Abstract
Background
Preterm birth, low birth weight, and infant catch-up growth seem associated with an increased risk of respiratory diseases in later life, but individual studies showed conflicting results.
Objectives
We performed an individual participant data meta-analysis for 147,252 children of 31 birth cohort studies to determine the associations of birth and infant growth characteristics with the risks of preschool wheezing (1-4 years) and school-age asthma (5-10 years).
Methods
First, we performed an adjusted 1-stage random-effect meta-analysis to assess the combined associations of gestational age, birth weight, and infant weight gain with childhood asthma. Second, we performed an adjusted 2-stage random-effect meta-analysis to assess the associations of preterm birth (gestational age <37 weeks) and low birth weight (<2500 g) with childhood asthma outcomes.
Results
Younger gestational age at birth and higher infant weight gain were independently associated with higher risks of preschool wheezing and school-age asthma (P < .05). The inverse associations of birth weight with childhood asthma were explained by gestational age at birth. Compared with term-born children with normal infant weight gain, we observed the highest risks of school-age asthma in children born preterm with high infant weight gain (odds ratio [OR], 4.47; 95% CI, 2.58-7.76). Preterm birth was positively associated with an increased risk of preschool wheezing (pooled odds ratio [pOR], 1.34; 95% CI, 1.25-1.43) and school-age asthma (pOR, 1.40; 95% CI, 1.18-1.67) independent of birth weight. Weaker effect estimates were observed for the associations of low birth weight adjusted for gestational age at birth with preschool wheezing (pOR, 1.10; 95% CI, 1.00-1.21) and school-age asthma (pOR, 1.13; 95% CI, 1.01-1.27).
Conclusion
Younger gestational age at birth and higher infant weight gain were associated with childhood asthma outcomes. The associations of lower birth weight with childhood asthma were largely explained by gestational age at birth.
Key words: Gestational age, low birth weight, infant growth, wheezing, asthma, children, cohort studies, epidemiology
Abbreviations used: BMI, Body mass index; ISAAC, International Study on Asthma and Allergy in Childhood; OR, Odds ratio; pOR, Pooled odds ratio; SDS, Standard deviation scores
Respiratory diseases have at least part of their origins in early life. It has been hypothesized that adverse exposures in fetal and early postnatal life might influence lung growth and development, which could lead to persistently smaller airways and impaired lung function. These developmental adaptations might predispose the subject to asthma and chronic obstructive pulmonary disease in childhood and adulthood.1-3 This hypothesis is supported by studies showing associations of low birth weight with an increased risk of wheezing and asthma in childhood4-7 and chronic obstructive pulmonary disease and lower pulmonary function in later life.8-11 Published findings are not consistent,4-7,12,13 which might be due to differences in study populations and in definitions of outcomes. Also, the observed associations of low birth weight with an increased risk of asthma-related outcomes might be confounded by preterm birth or catch-up growth in infancy. The lungs of preterm children have not yet been fully developed, which makes them prone to suboptimal further development.14-16
Most children with low birth weight show catch-up growth in infancy.17 Recent studies suggested that catch-up growth is associated with lower pulmonary function and an increased risk of childhood asthma.18-20 Whether and to what extent the previously reported associations of low birth weight with higher risks of asthma-related outcomes are explained by preterm birth and infant catch-up growth is not known.
Therefore we conducted a meta-analysis of individual data from 147,252 children up to the age of 10 years participating in 31 European cohort studies to assess the strength, consistency, and independence of the associations of gestational age, birth weight, and infant weight gain with the risk of preschool wheezing and school-age asthma. We specifically explored the combined effects of gestational age, birth weight, and infant growth.
Methods
Inclusion criteria and participating cohorts
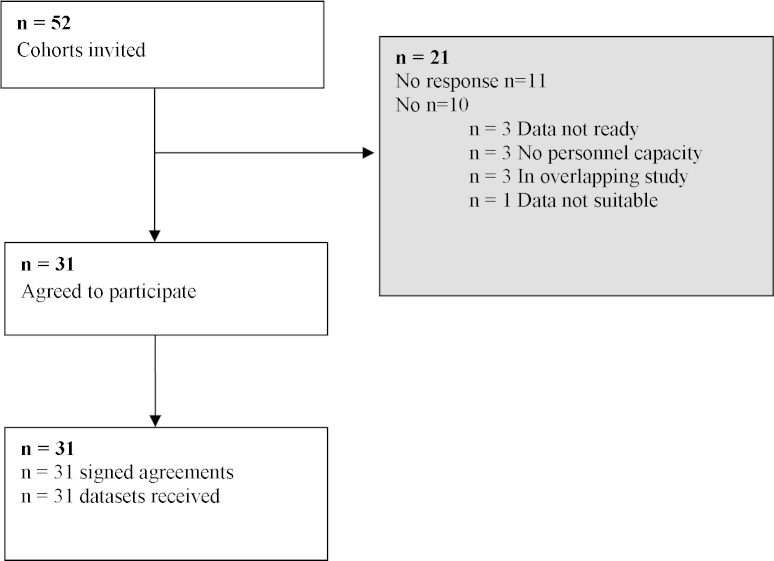
European population-based birth and mother-child cohorts participated if they included children born between 1989 and 2011, had information available on at least gestational age and weight at birth and preschool wheezing (1-4 years) or school-age asthma (5-10 years), and were willing and able to exchange original data. We identified 52 European cohorts selected from the existing collaborations on childhood health or asthma-related outcomes (www.chicosproject.eu, www.birthcohortsenrieco.net, www.ga2len.org, and www.birthcohorts.net assessed until May 29, 2012). We invited the 52 potentially eligible cohorts, of which 41 responded to our invitation. From those, 31 cohorts agreed to participate, leading to 147,252 children with information on at least 1 early growth characteristic and respiratory outcome (see the flow chart shown in Fig E1 in this article's Online Repository at www.jacionline.org). All original cohort studies were approved by their local institutional review boards and provided written informed consent for using their data. Anonymized data sets were stored on a single central secured data server with access for the main analysts (A.M.M.S., L.R.A., and L.D.) only.
Birth characteristics and infant growth
Information about birth weight, gestational age at birth, and weight in the first year of life per cohort was obtained by using measurements, medical registries, or parental questionnaires (cohort-specific information is shown in Table E1 in this article's Online Repository at www.jacionline.org) and used as continuous and categorical variables. Infant weight gain in the first year was defined as the difference between weight at 1 year (range, 6-18 months) and weight at birth divided by the exact number of months between those 2 measurements. We created gestational age–adjusted birth weight standard deviation scores (SDS) based on a North-European reference chart.21 No general European or World Health Organization reference curves of birth weight for gestational age are available. To test nonlinear and dose-response associations, we categorized gestational age (<28.0, 28.0-29.9, 30.0-31.9, 32.0-33.9, 34.0-35.9, 36.0-37.9, 38.0-39.9, 40-41.9, and ≥42 weeks), birth weight SDS (<−4, −4 to −3.01, −3 to −2.01, −2 to −1.01, −1 to −0.01, 0 to 0.99, 1 to 1.99, 2 to 2.99, 3 to 3.99, and ≥4 SDS), and infant weight gain (<300, 300-399, 400-499, 500-599, 600-699, 700-799, 800-899, 900-999, and ≥1000 g/mo). To test the combined associations of gestational age, birth weight SDS, and infant weight gain with childhood asthma outcomes, we used a smaller number of groups to have sufficient children per group (for gestational age: <32, 32-35.9, 36-39.9, and ≥40 weeks; for birth weight SDS: <−2; −2 to −1.01, −1 to 0.99, 1 to 1.99, and ≥2 SDs; and for infant weight gain: <500, 500-599, 600-699, and ≥700 g/mo). Finally, we dichotomized gestational age at birth into term birth (≥37 weeks) and preterm birth (gestational age, <37 weeks) and birth weight into normal birth weight (≥2500 g) and low birth weight (<2500 g) to test the effects of clinical birth complications on childhood asthma outcomes. Cohort-specific characteristics of determinants are shown in Table E2 in this article's Online Repository at www.jacionline.org.
