Abstract
Helicobacter pylori infection is highly prevalent, affecting approximately half of the world’s population. While the majority of infected individuals are asymptomatic, H. pylori infection is associated with certain diseases, including peptic ulcers (either duodenal or gastric), gastritis, and 2 malignancies—gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma. Many of the epidemiologic associations between these diseases and H. pylori infection have been further validated by treatment studies, which show that effective eradication therapy correlates with a decreased risk of disease. A variety of testing strategies are used to detect H. pylori infection. Serologic techniques are widely available and inexpensive, but they are no longer preferred as they have low sensitivities and specificities, and they may show a positive result for a long period following effective therapy. The remaining testing methods are divided into 2 categories: invasive tests (which require endoscopy) and noninvasive tests. Noninvasive test methods such as the urea breath test and stool antigen test have gained popularity due to their high sensitivities and specificities. Further, both of these methods may be used to confirm the absence of infection following eradication therapy. Due to the increasing incidence of treatment failure (caused in part by antibiotic resistance), post-treatment testing is recommended to confirm H. pylori eradication.
Helicobacter pylori: History, Prevalence, and Association with Disease
Helicobacter pylori was first identified in 1982 by Robin Warren and Barry Marshall in Perth, Western Australia. These physicians observed a small, curved bacteria colonizing the antrum of patients with gastric inflammation; they reported the successful isolation of this unidentified bacilli in a Letter to the Editor published in a 1983 edition of The Lancet.1 They further noted the strong association between the presence of this bacteria and the presence of gastritis or duodenal ulcer. Shortly thereafter, in 1983, Marshall inoculated himself with an isolate of this bacteria taken from a 66-year-old male with known dyspepsia.2 Subsequent to his self-inoculation, Marshall developed a mild gastrointestinal illness in the following weeks; a biopsy confirmed the presence of gastritis.
At the turn of the next decade, in 1990, researchers in the Netherlands made a key discovery. They reported the cure of duodenal ulcers with eradication of H. pylori infection.3 A total of 50 patients were included in this study, all of whom had intractable duodenal ulcers. Patients were randomly assigned to 4 weeks of treatment with colloidal bismuth subcitrate, given alone or in combination with amoxicillin and metronidazole. Five patients in the triple therapy group withdrew from the study due to side effects. Among the remaining 45 patients, 17 experienced eradication of H. pylori infection with no ulcer relapse during 12 months of follow-up. Among 21 patients who remained positive for H. pylori, the 12-month ulcer relapse rate was 89%. Nine of these patients were subsequently treated with the triple therapy regimen, 7 of whom demonstrated H. pylori eradication and no ulcer relapse in the 12-month follow-up period. The study results caused the authors to conclude that H. pylori eradication would become a key component in the treatment and cure of duodenal ulcers.
This conclusion was supported in 1994, when the National Institutes of Health convened an expert consensus panel to develop recommendations for the treatment of patients with H. pylori infection.4 A key component of the panel’s recommendations was the suggestion that ulcer patients with H. pylori infection be treated with antimicrobial agents in addition to antisecretory drugs. Further, the panel concluded that there appeared to be an “interesting relationship” between H. pylori infection and gastric cancers, an association that would require further research.
The key contributions made by Warren and Marshall were recognized in 2005, when the 2 researchers were awarded the Nobel Prize in Physiology or Medicine “for their discovery of H. pylori and its role in gastritis and peptic ulcer disease.”5 This award noted that, thanks to their discovery, peptic ulcer disease was no longer a chronic and frequently disabling condition, but instead could often be cured with treatment. Ongoing research continues to evaluate the importance of H. pylori infection as a contributing factor in various conditions. In addition to peptic ulcer disease, H. pylori is also associated with gastric cancer and gastric mucosa—associated lymphoid tissue (MALT) lymphoma, both of which are discussed in further detail below.6
Prevalence of Helicobacter pylori Infection
At least half of the world’s population is estimated to be infected with H. pylori.7 However, the prevalence of this infection varies widely across both geographic regions and ethnic groups. Overall, rates of H. pylori infection are markedly higher in developing countries compared to developed countries. For example, prevalence rates that approach or even exceed 90% have been reported in multiple studies conducted in Bangladesh, Egypt, Russia, Siberia, and Africa. In contrast, prevalence rates are much lower in developed regions, including the United States (6.8-79%), Europe (7.3-70%), and Australia (15.5—23%). These patterns may reflect the known association between low socioeconomic status and increased risk of H. pylori infection.
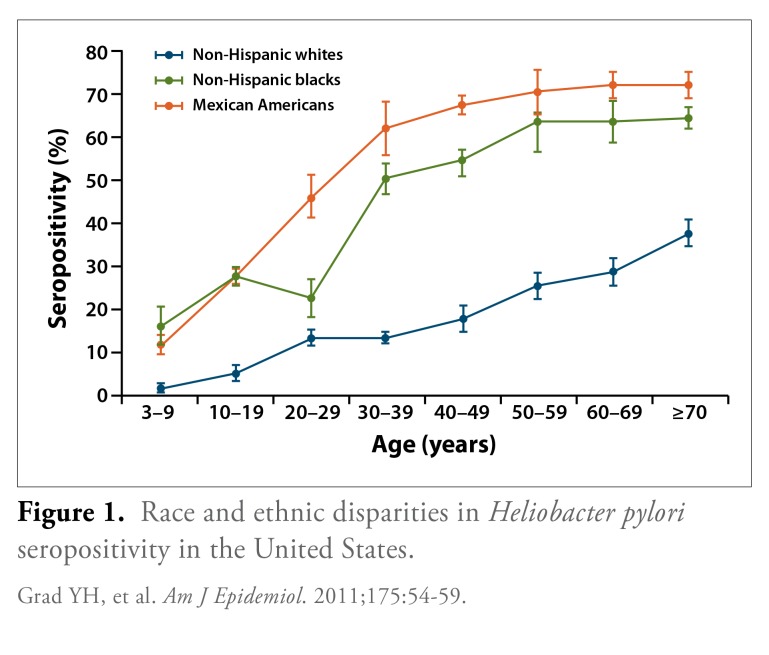
Substantial disparities in the prevalence of H. pylori infection occur across racial and ethnic groups. This disparity was demonstrated in an analysis of H. pylori seroprevalence rates among adults (aged 20 years or older) enrolled in the National Health and Nutrition Examination Survey (1999-2000).8 In an analysis of 4,145 participants, the age-standardized proportion of seropositivity for H. pylori was 30.7% (95% confidence interval [CI], 27.9-33.6). When the population was stratified by racial and ethnic groups, a higher seropositivity rate was observed among non-Hispanic blacks and Mexican Americans compared to non-Hispanic whites (Figure 1). Further, a multivariate analysis demonstrated that non-Hispanic blacks and Mexican Americans had a significantly higher odds ratio (OR) for H. pylori infection even after adjusting for factors such as age, socioeconomic status, and country of origin. The OR for H. pylori infection was 3.12 among non-Hispanic blacks (95% CI, 2.46-3.94) and 3.05 among Mexican Americans (95% CI, 2.10-4.44; both comparisons versus non-Hispanic whites). The factors driving the higher prevalence of seropositivity among non-Hispanic blacks and Mexican Americans remain unclear and require further exploration.
Figure 1.
Race and ethnic disparities in Heliobacter pylori seropositivity in the United States.
Grad YH, et al. Am J Epidemiol. 2011;175:54-59.
