Abstract
Symbiotic interactions are ubiquitous in nature and play a major role in driving the evolution of life. Interactions between partners are often mediated by shared signalling pathways, which strongly influence both partners' biology and the evolution of the association in various environments. As an example of ‘common language’, the regulation of the oxidative environment plays an important role in driving the evolution of symbiotic associations. Such processes have been occurring for billions of years, including the increase in Earth's atmospheric oxygen and the subsequent evolution of mitochondria. The effect of reactive oxygen species and reactive nitrogen species (RONS) has been characterized functionally, but the molecular dialogue between partners has not been integrated within a broader evolutionary context yet. Given the pleiotropic role of RONS in cell–cell communication, development and immunity, but also their associated physiological costs, we discuss here how their regulation can influence the establishment, the maintenance and the breakdown of various symbiotic associations. By synthesizing recent developments in redox biology, we aim to provide an interdisciplinary understanding of the influence of such mediators of interspecies communication on the evolution and stability of symbioses, which in turn can shape ecosystems and play a role in health and disease.
Keywords: symbiotic interactions, oxidative environment, specificity, extended phenotype, coevolution, resistance/tolerance
1. Introduction
Symbiosis is defined as a long-term interaction between two organisms belonging to different species [1] and is considered as a major evolutionary force. Indeed, the intimate interactions between partners can profoundly affect many aspects of the partners' biology, through the perturbation of an initial physiological state to the provision of new functions [2]. A striking example is the evolution of organelles (mitochondria and chloroplasts) and eukaryotic cells, from an early association between unicellular prokaryotes [3]. More generally, interactions between partners can influence the evolution of symbioses along a dynamic continuum between parasitism and mutualism, but can also impact the stability of the association and the interdependence between the two partners.
Evolution of symbioses is possible because parasitic and mutualistic interactions mainly use shared signalling pathways, as a common ‘language’ [4]. Both partners can influence the evolution of symbiosis, and selection can act at the ‘holobiont’ level, which includes the host and its associated symbionts [5]. From the host side, the intensity of the immune response, and the pleiotropy and trade-offs between different phenotypic traits can play major roles in the evolution of symbioses. From the symbiont side, the virulence and transmission mode can affect the degree of biological perturbations inflicted on the host, but also the efficiency of the symbiont transmission and thus its prevalence into host populations. Finally, the presence of a symbiont can also influence the host phenotype, hence defined as the ‘extended phenotype’ [6], and affect for instance the host morphology or behaviour.
As an example of shared functions that could influence symbioses, we propose to focus on the control of the oxidative environment. Coming back to the origin of eukaryotic cells, mitochondria enabled cells to respire aerobically, via oxidative phosphorylation, but initiated in the meantime the production of oxidant radicals, such as H2O2,  and HO•, commonly called reactive oxygen species (ROS). In combination with nitric oxide (•NO),
and HO•, commonly called reactive oxygen species (ROS). In combination with nitric oxide (•NO),  radicals produce reactive nitrogen species (RNS), such as ONOO−. Although ROS and RNS have distinct chemical and biological properties [7,8], they are collectively called reactive oxygen species and reactive nitrogen species (RONS) because of their role in redox biology and their high toxicity in the cell. The biochemical and biological properties of RONS have been detailed in numerous comprehensive reviews, which we recommend for the interested reader (e.g. [8–10]). Briefly, ROS are mainly produced by the mitochondrial respiratory chain and by NADPH oxidases (NOXs), whereas RNS are principally produced by nitric oxide synthases (NOSs; figure 1). In parallel, various anti-oxidant mechanisms have been developed by bacteria and eukaryotic cells and include enzymes involved in ROS scavenging (e.g. superoxide dismutase, catalase, peroxidase) or disulfide reduction (e.g. thioredoxin, glutaredoxin), but also small anti-oxidant molecules (e.g. ascorbate and glutathione). Hence, the balance between RONS production and catabolism, also called ‘redox homeostasis’, is tightly controlled but can lead to ‘oxidative stress’ when deregulated [11].