Asthma-related outcomes in childhood
We used preschool wheezing and school-age asthma as the main outcomes. These data were mainly obtained by using questionnaires adapted from the International Study on Asthma and Allergy in Childhood (ISAAC).22 Cohort-specific information is shown in Table E1. We defined preschool wheezing as “ever reported wheezing during the first 4 years of life (no, yes)” and school-age asthma as “asthma diagnosis reported between 5 and 10 years (no, yes),” preferably physician diagnosed. If cohorts had repeatedly collected data on ever wheezing in the first 4 years or asthma diagnosis between 5 and 10 years of life, we used data collected at the oldest age.
Covariates
We included covariates based on known associations with childhood asthma from previous studies.23-27 Information on covariates was mostly assessed by using questionnaires (see Table E1). The individual cohort analyses were adjusted for potential confounders, including maternal educational level (low, medium, high), smoking during pregnancy (no, yes), history of asthma (no, yes), smoking during infancy of their offspring (no, yes), child's sex (female, male), siblings (no, yes), and attending day care in the first 2 years of lice (no, yes; description of available covariates per cohort is shown in Table E3 in this article's Online Repository at www.jacionline.org). We considered breast-feeding status (never, ever), lower respiratory tract infections (no, yes), and eczema (no, yes) in the first 2 years of life as potential intermediates (description of available intermediates per cohort is shown in Table E4 in this article's Online Repository at www.jacionline.org).
Statistical analysis
First, we performed a 1-stage random-effect meta-analysis on an individual participant's data to examine the separate and combined associations of gestational age, birth weight, and infant weight gain with preschool wheezing and school-age asthma. For this analysis, individual participants' data from all cohorts were included in 1 multilevel analysis and were analyzed, simultaneously taking into account clustering of participants within studies.28 Because we used a Northern European reference curve for birth weight for gestational age (birth weight SDS), we performed a sensitivity analysis to explore whether the association was different in Northwest European subjects only (Denmark, France, Germany, Ireland, The Netherlands, Norway, Sweden, Switzerland, and United Kingdom).29 Numbers were too low to perform these analyses separately in other European regions.
Second, we performed a 2-stage random-effect meta-analysis to examine the associations of gestational age at birth, birth weight, and infant weight gain and dichotomized preterm birth and low birth weight with the risks of preschool wheezing and school-age asthma. For this analysis, which was used for the clinically relevant associations of preterm birth and low birth weight, we first used logistic regression models to calculate effect estimates per cohort and then used calculated pooled odds ratios (pORs) from the per-cohort effect estimates.28 To enable comparison of effect estimates, results for birth weight and infant weight gain are presented as pORs per 500 g/mo and 100 g/mo increase, respectively, which reflect the corresponding SDs. We tested for heterogeneity by calculating the Cochran Q and I2 values, which varied per analysis.30 We used random-effects models, which take into account the potential between-study variation next to the within-study variation.31 To determine the influence of any particular cohort on overall results, we repeated each meta-analysis, leaving out 1 cohort at the time. The first model was adjusted for the child's sex (crude model), the second model was additionally adjusted for potential confounders (confounder model), and the third model was additionally adjusted for potential intermediates (intermediate model). We considered the confounder model as the main model. Results are presented as forest plots or tables with central point estimates from the random-effect models with their 95% CIs. The number of cohorts and children per meta-analysis differed because of differences in data availability. For all analyses, missing values in covariates were used as an additional group in the categorical variables to prevent exclusion of noncomplete cases. We also performed a complete-case sensitivity analysis to explore any differences with complete-case analyses and sensitivity analyses in which we first excluded children with parent-reported birth weight and then excluded children without ISAAC-based questionnaires on wheezing. Statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, NC) and Comprehensive Meta-Analysis (Biostat).
Results
Subjects' characteristics
The cohort-specific information about the main exposures and outcomes are shown in Table I. The overall prevalences of preterm birth (gestational age <37 weeks) and low birth weight (<2500 g) were 5.1% and 3.9%, respectively. Overall preschool wheezing prevalence was 31.6%, and overall school-age asthma prevalence was 12.8%.
Table I.
Characteristics of the participating European birth cohorts
| Cohort name (country) | No. (total = 147,252) | Birth years | Birth weight (g), mean (SD) |
Gestational age at birth (wk), median (5% to 95% range) |
Preschool wheezing, % (no.) | School-age asthma, % (no.) | Available covariates |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal education | Prenatal smoke | Maternal asthma | Postnatal smoke | Sex | Siblings | Day care | |||||||
| ABIS (Sweden) | 6,829 | 1997-1998 | 3,576 (537) | 40 (37-42) | 32.6 (2,200) | 9.9 (258) | √ | √ | √ | √ | √ | √ | |
| ALSPAC (United Kingdom) | 12,485 | 1991-1992 | 3,403 (554) | 40 (36-42) | 45.8 (5,683) | 21.7 (1,622) | √ | √ | √ | √ | √ | √ | √ |
| BILD (Switzerland) | 432 | 1999 | 3,382 (441) | 39 (37-41) | 20.6 (89) | — | √ | √ | √ | √ | √ | ||
| CONER (Italy) | 389 | 2004-2005 | 3,321 (448) | 39 (37-41) | 41.4 (161) | — | √ | √ | √ | √ | √ | √ | |
| COPSAC (Denmark) | 384 | 1998-2001 | 3,513 (524) | 40 (37-42) | 89.5 (331) | 18.2 (62) | √ | √ | √ | √ | √ | √ | √ |
| CZECH (Czech Republic) | 1,830 | 2001-2004 | 3,331 (519) | 40 (36-41) | — | 13.3 (244) | √ | √ | √ | √ | √ | √ | |
| DNBC (Denmark) | 76,810 | 1996-2001 | 3,594 (555) | 40 (37-42) | 26.9 (17,671) | 12.4 (6,498) | √ | √ | √ | √ | √ | √ | √ |
| EDEN (France) | 1,774 | 2003-2005 | 3,285 (506) | 40 (36-41) | 32.3 (573) | 12.8 (227) | √ | √ | √ | √ | √ | √ | √ |
| GASPII (Italy) | 694 | 2003-2004 | 3,313 (529) | 40 (36-41) | 43.7 (303) | — | √ | √ | √ | √ | √ | √ | √ |
| GECKO Drenthe (The Netherlands) | 1,718 | 2006-2007 | 3,557 (544) | 40 (37-42) | 29.2 (501) | — | √ | √ | √ | √ | √ | √ | |
| GENERATION R (The Netherlands) | 5,815 | 2002-2006 | 3,428 (575) | 40 (37-42) | 29.3 (1,505) | 6.0 (263) | √ | √ | √ | √ | √ | √ | √ |
| GENERATION XXI (Portugal) | 7,053 | 2005-2006 | 3,149 (533) | 39 (35-41) | 53.0 (2,970) | 4.4 (305) | √ | √ | √ | √ | √ | ||
| HUMIS (Norway) | 2,001 | 2003-2008 | 3,534 (677) | 40 (34-42) | 15.0 (301) | — | √ | √ | √ | √ | √ | √ | √ |
| INMA Gipuzkoa (Spain) | 478 | 2006-2008 | 3,298 (446) | 40 (37-42) | 35.8 (171) | — | √ | √ | √ | √ | √ | √ | √ |
| INMA Menorca (Spain) | 474 | 1997-1998 | 3,186 (498) | 40 (37-41) | 47.9 (227) | 6.4 (27) | √ | √ | √ | √ | √ | √ | √ |
| INMA Sabadell (Spain) | 502 | 2004-2007 | 3,253 (412) | 40 (37-42) | 59.8 (300) | — | √ | √ | √ | √ | √ | √ | √ |
| INMA Valencia (Spain) | 604 | 2003-2005 | 3,247 (501) | 40 (37-42) | 25.7 (155) | — | √ | √ | √ | √ | √ | √ | √ |
| ISLE OF WIGHT (United Kingdom) | 1,405 | 1989-1990 | 3,411 (523) | 40 (38-42) | 24.2 (263) | 20.1 (272) | √ | √ | √ | √ | |||
| KOALA (The Netherlands) | 2,151 | 2000-2003 | 3,525 (499) | 40 (38-42) | 24.7 (494) | 7.6 (134) | √ | √ | √ | √ | √ | √ | |
| LEICESTER 1990 (United Kingdom) | 1,231 | 1990 | 3,381 (555) | 40 (36-41) | 15.0 (156) | 30.6 (136) | √ | √ | √ | ||||
| LEICESTER 1998 (United Kingdom) | 6,836 | 1998 | 3,289 (582) | 39 (36-41) | 38.0 (2,242) | 22.3 (1,029) | √ | √ | √ | √ | √ | ||
| LIFEWAYS (Ireland) | 421 | 2001-2002 | 3,526 (565) | 40 (38-42) | — | 26.4 (111) | √ | √ | √ | √ | √ | ||
| MAS (Germany) | 1,263 | 1990 | 3,412 (463) | 40 (37-42) | 18.8 (237) | 6.6 (44) | √ | √ | √ | √ | √ | √ | √ |
| NINFEA (Italy) | 1,922 | 2005-2010 | 3,215 (508) | 40 (36-42) | 23.9 (460) | — | √ | √ | √ | √ | √ | √ | √ |
| PCB (Slovakia) | 429 | 2001-2004 | 3,359 (492) | 40 (38-41) | 5.6 (24) | — | √ | √ | √ | √ | |||
| PIAMA (The Netherlands) | 3,631 | 1996-1997 | 3,515 (543) | 40 (37-42) | 27.3 (964) | 10.1 (327) | √ | √ | √ | √ | √ | √ | √ |
| REPRO PL (Poland) | 314 | 2007-2011 | 3,349 (480) | 40 (37-41) | 12.4 (39) | — | √ | √ | √ | √ | √ | √ | |
| RHEA (Greece) | 1,046 | 2007-2008 | 3,179 (437) | 38 (36-40) | 25.7 (269) | — | √ | √ | √ | √ | √ | √ | √ |
| SEATON (United Kingdom) | 1,891 | 1997 | 3,414 (610) | 40 (35-42) | 27.3 (517) | 14.7 (131) | √ | √ | √ | √ | √ | √ | √ |
| SWS (United Kingdom) | 2,291 | 1998-2007 | 3,442 (555) | 40 (37-42) | 70.9 (1,614) | 15.4 (145) | √ | √ | √ | √ | √ | √ | |
| WHISTLER (The Netherlands) | 2,149 | 2001-2012 | 3,525 (513) | 40 (37-42) | 27.2 (577) | 7.7 (43) | √ | √ | √ | √ | √ | √ | |
No., Number of participants with information on at least birth weight or gestational and a respiratory outcome.