Studies have also consistently found the prevalence of H. pylori infection to be higher in adults than in children. This observation is likely due to 2 factors: First, infection with H. pylori typically occurs early in life and, second, becomes chronic if the bacteria are not eradicated, allowing infection to persist into adulthood. This trend may also reflect a birth cohort effect, with poor living conditions and sanitation in prior decades resulting in a higher prevalence of H. pylori infection in the past.
Risk Factors for Infection
The primary mode of H. pylori transmission is person-to-person passage, occurring via gastric-oral, oral-oral, or fecal-oral routes. Thus, risk factors associated with person-to-person transmission of infection have been linked to H. pylori; these factors include infected family members, increased number of siblings, crowded living conditions, and poor sanitation and hygiene.9,10 For example, when the concordance of H. pylori infection was compared among 241 sibling and nonsibling children (aged 2-18 years), the odds of infection increased from 1.2 (95% CI, 0.52-2.9) among children residing with at least 1 infected nonsibling to 3.2 (95% CI, 1.14-9.1) among children residing with at least 1 infected sibling and to 9.4 (95% CI, 3.1-28.5) among children residing with at least 1 infected sibling and 1 infected nonsibling.11
Association Between Helicobacter pylori Infection and Disease
Although the vast majority of infected individuals are asymptomatic, H. pylori infection is a cofactor in the development of several diseases. Peptic ulcers (either duodenal or gastric) occur in 10-15% of individuals infected with H. pylori.12 Ford and colleagues conducted a systemic review of eradication therapy for the treatment of H. pylori-positive peptic ulcer disease; a total of 52 trials were included in the final meta-analysis.13 For healing duodenal ulcers, eradication therapy proved to be superior to treatment with either an ulcer-healing drug only or no treatment; the relative risks for the persistence of the ulcer were 0.66 (95% CI, 0.58-0.76) and 0.37 (95% CI, 0.26-0.53), respectively. For gastric ulcers, in contrast, the relative risk of the ulcer persisting was not significantly superior for eradication therapy versus treatment with an ulcer-healing drug only (relative risk, 1.32; 95% CI, 0.92-1.90).
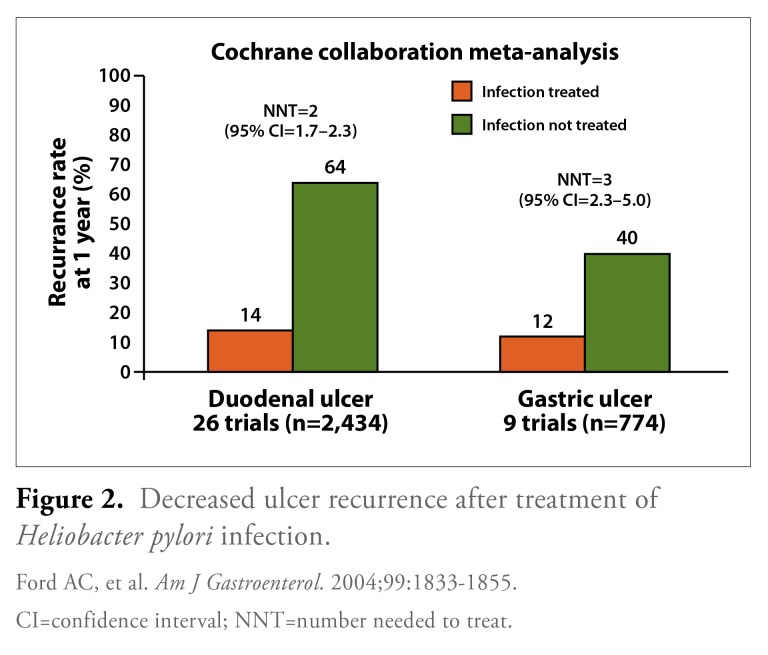
The study by Ford and colleagues also showed that eradication therapy was associated with markedly lower recurrence rates for both duodenal ulcers (14% with treatment vs 64% with no treatment) and gastric ulcers (12% with treatment vs 40% with no treatment; Figure 2). Additionally, the number needed to treat (NNT) was calculated as the reciprocal of the risk difference from the meta-analysis; the NNT to prevent 1 duodenal ulcer recurrence within 1 year was 2 (95% CI, 1.7-2.3), and the NNT to prevent 1 gastric ulcer recurrence was 3 (95% CI, 2.3—5.0). However, even with treatment of H. pylori infection, an appreciable recurrence rate remained; these recurrences were likely due to failed H. pylori eradication as well as other ulcerogenic factors, such as use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs).
Figure 2.
Decreased ulcer recurrence after treatment of Heliobacter pylori infection.
CI=confidence interval; NNT=number needed to treat.
Ford AC, et al. Am J Gastroenterol. 2004;99:1833-1855.
Mounting epidemiologic evidence suggests that H. pylori infection has an important role in the etiology of 2 malignancies: gastric adenocarcinoma and gastric MALT lymphoma. H. pylori infection may initiate the critical sequence of phenotypic changes in gastric mucosa that lead to cancer, which begins with inflammation and then proceeds to superficial gastritis, chronic atrophic gastritis, intestinal metaplasia, dysplasia, and, finally, carcinoma. Given its role in the pathogenesis of these malignancies, H. pylori infection is recognized by the World Health Organization as a Group 1 carcinogen.14 Overall, H. pylori infection is associated with a 2-fold to 3-fold increased risk for the development of gastric adenocarcinoma, although the lifetime risk of developing the disease still remains relatively low (<1%).12,15 The risk of developing gastric MALT lymphoma, a rarer malignancy, is even lower.6,16
Wong and colleagues conducted a prospective, randomized, placebo-controlled, population-based trial to determine whether treatment of H. pylori infection could reduce the incidence of gastric cancer.17 A total of 1,630 healthy, H. pylori-positive, Chinese patients were included in this study and were followed for a mean of 7.5 years. Patients were randomized to receive H. pylori eradication therapy (combination therapy with omeprazole, amoxicillin, clavulanate potassium, and metronidazole) or placebo. Eighteen new cases of gastric cancer developed during the follow-up period, but the incidence of gastric cancer did not differ significantly between patients who received eradication therapy and those who did not (7 vs 11 cases, respectively; P=.33). However, a subgroup analysis of the patients who had no precancerous lesions at the time of study enrollment (N=988) demonstrated that no cases of gastric cancer developed among patients who were treated with eradication therapy, compared to 6 cases of cancer occurring among patients in this subgroup who were treated with placebo (P=.02).
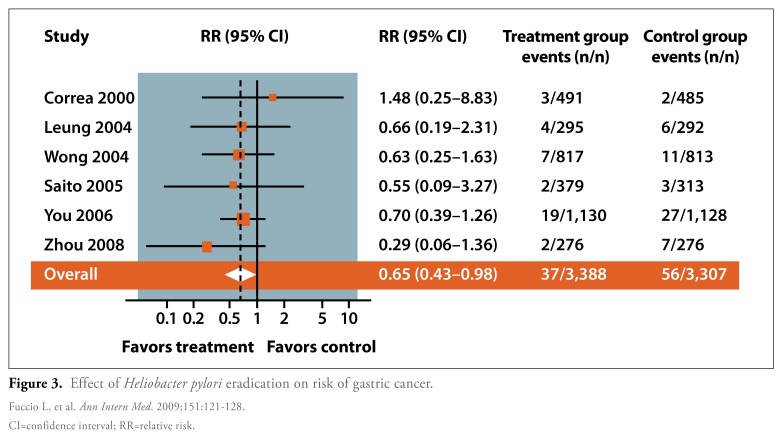
More recently, Fuccio and coauthors conducted a meta-analysis of randomized trials in which they aimed to determine the ability of H. pylori eradication therapy to reduce the risk of gastric cancer (Figure 3).18 This meta-analysis included 6 studies, all of which were conducted in areas that had a relatively high incidence of gastric cancer. In total, 3,388 patients received eradication therapy, while 3,307 untreated patients served as a control group. The authors reported that 1.1% of patients treated with eradication therapy developed gastric cancer, compared to 1.7% of untreated patients. This difference translated to a relative risk of 0.65 (95% CI, 0.43-0.98) for gastric cancer developing after eradication therapy.