radicals produce reactive nitrogen species (RNS), such as ONOO−. Although ROS and RNS have distinct chemical and biological properties [7,8], they are collectively called reactive oxygen species and reactive nitrogen species (RONS) because of their role in redox biology and their high toxicity in the cell. The biochemical and biological properties of RONS have been detailed in numerous comprehensive reviews, which we recommend for the interested reader (e.g. [8–10]). Briefly, ROS are mainly produced by the mitochondrial respiratory chain and by NADPH oxidases (NOXs), whereas RNS are principally produced by nitric oxide synthases (NOSs; figure 1). In parallel, various anti-oxidant mechanisms have been developed by bacteria and eukaryotic cells and include enzymes involved in ROS scavenging (e.g. superoxide dismutase, catalase, peroxidase) or disulfide reduction (e.g. thioredoxin, glutaredoxin), but also small anti-oxidant molecules (e.g. ascorbate and glutathione). Hence, the balance between RONS production and catabolism, also called ‘redox homeostasis’, is tightly controlled but can lead to ‘oxidative stress’ when deregulated [11].
Figure 1.

Origin and pleiotropic effects of reactive oxygen (ROS) and nitrogen species (RNS). Superoxide anion ( ) is generated by the mitochondrial respiratory chain and by NOXs.
) is generated by the mitochondrial respiratory chain and by NOXs. 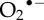 is then converted to hydrogen peroxide (H2O2) by the superoxide dismutase enzyme. H2O2 can further react with Fe2+ to produce hydroxyl radical (HO•) by the Fenton reaction. Nitric oxide (•NO) is synthesized from l-arginine by the action of NOSs. Nitric oxide can react with
is then converted to hydrogen peroxide (H2O2) by the superoxide dismutase enzyme. H2O2 can further react with Fe2+ to produce hydroxyl radical (HO•) by the Fenton reaction. Nitric oxide (•NO) is synthesized from l-arginine by the action of NOSs. Nitric oxide can react with  to produce peroxynitrite (OONO−). ROS (H2O2, HO•,
to produce peroxynitrite (OONO−). ROS (H2O2, HO•,  ) and RNS (•NO, OONO−) modulate various cellular processes. (Online version in colour.)
) and RNS (•NO, OONO−) modulate various cellular processes. (Online version in colour.)
Because adaptation to the oxidative environment has occurred together with organisms' diversification, RONS might be ancestral shared signals between kingdoms. The biochemical instability of RONS makes them versatile enough to be good signalling molecules, despite the potential toxicity associated with radicals [12]. ROS are players of conserved pathways initially used for signalling, such as the two-components systems [13], and •NO is a transmitter mechanism for cell–cell communication [14,15]. RONS also influence various cellular mechanisms (figure 1) [11]. In particular, RONS directly affect cell division and differentiation, apoptosis [13,16,17] and multicellular behaviours in various organisms (e.g. [18,19]). Finally, ROS are strongly involved in microbial killing processes, such as ‘oxidative bursts’ or ‘hypersensitive reactions’, because of their highly toxic properties when they are not buffered in specific compartments of the cell [8]. As the concentration, timing and combined effects of RONS are tightly controlled in the organism and play a critical role in signalling and cellular processes, any variation of the RONS balance and the associated proximal mechanisms is expected to impact more generally the symbiotic association.
Although the importance of RONS has been thoroughly characterized functionally, the associated molecular dialogue between symbiotic partners has not yet been integrated within a broader evolutionary context. Focusing on the oxidative environment, we thus propose to illustrate how interspecies communication can drive the evolution of symbiotic associations and impact on their stability. In particular, we will develop how the regulation of the oxidative environment plays a critical role in the establishment, the maintenance, but also in the breakdown of symbioses.
2. Pleiotropic roles of reactive oxygen species and reactive nitrogen species during the establishment of symbioses
(a). Role of reactive oxygen species and reactive nitrogen species in establishing the specificity
RONS modulation plays a critical role during the initiation of horizontal symbioses, especially when it affects bacterial homeostasis in complex microbiota or selection of specific partners in binary associations.
The midgut of insects is protected by a peritrophic membrane and colonized by a large resident bacterial community [20]. In response to contact with microbe-contaminated food, an oxidative burst controlled by the H2O2-producing dual oxidase (DUOX) is detected in the midgut of Drosophila melanogaster [21], and the host defence strongly depends on an immune-regulated catalase [22]. Interestingly, the signalling pathway associated with DUOX is negatively regulated in response to colonization by resident bacteria [23], suggesting that the regulation of H2O2 production plays an important role in the homeostasis of the gut microbiota, through the tolerance of resident bacteria and the elimination of pathogenic ones.