Gestational age, birth weight, and infant weight gain
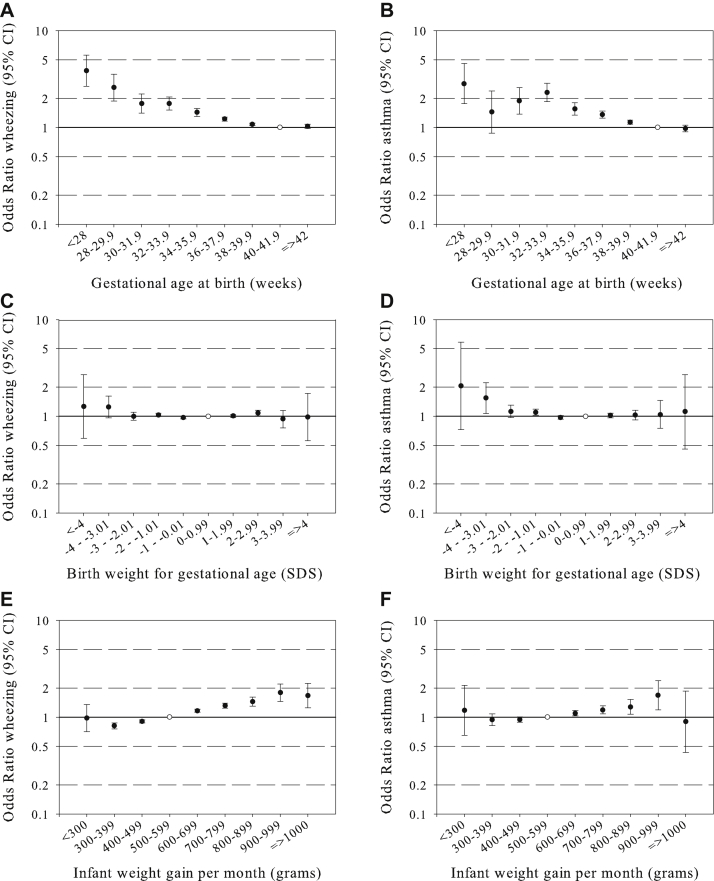
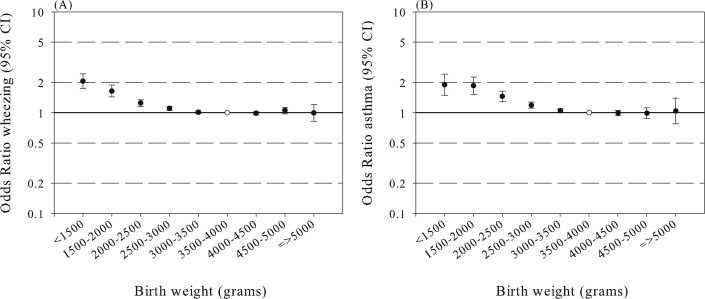
In the 1-stage meta-analysis of individual participants' data, we observed consistent inverse associations of gestational age at birth with the risk of preschool wheezing and school-age asthma. Compared with term-born children, children born before 28 weeks of gestation had the highest risk of preschool wheezing (odds ratio [OR], 3.87; 95% CI, 2.70-5.53) and school-age asthma (OR, 2.92; 95% CI, 1.84-4.62; Fig 1, A and B). Almost all children born before a gestational age of 40.0 weeks had an increased risk of preschool wheezing and school-age asthma. Birth weight SDS were not consistently associated with childhood asthma outcomes (Fig 1, C and D). Results for birth weight in grams without taking gestational age into account are shown in Fig E2 in this article's Online Repository at www.jacionline.org, showing an inverse association. We observed a positive association of infant weight gain with preschool wheezing and school-age asthma. Compared with children with a weight gain of between 500 and 600 g/mo (largest group), children with a mean infant weight gain of between 900 and 1000 g/mo had the highest risk of preschool wheezing (OR, 1.79; 95% CI, 1.45-2.21) and school-age asthma (OR, 1.69; 95% CI, 1.19-2.38; Fig 1, E and F). The overall results for the linear associations of gestational age at birth, birth weight, and infant weight gain from the 1-stage meta-analysis of individual participants' data were similar to those from the 2-stage meta-analysis of individual participants' data (results are shown in Table E5 in this article's Online Repository at www.jacionline.org). The results from the confounder model were not materially different from those from the crude model. Also, additionally adjusting the confounder model for potential intermediates (breast-feeding, lower respiratory tract infections, and eczema) did not materially change the effect estimates (results are shown in Tables E6 and E7 in this article's Online Repository at www.jacionline.org). Also, we observed similar effect estimates for preschool wheezing and school-age asthma after excluding cohorts one by one, indicating no disturbing effect of any particular population (data not shown). After exclusion of the Danish National Birth Cohort, the largest cohort in our meta-analysis (COPSAC) and a high-risk asthma and atopy cohort, we also did not observe major changes in effect estimates (data not shown).
Fig 1.
Associations of gestational age at birth, birth weight, and infant weight gain with preschool wheezing and school-age asthma. Values are ORs (95% CIs) from multilevel models for the associations of gestational age at birth (A and B), gestational age–adjusted birth weight (C and D), and gestational age–and birth weight–adjusted infant weight gain (E and F) with asthma outcomes. Models are adjusted for confounders (see the Methods section). Reference groups are represented by open circles.