Figure 3.
Effect of Heliobacter pylori eradication on risk of gastric cancer.
CI=confidence interval; RR=relative risk.
Fuccio L, et al. Ann Intern Med. 2009;151:121-128.
Development of Gastritis
Based on histologic evidence, all patients with H. pylori infection develop gastritis. Other gastrointestinal conditions may also be related to H. pylori infection, although these associations are more controversial; for example, H. pylori infection and associated inflammation have been reported in biopsy specimens of gastric mucosa from patients with nonulcerative dyspepsia. However, this finding is mitigated by the fact that signs of H. pylori infection are commonly observed in asymptomatic patients.
Once H. pylori infection occurs, several factors are important in determining the outcome of the infection— notably, the level of acid secretion and the distribution of the gastritis. An antral-predominant distribution is associated with a high level of gastric acid secretion, which leads to an increased risk for the development of duodenal ulcers. In contrast, corpus-predominant gastritis (or pangastritis) is associated with a lower level of gastric acid secretion and increased risk for both gastric ulcers and gastric cancer.
Importantly, the level of acid secretion and the gastritis distribution are themselves affected by multiple factors acting at various levels: the host level (such as changes in immune response and gene polymorphisms), the bacteria level (such as virulence factors), and the environmental level (including smoking, alcohol use, NSAID use, and proton pump inhibitor [PPI] use). At the host level, an emerging determinant of gastric distribution and acid secretion is the proinflammatory cytokine interleukin (IL)-1β.19 Polymorphisms in the IL-1β gene have been shown to be linked to the risk of developing H. pylori— induced gastric cancer. At the environmental level, an important determinant may be the use of PPIs, which are pharmacologic inhibitors of acid secretion. Among H. pylori-infected patients, PPIs have been shown to result in redistribution of gastritis from an antral-predominant location to a corpus-predominant location.20
Acknowledgement
Adam B. Elfant, MD, FACG, was paid by Otsuka America Pharmaceutical, Inc. for participation in this roundtable and development of this monograph, and he is a promotional speaker for BreathTek® UBT for Helicobacter pylori (H. pylori) Kit.
References
- 1.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 2.Gustafson J, Welling D. “No acid, no ulcer”—100 years later: a review of the history of peptic ulcer disease. J Am Coll Surg. 2010;210:110–116. doi: 10.1016/j.jamcollsurg.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of. Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 4.Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–69. NIH Consensus Conference. [PubMed] [Google Scholar]
- 5.www.nobelprize.org/nobel_prizes/medicine/laureates/2005/press.html The Nobel Prize in Physiology or Medicine 2005 [press release]. October 3, 2005.
- 6.Aliment Pharmacol Ther. 2002;16(Suppl 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. Go MR Review article: natural history and epidemiology of Helicobacter pylori infection. [DOI] [PubMed] [Google Scholar]
- 7.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–578. doi: 10.1016/s0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 8.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16(Suppl 1):1–9. doi: 10.1111/j.1523-5378.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vale FF, Vitor JM. Transmission pathway of Helicobacter pylori: does food play a role in rural and urban areas? Int J Food Microbiol. 2010;138:1–12. doi: 10.1016/j.ijfoodmicro.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Garg PK, Perry S, Sanchez L, Parsonnet J. Concordance of Helicobacter pylori infection among children in extended-family homes. Epidemiol Infect. 2006;134:450–459. doi: 10.1017/S0950268805005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833–1855. doi: 10.1111/j.1572-0241.2004.40014.x. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. Infection with Helicobacter pylori. In: IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. IARC monographs on the evaluation of carcinogenic risks to humans; vol 61. Lyon, France: International Agency for Research on Cancer, 1994:177-240.
- 15.Malfertheiner P, Bornschein J, Selgrad M. Role of Helicobacter pylori infection in gastric cancer pathogenesis: a chance for prevention. J Dig Dis. 2010;11:2–11. doi: 10.1111/j.1751-2980.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 16.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 17.Wong BC, Lam SK, Wong WM, et al. China Gastric Cancer Study Group Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 18.Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151:121–128. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto M, Yamaoka Y, Furuta T. Influence of interleukin polymorphisms on development of gastric cancer and peptic ulcer. World J Gastroenterol. 2010;16:1188–1200. doi: 10.3748/wjg.v16.i10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigneri S, Termini R, Savarino V, Pace F. Review article: is Helicobacter pylori status relevant in the management of GORD? Aliment Pharmacol Ther. 2000;l4(Suppl 3):31–42. doi: 10.1046/j.1365-2036.2000.00398.x. [DOI] [PubMed] [Google Scholar]
Testing for Helicobacter pylori Infection
According to guidelines from the American College of Gastroenterology (ACG), established indications for H. pylori testing include: peptic ulcer disease (either duodenal or gastric ulcer, regardless of whether the patient has active disease or a documented history of previously untreated disease), a diagnosis of low-grade gastric MALT lymphoma, and following endoscopic resection of early gastric cancer.1 Importantly, these guidelines note that testing should only be performed in patients who will subsequently be offered H. pylori eradication therapy.
Other guidelines from the ACG list possible or probable indications for H. pylori testing. For example, while there is no established evidence that eradication of H. pylori infection will cure nonulcer dyspepsia, testing may be performed in such patients on a case-by-case basis, and treatment may be offered to patients with a positive test result.2 Similarly, testing and treating for H. pylori infection may be beneficial for preventing NSAID-related ulcer complications, especially in patients planning to undergo long-term NSAID therapy.3
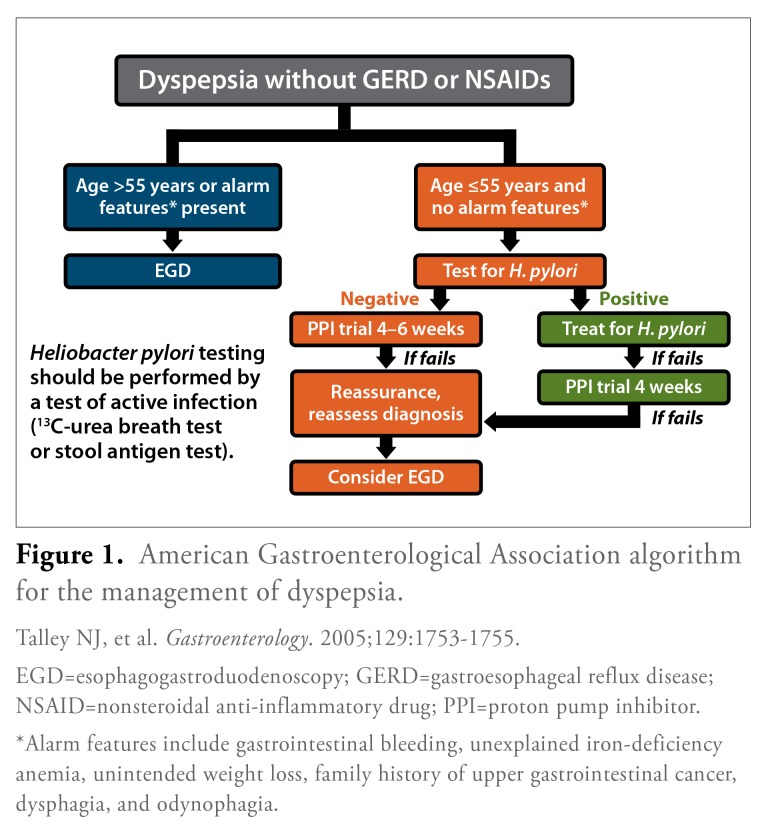
The American Gastroenterological Association (AGA) has also published official recommendations on the evaluation of dyspepsia, which include an algorithm for determining which patients should undergo H. pylori testing (Figure 1).4 According to this algorithm, patients who present with dyspepsia in the absence of gastroesophageal reflux disease or NSAID use are first divided into 2 groups: those who require immediate endoscopy and those who are suitable candidates for noninvasive testing. Patients who are over the age of 55 years or who exhibit certain alarm features should undergo immediate investigation with endoscopy in order to rule out peptic ulcer diseases or upper gastrointestinal tract malignancy. Alarm features include gastrointestinal bleeding, unexplained iron-deficiency anemia, unintended weight loss, family history of upper gastrointestinal cancer, dysphagia, and odynophagia. In contrast, dyspeptic patients who are 55 years or younger and who have no alarm features should undergo H. pylori testing using a validated, noninvasive test. H. pylori-positive patients should then undergo eradication therapy and a trial of PPI therapy, if symptoms persist. In all cases, endoscopy may be considered if patients do not respond to therapy.