RONS also play a role in the selection of the symbiotic partner of the bobtail squid Euprymna scolopes. Indeed, the squid light-organ can only be efficiently colonized by the luminescent bacterium Vibrio fischeri, even though it represents less than 0.1% of the bacterioplankton. The selection of V. fischeri and the exclusion of non-symbiotic bacteria, or winnowing [24], is partly linked to the chemical properties of the mucus coating the light-organ epithelium, which contains antimicrobial components. •NO in particular is detectable in the mucus immediately after the squid hatches [25], and haemocyanin, whose phenoloxidase activity produces toxic quinones, could also participate to the selection process [26]. Only Gram-negative bacteria attach to host tissues, and very few bacterial species aggregate on the ciliated epithelium [24]. Interestingly, the size of the aggregate is strongly dependent on •NO levels [25,27]. After aggregation in the mucus, V. fischeri is the only species successfully migrating through the pores, the ducts and the antechamber before reaching its final site of residence, the crypts. All along the way, and especially in the duct and antechamber regions, strong expression of •NO and ROS is detected ([25], N Kremer and MJ McFall-Ngai 2013, unpublished results), suggesting that V. fischeri faces significant nitroxidative stress. Vibrio fischeri is able to detect •NO through an H-NOX protein in vitro, and adjusts gene expression accordingly [28]. Vibrio fischeri also expresses a variety of •NO-detoxifying enzymes, especially a flavohaemoglobin, whose deletion leads to a reduced competitiveness in colonization of the squid, in comparison to wild-type bacteria [27]. Interestingly, a pre-treatment with •NO increases the resistance of V. fischeri to an acute •NO stress, suggesting that bacteria exhibit a ‘pre-adaptation’ phase in the aggregate [27]. This process must increase their resistance to RONS during the later stages of their symbiotic journey, and play a role in the establishment of the specificity.
Finally, RONS play a pleiotropic role in signalling and immunity in the mutualistic associations between leguminous plants and rhizobia, soil nitrogen-fixing bacteria. A complex cross-talk between partners leads to the development of a specialized symbiotic organ, the nodule, which houses the symbionts. This ‘conversation’ starts with the secretion by root exudates of flavonoids that activate bacterial nodulation-genes and lead to the production of lipo-chito-oligosaccharides (nodulation factors or NFs) that will, in turn, be recognized by the plant [29]. The establishment of the symbiosis is highly specific, as a rhizobia species will only interact with a specific legume species. Specificity is greatly influenced by the Nod signalling pathway [30], but H2O2 and •NO also play a role in the proximal mechanisms associated with the specific establishment of the symbiosis [31]. Indeed, in the common bean Phaseolus vulgaris, the contact of roots with NF rapidly triggers a transient burst of intracellular ROS at the tip of the hairs [32]. After a few minutes, however, the exposure to NF has an opposite effect on ROS production in legumes, and limits the H2O2 production in root cells [32]. Interestingly, this specific decrease in intracellular ROS production is not detectable when roots are put in contact with fungal derivatives [32]. The contact with NF also suppresses the early oxidative burst response observed a few hours after legumes are exposed to a pathogenic elicitor [33]. All these results suggest that cells are capable of distinguishing specific signals within seconds, and that this transient ROS signal plays a role in modulating the symbiotic response. When the symbiosis is established, ROS are produced in the infection thread under the control of NF [35], and this production is correlated with the suppression of pathogenesis-related genes in the compatible interaction between Medicago sp. and Sinorhizobium meliloti [36]. Similar to ROS, •NO is transiently produced at 4 h post-inoculation of Lotus japonicus with a compatible strain but not with incompatible ones. By contrast, •NO is continuously produced after an inoculation with a pathogen [34], suggesting that •NO is part of the defence response, and is modulated by symbiotic signals. From the bacterial point of view, transgenic S. meliloti unable to scavenge •NO are less competitive than wild-type rhizobia in the nodulation process [37], suggesting that •NO is necessary for an optimal establishment of the association. Similar to the squid–vibrio symbiosis [27], it has been proposed that the initial exposure to •NO primes the symbionts to resist against stressful conditions encountered during later symbiotic stages [31].