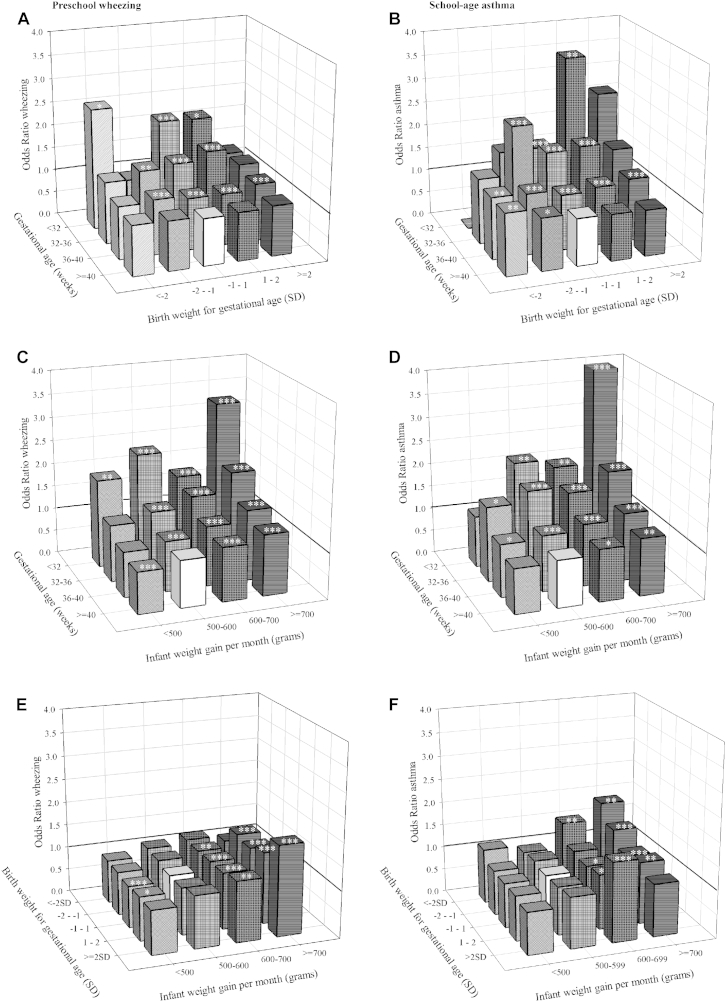
Next, we explored the combined effects of gestational age at birth, birth weight SDS, and infant weight gain. The significant correlations were between gestational age and birth weight (r = 0.58, P < .001), between gestational age and infant weight gain (r = −0.16, P < .001), and between birth weight and infant weight gain (r = −0.12, P < .001). We performed stratified analyses and an overall test for interaction. In each analysis the largest group was used as the reference group. For the combined effect analysis of gestational age at birth and birth weight SDS, we observed a higher risk of preschool wheezing among children born at an earlier age with higher birth weight SDS, but the overall interaction term with birth weight SDS was not significant (Fig 2, A). Similarly, we observed a tendency toward a higher risk of school-age asthma in children born at an earlier gestational age with higher birth weight SDS (P for interaction = .04; Fig 2, B). The highest risks for school-age asthma were observed for children born before 32 weeks of gestation with a moderately high birth weight SDS (OR, 3.47; 95% CI, 1.65-7.31) and with a high birth weight SDS (OR, 2.63; 95% CI, 0.53-13.13) compared with children born at term with a normal birth weight SDS. The P value for interaction between gestational age at birth and infant weight gain for the associations with preschool wheezing and school-age asthma were 0.05 and 0.23, respectively (Fig 2, C and D). We observed the highest risk of preschool wheezing and school-age asthma among children born before 32 weeks of gestation with an infant weight gain of more than 700 g compared with children born at term with a normal weight gain (ORs of 3.27 [95% CI, 2.06-5.19] and 4.47 [95% CI, 2.58-7.76], respectively). The interactions between birth weight SDSs and infant weight gain for the associations with preschool wheezing and school-age wheezing were not significant (Fig 2, E and F). As a sensitivity analysis, we performed our analysis in Northwest European cohorts only and observed similar results (results are shown in Tables E8 and E9 in this article's Online Repository at www.jacionline.org). The results of complete case analyses were similar (data not shown). Also, we observed similar effect estimates for preschool wheezing and school-age asthma after excluding cohorts that used parental reports of birth weight or non–ISAAC-based questions on wheezing. With this exclusion, only the youngest and lowest weight-for-gestational-age groups tended to show stronger effects (see Tables E10 and E11 in this article's Online Repository at www.jacionline.org). This indicates that differences in data collection did not lead to systematic differences in effect estimates.
Fig 2.
Combined associations of gestational age at birth, birth weight, and infant weight gain with preschool wheezing and school-age asthma. Values are ORs (95% CIs) from multilevel models for the associations of gestational age at birth and birth weight SDSs (A and B), gestational age at birth and infant weight gain (C and D), and birth weight SDSs and infant weight gain (E and F) with asthma outcomes. Models are adjusted for confounders (see the Methods section). Reference groups are represented by a white bar. P values for gestational age*SD birth weight interactions are as follows: wheezing, .97; asthma, .04. P values for gestational age*weight gain interaction are as follows: wheezing, .05; asthma, .23. P values for birth weight SDS*weight gain interactions are as follows: wheezing, .15; asthma, .57. *P < .05, **P < .01, and ***P < .001.
Preterm birth, low birth weight, and childhood asthma outcomes
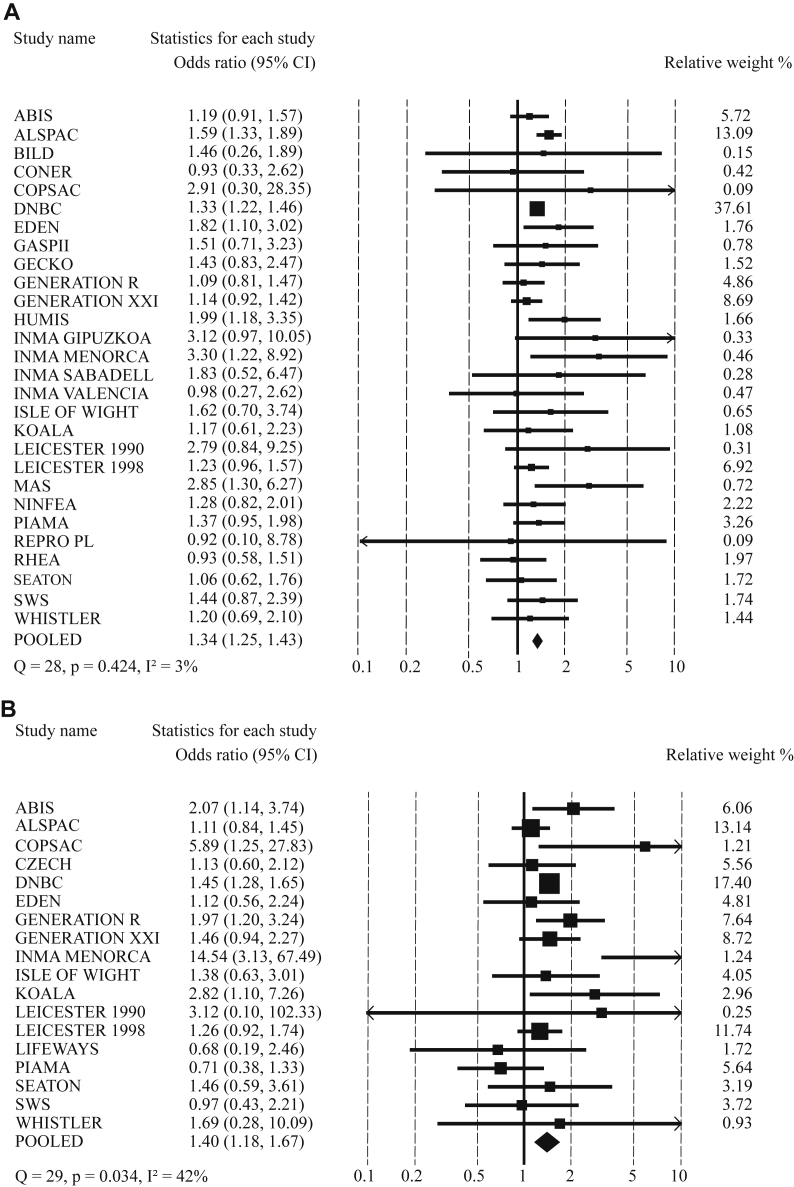
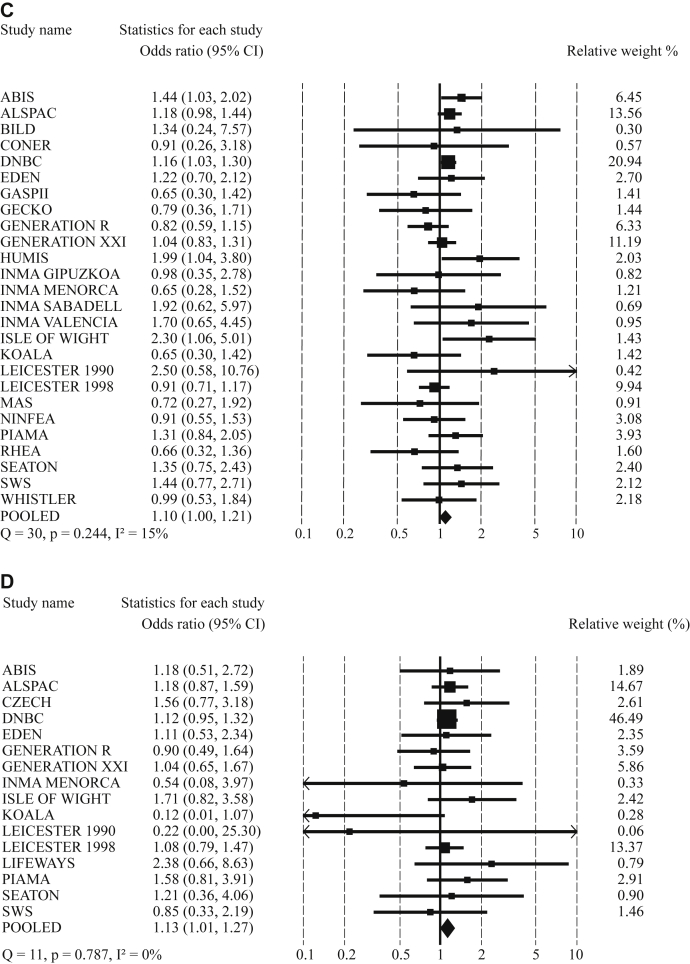
Results from the 2-stage meta-analysis focused on the associations of preterm birth and low birth weight with childhood asthma outcomes are shown in Fig 3. Compared with term-born children, preterm-born children had an increased risk of preschool wheezing (pOR, 1.34; 95% CI, 1.25-1.43) and school-age asthma (pOR, 1.40; 95% CI, 1.18-1.67; Fig 3, A and B). These associations were independent of birth weight. The population-attributable risk of preterm birth was 1.96% for preschool wheezing and 2.14% for school-age asthma. Compared with children with a normal birth weight, those with a low birth weight (<2500 g) had an increased risk of preschool wheezing (pOR, 1.10; 95% CI, 1.00-1.21) and school-age asthma (pOR, 1.13; 95% CI, 1.01-1.27; Fig 3, C and D). These associations were stronger without adjustment for gestational age at birth (results are shown in Table E6).
Fig 3.
Meta-analysis for associations of preterm birth and low birth weight with preschool wheezing and school-age asthma. A, Preterm birth and preschool wheezing. B, Preterm birth and school-age asthma. C, Low birth weight and preschool wheezing. D, Low birth weight and school-age asthma. Values from random-effects models reflect ORs (95% CIs) of preschool wheezing and school-age asthma in preterm children (<37 weeks) compared with those in children born at term (≥37 weeks) adjusted for birth weight (A and B) and of preschool wheezing and school-age asthma in low-birth-weight children (<2500 g) compared with children born with a normal birth weight (≥2500 g) adjusted for gestational age at birth (C and D). Arrows represent 95% CIs that exceed the outer limits (0.1-10). Models are adjusted for confounders (see the Methods section).
Discussion