Figure 1.
American Gastroenterological Association algorithm for the management of dyspepsia.
EGD=esophagogastroduodenoscopy; GERD=gastroesophageal reflux disease; NSAID=nonsteroidal anti-inflammatory drug; PPI=proton pump inhibitor.
*Alarm features include gastrointestinal bleeding, unexplained iron-deficiency anemia, unintended weight loss, family history of upper gastrointestinal cancer, dysphagia, and odynophagia.
Talley NJ, et al. Gastroenterology. 2005;129:1753-1755.
Current Strategies for Helicobacter pylori Testing
A variety of testing strategies are available to assess for H. pylori infection (Tables 1 and 2).1 Of these strategies, serologic tests are capable of determining whether the patient has a history of previous exposure to H. pylori, as these tests measure the formation of anti—H. pylori antibodies. They are widely available and are the least expensive of the testing strategies currently in use. However, because positive results may reflect a previous, rather than current infection, use of these tests is not recommended in low-prevalence populations due to their low positive predictive value, nor when clinicians are seeking to confirm the success of H. pylori eradication therapy.
Table 1.
Methods of testing for Helicobacter pylori infection.
| Active infection | History of exposure | |
|---|---|---|
| Endoscopic |
|
— |
| Nonendoscopic |
|
Serology* |
American College of Gastroenterology practice guidelines: In populations with a low pretest probability of H. pylori infection, nonendoscopic tests such as the urea breath test and stool antigen test offer superior positive predictive values compared to antibody tests.
Chey WD, et al. Am J Gastroenterol. 2007;102:1808-1825.
Table 2.
Nonendoscopic tests for Heliobacter pylori.
| Test | Advantages | Disadvantages |
|---|---|---|
| Serology |
|
|
| Urea breath test |
|
|
| Stool antigen test |
|
|
Adapted from McColl KE. N Engl J Med. 2010;362:1597-1604.
PPI=proton pump inhibitor.
The nonserologic tests can be divided into 2 categories: invasive (or endoscopic) versus noninvasive (nonendoscopic).5 Endoscopic detection methods include biopsies, histology, or culture. Nonendoscopic methods for detecting H. pylori—which are more commonly used for routine testing—include the urea breath test (UBT) or a stool antigen test. For the UBT assay, the patient drinks a solution containing 13C-labeled or 14C-labeled urea, which is converted to labeled carbon dioxide by the urease in H. pylori. The labeled gas is then measured in a breath sample. The other noninvasive test, the stool antigen detection method, uses polyclonal or monoclonal antibodies to identify H. pylori-specific antigens in a stool sample.
Both UBT and stool antigen detection assays are associated with high negative and positive predictive values, and both are useful either prior to or following eradication therapy. Also, the ACG practice guidelines note that the UBT and stool antigen detection tests both offer superior positive predictive values compared to serologic testing when used in patients with a low pretest probability of H. pylori infection.1 This group includes individuals from high socioeconomic status areas and/or regions with a low background prevalence of ulcer or H. pylori infection.
The disadvantage of the UBT method is that it requires specialized equipment to perform the test; the disadvantage of stool antigen detection methods is their requirement that patients provide stool samples, which can be pragmatically challenging for some patients. Another disadvantage of both UBT and stool antigen detection assays is that recent treatment with a PPI, an antibiotic, or a bismuth preparation may lead to a false-negative result.
Despite their various advantages and disadvantages, all of these testing strategies have relatively high rates of sensitivity and specificity (Table 3).6-8 For example, the BreathTek® UBT for H. pylori Kit (Otsuka America Pharmaceutical, Inc.) is associated with sensitivity and specificity rates of 95% and 90%, respectively, for initial diagnosis with the BreathTek UBT Kit. Similarly, the HpSA stool antigen assay has a 93% sensitivity rate and a 93% specificity rate. Similarly, endoscopic biopsy followed by routine histology has a 93% sensitivity rate and a 90% specificity rate. Because of a higher propensity for false positives with serologic techniques, this method is associated with slightly lower sensitivity and specificity rates: 85% and 79%, respectively.
Table 3.
Sensitivity and specificity of tests for Heliobacter pylori.
| Urea breath test (BreathTek UBT for H. pylori)*† | Stool antigen test (HpSA)* | Endoscopic biopsy‡ (routine histology) | Serology (ELISA)* | ||
|---|---|---|---|---|---|
| Initial diagnosis | Post-eradication | ||||
| Sensitivity | 95% | 95.5% | 93% | 93% | 85% |
| Specificity | 90% | 96% | 93% | 90% | 79% |
ELISA=enzyme-linked immunosorbent assay.
Vaira D, et al. Gut. 2001;48:287-289;
BreathTek UBT [package insert]; Rockville, Md; Medical Device Division of Otsuka America Pharmaceutical, Inc.; 2012;
Maconi G, et al. Aliment Pharmacol Ther. 1999;13:327-331.
BreathTek® UBT for H. pylon Testing
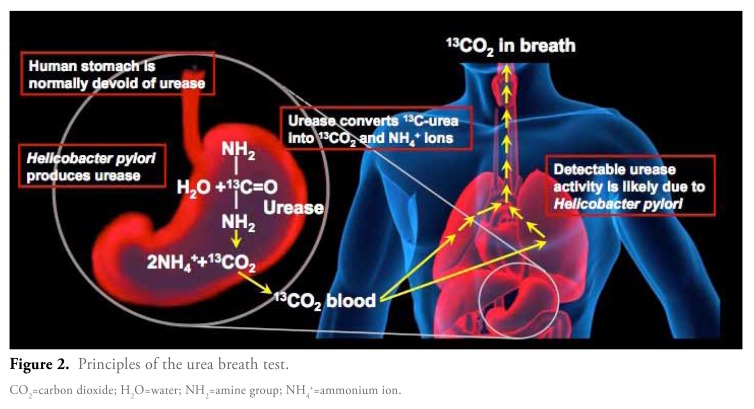
The BreathTek UBT Kit is intended to be used for the qualitative detection of urease activity associated with H. pylori infection present in the human stomach and is indicated as an aid in the initial diagnosis and post-treatment monitoring of H. pylori infection in adult patients. It is for administration by a healthcare professional, as prescribed by a physician.8 This test is based on the fact that all H. pylori strains produce the enzyme urease, which cleaves urea into ammonium ions and carbon dioxide. Normally, the human stomach is devoid of urease, so any detectable urease activity in the human stomach is likely due to the presence of H. pylori. The BreathTek UBT is indicated for use in adult patients both to aid in the initial diagnosis of H. pylori infection and for post-treatment monitoring.
