between ciliary body and retina. IFNγ producing activated microglia were observed in the anterior segment of the injected eye on or before day 3 p.i. While these studies suggest that microglia and other early responders play a role in protecting the retina of the injected eye from infection by preventing direct anterior to posterior spread of the virus, they do not define the mechanism by which such protection is accomplished. Now that the infiltrating cells have been described, additional studies will be needed to fully understand the role of each cell type and of the role of IFNγ in the process of virus infection in the injected eye.
ACKNOWLEDGMENTS
The authors are grateful to Mrs. Jeanene Pihkala and Dr. Babak Baban for their assistance with flow cytometry and Mr. Robert Smith, Mrs. Libby Perry and Mrs. Penny Roon for their assistance with immunoelectron microscopy. These studies were supported by National Institutes of Health Grant EY006012 (SSA) and EY015392 (MAF).
REFERENCES
- 1.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive committee of the American Uveitis Society. Am J Ophthalmol. 1996;117:663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 2.Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35:327–343. doi: 10.1016/0039-6257(91)90183-g. [DOI] [PubMed] [Google Scholar]
- 3.Whittum J, McCulley J, Niederkorn JY, Streilein J. Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Invest Ophthalmol Vis Sci. 1984;25:1065–1073. [PubMed] [Google Scholar]
- 4.Vann VR, Atherton SS. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32:2462–2472. [PubMed] [Google Scholar]
- 5.Bosem ME, Harris R, Atherton SS. Optic nerve involvement in viral spread in herpes simplex virus type 1 retinitis. Invest Ophthalmol Vis Sci. 1990;31:1683–1689. [PubMed] [Google Scholar]
- 6.Atherton S. Acute retinal necrosis: Insights into pathogenesis from the mouse model. Herpes. 2001;8:69–73. [PubMed] [Google Scholar]
- 7.Atherton S, Altman N, Streilein J. Histopathologic study of herpes virus-induced retinitis in athymic BALB/c mice: Evidence for an immunopathogenic process. Curr Eye Res. 1989;8:1179–1191. doi: 10.3109/02713688909000043. [DOI] [PubMed] [Google Scholar]
- 8.Atherton S, Vann V. Recent Advances in Uveitis. Brussels, Belgium: 1992. Immunologic control of neural spread of herpes simplex virus type 1 (HSV-1) following anterior chamber inoculation; pp. 23–28. [Google Scholar]
- 9.Matsubara S, Atherton SS. Spread of HSV-1 to the suprachiasmatic nuclei and retina in T cell depleted BALB/c mice. J Neuroimmunol. 1997;80:165–171. doi: 10.1016/s0165-5728(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 10.Azumi A, Atherton SS. Sparing of the ipsilateral retina after anterior chamber inoculation of HSV-1: Requirement for either CD4+ or CD8+ T cells. Invest Ophthalmol Vis Sci. 1994;35:3251–3259. [PubMed] [Google Scholar]
- 11.Tanigawa M, Bigger JE, Kanter MY, Atherton SS. Natural killer cells prevent direct anterior to posterior spread of herpes simplex virus type 1 in the eye. Invest Ophthalmol Vis Sci. 2000;41:132–137. [PubMed] [Google Scholar]
- 12.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: An overview of signals, mechanism and functions. J Leukoe Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 13.Takaoka A, Yanai H. Interferon signaling network in innate defense. Cellular Microbiology. 2006;8:907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 14.Zheng M, Atherton SS. Cytokine profiles and inflammatory cells during HSV-1 induced acute retinal necrosis. Invest Ophthalmol Vis Sci. 2005;46:1356–1363. doi: 10.1167/iovs.04-1284. [DOI] [PubMed] [Google Scholar]
- 15.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998;12:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ethuin F, Gerard B, Benna JE, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 17.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Ann Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 18.Geiger K, Howes E, Sarvetnick N. Ectopic expression of gamma interferon in the eyes protects transgenic mice from intraocular herpes simplex virus type 1 infections. J Virol. 1994;68:5556–5567. doi: 10.1128/jvi.68.9.5556-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouley D, Kanangat S, Wire W, Rouse B. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 20.Geiger K, Nash T, Sawyer S, et al. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 21.Hooks J, Wang Y, Detrick B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Invest Ophthalmol Vis Sc. 2003;44:3402–3408. doi: 10.1167/iovs.02-1106. [DOI] [PubMed] [Google Scholar]
- 22.Cantin E, Tanamachi B, Openshaw H, Mann J, Clarke K. Gamma interferon (IFNG) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFNG ligand null-mutant mice. J Virol. 1999;73:5196–5200. doi: 10.1128/jvi.73.6.5196-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng M, Fields MA, Lui Y, et al. Neutrophils protect the retina from infection after anterior chamber inoculation of HSV-1 in BALB/c mice. Invest Ophthalmol Vis Sc. 2008;9:4018–4025. doi: 10.1167/iovs.08-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdan C, Schleicher U. Production of interferon-gamma by myeloid cells- fact or fancy? Trends Immunol. 2006;27:282–290. doi: 10.1016/j.it.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kawanokuchi J, Mizuno T, Takeuchi H, et al. Production of interferon-gamma by microglia. Mult Scler. 2006;12:558–564. doi: 10.1177/1352458506070763. [DOI] [PubMed] [Google Scholar]
- 26.Atherton S, Streilein J. Two waves of virus following anterior chamber inoculation of HSV-1. Invest Ophthalmol Vis Sci. 1987;28:571–579. [PubMed] [Google Scholar]
- 27.Roizman B, Knipe D. Fields virology. 4th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. pp. 2399–2459. [Google Scholar]
- 28.Nolte J. The human brain: An introduction to its functional anatomy. 5th ed. St. Louis, MO: Mosby; 2002. pp. 37–52. [Google Scholar]
- 29.Archin NM, van den Boom L, Perelygina L, Hilliard JM, Atherton SS. Delayed spread and reduction in virus titer after anterior chamber inoculation of a recombinant of HSV-1 expressing IL-16. Invest Ophthalmol Vis Sc. 2003;44:3066–3076. doi: 10.1167/iovs.02-1071. [DOI] [PubMed] [Google Scholar]
- 30.Langmann T. Microglia activation in retinal degeneration. J Leukoe Biol. 2007;31:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- 32.Springer T, Galfre G, Secher DS, Milstein C. Mac-1: A macrophage differentiation antigen identified by monoclonal antibody. Eur J of Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 33.Ault KA, Springer TA. Cross-reaction of a rat-anti-mouse phagocyte-specific monoclonal antibody (anti-Mac-1) with human monocytes and natural killer cells. J Immunol. 1981;126:359–364. [PubMed] [Google Scholar]
- 34.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 36.Reading PC, Whitney PG, Barr DP, Smyth MJ, Brooks AG. NK cells contribute to the early clearance of HSV-1 from the lung but cannot control replication in the central nervous system following intranasal infection. Eur J of Immunol. 2006;36:897–905. doi: 10.1002/eji.200535710. [DOI] [PubMed] [Google Scholar]
- 37.Vollstedt S, Arnold S, Schwerdel C, et al. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in defense against herpes simplex virus type 1. J Virol. 2004;78:3846–3850. doi: 10.1128/JVI.78.8.3846-3850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. 1999;83:598–604. doi: 10.1136/bjo.83.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streilein JW. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J Leukoe Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 40.Colonna M, Trinchieri G, Liu Y-J. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]