Results from this large-scale meta-analysis of individual participants' data suggested that younger gestational age at birth and higher infant weight gain were associated with increased risks of preschool wheezing and school-age asthma. The associations of low birth weight with childhood asthma outcomes were largely explained by gestational age at birth. The highest risk for childhood asthma outcomes was observed among children born before a gestational age of 32 weeks with high infant weight gain.
Comparison with earlier studies
Adverse exposures in fetal and early postnatal life can lead to developmental lung adaptations, such as persistently smaller airways and impaired lung function. These developmental adaptations might predispose the patients to obstructive pulmonary diseases in childhood and adulthood.1-3 This hypothesis is supported by studies showing associations of low birth weight with an increased risk of wheezing and asthma in childhood.4-11 Because low birth weight is correlated with gestational age at birth and infant weight gain, we aimed to disentangle the associations of both gestational age at birth, gestational age–adjusted birth weight, and infant weight gain with childhood asthma outcomes.
Jaakkola et al16 performed a meta-analysis on the associations of preterm birth with asthma based on 19 published cohort, case-control, and cross-sectional studies. They observed that preterm-born children, which were defined as those born before 37 weeks of gestation, had an increased risk of asthma between 1 and 24 years of age, with a similar effect estimate as observed in our group of 5- to 10-year-olds. They did not assess associations of birth weight with asthma outcomes. Also, Flaherman and Rutherford32 performed a meta-analysis on 12 previously published prospective and retrospective studies and suggested that children with a high weight at birth had an increased risk of asthma between 6 months and 31 years of age. They were not able to explore the role of confounders or the effect of gestational age at birth. No association of gestational age with childhood asthma was presented. Because these reports were based on published results, they might be biased and unable to take account of differences in adjustment. A recent analysis by Rzehak et al33 of 8 European cohort studies with 12,050 participants observed an increased incidence of asthma until the age of 6 years in children with a high gain of body mass index (BMI) in the first 2 years. In line with this study, we observed increased risk for wheezing and asthma in children with an increased infant weight gain.
Combining childhood asthma outcomes from different age periods is not easy. Asthma is a difficult clinical diagnosis and cannot easily be diagnosed in children younger than 5 years. Many studies used asthma-related outcomes, such as wheezing and shortness of breath, as main outcomes in children. Wheezing seems to be the strongest risk factor for childhood asthma.34 Still, wheezing in different age periods might reflect different physiologic mechanisms.35 For example, wheezing in infants might reflect viral airway infections instead of asthma.35 Therefore we used both wheezing in preschool children and asthma diagnosis in school-age children as outcomes. We observed that both a younger gestational age at birth and higher infant weight gain were associated with an increased risk of preschool wheezing and school-age asthma. For both gestational age at birth and infant weight gain, we observed dose-response associations with childhood asthma outcomes. The associations were not restricted to the extremes of the distribution but present across the full range of gestational age at birth and infant weight gain. To the best of our knowledge, this study is the first showing these associations within the normal ranges. Our results also suggest that the previously observed associations of low birth weight with childhood asthma were largely explained by gestational age at birth. We observed the highest risk of childhood asthma outcomes among children born before a gestational age of 32 weeks with high weight gain in infancy.
Interpretation of main findings
Mechanisms underlying the associations of factors in early life with asthma outcomes in later childhood might include smaller airways and lungs.36 The highest rates of airway and alveolar development occur in early life, and growth and development of the airways and alveoli might continue until the age of 21 years.37,38 Extremely premature-born children with respiratory distress syndrome or chronic lung disease commonly have impaired lung function in later life.39,40 Follow-up studies in preterm children showed persistently lower lung volumes and reduced airway caliber in later life.40-44 However, these extremes do not explain our associations within the less extreme range of gestational age. Children born preterm also have higher chemokine and cytokine levels in nasopharyngeal aspirates at 1 year compared with term-born children, which suggests that preterm-born children are more responsive to proinflammatory stimuli.45 The observed associations of high infant weight gain with childhood asthma outcomes are in line with previous studies reporting associations of BMI or adiposity with asthma.33,46,47 These associations might be explained by immunologically active factors from adipose tissue, such as leptin.48 In mice leptin has been shown to enhance airway hyperresponsiveness, suggesting an immunomodulatory role.49 Results in human subjects are inconsistent.50-52 High infant weight gain might also have a direct mechanical effect on lung function.53 Further studies are needed to identify the developmental adaptations of the lungs and immune system that might explain the associations of preterm birth and infant weight gain with childhood asthma.