The diagnostic pharmacologic agent in the BreathTek UBT Kit is 13C-urea. This agent is a synthetic urea that becomes orally available after reconstitution with water. The 13C component of this compound is a stable, naturally occurring, nonradioactive carbon isotope. Taken orally, the 13C-urea is degraded by the bacterial urease, if present. The resulting, isotopically-labeled carbon dioxide product will then equilibrate with body fluids and will ultimately be expired from the lungs (Figure 2). In the absence of H. pylori, the 13C-urea is not converted into labeled carbon dioxide, and the ratio of 13C-labeled carbon dioxide in the post-dose breath sample remains similar to the level detected in the baseline sample.
Figure 2.
Principles of the urea breath test.
CO2=carbon dioxide; H2O=water; NH2=amine group; NH4+=ammonium ion.
In clinical practice, the testing process begins with the patient providing a baseline breath sample. The patient then ingests a solution containing the 13C-urea agent, and 15 minutes later the patient provides a post-test breath sample. Both the baseline and post-test samples can be analyzed immediately, or they can be stored and tested at any time within 7 days. Once the samples are ready for testing, the BreathTek UBT assay uses an infrared spectrophotometer to measure the ratio of 13C-labeled carbon dioxide to 12C-labeled carbon dioxide in both the pretest and post-test breath samples.
Before undergoing the BreathTek UBT, patients must fast for at least 1 hour. Additionally, patients must wait at least 4 weeks after the end of H. pylori eradication therapy before undergoing post-treatment testing. False-negative results may occur in patients who recently received PPI acid suppression therapy and/or bismuth compounds or antibiotics; therefore, patient should not have received any of these agents within the 2 weeks prior to use of the BreathTek UBT. Another potential cause of false-negative test results is a premature post-dose breath collection. In contrast, false-positive results may be caused by the presence of urease produced by other gastric spiral organisms, such as H. heilmannii. Conditions such as achlorhydria may also lead to a false-positive test result.
Several precautions should be followed when administering this test. Patients with phenylketonuria should be cautioned that the test solution contains phenylalanine in an amount approximately equivalent to a typical 12-ounce diet cola soft drink. Additionally, because the drug solution contains Aspartame, it should be used with caution in diabetic patients. Patients who are hypersensitive to mannitol, citric acid, or Aspartame should also avoid the drug solution. Use with caution in patients with difficulty swallowing or who may be at a high risk of aspiration due to medical or physical conditions. No information is available on the use of the 13C drug agent during preganancy. Finally, several adverse events have been identified in postapproval use of the BreathTek UBT. These voluntarily reported reactions included anaphylactic reaction, hypersensitivity, rash, a burning sensation in the stomach, tingling in the skin, vomiting, and diarrhea.
Acknowledgement
Neil Stollman, MD, FACP, FACG, AGAF, was paid by Otsuka America Pharmaceutical, Inc. for participation in this roundtable and development of this monograph, and he is a promotional speaker for BreathTek® UBT for Helicobacter pylori (H. pylori) Kit. He has also received speaking honoraria from Aptalis Pharmaceuticals, Optimer Pharmaceuticals, and Warner Chilcott, and he receives research support from Warner Chilcott.
References
- 1.Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Vakil N. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–2337. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 3.Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129:1753–1755. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 5.McColl KE. Clinical practice Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 6.Vaira D, Vakil N. Blood, urine, stool, breath, money, and Helicobacter pylori. Gut. 2001;48:287–289. doi: 10.1136/gut.48.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maconi G, Vago L, Galletta G, et al. Is routine histological evaluation an accurate test for Helicobacter pylori infection? Aliment Pharmacol Ther. 1999;13:327–331. doi: 10.1046/j.1365-2036.1999.00469.x. [DOI] [PubMed] [Google Scholar]
- 8. BreathTek UBT [package insert]. Rockville, Md: Medical Device Division of Otsuka America Pharmaceutical, Inc.; 2012.
Treatment and Post-Treatment Testing for Helicobacter pylori Infection
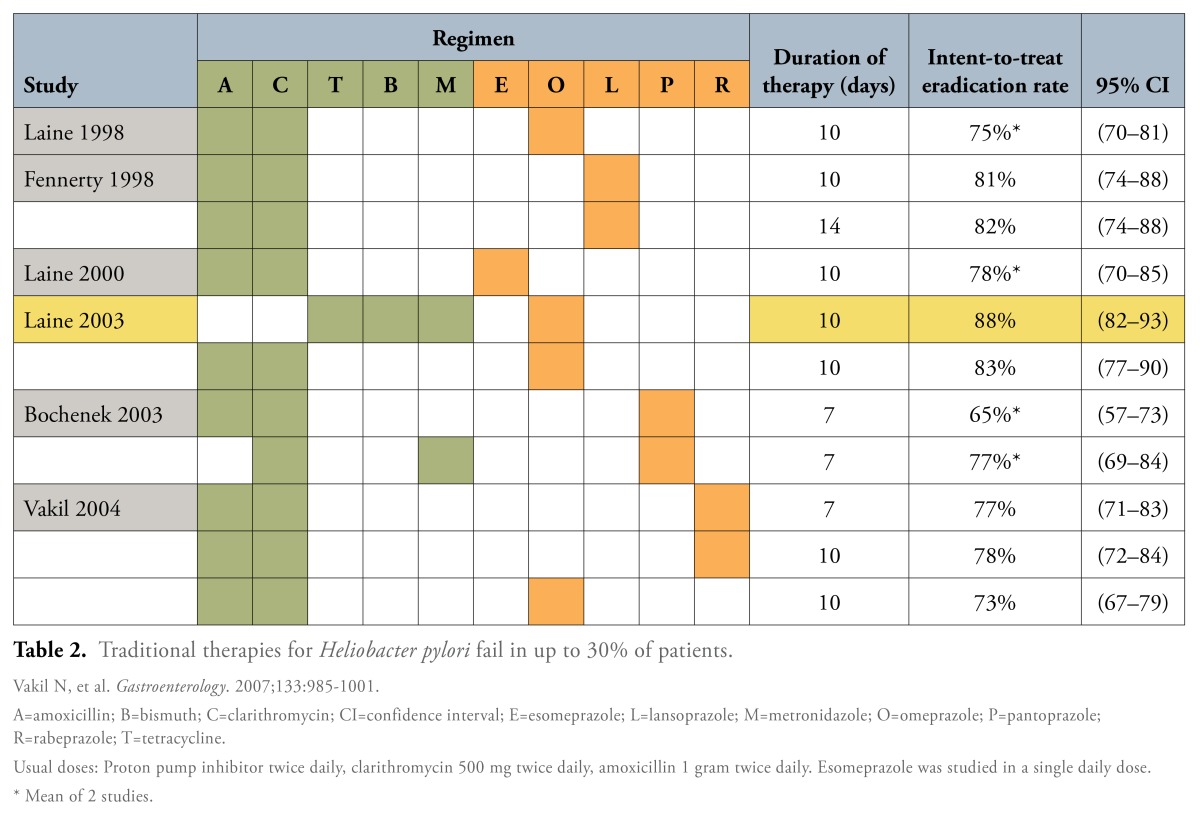
A variety of drug regimens are used in the treatment of H. pylori infection.1 The most widely used is a triple combination of a PPI, clarithromycin, and amoxicillin, each of which is dosed twice daily for 10 or 14 days. For patients who are allergic to amoxicillin, metronidazole can be substituted. Especially in areas with a high prevalence of clarithromycin resistance, a quadruple drug regimen may also be used for H. pylori eradication; this option includes a PPI plus tetracycline, metronidazole, and a bismuth salt.
