Summary
The emergence of limb-driven locomotor behaviors was a key event in the evolution of vertebrates and fostered the transition from aquatic to terrestrial life. We show that the generation of limb-projecting lateral motor column (LMC) neurons in mice relies on a transcriptional autoregulatory module initiated via transient activity of multiple genes within the HoxA and HoxC clusters. Repression of this module at thoracic levels restricts expression of LMC determinants, thus dictating LMC position relative to the limbs. This suppression is mediated by a key regulatory domain that is specifically found in the Hoxc9 proteins of appendage-bearing vertebrates. The profile of Hoxc9 expression inversely correlates with LMC position in land vertebrates, and likely accounts for the absence of LMC neurons in limbless species such as snakes. Thus, modulation of both Hoxc9 protein function and Hoxc9 gene expression likely contributed to evolutionary transitions between undulatory and ambulatory motor circuit connectivity programs.
Introduction
Locomotion is a basic behavior exhibited by virtually all animals. While species display a wide variety of motor capabilities, land and water-based locomotion typically employs spinal neural networks whose outputs can be classified as being either ambulatory or undulatory. Undulatory motor behaviors, driven by sinusoidal waves of muscle contraction along the body axis, are observed in a large number of vertebrate and invertebrate species including anguilliform fish, snakes, worms, and insect larvae. Ambulatory behaviors, such as walking, are prominent in tetrapod vertebrates and require the coordinate activation of limb muscle groups by spinal motor neurons. The appearance of a limb innervation program was a significant step in expanding the repertoire of motor functions in vertebrates, allowing for a diverse array of behavioral innovations extending beyond locomotion, as exemplified by the range of articulations that can be performed by the human hand.
All motor behaviors rely on the selective innervation of muscles by motor neurons (MNs) residing in the brainstem and spinal cord. The basic program for muscle innervation is conserved across many species and determines features common to all MNs, such as the trajectory of axons towards muscle and the establishment of neuromuscular synapses (Thor and Thomas, 2002; Tripodi and Arber, 2012). Although both vertebrates and invertebrates are capable of walking, the pathway leading to limb innervation is thought to have originated independently in the vertebrate lineage (Murakami and Tanaka, 2011). Vertebrates bearing paired appendages (i.e. fins or limbs) evolved from marine species that lacked appendages and displayed undulatory-type motor behaviors. This locomotor strategy is present in modern representatives of basal chordate lineages including cephalochordates (e.g. amphioxus) and cyclostomes (e.g. lamprey and hagfish) (Grillner and Jessell, 2009). How spinal neuronal circuits evolved to implement limb-based motor strategies remains poorly understood.
The foundation of tetrapod limb innervation programs emerged in species that used fins to balance and modulate axial muscle-driven swimming behaviors. Studies in ray-finned fish suggest this program originated through adaptive changes in hindbrain-derived MNs that were initially involved in head bending (Ma et al., 2010). Aspects of the tetrapod limb innervation program, such as expression of the retinaldehyde dehydrogenase-2 (Raldh2) gene by limb-level MNs, are also present in pectoral MNs of zebrafish embryos (Begemann et al., 2001). Moreover, certain modern and ancient fish species appear to have utilized pectoral appendages for transient excursions on land (Daeschler et al., 2006; Kawano and Blob, 2013), suggesting the invasion of terrestrial environments by vertebrates was mediated by adaptive changes within forelimb-level locomotor circuits.
In quadrupeds, forelimb and hindlimb muscles are innervated by a column of MNs spanning 4–6 segments generated in registry with the developing limbs (Landmesser, 2001). Although they arise at distinct levels, brachial and lumbar LMC neurons share identical early features. Both populations are defined by expression of the Foxp1 and Raldh2 genes, and exhibit similar codes of Lim homeodomain (HD) protein expression (Dasen and Jessell, 2009; Sockanathan and Jessell, 1998; Tsuchida et al., 1994). A key step in LMC specification in mice is the activation of the Foxp1 gene, encoding a transcription factor required for LMC subtype diversification, and the selection of limb muscles (Dasen et al., 2008; Rousso et al., 2008). Initiation of the Foxp1→Raldh2→Lim HD pathway at limb levels is dictated by Hox proteins expressed by MNs at specific rostrocaudal coordinates. Hox6 and Hox10 proteins contribute to the positioning of brachial and lumbar LMC neurons, respectively, while Hoxc9 defines intervening thoracic MN populations, including preganglionic and hypaxial motor column (PGC and HMC) neurons (Figure 1A) (Jung et al., 2010). An additional network of ~20 Hox proteins acts within these columnar groupings to specify the identity of MN pools targeting individual muscles (Dasen et al., 2005). Given the critical roles of Hox genes in tetrapod MN specification, it is plausible that they contributed to the appearance of limb-based motor networks, as well as the variations in MN organization observed amongst vertebrate species (Fetcho, 1992).
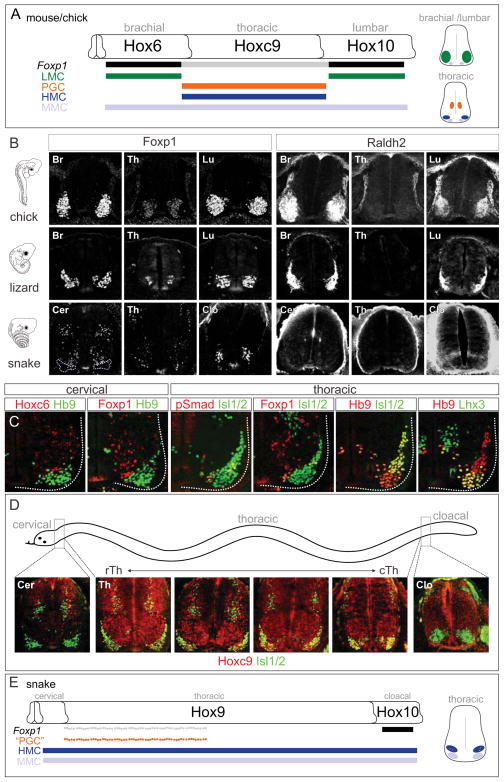
Figure 1. Analysis of MN Columnar Organization and Hox Gene Profiles in Vertebrates.
(A) Summary of MN columnar subtypes and their key determinants in mouse and chick spinal cord. Hox-dependent LMC and PGC neurons are specified through high and low levels of Foxp1, respectively. Right: Schematics of transverse sections showing MN position. (B) Comparison of Foxp1 and Raldh2 patterns in chick, lizard and snake embryos. In snake, Foxp1high;Raldh2+ MNs were not found at any spinal level. Chick embryos are shown at HH (Hamburger Hamilton) stage 27; lizard embryos at 10–11 dpo (day post-oviposition); snake embryos at 8–9 and 9–10 dpo. (C) MN columnar organization in snake embryos at 9–10 and 10–11 dpo. MNs were labeled by Hb9 or Isl1/2 in each panel. At cervical levels, Hoxc6 and Foxp1 are restricted to dorsal interneuron populations. At thoracic levels, PGC-like MNs (pSmad1/5/8+, Foxp1low) were detected in a scattered distribution, and HMC neurons (Hb9+, Isl1/2+) and MMC (Hb9+, Lhx3+) neurons were present. (D) Expansion of Hoxc9 expression throughout thoracic levels in snake. Hoxc9 expression was observed in cervical interneurons and thoracic MNs, but was absent from cloacal levels at 9–10 dpo. (E) Summary of Hox patterns and MN columnar organization in snake embryos. The scattered distribution of PGC-like MNs likely reflects cross-repressive interactions between Foxp1 and Hb9 within Hoxc9+ MNs (Dasen et al., 2008).
See also Figure S1.
We reasoned insights into the evolution of spinal circuits could emerge by analyzing Hox profiles in species that display distinct motor behaviors, and by assessing the activities of Hox proteins derived from more “primitive” vertebrate species. We show here that LMC neurons are specified through induction of the Foxp1 gene by transient, and somewhat generic, Hox activity. This program is maintained at limb levels through positive Foxp1 autoregulation, while LMC position relative to limbs is defined through Hoxc9-mediated suppression of Foxp1 at non-limb levels. This regulatory strategy appears to have emerged early in the development of paired appendages. These findings suggest that modulation in the spatiotemporal profiles and activities of Hox proteins can facilitate nervous system adaptations.