Strengths and limitations
We performed a large meta-analysis of individual participants' data from many birth cohorts throughout Europe. We did not rely on published data, which limits any potential publication bias. The large number of participants enabled us to assess small effects and to adjust for various potential confounders. We presented results from random-effects models, which allow heterogeneity in the true effect estimates between different populations and take between-study variation into account. Another strength is that information on exposures in early life was collected from records and did not depend on long-term participant recall. Misclassification of gestational age is always possible because of the large number of pregnant women who did not know their exact gestational duration.54,55 Misclassification of gestational age might have increased the number of children born postterm with a small size for gestational age and children born preterm with a large size for gestational age. Most cohorts used standardized and validated questionnaires to assess wheezing and asthma. This method is widely accepted in epidemiologic studies and reliably reflects the incidence of wheezing and asthma in children.22,56 Multiple imputation has been suggested to be the preferable method to deal with missing values.57 However, we did not have additional data on patterns of missing values and were therefore unable to perform multiple imputations within cohorts. We used missing values in covariates as an additional group to prevent exclusion of noncomplete cases. No differences in results were observed between the missing as extra category and complete case analyses. In the current study we were not able to assess the effects of early growth characteristics on other objective asthma-related outcomes, such as lung function or bronchial hyperresponsiveness. Although we did take major potential confounders into account, residual confounding might still be an issue. For example, although cohorts comprised predominantly white children, we were unable to adjust for ethnicity. Also, we were unable to adjust for maternal BMI or chorioamnionitis, which might influence growth and inflammatory factors associated with childhood asthma.58,59 We were not able to take BMI at the time of obtaining information on childhood asthma outcomes into account. Especially the associations of infant weight gain with childhood asthma outcomes might be explained by later adiposity. Childhood adiposity might be an intermediate in this association.
Conclusions
Younger gestational age at birth and higher weight gain in infancy were associated with childhood asthma outcomes. The association of lower birth weight with childhood asthma outcomes was largely explained by gestational age at birth. Further studies are needed to evaluate the effects of early-life characteristics on specific asthma-related outcomes, such as lung function, airway size, and airway inflammation.
Clinical implications.
Children born at a younger gestational age at birth or higher infant weight gain have increased risks of childhood asthma.
Acknowledgments
Per cohort
ABIS
Data used for this research was provided by the Cohort Study, which is supported in part by JDRF-Wallenberg foundations (K 98-99D-12813-01A), the Swedish Medical Research Council (MFR; Vetenskapsrådet; K99-72X-11242-05A), the Swedish Child Diabetes Foundation (Barndiabetesfonden), and the Swedish Diabetes Association, Medical Research Council of South East Sweden (FORSS), Novo Nordisk Foundation, Prevention of Diabetes, and its Complications Strategic Area-LiU.
ALSPAC
We are extremely grateful to all the families who took part in the study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionist, and nurses. The UK Medical Research Council and the Wellcome Trust (grant reference 092731) and the University of Bristol provide core support for ALSPAC.
BILD
Data used for this research were provided by the Cohort Study, which is supported in part by funds of the Swiss National Science Foundation; the European Respiratory Society (ERS); the Austrian, German and Swiss Paediatric respiratory Society; and the Swiss Governmental Anti-Tobacco Fund.
CONER
Data used for this research were provided by the Cohort Study, which is supported in part by funds of the Italian ministry of health.
COPSAC
COPSAC is funded by private and public research funds listed on www.copsac.com. The Lundbeck Foundation, the Danish Strategic Research Council, the Pharmacy Foundation of 1991, the Augustinus Foundation, the Danish Medical Research Council, and the Danish Pediatric Asthma Centre provided the core support for the COPSAC research center. No pharmaceutical company was involved in the study. The funding agencies did not have any role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, or approval of the manuscript.
CZECH
Data used for this research was provided by the Cohort Study, which is supported in part by funds of the Ministry of Environment of the Czech Republic (SP/1b3/8/08).
DNBC
The Danish National Research Foundation has established the Danish Epidemiology Science Centre that initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this foundation. Additional support for the Danish National Birth Cohort is obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, and the Augustinus Foundation.
EDEN
We acknowledge all the funding sources for the EDEN study: Fondation pour la Recherche Médicale (FRM), the French Ministry of Research: IFR program, the INSERM Nutrition Research program, the French Ministry of Health Perinatality Program, the French Agency for Environment security (AFFSET), the French National Institute for Population Health Surveillance (INVS), Paris-Sud University, the French National Institute for Health Education (INPES), Nestlé, Mutuelle Générale de l'Education Nationale {MGEN), the French-speaking Association for the Study of Diabetes and Metabolism (Alfediam), and the National Agency for Research (ANR).
GASPII
Data used for this research was provided by the Cohort Study, which is supported in part by funds of the Italian Ministry of Health, 2001.
GECKO Drenthe
The GECKO Drenthe cohort is supported and funded by an unrestricted grant from Hutchison Whampoa, the University of Groningen, and Well Baby Clinic Foundation Icare.
GENERATION R
The Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam; the Erasmus University Rotterdam; and the Netherlands Organization for Health Research and Development. The researchers are independent from the funders. The study sponsors had no role in study design, data analysis, interpretation of data, or writing of this report. Additional support was available from the Netherlands Organization for Health Research and Development (VIDI) and the Dutch Asthma Foundation.
GENERATION XXI
Data used for this research were provided by the Cohort Study, which is supported in part by funds of the Programa Operacional de Saúde–Saúde XXI, Quadro Comunitário de Apoio III (FEDER), the Northern Regional Administration of Health, the Portuguese Foundation for Science and Technology (PTDC/SAUESA/105033/2008), and the Calouste Gulbenkian Foundation.
HUMIS
The research leading to these results has received funding from the Norwegian Research Council under grant agreement 213148 (MILPAAHEL) and the European Union's Seventh Framework Programme (FP7/2007-2013), project Early Nutrition under grant agreement number 289346, and project OBELIX under grant agreement number 22739.
INMA
Gipuzkoa/Sabadell/Valencia/Menorca Data used for this research were provided by the INMA–Environment and Childhood Project (www.proyectoinma.org), which is supported in part by funds. This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176 and CB06/02/0041), the Spanish Ministry of Health (FIS- PI041436, PI042018, PI06/0867 PI07/0252, PI081151, and PI09/02311,and FIS-FEDER 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314, and 09/02647), Generalitat de Catalunya-CIRIT 1999SGR 00241, the Conselleria de Sanitat Generalitat Valenciana, the Department of Health of the Basque Government (2005111093 and 2009111069), the Provincial Government of Gipuzkoa (DFG06/004 and DFG08/001), Obra Social Cajastur, Universidad de Oviedo, the EU Commission (QLK4-1999-01422, QLK4-2002-00603 and CONTAMED FP7-ENV-212502), Consejería de Salud de la Junta de Andalucía (grant number 183/07), and Fundació Roger Torné.
ISLE OF WIGHT
Data used for this research were provided by the Cohort Study, which is supported in part by funds of the National Institute of Health, the British Medical Association, and David Hide Asthma and Allergy Research Centre Trustees.
KOALA
Data used for this research were provided by the Cohort Study, which is supported in part by funds from the Netherlands Asthma Foundation (grant nos. 3.2.03.48 and 3.2.07.022).
LEICESTER 1990/1998
Data used for this research were provided by the Leicester Cohort Studies, which are supported by funds from Asthma UK (grant no. 07/048), the Swiss National Science Foundation (grant no. 32003B-144068), the Wellcome Trust, and many others.
LIFEWAYS
Data used for this research were provided by the Cohort Study, which is supported in part by funds of the Health Research Board, Republic of Ireland.
MAS
Data for this research question were obtained by the study centre of the cohort study. The Multicentre Allergy Study (1990) was supported by grants from the German Federal Ministry for Education and Research (BMBF) under reference numbers 07015633, 07 ALE 27, 01EE9405/5, and 01EE9406.
NINFEA
Data used for this research were provided by the Cohort Study, which is supported in part by funds of Compagnia di SanPaolo Foundation, Piedmont Region, and the Italian Ministry of University and Research.
PCB
Data used for this research was provided by the Cohort Study, which is supported in part by funds from National Institutes of Health grant R01-CA096525 and EU project OBELIX (no. 227391).