The success rates for H. pylori eradication are relatively similar with triple and quadruple therapy; per-protocol analyses show eradication rates of 85% and 87%, respectively, and intent-to-treat analyses show eradication rates of 79% and 80%, respectively (Table 1).2 To further compare triple versus quadruple drug regimens, Fischbach and colleagues conducted a meta-analysis of 93 studies (N=10,178).3 This study found a higher rate of eradication with quadruple therapy among populations with higher rates of resistance to either clarithromycin or metronidazole. Quadruple therapy regimens were consistently found to eradicate over 80% of H. pylori infections.
Table 1.
Success rates of standard treatments for Heliobacter pylori infection.
| Per-protocol eradication rate | Intent-to-treat eradication rate | |
|---|---|---|
| PPI + clarithromycin + amoxicillin/metronidazole | 85% | 79% |
| PPI + bismuth + tetracycline + metronidazole | 87% | 80% |
Saad RJ, et al. Natl Clin Pract Gastroenterol Hepatol. 2006;3:20-21.
PPI=proton pump inhibitor.
A more recent meta-analysis of 9 studies (N=1,679), which was conducted by Luther and colleagues, demonstrated that per-protocol eradication rates were similar for triple and quadruple therapy; the intent-to-treat eradication rates were also similar for the 2 regimens (77.0% vs 78.3%, respectively; risk ratio, 1.00; 95% CI, 0.94-1.07).4 This same meta-analysis reported no statistically significant differences between triple and quadruple drug regimens in terms of either patient compliance or side effects.
Benefits of Helicobacter pylori Eradication
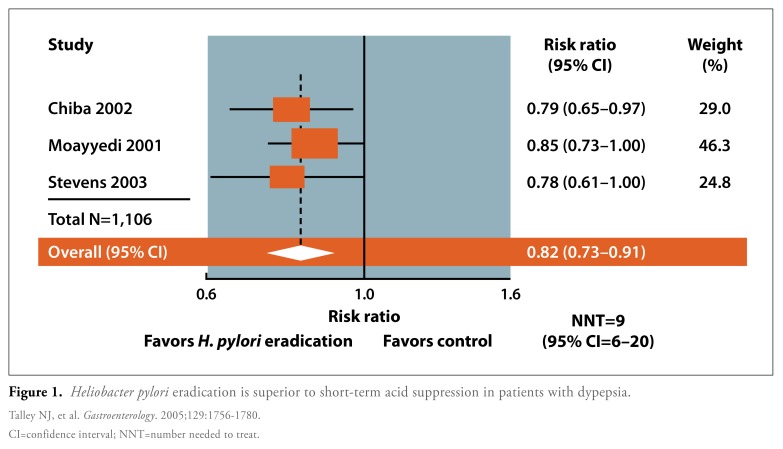
Several randomized, controlled studies have demonstrated that H. pylori eradication therapy is superior to short-term treatment with placebo or an acid-suppressing drug for the treatment of dyspepsia among patients in the primary care setting. Ameta-analysis of 3 such studies (N= 1,106) reported a relative risk of 0.82 (95% CI, 0.73-0.91) and an NNT of 9 (95% CI, 6-20; Figure 1).5 In another study, Chiba and colleagues used a test-and-treat strategy to assess improvements in symptoms among primary care patients with uninvestigated dyspepsia.6 A total of 294 H. pylori-positive patients with moderately severe dyspepsia were randomized to either omeprazole, metronidazole, and clarithromycin or to omeprazole and placebo. Compared to the omeprazole-placebo combination, eradication therapy resulted in a significantly higher rate of treatment success (36% vs 50%, P=.02), defined as no or minimal dyspepsia at the end of 1 year. Eradication therapy successfully cured H. pylori infection in 80% of evaluable patients.
Figure 1.
Heliobacter pylori eradication is superior to short-term acid suppression in patients with dypepsia.
CI=confidence interval; NNT=number needed to treat.
Talley NJ, et al. Gastroenterology. 2005;129:1756-1780.
In another study, Stevens and colleagues evaluated H. pylori eradication therapy among primary care patients presenting with ulcer-like symptoms; this study excluded patients with a confirmed ulcer.7 Of the 543 patients enrolled in this study, 364 were found to be positive for H. pylori infection. These patients were then randomized to receive either lansoprazole, clarithromycin, and amoxicillin or lansoprazole and placebo. The 179 patients who were negative for H.pylori infection underwent open-label treatment with lansoprazole. Over the 1-year follow-up period, patients who received H. pylori eradication therapy demonstrated significantly lower average numbers of gastrointestinal consultations (P<.02) and prescriptions (P=.05) compared to patients who received lansoprazole and placebo. Further, the relapse rate was significantly lower among patients who received eradication therapy compared to patients who received lansoprazole and placebo (37.0% vs 51.4%; P=.02).
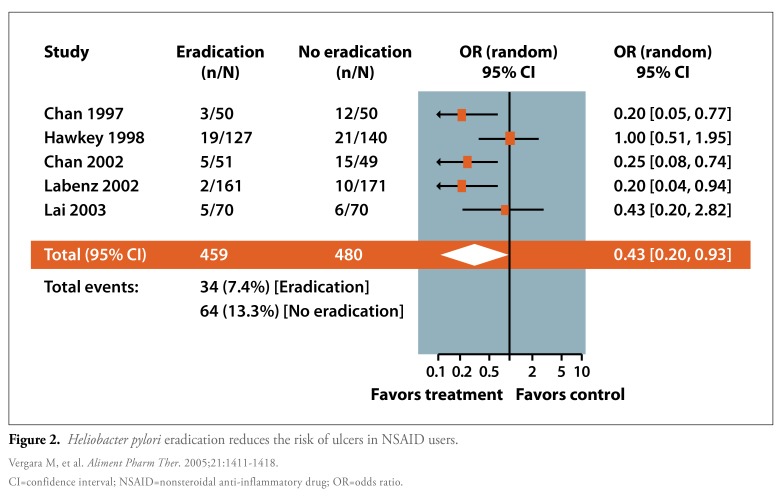
Finally, a meta-analysis by Vergara and colleagues sought to determine whether H. pylori eradication therapy was associated with the prevention of peptic ulcers among NSAID users (Figure 2).8 Five studies with a total of 939 patients were included in this meta-analysis. All of these studies compared eradication therapy with either no therapy or PPI therapy. Nearly twice as many patients in the control group developed a peptic ulcer compared to patients in the eradication therapy group (13.3% vs 7.4%; OR, 0.43; 95% CI, 0.20-0.93). Interestingly, a subanalysis of the data demonstrated a significant reduction in the risk of developing a peptic ulcer among NSAID-naïve patients (OR, 0.26; 95% CI, 0.14-0.49) but not among patients who had been previously treated with NSAIDs (OR, 0.95; 95% CI, 0.53-1.72).
Figure 2.