Results
Hox Genes and the Diversity of Vertebrate MN Columnar Organization
To explore the relationship between Hox gene profiles and MN organization in vertebrates, we compared MN columnar subtypes in three representative species of appendage-bearing tetrapod classes: mammals (mice), birds (chicks), and reptiles (whiptail lizards, Aspidoscelis uniparens). We also analyzed MN organization in two species of snake (Corn snake, Pantherophis guttatus and African house snake, Lamprophis fuliginosus), which lack the limb appendages targeted by LMC neurons. We examined the profile of markers for columnar subtypes dependent on Hox genes; LMC neurons at limb levels, PGC and HMC neurons at thoracic levels, and Hox-independent medial motor column (MMC) neurons (Figure 1A). In lizard embryos LMC neurons were present, as limb-level MNs settled in a ventrolateral position and expressed high levels of Foxp1 and Raldh2 (Figure 1B). At thoracic levels a subset of MNs migrated dorsally, expressed low levels of Foxp1 and was labeled by phospho (p) Smad1/5/8, indicative of a PGC identity (Figure 1B and S1A). While HMC (Hb9+, Isl1/2+) neurons were predominantly found at thoracic levels, axial projecting MMC neurons (Hb9+, Lhx3+) were present at all rostrocaudal levels (Figure S1B and S1C). These analyses reveal that the basic program for columnar organization is largely conserved in tetrapod species.
In contrast, snake embryos lacked discernible LMC populations, as Foxp1high; Raldh2+ MNs were not detected at any level (Figure 1B and 1C). Instead snakes displayed an extended thoracic columnar organization, as MMC and HMC neurons were found throughout the spinal cord (Figure 1C, S1B, and S1C). PGC-like neurons (pSmad1/5/8+, Foxp1low, Hb9−, Isl1/2+) were present at thoracic levels but were scattered within the ventral horn, suggesting an alternative organization for this population (Figure 1B, 1C, and S1A). At cloacal levels, a ventrolateral cluster of Foxp1+ MNs was observed in a region that occupied the same segments as the genital tubercles (Figure 1B). These MNs expressed the lumbar determinant Hoxd10, but did not express Raldh2 or display the Lim-HD profile characteristic of LMC neurons (Figure 1B, S1F, and S1G).
Profiles of Hox expression paralleled the marked differences in columnar organization observed between snakes and other tetrapods. Lizards displayed a pattern of Hox expression in MNs similar to chick and mice (Figure S1D–S1F). In contrast Hoxc6 was not expressed by MNs in snakes, and most of ~200 thoracic segments expressed Hoxc9, indicating a broad rostrocaudal extension in its expression domain (Figure 1C, 1D, S1D, and S1E). These observations indicate that the lack of a forelimb LMC program, in conjunction with an increase in the number of thoracic segments, is associated with an expanded domain of Hoxc9 (Figure 1E).
Erosion of Motor Neuron Columnar Identities in Hox Cluster Mutants
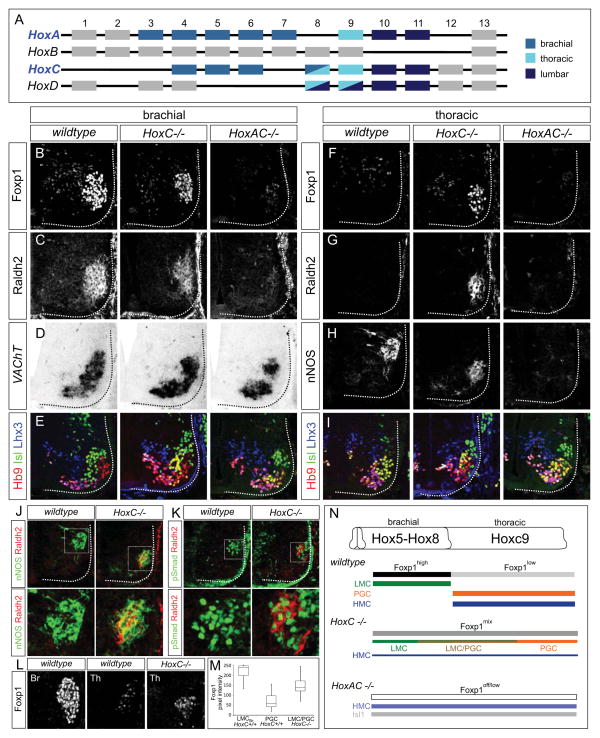
The absence of LMC neurons in snake embryos is consistent with the idea that Hoxc9 represses limb innervation programs. This observation is also in agreement with the finding that in Hoxc9 mutant mice all thoracic MNs are transformed to a brachial LMC identity (Jung et al., 2010). To understand how Hoxc9 mediates LMC suppression, we first sought to resolve the Hox-dependent mechanisms through which limb-innervating MNs are normally specified. Misexpression studies in chick indicate Hox5-Hox8 paralogs can impose an LMC identity onto thoracic MNs, suggesting multiple Hox genes contribute to LMC fate (Lacombe et al., 2013). To definitively assess Hox function in MN columnar specification, we determined the consequences of eliminating several Hox genes in mice. Since the majority of Hox genes expressed by brachial and thoracic MNs are concentrated within the HoxA and HoxC gene clusters (Figure 2A) (Dasen et al., 2005), we analyzed MN specification in HoxA and HoxC gene cluster mutants at embryonic day (e) 12.5 (Kmita et al., 2005; Suemori and Noguchi, 2000).
Figure 2. Loss of MN Columnar Identities in Hox Cluster Mutants.
(A) Summary of Hox expression profiles in MNs of limb-bearing tetrapods. Hox genes expressed by MNs at different levels are color-coded. (B–I) Defects in MN columnar specification in HoxC and HoxA/HoxC cluster mutants at brachial (B–E) and thoracic (F–I) levels at e11.5. (B and C) LMC neurons (Foxp1high, Raldh2+) were reduced in HoxC mutants, and lost in HoxA/HoxC mutants. (D) The total number of brachial MNs remained grossly unchanged in HoxC mutants, but was reduced by ~30% in HoxA/HoxC mutants, similar to the loss observed in Foxp1 mutants, in which LMC specification is similarly affected (Dasen et al., 2008). (E) MNs with an HMC molecular profile (Hb9+, Isl1/2+, Lhx3−) were increased in both HoxC and HoxA/HoxC mutants. (F–H) Both LMC and PGC markers were detected in HoxC mutants, but were absent in HoxA/HoxC mutants. (I) Analysis of MN molecular profiles (Hb9+, Isl1/2+, Lhx3+) revealed that total thoracic MN numbers were similar between wildtype, HoxC and HoxA/HoxC mutants. MMC neurons (Hb9+, Isl1/2low, Lhx3+) were grossly unaffected in both mutants. (J and K) Analysis of LMC/PGC hybrid MNs in HoxC mutants at thoracic levels. Raldh2+ MNs co-express the PGC markers nNOS and pSmad1/5/8. Bottom panels are magnified images of boxed area in upper panels. (L and M) Comparison of Foxp1 expression levels at brachial and thoracic in wildtype and at thoracic spinal cord in HoxC mutants. (N) Summary of altered MN columnar organization in HoxC and HoxA/HoxC cluster mutants. In HoxA/HoxC mutants a population of Isl1+;Hb9− MNs is also present.
See also Figure S2.
In the absence of the HoxC cluster, the number of forelimb LMC neurons was reduced by ~40%, assessed by the number of Foxp1high; Raldh2+ MNs (Figure 2B, 2C, and S2A). Total MN number was grossly unchanged in HoxC mutants, and the specification of Hox-independent, axially projecting MMC neurons was unaffected (Figure 2D, 2E, and 2I). Hindlimb-innervating LMC neurons developed normally, consistent with a prominent role for HoxD genes in their specification (Figure S2B) (Shah et al., 2004; Wu et al., 2008). In contrast, brachial MN pools, defined by expression of the transcription factors Pea3 and Scip were markedly depleted in HoxC cluster mutants (Figure S2C), consistent with a requirement for Hoxc6 and Hoxc8 in these subtypes (Lacombe et al., 2013; Vermot et al., 2005).
Genes in the HoxA cluster have been implicated in LMC specification (Lacombe et al., 2013) and could be responsible for its perseverance in HoxC mutants. In support of this idea, we found that there is an elevation in HoxA expression in HoxC mutants (Figure S2D and S2E). We therefore analyzed mice mutant for both the HoxA and HoxC clusters. Analysis of Foxp1 and Raldh2 expression at e12.5 in HoxA/HoxC mutants revealed a marked loss of brachial LMC neurons (Figure 2B and 2C). Low levels of Foxp1 were detected in HoxA/HoxC mutants, although was apparently insufficient to promote critical aspects of LMC identity, such as Raldh2 expression (Figure S2F). Thoracic PGC neurons were also absent in HoxA/HoxC mutants, consistent with a requirement for Hoxc9 and Hoxa9 (Figure 2H) (Dasen et al., 2003). These observations indicate that only through combined deletion of the HoxA and HoxC clusters is brachial LMC identity effectively erased from MNs.