PIAMA
The PIAMA study has been funded by the Netherlands Organization for Health Research and Development; the Netherlands Organization for Scientific Research; the Netherlands Asthma Fund; the Netherlands Ministry of Spatial Planning, Housing, and the Environment; and the Netherlands Ministry of Health, Welfare and Sport.
REPRO PL
Data used for this research were provided by the Cohort Study, which is supported in part by funds from the National Center for Research and Development, Poland (grant no. PBZ-MEiN-/8/2//2006; contract no. K140/P01/2007/1.3.1.1.) and grant PNRF-218-AI-1/07 from Norway through the Norwegian Financial Mechanism within the Polish-Norwegian Research Fund.
RHEA
Data used for this research were provided by the Cohort Study, which is supported in part by funds of European Commission.
SEATON
Data used for this research were provided by the university, which is supported in part by funds from Asthma UK and the Medical Research Council.
SWS
The Southampton Women's Survey is supported by grants from the Medical Research Council, the British Heart Foundation, the Food Standards Agency, the British Lung Foundation, Arthritis Research UK, NIHR Southampton Biomedical Research Centre, the University of Southampton and University Hospital Southampton NHS Foundation Trust, and the Commission of the European Community, specific RTD Programme “Quality of Life and Management of Living Resources,” within the 7th Framework Programme, research grant no. FP7/2007-13 (Early Nutrition Project). This manuscript does not necessarily reflect the views of the funders and in no way anticipates the future policy in this area.
WHISTLER
Data used for this research were provided by the Cohort Study, which is supported in part by funds from the Netherlands Organization for health Research and Development (ZON-MW), the University Medical Center Utrecht, and an unrestricted research grant from GlaxoSmithKline, The Netherlands.
Footnotes
Supported by the European Community's Seventh Framework ProgrammeFP7/2007-2013, project CHICOS. The research leading to these results has received funding from the European Respiratory Society and the European Community's Seventh Framework ProgrammeFP7/2007-2013–Marie Curie Actions under grant agreement RESPIRE, PCOFUND-GA-2008-229571.
Disclosure of potential conflict of interest: I. Annesi-Maesano has received one or more grants from or has one or more grants pending with UE. S. H. Arshad has been supported by one or more grants from the National Institute of Health and Asthma UK and has received one or more payments for lecturing from or is on the speakers' bureau for Thermo Fisher and GlaxoSmithKline. H. Bisgaard has been supported by one or more grants from the Danish State Budget and the Lundbeck Foundation, has consultancy arrangements with Chiesi Pharmaceuticals, and has received one or more grants from or has one or more grants pending with the Danish Strategic Research Council, the Capital Region of Denmark, the Oticon Foundation, the European Research Council, and the Danish Council for Independent Research, Medical Sciences. C. Dogaru and C. E. Kuehni are employed by the University of Bern. M. Eggesbø has been supported by one or more grants from and has received support for travel from Chicos. A. J. Henderson has been supported by one or more grants from the Wellcome Trust and the Medical Research Council. H. M. Inskip has been supported by one or more grants from the UK Medical Research Council and many funding bodies and is a Board member for the UK Medical Research Council. T. Keil has received one or more grants from or has one or more grants pending with the European Commission. K. Lancz and L. Palkovicova have been supported by one or more grants from the NIH (NCI and Fogarty), the European Union, and the Slovak Ministry of Health. S. Lau has been supported by one or more grants from the German Research Foundation DFG, is a member of the Merck Drug monitoring committee, has consultancy arrangements with Allergopharma (Reinbek, Germany), has received one or more grants from or has one or more grants pending with Symbiopharm (Herborn, Germany), and has received one or more payments for lecturing from or is on the speakers' bureau for Symbiopharm, GlaxoSmithKline, CSL Behring. M. Mommers has been supported by one or more grants from the Netherlands Asthma Foundation and has received support for travel from the European Community Seventh Framework Programme. K. C. Pike has been supported by one or more grants from FSA BLF and has received various travel grants and bursaries, most recently from the European Respiratory Society. G. Roberts has been supported by one or more grants from the NIH and BMA. A. Schmidt has been supported by one or more grants from the Swiss National Science Foundation. C. Thijs has been supported by one or more grants from the Netherlands Asthma Foundation and has received support for travel from European Community's Seventh Framework Programme. M. Vrijheid has been supported by one or more grants from and has received support for travel from the European Community's Seventh Framework Programme FP7/2007-2013 (Project CHICOS). L. Duijts has received research support from the European Community's Seventh Framework Programme FP7/2007-2013 (Project CHICOS) and is the recipient of a European Respiratory Society/Marie Curie Joint Research Fellowship (no. MC 1226-2009). The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
Fig E1.
Fig E2.
References
- 1.Barker D.J., Godfrey K.M., Fall C., Osmond C., Winter P.D., Shaheen S.O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27:5–14. doi: 10.1007/s10654-012-9657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caudri D., Wijga A., Gehring U., Smit H.A., Brunekreef B., Kerkhof M. Respiratory symptoms in the first 7 years of life and birth weight at term: the PIAMA Birth Cohort. Am J Respir Crit Care Med. 2007;175:1078–1085. doi: 10.1164/rccm.200610-1441OC. [DOI] [PubMed] [Google Scholar]
- 5.Kindlund K., Thomsen S.F., Stensballe L.G., Skytthe A., Kyvik K.O., Backer V. Birth weight and risk of asthma in 3-9-year-old twins: exploring the fetal origins hypothesis. Thorax. 2010;65:146–149. doi: 10.1136/thx.2009.117101. [DOI] [PubMed] [Google Scholar]
- 6.Taveras E.M., Camargo C.A., Jr., Rifas-Shiman S.L., Oken E., Gold D.R., Weiss S.T. Association of birth weight with asthma-related outcomes at age 2 years. Pediatr Pulmonol. 2006;41:643–648. doi: 10.1002/ppul.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan W., Basso O., Sorensen H.T., Olsen J. Fetal growth and hospitalization with asthma during early childhood: a follow-up study in Denmark. Int J Epidemiol. 2002;31:1240–1245. doi: 10.1093/ije/31.6.1240. [DOI] [PubMed] [Google Scholar]
- 8.Canoy D., Pekkanen J., Elliott P., Pouta A., Laitinen J., Hartikainen A.L. Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax. 2007;62:396–402. doi: 10.1136/thx.2006.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor D.A., Ebrahim S., Davey Smith G. Association of birth weight with adult lung function: findings from the British Women's Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopuhaa C.E., Roseboom T.J., Osmond C., Barker D.J., Ravelli A.C., Bleker O.P. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax. 2000;55:555–561. doi: 10.1136/thorax.55.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancox R.J., Poulton R., Greene J.M., McLachlan C.R., Pearce M.S., Sears M.R. Associations between birth weight, early childhood weight gain and adult lung function. Thorax. 2009;64:228–232. doi: 10.1136/thx.2008.103978. [DOI] [PubMed] [Google Scholar]
- 12.Sevelsted A., Bisgaard H. Neonatal size in term children is associated with asthma at age 7, but not with atopic dermatitis or allergic sensitization. Allergy. 2012;67:670–675. doi: 10.1111/j.1398-9995.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 13.Latzin P., Frey U., Roiha H.L., Baldwin D.N., Regamey N., Strippoli M.P. Prospectively assessed incidence, severity, and determinants of respiratory symptoms in the first year of life. Pediatr Pulmonol. 2007;42:41–50. doi: 10.1002/ppul.20542. [DOI] [PubMed] [Google Scholar]
- 14.Boyle E.M., Poulsen G., Field D.J., Kurinczuk J.J., Wolke D., Alfirevic Z. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. 2012;344:e896. doi: 10.1136/bmj.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogt H., Lindstrom K., Braback L., Hjern A. Preterm birth and inhaled corticosteroid use in 6- to 19-year-olds: a Swedish national cohort study. Pediatrics. 2011;127:1052–1059. doi: 10.1542/peds.2010-3083. [DOI] [PubMed] [Google Scholar]