Heliobacter pylori eradication reduces the risk of ulcers in NSAID users.
CI=confidence interval; NSAID=nonsteroidal anti-inflammatory drug; OR=odds ratio.
Vergara M, et al. Aliment Pharm Ther. 2005;21:1411-1418.
Based on these and other data, ACG practice guidelines recognized a potential advantage for H. pylori testing among individuals who will require long-term NSAID therapy, with treatment of the infection in patients who test positive.9 Thus, the ACG recommendation is to consider H. pylori testing (and treatment, if positive) for all patients prior to initiation of long-term NSAID therapy.
Importance of Confirmatory Post-Treatment Testing
Following therapy, symptoms alone are not a reliable indicator of whether a patient’s H. pylori infection has been eradicated. The ACG’s updated practice guidelines for the management of H. pylori infection recommend post-treatment testing for patients with H. pylori—associated ulcers, patients with persistent dyspepsia, patients with H. pylori—associated MALT lymphoma, and patients who have undergone endoscopic resection of lesions of early gastric cancer.10 Post-treatment testing should be performed with a test of active infection a minimum of 4 weeks following the completion of therapy.
Strategies for post-treatment testing are generally similar to those used prior to eradication therapy, with the important exception that serology is definitely not appropriate in the post-treatment setting. For patients in whom endoscopic follow-up is necessary, post-treatment H. pylori testing can be performed endoscopically. For all other patients, the ACG guidelines recommend use of the UBT, as this method is the most reliable nonendoscopic test for proving eradication of H. pylori infection. Alternatively, the stool antigen test may be used for post-treatment testing, although the ACG guidelines note that the timing and reliability of this method have not been demonstrated as clearly as they have been for the UBT.
When Initial Treatment Fails
H. pylori eradication rates in recent trials are lower than those reported in the late 1990s (Table 2). The primary causes of initial treatment failure are antimicrobial resistance and poor adherence.11 Indeed, eradication rates with triple therapy have been declining as resistance rates to commonly used antimicrobials have risen. One study reported clarithromycin resistance in approximately one third of patients who failed therapy.12
 |
To address concerns about antibiotic resistance in the United States, researchers and clinicians formed the Helicobacter pylori Antimicrobial Resistance Monitoring Program, a prospective, multicenter network that tracked national incidence rates of H. pylori antimicrobial resistance.13 Among 347 clinical isolates collected through this network between 1998 and 2002, 29.1% were found to be resistant to 1 antimicrobial agent, and 5% to 2 or more. When resistance to specific drugs was analyzed, 25.1% of isolates were found to be resistant to metronidzole, 12.9% to clarithromycin, and only 0.9% to amoxicillin; none was resistant to tetracycline. A multivariate analysis found that black race was the only significant risk factor for infection with an antibiotic-resistant strain of H. pylori.13
Antimicrobial resistance to H. pylori is particularly prevalent in Alaska, as shown by data from the Alaska Sentinel Surveillance Study conducted by the Centers for Disease Control and Prevention; this study involved hospitals in 5 Alaska regions.14 An analysis of isolates collected from 531 H. pylori-positive patients between 2000 and 2008 showed high rates of resistance to metronidazole (42%), clarithromycin (30%), and levofloxacin (19%), while resistance to amoxicillin was only 2%. Again, no isolates were resistant to tetracycline. Fifteen percent of clinical isolates were found to be resistant to both metronidazole and clarithromycin. Of note, the rates of resistance to metronidazole and clarithromycin reported in this Alaskan study were approximately 2-fold and 3-fold higher, respectively, than the rates observed in the general US population.
When initial eradication therapy for H. pylori infection fails, several subsequent treatment options may be considered. If a patient has already been treated with a clarithromycin-containing regimen, this drug should be avoided in subsequent therapy. Salvage regimens may include amoxicillin even if it had been previously used, due to the very low prevalence of amoxicillin resistance. Finally, although antimicrobial susceptibility testing might appear logical for the evaluation of treatment failure, such testing is not widely available in the United States.
Acknowledgement
Colin W. Howden, MD, was paid by Otsuka America Pharmaceutical, Inc. for participation in this roundtable and development of this monograph, and he is a promotional speaker for BreathTek® UBT for Helicobacter pylori (H. pylori) Kit. He has also been a paid consultant for Perrigo and Takeda, and he has received speaking honoraria from GlaxoSmithKline International and Takeda.
References
- 1.McColl KE. Clinical practice Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Saad R, Chey WD. What is the optimal therapy for patients with Helicobacter pylori-negative dyspepsia? Nat Clin Pract Gastroenterol Hepatol. 2006;3:20–21. doi: 10.1038/ncpgasthep0362. [DOI] [PubMed] [Google Scholar]
- 3.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 4.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ. American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129:1753–1755. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Chiba N, Van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric trealment-Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ. 2002;324:1012–1016. doi: 10.1136/bmj.324.7344.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens R, Baxter G. Benefit of Helicobacter pylori eradication in the treatment of ulcer-like dyspepsia in primary care. Gastroenterology. 2001;120(5) Suppl 1:A50. [Google Scholar]
- 8.Vergara M, Catalàn M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21:1411–1418. doi: 10.1111/j.1365-2036.2005.02444.x. [DOI] [PubMed] [Google Scholar]
- 9.Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 10.Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 11.Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United Stales. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 13.Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088–1094. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tveit AH, Bruce MG, Bruden DL, et al. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska Native persons from 2000 to 2008. J Clin Microbiol. 2011;49:3638–3643. doi: 10.1128/JCM.01067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Question-and-Answer Forum
G&H A 45-year-old man is referred for epigastric pain. He had a bleeding duodenal ulcer that required hospitalization and transfusion years ago. He takes intermittent ibuprofen for headaches. There is no nausea or evidence of gastrointestinal bleeding, and a physical examination reveals only mild epigastric tenderness. What would you do next?
Colin W. Howden, MD The patient must be tested for H. pylori infection in accordance with practice guidelines. Assuming he is not currently taking a PPI, he could be tested at the time of referral using the UBT or a stool antigen test. If he is taking a PPI, that drug would need to be stopped for at least 14 days before testing. There is no pressing need for a repeat endoscopy at this stage. If he tests positive for H. pylori, the infection should be treated appropriately, and a post-treatment test of eradication should be performed at least 4 weeks after completion of therapy.
Neil Stollman, MD, FACP, FACG, AGAF I agree. The indication for testing is very clear in this case. The fact that he is taking ibuprofen is also a consideration, especially if testing shows that he is negative for H. pylori. He should be educated about minimizing his NSAID use regardless of his test result, but if he is positive for H. pylori, then eradicating this infection will drastically lower his risk of recurrent ulcers even if he continues to use NSAIDs occasionally.
Adam B. Elfant, MD, FACG There is no question that this patient requires testing for H. pylori. Given his history of a clinically significant gastrointestinal bleed, I would also ask him to discontinue NSAID use and consider less ulcerogenic agents instead.
G&H How did the discovery of H. pylori by Warren and Marshall change physicians’ understanding of peptic ulcer disease?
CWH The discovery of H. pylori—which was initially called a “ Campylobacter-like organism”—ultimately proved to be revolutionary. Although the association between H. pylori and peptic ulcer disease was initially viewed with great skepticism, H. pylori infection is now universally accepted as the most common (but not the only) cause of peptic ulcers.
NS Physicians used to think that ulcers were due to stress, alcohol, and tobacco use. Thus, we blamed patients for their ulcers: We thought that ulcers were caused by what the patient was doing, and we believed that if patients changed their lifestyle, then they would not get ulcers. As we later learned, ulcers had little to do with lifestyle and had everything to do with bacteria. Ultimately, this discovery led to a sea change in our conception of ulcer disease.