Hybrid Motor Neuron Columnar Identities in HoxC cluster mutants
In contrast to the multiple Hox inputs controlling MN identity at brachial levels, thoracic fates are determined by the single Hoxc9 gene, which represses brachial Hox4-Hox8 genes at thoracic levels and sets low Foxp1 levels in PGC neurons (Jung et al., 2010). In HoxC mutants we expected that ectopic LMC neurons would be generated throughout thoracic levels, due to derepression of HoxA genes. Surprisingly, we detected markers of both LMC and PGC neurons at thoracic levels in HoxC mutants (Figure 2F–2H). These MNs occupied the same ventrolateral position, suggesting some acquired a “hybrid” columnar identity. Consistent with this idea Raldh2+/pSmad+ and Raldh2+/nNOS+ neurons were observed in HoxC mutants (Figure 2J and 2K). The extent of this phenotype varied along the rostrocaudal axis, with MNs co-expressing LMC and PGC determinants extending from caudal brachial to rostral thoracic levels, likely reflecting differential compensation by HoxA genes.
Foxp1 has been suggested to act as a dose-dependent determinant of LMC and PGC identities, and Foxp1 overexpression can convert PGC and HMC neurons to an LMC fate (Dasen et al., 2008). To understand why hybrid LMC/PGC neurons were generated, we analyzed Foxp1 levels in HoxC mutants. We observed Foxp1 levels that were intermediate to that of wildtype brachial LMC and thoracic PGC neurons (Figure 2L and 2M). Thus attenuation of the normal Hox inputs in MNs generates cells with inappropriate Foxp1 levels and hybrid molecular identities. Collectively, these results indicate that a primary function of Hox genes in tetrapod MNs is to set Foxp1 levels, with multiple Hox proteins promoting high levels in LMC neurons at limb-levels, while Hoxc9 dampens Foxp1 at thoracic levels (Figure 2N).
Conservation and Variation of Hox9 Paralog Activities in Motor Neurons
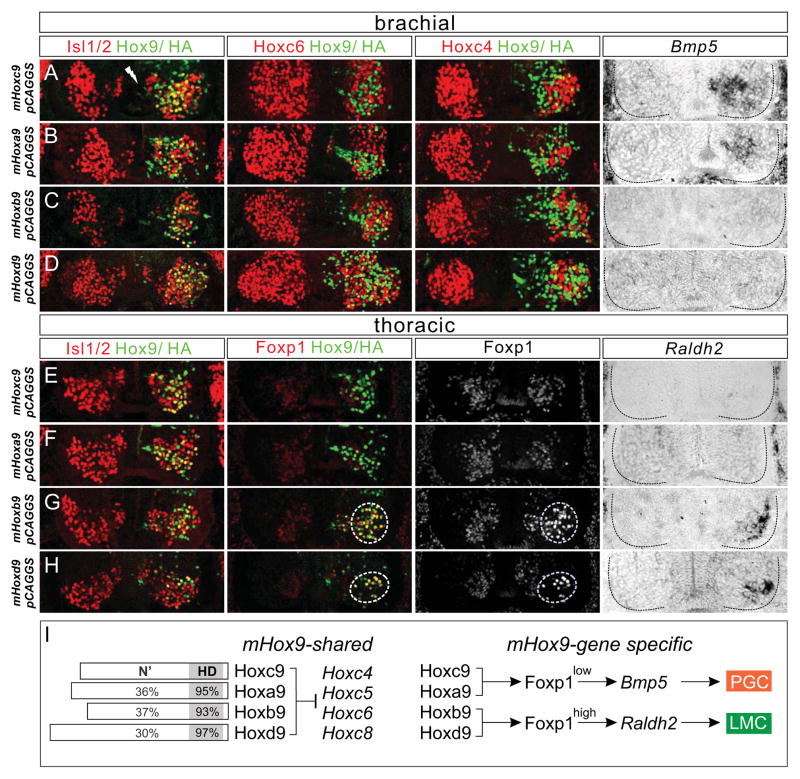
Analysis of Hox cluster mutant animals indicates that programming of LMC fate involves an activity shared by many Hox genes, whereas Hoxc9 has a selective function in preventing thoracic MNs from acquiring an LMC fate. These findings support the idea that the Hoxc9 gene has a central role in determining MN columnar organization in vertebrates. In principle the restricted actions of Hoxc9 could reflect selective binding to specific MN gene targets, or target recognition-independent activities mediated by regions outside the homeodomain. To examine this question in vivo, we first determined whether the activities of Hoxc9 are displayed by additional Hox9 paralogs (Hoxa9, Hoxb9, Hoxd9) (Figure 3). All murine (m) Hox9 proteins share highly conserved homeodomains, but display limited homology outside this region (Figure 3I and S3A). To test the specificity of Hox9 paralog activity, we compared their function in relation to two activities of Hoxc9: 1) a repressive activity towards Hox4-Hox8 genes, and 2) the ability to attenuate Foxp1 expression levels in PGC neurons.
Figure 3. Shared and Distinct Activities of Hox9 Proteins in MNs.
(A–H) Effects of murine Hox9 gene misexpression via in ovo electroporation at brachial (A–D) and thoracic (E–H) levels. To determine whether Hox9 proteins were expressed in MNs, Isl1/2 and Hox9/HA co-staining is shown in the left panels. (A–D) All four mHox9 paralogs cell autonomously repressed Hoxc6 and Hoxc4 at brachial levels. Hoxa9 and Hoxc9 misexpression lowered Foxp1 levels (See Figure S3B and S3C) and induced ectopic PGC (Bmp5+) neurons at brachial levels. (E–H) Expression of Hoxb9 and Hoxd9 at thoracic levels induced ectopic LMC neurons as assessed by high levels of Foxp1 and Raldh2 and these MNs migrated to a ventrolateral position. (I) Summary of shared and gene-specific functions of the four mHox9 paralogs in MNs. Schematics on left indicate percentage of amino acids at the N-terminus (N′) and homeodomain (HD) conserved between indicated paralog and mHoxc9.
See also Figure S3.
We used in ovo chick electroporation to misexpress murine Hox9 genes at brachial and thoracic levels and determined their effects on Hox expression and columnar differentiation. Each of the four mHox9 paralogs repressed brachial Hox genes as assessed by their ability to cell-autonomously extinguish Hoxc4 and Hoxc6 expression (Figure 3A–3D). Consistent with previous studies Hoxa9 activity was identical to Hoxc9 and promoted PGC fates (Bmp5+, Foxp1low) at brachial levels (Figure 3A, 3B, 3E, 3F, S3B, and S3C) (Dasen et al., 2003; Jung et al., 2010). In contrast, Hoxb9 and Hoxd9 failed to induce PGC identity or suppress LMC specification at brachial levels (Figure 3C and 3D). Remarkably, MNs expressing Hoxb9 or Hoxd9 at thoracic levels migrated to a ventrolateral position and induced high levels of Foxp1 and Raldh2, indicative of a conversion to an LMC fate (Figure 3G and 3H). After Hoxb9/d9 misexpression, Hoxc9 expression was retained, indicating LMC induction is not simply due to the extinction of endogenous Hoxc9 (Figure S3D and S3E). These data indicate that the repressive activity toward brachial Hox genes is conserved in all murine Hox9 paralogs, while the promotion of PGC identity and suppression of LMC fate are specific activities of Hoxa9 and Hoxc9, likely reflecting divergence of functional motifs amongst Hox9 proteins (Figure 3I).
Tetrapod Hoxc9 Contains a Latent LMC Promoting Activity
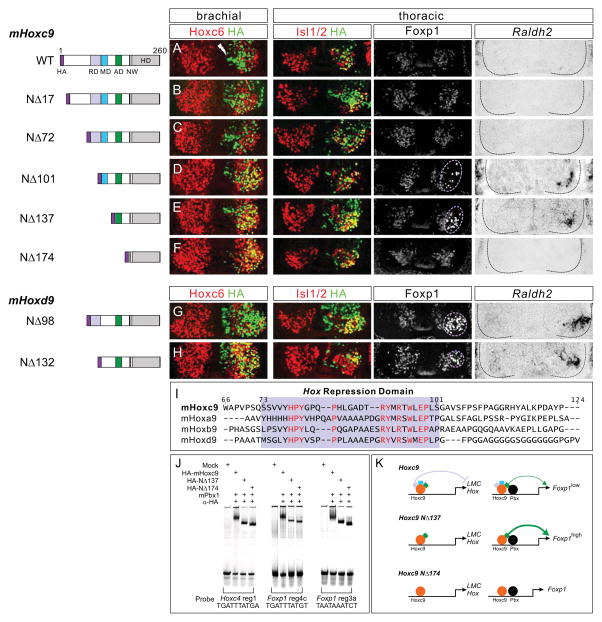
The ability of each Hox9 paralog to repress brachial Hox genes indicates that they are capable of regulating the same set of target genes; implying regions outside the DNA recognition motif are responsible for their in vivo specificities. To define functional domains in Hoxc9 we generated a series of N-terminal deletion constructs and tested their activities in vivo. We first mapped the peptide sequences within the mHoxc9 protein required for repression of Hox4-Hox8 genes. This analysis revealed a repression domain positioned between amino acids 73 and 101, as expression of mHoxc9NΔ101 failed to repress Hoxc4 and Hoxc6 at brachial levels, while mHoxc9NΔ72 retained repressive activity (Figure 4A–4F and S4A–S4F).