- 16.Jaakkola J.J., Ahmed P., Ieromnimon A., Goepfert P., Laiou E., Quansah R. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Taal H.R., Vd Heijden A.J., Steegers E.A., Hofman A., Jaddoe V.W. Small and large size for gestational age at birth, infant growth, and childhood overweight. Obesity (Silver Spring) 2013;21:1261–1268. doi: 10.1002/oby.20116. [DOI] [PubMed] [Google Scholar]
- 18.Pike K.C., Crozier S.R., Lucas J.S., Inskip H.M., Robinson S., Southampton Women's Survey Study Group The. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax. 2010;65:1099–1106. doi: 10.1136/thx.2010.134742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai A., Volkmer R., Burton A. Association between asthma symptoms and obesity in preschool (4-5 year old) children. J Asthma. 2009;46:362–365. doi: 10.1080/02770900902759260. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenschein-van der Voort A.M., Jaddoe V.W., Raat H., Moll H.A., Hofman A., de Jongste J.C. Fetal and infant growth and asthma symptoms in preschool children: the Generation R Study. Am J Respir Crit Care Med. 2012;185:731–737. doi: 10.1164/rccm.201107-1266OC. [DOI] [PubMed] [Google Scholar]
- 21.Niklasson A., Ericson A., Fryer J.G., Karlberg J., Lawrence C., Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981) Acta Paediatr Scand. 1991;80:756–762. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 22.Asher M.I., Keil U., Anderson H.R., Beasley R., Crane J., Martinez F. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 23.Caudri D., Wijga A., Scholtens S., Kerkhof M., Gerritsen J., Ruskamp J.M. Early daycare is associated with an increase in airway symptoms in early childhood but is no protection against asthma or atopy at 8 years. Am J Respir Crit Care Med. 2009;180:491–498. doi: 10.1164/rccm.200903-0327OC. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenschein-van der Voort A.M., Jaddoe V.W., van der Valk R.J., Willemsen S.P., Hofman A., Moll H.A. Duration and exclusiveness of breastfeeding and childhood asthma-related symptoms. Eur Respir J. 2012;39:81–89. doi: 10.1183/09031936.00178110. [DOI] [PubMed] [Google Scholar]
- 25.Lim R.H., Kobzik L., Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5:e10134. doi: 10.1371/journal.pone.0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duijts L., Jaddoe V.W., van der Valk R.J., Henderson J.A., Hofman A., Raat H. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children. The Generation R Study. Chest. 2012;141:876–885. doi: 10.1378/chest.11-0112. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20:682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 28.Debray T.P., Moons K.G., Abo-Zaid G.M., Koffijberg H., Riley R.D. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One. 2013;8:e60650. doi: 10.1371/journal.pone.0060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. 2013.] Available at: http://unstats.un.org/unsd/methods/m49/m49regin.htm - europe. Accessed May 31, 2013.
- 30.Normand S.L. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.van Houwelingen H.C., Arends L.R., Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 32.Flaherman V., Rutherford G.W. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rzehak P., Wijga A.H., Keil T., Eller E., Bindslev-Jensen C., Smit H.A. Body mass index trajectory classes and incident asthma in childhood: results from 8 European Birth Cohorts—a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. 2013;131:1528–1536. doi: 10.1016/j.jaci.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Sears M.R., Greene J.M., Willan A.R., Wiecek E.M., Taylor D.R., Flannery E.M. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 35.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 36.Cadman R.V., Lemanske R.F., Jr., Evans M.D., Jackson D.J., Gern J.E., Sorkness R.L. Pulmonary 3He magnetic resonance imaging of childhood asthma. J Allergy Clin Immunol. 2013;131:369–376. doi: 10.1016/j.jaci.2012.10.032. e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayanan M., Owers-Bradley J., Beardsmore C.S., Mada M., Ball I., Garipov R. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotecha S. Lung growth for beginners. Paediatr Respir Rev. 2000;1:308–313. doi: 10.1053/prrv.2000.0069. [DOI] [PubMed] [Google Scholar]
- 39.Baraldi E., Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 40.Colin A.A., McEvoy C., Castile R.G. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks' gestational age. Pediatrics. 2010;126:115–128. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jobe A.H. An unknown: lung growth and development after very preterm birth. Am J Respir Crit Care Med. 2002;166:1529–1530. doi: 10.1164/rccm.2209012. [DOI] [PubMed] [Google Scholar]
- 42.Friedrich L., Stein R.T., Pitrez P.M., Corso A.L., Jones M.H. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med. 2006;173:442–447. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 43.Hoo A.F., Dezateux C., Henschen M., Costeloe K., Stocks J. Development of airway function in infancy after preterm delivery. J Pediatr. 2002;141:652–658. doi: 10.1067/mpd.2002.128114. [DOI] [PubMed] [Google Scholar]
- 44.Kotecha S.J., Watkins W.J., Paranjothy S., Dunstan F.D., Henderson A.J., Kotecha S. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax. 2012;67:54–61. doi: 10.1136/thoraxjnl-2011-200329. [DOI] [PubMed] [Google Scholar]
- 45.Matias V., San Feliciano L., Fernandez J.E., Lapena S., Garrido E., Ardura J. Host and environmental factors influencing respiratory secretion of pro-wheezing biomarkers in preterm children. Pediatr Allergy Immunol. 2012;23:441–447. doi: 10.1111/j.1399-3038.2012.01269.x. [DOI] [PubMed] [Google Scholar]
- 46.Mamun A.A., Lawlor D.A., Alati R., O'Callaghan M.J., Williams G.M., Najman J.M. Increasing body mass index from age 5 to 14 years predicts asthma among adolescents: evidence from a birth cohort study. Int J Obes (Lond) 2007;31:578–583. doi: 10.1038/sj.ijo.0803571. [DOI] [PubMed] [Google Scholar]
- 47.Scholtens S., Wijga A.H., Seidell J.C., Brunekreef B., de Jongste J.C., Gehring U. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. J Allergy Clin Immunol. 2009;123:1312–1318.e2. doi: 10.1016/j.jaci.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 48.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 49.Shore S.A., Schwartzman I.N., Mellema M.S., Flynt L., Imrich A., Johnston R.A. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Baek H.S., Kim Y.D., Shin J.H., Kim J.H., Oh J.W., Lee H.B. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107:14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Jartti T., Saarikoski L., Jartti L., Lisinen I., Jula A., Huupponen R. Obesity, adipokines and asthma. Allergy. 2009;64:770–777. doi: 10.1111/j.1398-9995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 52.Nagel G., Koenig W., Rapp K., Wabitsch M., Zoellner I., Weiland S.K. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20:81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 53.Beuther D.A., Weiss S.T., Sutherland E.R. Obesity and asthma. Am J Respir Crit Care Med. 2006;174:112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tunon K., Eik-Nes S.H., Grottum P. A comparison between ultrasound and a reliable last menstrual period as predictors of the day of delivery in 15,000 examinations. Ultrasound Obstet Gynecol. 1996;8:178–185. doi: 10.1046/j.1469-0705.1996.08030178.x. [DOI] [PubMed] [Google Scholar]
- 55.Tunon K., Eik-Nes S.H., Grottum P., Von During V., Kahn J.A. Gestational age in pregnancies conceived after in vitro fertilization: a comparison between age assessed from oocyte retrieval, crown-rump length and biparietal diameter. Ultrasound Obstet Gynecol. 2000;15:41–46. doi: 10.1046/j.1469-0705.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 56.Jenkins M.A., Clarke J.R., Carlin J.B., Robertson C.F., Hopper J.L., Dalton M.F. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–616. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]
- 57.Koopman L., van der Heijden G.J., Grobbee D.E., Rovers M.M. Comparison of methods of handling missing data in individual patient data meta-analyses: an empirical example on antibiotics in children with acute otitis media. Am J Epidemiol. 2008;167:540–545. doi: 10.1093/aje/kwm341. [DOI] [PubMed] [Google Scholar]
- 58.Getahun D., Strickland D., Zeiger R.S., Fassett M.J., Chen W., Rhoads G.G. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164:187–192. doi: 10.1001/archpediatrics.2009.238. [DOI] [PubMed] [Google Scholar]
- 59.Kumar R., Yu Y., Story R.E., Pongracic J.A., Gupta R., Pearson C. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121:878–884.e6. doi: 10.1016/j.jaci.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.