ABE Acid was also thought to be a prime culprit in the development of peptic ulcers. In fact, the old dogma of “no acid no ulcer” was held for many years. Now, the important etiologic role of H. pylori is no longer disputed, and eradication has become the mainstay of successful management of this disease.
G&H Of the various types of H. pylori tests available—UBT, stool antigen test, endoscopy, and serology—which do you most commonly use in your practice?
NS I use breath testing almost exclusively, in part, because it is my most convenient option; my office has an instrument for analyzing breath samples from the BreathTek UBT test, which allows me to get a result in just 20 minutes. Stool testing is accurate, but compliance can be a problem because of the substantial patient effort required to collect and transport a sample. Serology testing for H. pylori is nearly obsolete, in my opinion; the specificity of serology is very poor, and a positive serology result can be a marker of prior exposure rather than active infection. Finally, endoscopy is a great way to test for H. pylori if the patient actually needs an endoscopy. I would never do an endoscopy just to look for H. pylori, but if the patient happens to need this procedure, then endoscopy is a perfect way to look for H. pylori; it is accurate, inexpensive, and safe.
ABE In my practice, we only utilize tests of active infection due to their higher sensitivity and specificity. If a patient requires endoscopy, then a biopsy will be obtained during the procedure. It is important to remember that H. pylori may colonize the human stomach in different areas based on ethnicity, acid exposure, and medication history. These factors need to be taken into consideration when obtaining biopsies from various areas of the stomach. Otherwise, I use both breath testing and stool antigen testing, based on patient preference.
G&H What are the advantages and disadvantages of the UBT when testing for H. pylori infection?
CWH Advantages of the UBT include its simplicity, its reliability, and the fact that it can be applied both before and after treatment for H. pylori infection. It is noninvasive, and, assuming the 13C-UBT is used, it does not involve the administration of any radioactive material. Also, it can be administered in the clinic setting by a healthcare professional, such as nursing personnel. A potential disadvantage of the UBT is the fact that it should not be used in patients who have been taking a PPI, an antibiotic, or a bismuth preparation within the preceding 14 days.
ABE I think that the major advantage is the ease of testing, whether you perform it in the office or send the patient to an outpatient lab. I would echo Dr. Howden’s comments on the disadvantages.
NS Urea breath testing has high sensitivity and specificity, and it is a test for active infection—these are important advantages. If clinicians have the testing instrument in their office, then it also offers patients the convenience of real-time results. There is a risk of false-negative test results due to premature post-dose breath collection. False-positive results may also be caused by the presence of urease produced by other gastric spiral organisms, such as H. heilmannii, or achlorhydria.
G&H Are you concerned about encountering antibiotic resistance when treating H. pylori infection? How can this concern be addressed?
NS Yes, antibiotic resistance is definitely a concern and, unfortunately, it is already a problem. Resistance is rapidly increasing, and we are already seeing very significant and clinically meaningful levels of clarithromycin resistance. We are also seeing metronidazole resistance, but this is less clinically relevant. The result of this resistance is markedly decreased eradication rates; when we first started treating H. pylori infection, we were eradicating the infection 90—95% of the time, but more recent numbers suggest we are now eradicating it only 60—75% of the time.
CWH I agree. Antimicrobial resistance is a major problem when treating H. pylori infection. The most important means of addressing this problem is to have some information about a particular patients antibiotic history. Patients who have received clarithromycin or another macrolide antibiotic in the past—either for the treatment of H. pylori infection or for some other reason—must not be given this drug as part of their H. pylori treatment regimen.
ABE Antibiotic resistance is a concern, but this is an issue that we deal with daily when treating H. pylori infection, as when treating many other infectious diseases. As physicians, we need to be cognizant that inappropriate prescribing practices exacerbate the problem of antibiotic resistance, and we need to be more vigilant in our use of broad-spectrum antibiotic therapy. Increasing resistance also means that we must take a careful medication history and try to utilize agents that the patient has not been treated with previously.
G&H What is your preferred first-line treatment for H. pylori infection? Your preferred second-line therapy?
CWH My preferred treatment depends upon which antibiotic(s) the patient has received previously. If a patient was given clarithromycin in the past, I would opt for a quadruple regimen consisting of a PPI, tetracycline, metronidazole, and bismuth as my first choice.
ABE My preferred regimen is also guided by the patient’s previous antibiotic exposure history. I will use a clarithromycin-based regimen if the patient has not previously been exposed to this drug. My alternatives would be either tetracycline or a quinolone antibiotic, along with bismuth and metronidazole.
NS Treatment of H. pylori infection is an ongoing evolution. Fifteen years ago, quadruple therapy was the standard regimen. Like most other practitioners, I then migrated to triple therapy, which includes clarithromycin, mainly for reasons of convenience and tolerability. Now that we are seeing such high rates of clarithromycin resistance, however, I am in the process of migrating back to quadruple therapy. My second-line therapy is whichever regimen I did not use initially; if the patient was previously treated with quadruple therapy, then I use triple therapy for the second-line regimen, and vice versa.
Acknowledgement
Drs. Elfant, Howden, and Stollman were paid by Otsuka America Pharmaceutical, Inc. for participation in the roundtable and development of this monograph and are promotional speakers for BreathTek® UBT for Helicobacter pylori (H. pylori) Kit. Dr. Howden has also been a paid consultant for Perrigo and Takeda, and he has received speaking honoraria from GlaxoSmithKline International and Takeda. Dr. Stollman has also received speaking honoraria from Aptalis Pharmaceuticals, Optimer Pharmaceuticals, and Warner Chilcott, and he receives research support from Warner Chilcott.
Biographies



Footnotes
Drs. Elfant, Howden, and Stollman are paid consultants of Otsuka America Pharmaceutical, Inc.
Supported through funding from Otsuka America Pharmaceutical, Inc., manufacturer and marketer of the BreathTek® UBT for H. pylori.
Disclaimer: This clinical round-table and its subsequent monograph were entirely funded by Otsuka America Pharmaceutical, Inc., manufacturer and marketer of the Breath Tek® UBT for Helicobacter pylori (H. pylori). Drs. Elfant, Howden, and Stollman were also paid by Otsuka America Pharmaceutical, Inc. for participation in the roundtable and development of this monograph and are promotional speakers for BreathTek® UBT for H. pylori Kit. Editorial support for the monograph was provided by Gastro-Hep Communications, Inc., which also received payment from Otsuka America Pharmaceutical, Inc. Otsuka America Pharmaceutical, Inc. is the distributor of the BreathTek® UBT for H. pylori Kit.
Gastro-Hep Communications, Inc.’s support of this monograph does not imply its agreement with the views expressed herein. Every effort has been made to ensure that drug and medical device usage, important safety information, and other information are presented accurately and completely; however, the ultimate responsibility for prescribing and use of any diagnostic test for H. pylori rests with the prescribing physician. Gastro-Hep Communications, Inc. and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug, compound, or medical device at present under clinical investigation.
Contributor Information
Adam B. Elfant, Associate Head, Division of Gastroenterology and Liver Disease Associate Professor of Medicine Cooper University Hospital Robert Wood Johnson Medical School at Camden Mount Laurel, New Jersey.
Colin W. Howden, Professor of Medicine Division of Gastroenterology and Hepatology Northwestern University Feinberg School of Medicine Chicago, Illinois.
Neil Stollman, Chairman of the Department of Medicine Alta Bates Summit Medical Center Oakland, California.