Figure 4. Hoxc9 Contains a Latent LMC Promoting Activity.
Identification of functional domains within Hox9 proteins. Schematics of mHoxc9/mHoxd9 truncations are shown on left. Mutant constructs were HA-tagged at the N-terminus. RD: Hox repression domain, MD: Foxp1 modulatory domain, AD: Foxp1 activation domain, NW: conserved Pbx interaction motif. (A–C) mHoxc9NΔ17 and mHoxc9NΔ72 retained normal repressive activity toward Hoxc6 at brachial levels. (D and E) mHoxc9NΔ101 and mHoxc9NΔ137 failed to repress Hoxc6 at brachial levels and induced ectopic LMC neurons (Foxp1high, Raldh2+) at thoracic levels. Ectopic LMC induction was not due to derepression of anterior Hox genes (See Figure S4I and S4J). (F) mHoxc9NΔ174 lost both Hox repressive and columnar promoting activities (See also Figure S4F). (G and H) mHoxd9NΔ132 failed to repress Hoxc6 at brachial levels, while mHoxd9NΔ98 retained repressive activity. Both constructs displayed LMC promoting activity at thoracic levels. (I) Sequence alignment of mHox9 paralogs revealed a conserved region between residues N73 and N101 in mHoxc9 (boxed in purple). Highly conserved residues are shown in red. (J) Binding of Hoxc9 mutant derivatives to elements in Hoxc4 and Foxp1 loci indicated that N-terminal deletions did not affect DNA binding activity. (K) A conserved repression domain (depicted in purple) is required to extinguish anterior Hox genes from thoracic levels. A latent activation domain (in green) is unleashed when the Hox repression domain is deleted. Models presume the mutant derivatives displace endogenous Hox proteins from the Foxp1 locus.
See also Figure S4.
Alignment of Hox9 protein sequences across the region required for Hox repression revealed a domain of sequence homology present in all four mHox9 paralogs and in other vertebrate Hox9 orthologs (Figure 4I and S5A) (Izpisua-Belmonte et al., 1991). To test functional conservation of this motif we generated mutant derivatives of mHoxd9 equivalent to mHoxc9NΔ72 and mHoxc9NΔ101 (mHoxd9NΔ98 and mHoxd9NΔ132, respectively) and tested their activities in vivo. Consistent with conserved activity, mHoxd9NΔ132 failed to repress brachial Hox genes, while mHoxd9NΔ98 repressed Hoxc4 and Hoxc6 (Figure 4G, 4H, S4G, and S4H).
We next tested the ability of Hox9 mutant derivatives to promote MN columnar identities. N-terminal deletions that retain Hox repressive functions exhibited normal activity; mHoxc9NΔ72 generated ectopic PGC neurons at brachial levels (Figure S4C) and mHoxd9NΔ98 promoted LMC fates at thoracic levels (Figure 4G). In contrast, mHoxc9NΔ101 and mHoxc9NΔ137 failed to induce ectopic PGC neurons at brachial levels (Figure S4D and S4E). Remarkably, both mHoxc9NΔ101 and mHoxc9NΔ137 induced LMC identity as assessed by the presence of ectopic Foxp1high and Raldh2+ MNs at thoracic levels (Figure 4D and 4E). In contrast a large deletion (mHoxc9NΔ174), which retains the region required for high affinity DNA binding, was inactive (Figure 4F and S4F).
Because the Hoxc9 mutant derivatives could influence the selectivity of target recognition, we also performed gel mobility shift assays to determine whether DNA binding is preserved. We tested mutant Hoxc9 proteins on a binding site located within the HoxC cluster, and two conserved sites within the Foxp1 gene that are occupied by Hoxc9 in vivo (Figure 4J and see Figure 7A below). We found that both mHoxc9NΔ137 and mHoxc9NΔ174 bound to each of these sites in the presence of Pbx cofactors (Figure 4J), indicating that Hoxc9 DNA binding activity is retained in the absence of its N-terminus.
Figure 7. Hoxc9 Dominantly Suppresses LMC Specification.
(A) ChIP-seq signal map of Hoxc9 binding near the Foxp1 locus in ES cell-derived MNs. Location of the BAC clone used for the Foxp1 reporter is indicated. (B) ChIP assays showing Hoxc6 binds to Foxp1 elements identified by Hoxc9 ChIP-seq. Error bars represent s.e.m. on duplicates. (C) Multiple Hox proteins including mHoxc6, mHoxc8 and mHoxc9 bound with mPbx1 to the Foxp1 sites, while mHoxb1 failed to bind in vitro. (D and E) Founder analysis of Foxp1::GFP-Δ3aΔ4c reporter lacking two Hox binding sites (see also Figure S7D). (D) At brachial levels, the GFP reporter was dramatically reduced relative to Foxp1::GFP. (E) At thoracic levels, ectopic GFP was detected in HMC neurons in Foxp1::GFP-Δ3aΔ4c embryos. (F) Expression of Hoxc9QN->AA DNA binding mutant at brachial levels failed to repress Hox genes or induce columnar identities. (G) Competition assays for DNA binding. Hoxc9 can displace Hoxc6 from binding sites within the Foxp1 gene. A Hoxc6 antibody was used to supershift the complex to distinguish it from Hoxc9 binding at the sites. Red arrow indicates supershifted Hoxc6 complex. (H) Co-electroporation showing that Hoxc9 dominantly suppressed LMC differentiation in the presence of Hoxc6, and induced ectopic PGC (Bmp5+) at brachial levels. Hoxc9 acts by displacing LMC promoting Hox proteins from the Foxp1 locus. When Hoxc6 was misexpressed with mHoxc9NΔ137, co-electroporated cells retained LMC identity.
See also Figure S7.
Collectively, these results demonstrate that while Hoxc9 normally promotes thoracic PGC fates by attenuating Foxp1 expression, it possesses a dormant LMC promoting activity which is unleashed after removal of the region containing the repression domain (Figure 4K).
The Emergence of Hox9 Activities in Chordates
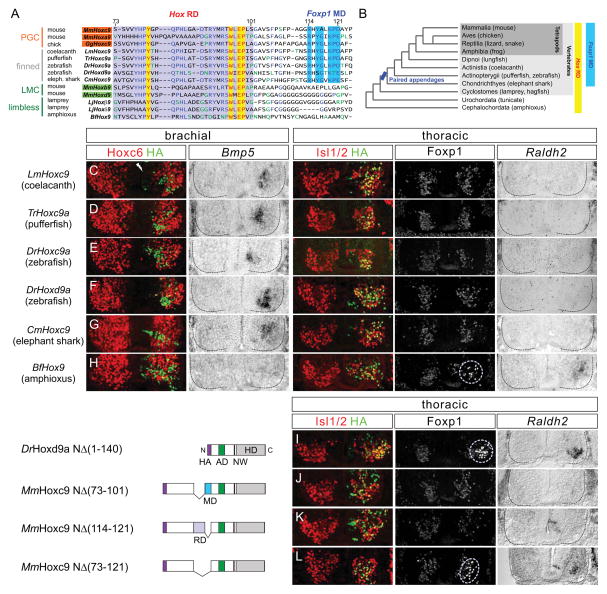
These observations raise the question of how the specific activities of Hoxc9 emerged in chordates, and what accounts for the differences in the columnar identities promoted by murine Hox9 paralogs. We considered the possibility that Hoxc9 acquired an LMC suppressing/PGC promoting activity concomitantly with the appearance of paired appendages. We therefore compared Hox9 amino acid sequences based on two criteria: 1) the presence or absence of paired appendages and 2) the ability of Hox9 proteins to promote either PGC or LMC fate in vivo. Inspection of sequences C-terminal to the core Hox repression domain revealed an additional motif present in Hox9 proteins that suppress LMC identity and in Hoxc9 proteins from species bearing paired-appendages (Figure 5A and 5B). In contrast this motif is either absent, degenerated, or shifted from its normal position in murine Hox9 genes that promote LMC fate, and in limbless chordates and cephalochordates (Figure 5A, 5B, and S5A). We refer to this motif as the Foxp1 modulatory domain (MD) to distinguish it from the repressive domain (RD) necessary to extinguish brachial Hox genes.
Figure 5. Evolution of Hox9 Function in Chordates.
(A) Alignment of Hox9 proteins from multiple chordate species revealed a conserved motif (boxed in blue) C-terminal to the Hox repression domain present in species with paired appendages. This motif is either absent or shifted C-terminally in other Hox9 proteins (See Figure S5A). Abbreviations: Mm, Mus musculus; Gg, Gallus gallus; Lm, Latimeria menadoensis; Tr, Takifugu rubripes; Dr, Danio rerio; Cm, Callorhinchus milii; Lj, Lethenteron japonicum; Bf, Branchiostoma floridae. The Hox repression domain is boxed in purple, and highly conserved amino acids in yellow. (B) Presence of Hox repression domain (RD) and Foxp1 modulatory domain (MD) are shown in relation to chordate phylogeny. (C–H) Analysis of Hox9 activities from various chordate species. Hox repressive activity was observed in all Hox9 homologs tested. (F) Unlike MmHoxd9, its ortholog DrHoxd9a has a Foxp1 MD in proximity to the RD (see also Figure 5A) and induced ectopic PGC (Bmp5+) at brachial levels. (H) Amphioxus Hox9 induced ectopic LMC (Foxp1high, Raldh2+) at thoracic levels. (I) DrHoxd9aNΔ140 lacking both Hox RD and Foxp1 MD failed to suppress Foxp1, and induced ectopic LMC neurons at thoracic levels. (J and K) Internal deletion of the RD region [MmHoxc9NΔ(73-101)] or MD [MmHoxc9NΔ(114-121)] failed to unmask LMC promoting activity. (L) LMC neurons were induced by the MmHoxc9NΔ(73-121), which lacks both the RD and MD.
See also Figure S5.
To test whether the presence of the MD mediates LMC suppression in vivo, we tested the activities of several Hox9 genes by in ovo chick electroporation. We isolated Hox9 genes from the limbless species amphioxus and lamprey, as well as pectoral fin-bearing species coelacanth, pufferfish, zebrafish, and elephant shark. Each species carried the core Hox RD (Figure 5A and S5A), and was capable of repressing Hoxc4 and Hoxc6 at brachial levels (Figure 5C–5H, S5B, and S5D). Hox9 genes from appendage-bearing vertebrates functioned as PGC determinants and suppressed LMC specification at brachial levels (Figure 5C–5G and S5B). In contrast the single Hox9 gene from amphioxus (BfHox9), which lacks the MD, acted as an LMC determinant as it induced Foxp1high and Raldh2 at thoracic levels (Figure 5H and S5B). Two of the Hox9 genes from lamprey acted as weak repressors of Foxp1, likely due to the presence of an MD-like region in these proteins (Figure S5A, S5D, and S5E).
Amongst vertebrate Hox9 homologs, the sequence of zebrafish Hoxd9 (DrHoxd9a) was distinct from that of mouse. DrHoxd9a contains the conserved MD in proximity to the Hox RD, and promoted PGC fate at brachial levels (Figure 5A and 5F). To test whether removal of MD would convert DrHoxd9a to an LMC determinant, we generated an N-terminal truncation in DrHoxd9a (NΔ140) equivalent to mHoxc9NΔ137, which lacks the MD. Consistent with a requirement for this motif to suppress LMC specification, DrHoxd9aNΔ140 induced Foxp1high and Raldh2 at thoracic levels (Figure 5I).
To further assess whether the modulatory domain contributes to the ability of mHoxc9 to suppress Foxp1, we generated internal deletion constructs lacking the Hox RD [mHoxc9NΔ (73-101)], the Foxp1 MD [mHoxc9NΔ (114-121)] and both the RD and MD [mHoxc9NΔ (73-121)] and tested their activities in vivo. With deletion of either the MD or RD, Hoxc9 derivatives retained LMC suppressing/PGC promoting activity, although the RD mutant failed to repress brachial Hox genes (Figure 5J, 5K, and S5C). Combined deletion of RD and MD converted Hoxc9 to an LMC inducer (Figure 5L), suggesting some degree of functional redundancy in these motifs with respect to Foxp1 regulation. Together, these observations indicate that Hoxc9 relies on specific motifs in its N-terminus to repress LMC specification at thoracic levels and suggest that this activity emerged at the time vertebrates acquired paired appendages.
Foxp1 Autoregulation Mediates LMC Specification
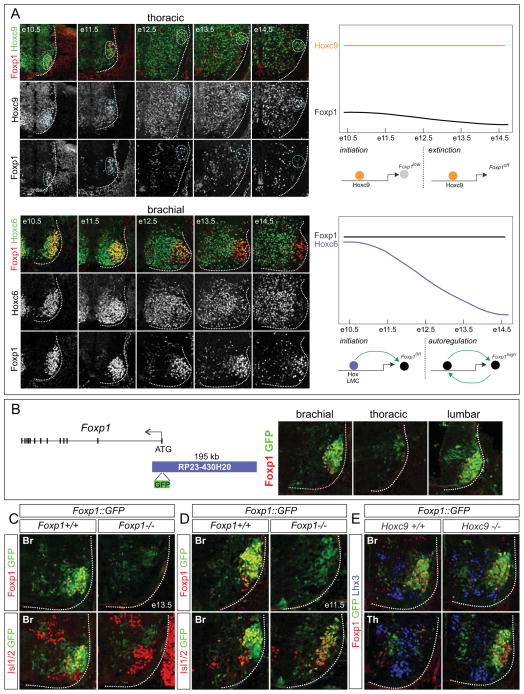
To resolve the mechanisms governing the actions of Hoxc9 during MN columnar organization, we focused on understanding how Hoxc9 suppresses activation of LMC determinants. Analysis of the spatial and temporal profiles of Hox proteins and Foxp1 expression provided some insight into this question. At thoracic levels, Hoxc9 expression is maintained by PGC neurons between e10.5 and e14.5, while Foxp1 is only transiently expressed (Figure 6A). In contrast in LMC neurons, Hox genes are transiently expressed by MNs between e10.5 and e12.5 while Foxp1 expression is sustained at high levels (Figure 6A and data not shown). These observations suggest a model in which LMC identity is promoted through transient expression of Hox proteins in MNs, which activates Foxp1. This pattern continues in the absence of Hox input, possibly through Foxp1 autoregulation. In contrast, the extended expression of Hoxc9 acts to dampen, and eventually silence Foxp1 expression in PGC neurons, effectively preventing deployment of the LMC-specific autoregulatory circuit (Figure 6A).
Figure 6. LMC Specification Relies on Foxp1 Autoregulation.
(A) Temporal patterns of Hox proteins and Foxp1 in LMC and PGC neurons. At thoracic levels, Hoxc9 expression was sustained between e10.5 and e14.5 while Foxp1 was undetectable by e14.5. Hoxc6 was transiently expressed by LMC neurons while Foxp1 was maintained. Model depicting differential regulation of Foxp1 in LMC and PGC neurons. Both LMC promoting Hox proteins and Hoxc9 are required for Foxp1 induction at e10.5-e11.5. At later embryonic stages, Foxp1 autoregulation maintains LMC identity while Hoxc9 suppresses Foxp1. (B) Generation of a Foxp1 reporter line using a BAC spanning ~195kb upstream of the Foxp1 start codon. Analysis of Foxp1::GFP transgenic mice showing GFP expression recapitulates both the spatial profile and relative Foxp1 levels at e11.5. (C–E) Analysis of Foxp1::GFP reporter mice in different mutant backgrounds. (C) In Foxp1 mutants, GFP was depleted from MNs (Isl1/2+) by e13.5, indicating a requirement for Foxp1 protein to maintain its own expression. (D) At e11.5, in Foxp1 mutants, GFP expression was detected in MNs indicating initial activation is retained. (E) In the absence of Hoxc9, GFP was expressed at high levels by ectopic LMC MNs at thoracic levels at e12.5.
See also Figure S6.
To test these models, we determined whether sustained expression of Foxp1 in LMC neurons relies on its autoregulation. To analyze Foxp1 regulation we generated a transgenic reporter line using a Bacterial Artificial Chromosome (BAC) containing ~195kb of 5′ Foxp1 sequence and inserted GFP at the initiating codon (Figure 6B). Analysis of e11.5 Foxp1::GFP embryos revealed robust expression of the reporter in LMC and low levels in PGC neurons, while GFP was absent from MMC and HMC neurons, thus recapitulating the endogenous Foxp1 pattern (Figure 6B and data not shown).
In principle, if Foxp1 autoregulates in LMC neurons, reporter expression in Foxp1::GFP mice would be lost in a Foxp1 mutant background. Consistent with this idea GFP expression was markedly depleted from MNs in Foxp1::GFP; Foxp1−/− mice at e13.5 (Figure 6C). Analysis of GFP between e10.5-e13.5 in Foxp1::GFP; Foxp1−/− mice indicated that the reporter was expressed in MNs between e10.5-e11.5, albeit at lower levels, indicating that Foxp1 is not needed for its initial activation (Figure 6D and S6). In addition, we introduced the Foxp1::GFP BAC reporter line into a Hoxc9 mutant background and analyzed embryos at e12.5. We observed ectopic GFP expression throughout the thoracic spinal cord (Figure 6E), consistent with the derepression of Hox5-Hox8 genes and the extension of high Foxp1 expression in Hoxc9 mutants. These data indicate that the Foxp1::GFP reporter contains cis elements necessary for regulation by Hox proteins and that high Foxp1 transcription relies on autoregulation.
Foxp1 Responds to Multiple Hox inputs
Because Foxp1 appears to respond to the activities of multiple Hox proteins, we assessed the role of Hox proteins in the direct regulation of Foxp1. Analysis of a previous genome-wide characterization of Hoxc9 binding in embryonic stem cell-derived MNs identified two regions within the Foxp1::GFP BAC containing potential Hox sites (Figure 7A) (Jung et al., 2010). Alignment of these candidate sites revealed high sequence conservation amongst vertebrates (Figure S7A). To determine whether these sites are occupied by LMC-promoting Hox proteins at limb levels, we performed chromatin immunoprecipitation (ChIP) assays and found Hoxc6 binds the same regions in vivo (Figure 7B). In vitro protein analysis of DNA binding revealed that multiple Hox proteins expressed by spinal MNs (Hoxc6, Hoxc8, Hoxc9) are capable of binding to these sites cooperatively with Pbx1 (Figure 7C). In contrast Hoxb1, which specifies MN subtype identity in the hindbrain (Studer et al., 1996), failed to effectively bind the Foxp1 sites, or affect columnar differentiation when misexpressed in vivo (Figure 7C and S7B). These results indicate that Hox sites in the Foxp1 gene can be engaged by a variety of Hox proteins expressed by spinal MNs, but are refractory to Hoxb1.
To investigate whether the Hox sites within the Foxp1::GFP BAC are functional in vivo, we deleted one or both elements and performed founder analysis at e11.5. Mutation of individual Hox sites in the Foxp1::GFP BAC did not alter reporter expression in MNs, suggesting functional redundancy (Figure S7C). After deletion of both sites, expression of GFP was markedly reduced in LMC neurons relative to wildtype Foxp1::GFP embryos generated under identical conditions (Figure 7D and S7D). In addition we detected ectopic GFP expression in HMC neurons at thoracic levels in the mutant construct, consistent with the idea that the sites are required for Hoxc9-mediated exclusion of Foxp1 from this population (Figure 7E and S7D). These results indicate that the Hox sites are essential for the regulation of Foxp1 in MNs.
Competitive Hox Interactions at the Foxp1 Locus
Our findings suggest that Hoxc9 suppresses LMC identity through blocking the ability of Hox proteins to initiate Foxp1 autoregulation. In principle Hoxc9 could accomplish this by neutralizing the activity of LMC-promoting Hox proteins via interactions off DNA, or by competing at shared target sites within the Foxp1 locus. To address these possibilities we first tested whether Hoxc9 DNA binding is necessary for its repressive actions. We introduced mutations in highly conserved DNA recognition sequences of the Hoxc9 homeodomain (Gln50→Ala and Asn51→Ala), which diminish DNA binding but preserve homeodomain structure (Remacle et al., 2002). Expression of this construct at brachial levels had no effect on Foxp1 or anterior Hox genes, indicating that DNA binding is required for Hoxc9 activities (Figure 7F). We also used gel-shift assays to test whether Hoxc9 can displace Hoxc6 from sites in the Foxp1 gene. This analysis revealed Hoxc9 was effective in competing with Hoxc6 at sites in the Foxp1 gene (Figure 7G). These data indicate Hoxc9 requires DNA binding to repress LMC fate, and that Hoxc9 is capable of excluding LMC-promoting Hox proteins from Foxp1.
We next tested whether Hoxc9 acts by blocking maintenance of Foxp1 expression. Ectopic expression of Hoxc9 at brachial levels inhibits LMC specification (Dasen et al., 2008); however, interpretation of this finding is confounded by the repressive effects of Hoxc9 on brachial Hox genes. To circumvent this issue, we co-expressed Hoxc6 and Hoxc9 at brachial levels, reasoning that if MNs were confronted with both Hoxc9 and Hoxc6, Hoxc9 would suppress Foxp1 autoregulation and favor PGC specification, independent of its repressive actions towards Hox genes. We optimized conditions so that the expression levels of each construct were similar to levels normally found in MNs (Figure S7F). MNs co-expressing Hoxc6 and Hoxc9 at brachial levels expressed low Foxp1 levels and acquired a PGC identity as assessed by Bmp5 induction (Figure 7H). In contrast MNs expressing Hoxc6 alone or in combination with the LMC-inducing Hoxc9 mutant derivative retained an LMC identity (Foxp1high, Raldh2+) (Figure 7H and S7E). These results indicate that Hoxc9 is capable of blocking activation of high levels of Foxp1, independent of its repressive activity towards Hox genes. Hoxc9 likely accomplishes this function in vivo by displacing Hox proteins that would otherwise promote LMC identity, many of which are expressed at low levels in thoracic segments (Jung et al., 2010; Lacombe et al., 2013). Thus the organization of spinal motor columns relies on the sustained binding of Hoxc9 to the Foxp1 locus which acts to prevent LMC induction at thoracic levels.
Discussion
Locomotion is a fundamental animal behavior, but the genetic programs that contributed to the emergence of limb-specific motor circuits are largely unexplored. In this study we defined the mechanisms controlling the specification and organization of MN subtypes required to coordinate limb muscles during ambulatory motor behaviors. We found that limb-innervating MNs are determined through a set of transient and permissive Hox inputs that initiate autoregulation of the Foxp1 gene, and that the registry between LMC and limb position is defined by Hoxc9-mediated suppression of Foxp1 at thoracic levels (Figure 8A). This specific activity is mediated through a modification in a subset of Hox9 proteins that appeared at the time vertebrates acquired paired appendages. Adjustment in the pattern of Hoxc9 expression in the neural tube likely contributes to the variety of columnar topographic arrangements amongst vertebrates. These studies thus offer insights into the strategies through which Hox genes facilitate evolution of the CNS.
Figure 8. Model for the Evolution of Hox-Dependent Motor Columns.
(A) Temporal regulation of Foxp1 by Hox proteins during MN columnar specification. In LMC neurons transient Hox activity initiates Foxp1 autoregulation. At thoracic levels Hoxc9 initiates low-level and transient Foxp1 expression in PGC neurons and represses Foxp1 in HMC neurons. (B) Model for the evolution of functional domains within Hox9 proteins. A domain capable of repressing anterior Hox genes is present in Hox9 proteins of early chordates. A modulatory domain within tetrapod Hoxa9 and Hoxc9 appeared in appendage-bearing vertebrates and is required for suppression of LMC identity at thoracic levels. (C) Speculative model for MN organization by evolving Hox activity profiles. MN columnar organization is controlled through species-specific Hox profiles to accommodate different vertebrate body plans. In zebrafish the posterior boundary of pectoral fin MNs (pec) corresponds to the anterior boundary of Hox9 expression (Ma et al., 2010). Hindbrain (HB)/spinal cord (SC) boundary is indicated. Skate is shown as a representative species having an extended pectoral fin that develops adjacent to the pelvic fin, while frogs bear the fewest number of thoracic segments amongst land vertebrates. In both species the position and distribution of LMC-like MNs may be defined by the profile of Hoxc9 activity. The expanded profile of Hoxc9 in snakes suppresses LMC differentiation at rostral levels. In mice mutant for the Hoxc9 gene LMC neurons extend from cervical to lumbar levels.
Hox Activity Regulation and the Diversity of MN Columnar Organization
Hox genes are key determinants of morphological diversity across animal species (Burke et al., 1995; Carroll et al., 2005). In Drosophila control of leg number is determined through a repressive motif in the Hox protein Ubx, which suppresses leg formation in abdominal segments (Galant and Carroll, 2002; Ronshaugen et al., 2002). Notably this motif is absent from crustaceans which bear appendages in trunk segments. In vertebrates, the pattern of Hox activity in the lateral plate mesoderm determines the number and position of ribs (Vinagre et al., 2010). In snakes mutation in a Hox-dependent cis element allows for rib formation in regions that would normally lack them (Guerreiro et al., 2013). Changes in the profiles of Hox expression are also correlated with the absence of limbs in snake embryos (Cohn and Tickle, 1999). Whether Hox proteins contribute to behavioral adaptations at the neural circuit level has not been addressed.
Our findings indicate that a key mechanism through which MN organization emerged involves modulation in Hox protein activities. We identified sequences within Hox9 proteins that confer differential effects on target gene regulation in vivo, and each motif appeared at a distinct phase of vertebrate evolution. All of the Hox9 proteins we tested possess a conserved N-terminal domain that can extinguish expression of brachial Hox genes in chick embryos. This repressive activity is present in the single Hox9 protein of amphioxus, suggesting a function at the base of the chordate lineage in establishing neuronal Hox profiles. In contrast Hox9 proteins of appendage-bearing vertebrates display distinct activities in MNs, and only a subset suppress LMC fates. Repression of LMC identity by Hoxc9 is mediated by a distinct region which plays a more restricted role through differential effects on the Foxp1 gene. The MD motif is active in the Hoxc9 protein of elephant shark, a representative of the most primitive appendage-bearing vertebrates, but is absent from the Hox9 protein of the limbless amphioxus. The presence of an MD or MD-like domain in three of the four mouse Hox9 paralogs suggests that this motif appeared prior to Hox cluster duplication events, implying that the first vertebrates bearing limb-like appendages may have contained a single Hox cluster.
At what stage during the evolution of limb innervation programs did this specific repressive activity of Hoxc9 arise? One model posits that basal fin-bearing vertebrates contained a single fin extending the length of the trunk (Freitas et al., 2006; Tanaka et al., 2002; Tanaka and Onimaru, 2012). Subsequently this elongate appendage was restricted to a rostral position, giving rise to the pectoral fins. One could imagine a scenario where primitive fin-bearing vertebrates contained an LMC-like population extending the length of the spinal cord, and its confinement to pectoral and pelvic levels was coordinated with changes in fin position. Conceivably, this could have been achieved through the appearance of a new repressive motif in Hoxc9. Thus we favor a model in which at the time vertebrates acquired paired-appendages a new activity emerged that allowed a subset of Hox9 genes to repress Foxp1 and/or other genes that promote fin innervation (Figure 8B). This hypothesis does not exclude alternative origins of the pectoral fin, such as the gill-arch (Gillis et al., 2009), which would have also necessitated a strategy for ensuring restriction of LMC-like populations.
CNS Organization as a Function of Modulation in Hox Expression Profiles
Our findings suggest a key mechanism governing variations in MN organization is mediated by alterations in Hox profiles. We found that in snake embryos the domain of Hoxc9 expression is extended along the rostrocaudal axis and likely contributes to the absence of brachial LMC neurons. In contrast species with relatively large appendages, such as the pectoral fins of stingrays and skates, could generate a broader distribution of LMC-like MN populations by attenuating the repressive influence of Hoxc9. Fin innervating MN populations in stingrays extend ~80 segments (Coggeshall et al., 1978; Droge and Leonard, 1983), and it is tempting to speculate that this organization is mediated through regulation of column-defining Hox genes. Certain skate species lack the HoxC cluster in its entirety (King et al., 2011), and removal of the Hoxc9 gene in this context could contribute to the extension of pectoral fin-innervating populations. Modification in the expression pattern of Hoxc9 would, in principle, allow for efficient reorganization of MN populations in registry with changes in the appendicular musculoskeletal system (Figure 8C).
There are also significant differences in the mechanisms of Hox gene regulation at limb and thoracic levels. At limb levels Hox determinants are only transiently expressed by LMC neurons, and identity is preserved through Foxp1 autoregulation. In contrast Foxp1 is transiently expressed by thoracic PGC neurons, while Hoxc9 is maintained throughout early embryogenesis. Hoxc9 is also distinct amongst Hox genes expressed by spinal MNs, as its function is required in MN progenitors, and is highly susceptible to alterations in the activities of early determinants that control Hox expression, including morphogens and Polycomb group proteins (Dasen et al., 2003; Golden and Dasen, 2012). The multiple pathways through which the Hoxc9 gene is regulated could serve to provide alternative strategies to modulate its spatial and temporal profile during adaptive changes in the CNS.
Hox genes are widely expressed in the nervous system (Philippidou and Dasen, 2013), and alterations in Hox activity profiles likely impact specification in multiple cell lineages, including the diverse interneuron populations which coordinate limb movement (Andersson et al., 2012; Lanuza et al., 2004). These ensembles of rhythmically active neurons are known to occupy specific rostrocaudal positions of the spinal cord (Ballion et al., 2001; Kjaerulff and Kiehn, 1996), and it is plausible that their connectivity is shaped by the same Hox networks that determine MN subtype identities. Hox-dependent programs could therefore exert a broader role in the evolution of motor circuits that foster behavioral adaptations.
Experimental Procedures
Mouse Genetics
HoxC cluster (Suemori and Noguchi, 2000), HoxA cluster (Kmita et al., 2005), Foxp1 (Wang et al., 2004), and Hoxc9 (McIntyre et al., 2007) mutant strains have been previously described. BAC transgenic mice were generated by pronuclear microinjection using standard procedures.
Generation of BAC Transgenic Mice
The Foxp1::GFP reporter line was generated using BAC clone RP23-430H20 corresponding to chr6:99,097K – 99,292K (mouse genome assembly mm9). Sequences flanking the Foxp1 ATG were cloned into the shuttle vector pLD53SC-AEB and introduced into the BAC by homologous recombination (Gong et al., 2003). To generate Foxp1::GFP-4cmut, a potential Hox binding site mutated into an NsiI site. A single region (3a) or both regions (3a and 4c) were deleted in the BAC to make Foxp1::GFP-Δ3a and Foxp1::GFP-Δ3aΔ4c transgenes, respectively. The genomic regions 3a and 4c were identified by Hoxc9 ChIP-seq (Jung et al., 2010): region 3a: chr6:99,286,209-99,286,532; region 4c: chr6:99,140,045-99,140,206 (mm9).
In Ovo Chick Electroporations
Expression constructs for murine Hox9 paralogs were generated as described previously (Dasen et al., 2003; Jung et al., 2010). Full-length zebrafish Hoxc9a and Hoxd9a cDNAs were obtained from Open Biosystems. To generate additional full-length Hox9 cDNAs, exons were amplified by PCR from genomic DNA. In ovo electroporation was performed in HH stage 13–15 and analyzed at HH stage 27. In each electroporation, the expression plasmid (pCAGGs) was used in the range of 50–200ng/μl with pBKS as carrier DNA (1μg/μl). We titrated the amount of HA-tagged mHoxc9 or mHoxd9 mutant derivatives before further analysis to ensure that their expression levels were similar to a HA-tagged wildtype mHoxc9 or mHoxd9. Results for each experiment are representative of at least three embryos in which the electroporation efficiency in MNs was >60%.
ChIP Assays
Chromatin Immunoprecipitation was performed as described previously (Jung et al., 2010) on e12.5-e13.5 mouse spinal cords using rabbit anti-Hoxc6 (Abcam, ab41587). Genomic regions were amplified using Power Sybr® Green PCR Master Mix (Applied Biosystems) and detected with Mx 3005P real-time PCR apparatus (Stratagene). Fold enrichment were calculated over IgG using the ΔΔCt method: fold enrichment = 2−(ΔΔCt), where ΔΔCt = (CtIP − CtInput) − (CtIgG − CtInput). Primer sequences used for the real-time PCR are as follows (5′-3′): Foxp1 reg3a_fw: GTCTCAAGGGAGGGGAAAAA; Foxp1 reg3a_rev: GGGATAGTGGCCGTTAATCA; Foxp1 reg4c_fw: ATGCGTCCCACCCATAAAG; Foxp1 reg4c_rev: ATCTCGGGTGTTGAGAATGA.
In Situ Hybridization and Immunohistochemistry
Fixed embryos were sectioned at 16μm by cryostat. In situ hybridization and immunohistochemistry were performed as described (Tsuchida et al., 1994). Antibodies against Hox proteins, LIM HD proteins and other proteins were generated or obtained as described (Dasen et al., 2005; Jung et al., 2010; Liu et al., 2001; Tsuchida et al., 1994). Additional antibodies used were: monoclonal anti-HA 1:10,000 (Covance), goat polyclonal anti-GFP 1:4,000 (Rockland).
Electrophoretic Mobility Shift Assays (EMSA)
EMSA assays were performed as described previously (Lacombe et al., 2013). 293T cells were transfected with expression constructs using Lipofectamine 2000 (Invitrogen) and nuclear extracts were prepared as described (Wadman et al., 1997). Protein amounts were estimated by Western blot. For competition assays, recombinant proteins were prepared as described (Lacombe et al., 2013). Protein amounts in Figure 7G were: mHoxc6, 4pmol; mHoxc9, 4pmol in lane 2/4/7, 12pmol in lane 8, 36pmol in lane 9/10; mPbx3, 4pmol in lane 4/5/6, 8pmol in lane 7, 16pmol in lane 8, 40pmole in lane 9/10/11. The sense sequences for probes are as follows: Hoxc4 reg1, ATTCCGCGAGACTGATTTATGACGTTTTACAGCC; Foxp1 reg3a, ATGCGGCATACATAATAAATCTAATCAAGTCTAC; Foxp1 reg4c, ATGTTGGAGGTCTGATTTATGTTGTCATTTCCTC. Hoxc9 binding sites are underlined in each oligo. 15bp of linker sequence (CCTCGTCCCACAGCT) was added to each probe for the IRDye-800 labeling. 0.5–1ug of anti-HA antibody (Covance) and 1ug of anti-Hoxc6 antibody (Abcam, ab41587) were used in supershifts.
Sequence Comparisons
Protein sequence alignments were generated using AlignX in Vector NTI (Invitrogen). The UCSC genome browser was used to compare vertebrate Foxp1 sequences.
Quantification of Protein Levels
Nuclear Foxp1, Hoxc6 and Hoxc9 levels were measured as described (Dasen et al., 2008). Mean pixel intensities for >100 MN nuclei are shown.
Supplementary Material
Highlights.
Early limb-innervation programs are induced and constrained by diverse Hox inputs
Hoxc9 restricts the position of limb-innervating motor neurons to the correct site
Selective Hoxc9 protein function emerged as vertebrates acquired paired appendages
Hoxc9 gene expression contributes to evolutionary diversification of neuronal pattern
Acknowledgments
We thank Olivier Pourquie for generously providing snake and whiptail lizard embryos; Chris Amemiya for coelacanth and amphioxus BAC clones; Martin Cohn for lamprey sequences; Tarang K. Mehta for lamprey gDNA and Hox9 sequences; Claude Desplan, Gord Fishell, Hyung Don Ryoo, and lab members for providing valuable feedback. We thank the NYU Mouse Transgenic Facility for BAC transgenics; Julie Lacombe and Doug Epstein for assistance with recombineering; Shaun Mahony for analysis of ChIP-seq data; Carolyn Walsh, Jonathan Grinstein and David Lee for technical assistance. D.D. is supported by grants from the Swiss National Research Foundation (SNSF) and the European Research Council (ERC). J.S.D. is supported by funding from HHMI and the NIH (R01 NS062822).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjalm G, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14:1727–1738. doi: 10.1046/j.0953-816x.2001.01794.x. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- Carroll SB, Grenier JK, Weatherbee SD. From DNA to diversity : molecular genetics and the evolution of animal design. 2. Malden, MA: Blackwell Pub; 2005. [Google Scholar]
- Coggeshall RE, Leonard RB, Applebaum ML, Willis WD. Organization of peripheral nerves and spinal roots of the Atlantic stingray, Dasyatis sabina. J Neurophys. 1978;41:97–107. doi: 10.1152/jn.1978.41.1.97. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Tickle C. Developmental basis of limblessness and axial patterning in snakes. Nature. 1999;399:474–479. doi: 10.1038/20944. [DOI] [PubMed] [Google Scholar]
- Daeschler EB, Shubin NH, Jenkins FA., Jr A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature. 2006;440:757–763. doi: 10.1038/nature04639. [DOI] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Droge MH, Leonard RB. Organization of spinal motor nuclei in the stingray, Dasyatis sabina. Brain Res. 1983;276:201–211. doi: 10.1016/0006-8993(83)90727-8. [DOI] [PubMed] [Google Scholar]
- Fetcho JR. The spinal motor system in early vertebrates and some of its evolutionary changes. Brain Behav Evol. 1992;40:82–97. doi: 10.1159/000113905. [DOI] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Cohn MJ. Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature. 2006;442:1033–1037. doi: 10.1038/nature04984. [DOI] [PubMed] [Google Scholar]
- Galant R, Carroll SB. Evolution of a transcriptional repression domain in an insect Hox protein. Nature. 2002;415:910–913. doi: 10.1038/nature717. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Dahn RD, Shubin NH. Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc Natl Acad Sci USA. 2009;106:5720–5724. doi: 10.1073/pnas.0810959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden MG, Dasen JS. Polycomb repressive complex 1 activities determine the columnar organization of motor neurons. Genes Dev. 2012;26:2236–2250. doi: 10.1101/gad.199133.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro I, Nunes A, Woltering JM, Casaca A, Novoa A, Vinagre T, Hunter ME, Duboule D, Mallo M. Role of a polymorphism in a Hox/Pax-responsive enhancer in the evolution of the vertebrate spine. Proc Natl Acad Sci USA. 2013;110:10682–10686. doi: 10.1073/pnas.1300592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisua-Belmonte JC, Falkenstein H, Dolle P, Renucci A, Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991;10:2279–2289. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Lacombe J, Mazzoni EO, Liem KF, Jr, Grinstein J, Mahony S, Mukhopadhyay D, Gifford DK, Young RA, Anderson KV, et al. Global control of motor neuron topography mediated by the repressive actions of a single Hox gene. Neuron. 2010;67:781–796. doi: 10.1016/j.neuron.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano SM, Blob RW. Propulsive forces of mudskipper fins and salamander limbs during terrestrial locomotion: implications for the invasion of land. Integrative and comparative biology. 2013;53:283–294. doi: 10.1093/icb/ict051. [DOI] [PubMed] [Google Scholar]
- King BL, Gillis JA, Carlisle HR, Dahn RD. A natural deletion of the HoxC cluster in elasmobranch fishes. Science. 2011;334:1517. doi: 10.1126/science.1210912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmita M, Tarchini B, Zakany J, Logan M, Tabin CJ, Duboule D. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435:1113–1116. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- Lacombe J, Hanley O, Jung H, Philippidou P, Surmeli G, Grinstein J, Dasen JS. Genetic and functional modularity of Hox activities in the specification of limb-innervating motor neurons. PLOS Genet. 2013;9:e1003184. doi: 10.1371/journal.pgen.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int J Dev Neurosci. 2001;19:175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Ma LH, Gilland E, Bass AH, Baker R. Ancestry of motor innervation to pectoral fin and forelimb. Nat Commun. 2010;1:49. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM. Hox patterning of the vertebrate rib cage. Development. 2007;134:2981–2989. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Tanaka M. Evolution of motor innervation to vertebrate fins and limbs. Dev Biol. 2011;355:164–172. doi: 10.1016/j.ydbio.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle S, Shaw-Jackson C, Matis C, Lampe X, Picard J, Rezsohazy R. Changing homeodomain residues 2 and 3 of Hoxa1 alters its activity in a cell-type and enhancer dependent manner. Nucleic acids research. 2002;30:2663–2668. doi: 10.1093/nar/gkf372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V, Drill E, Lance-Jones C. Ectopic expression of Hoxd10 in thoracic spinal segments induces motoneurons with a lumbosacral molecular profile and axon projections to the limb. Dev Dyn. 2004;231:43–56. doi: 10.1002/dvdy.20103. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Suemori H, Noguchi S. Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Dev Biol. 2000;220:333–342. doi: 10.1006/dbio.2000.9651. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Munsterberg A, Anderson WG, Prescott AR, Hazon N, Tickle C. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature. 2002;416:527–531. doi: 10.1038/416527a. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Onimaru K. Acquisition of the paired fins: a view from the sequential evolution of the lateral plate mesoderm. Evolution & development. 2012;14:412–420. doi: 10.1111/j.1525-142X.2012.00561.x. [DOI] [PubMed] [Google Scholar]
- Thor S, Thomas JB. Motor neuron specification in worms, flies and mice: conserved and ‘lost’ mechanisms. Curr Opin Genet Dev. 2002;12:558–564. doi: 10.1016/s0959-437x(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Tripodi M, Arber S. Regulation of motor circuit assembly by spatial and temporal mechanisms. Curr Opin Neurobiol. 2012;22:615–623. doi: 10.1016/j.conb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Vermot J, Schuhbaur B, Le Mouellic H, McCaffery P, Garnier JM, Hentsch D, Brulet P, Niederreither K, Chambon P, Dolle P, et al. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development. 2005;132:1611–1621. doi: 10.1242/dev.01718. [DOI] [PubMed] [Google Scholar]
- Vinagre T, Moncaut N, Carapuco M, Novoa A, Bom J, Mallo M. Evidence for a myotomal Hox/Myf cascade governing nonautonomous control of rib specification within global vertebral domains. Dev Cell. 2010;18:655–661. doi: 10.1016/j.devcel.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 1997;16:3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, Tucker PW. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang G, Scott SA, Capecchi MR. Hoxc10 and Hoxd10 regulate mouse columnar, divisional and motor pool identity of lumbar motoneurons. Development. 2008;135:171–182. doi: 10.1242/dev.009225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.