These studies suggest that ROS and •NO constitute key proximal factors to determine the specificity in symbioses, and that the regulation of the timing and localization of these diffusible molecules is critical. Whether these molecules work in synergy has not been fully determined yet, but the association between ROS and •NO could be part of an antimicrobial response whose regulation favours the establishment of compatible associations.
(b). Role of reactive oxygen species and reactive nitrogen species in building the extended phenotype
RONS, by inducing the cellular apoptotic programme and other signalling cascades [13,17], can affect the developmental programme of both partners, and as such play a role in the establishment of the extended phenotype.
The role of ROS in the renewal of the gut epithelium in the presence of resident bacteria or pathogenic ones is a clear example of how ROS can affect development in a constantly challenged organ. Indeed, the defence response associated with oxidative burst in the Drosophila gut induces strong damage to the epithelial cells because of the production of H2O2 by DUOX [21,23]. Interestingly, ROS also act as signalling molecules, by activating the JAK-STAT and JNK pathways. These pathways play a role in cell delamination and compensatory epithelium renewal, like proliferation or differentiation, and participate to the repair of the gut epithelium after injury [38].
The developmental programme might be even more affected by RONS, as is the case in the well-studied squid–vibrio and legume–rhizobia symbioses, when the shape and the functionality of the dedicated symbiotic organ are directly modified by the presence/absence of the symbiont. In response to symbiosis, the ciliated epithelium of the squid light organ, along which the recruitment of V. fischeri takes place, drastically regresses after an irreversible signal 12 h after infection [24]. As soon as 6 h post hatching, •NO production is reduced in the pores, ducts and antechambers of symbiotic light organs but not in aposymbiotic ones [25]. In fact, the reduction of NOS activity and •NO production induced by symbionts is associated with early-stage apoptosis and chromatin condensation in the epithelium of symbiotic appendages [39]. This restructuration of the organ might limit secondary infections by reducing the host–bacterial interface.
ROS regulation also plays a key role in the establishment of functional nodules. Recently, the decrease in ROS production in roots after NF signalling has been linked to root hair deformation, including hair tip swelling, polar hair growth and branching in Medicago [40]. The long-term inhibition of ROS, however, prevents root hair curling and formation of infection threads [41] and mutants over-expressing catalase delay nodulation because of an enlargement of infection threads [42]. A decrease in •NO delays nodule formation [37], suggesting that •NO plays a role in cell cycle regulation and root organogenesis as in lateral root primordia [43]. From the bacterial side, studies using the catalase mutant of S. meliloti highlight a role of H2O2 in the bacterial differentiation process into the symbiotic form [44].
These examples show how RONS play a role in building the extended phenotype during the establishment of the symbiosis, through their effect as signalling molecules in developmental pathways. Whether these developmental changes have been selected for in coevolved mutualistic associations or are the by-product of the pleiotropic role of RONS in immunity, signalling and apoptosis remains to be determined.
3. Influence of the oxidative stress in the maintenance and evolution of symbioses
(a). Oxidative stress, life-history traits and the evolution of symbioses
The presence of a symbiont can alter the physiology of the host by disturbing the oxidative homeostasis. As this perturbation can affect the life history of the host, the selective pressures acting on the symbiotic partners will be modified, and will impact the evolution of the association. Life history corresponds to the set of traits directly contributing to an organism's fitness, and the presence of negative correlations between traits, or ‘evolutionary trade-offs’, explain why an organism cannot maximize all those traits at once [45]. Because of their antagonistic pleiotropic effects, oxidative reactive molecules have been recently proposed as a key factor underlying evolutionary trade-offs between life-history traits, such as fecundity, immunity, growth and longevity [46,47]. Notably, the production of RONS may underlie the trade-off between immune competence and other life-history traits, because those molecules are both a component of the immune response and a cause of damage to the host tissues. In the Anopheles–Plasmodium interaction, a state of chronic oxidative stress in the mosquito is indeed associated with both an increase in immune competence and a decrease in fecundity [48,49]. Hence, different strategies will be under selection to limit potential deleterious effects associated with symbiosis, depending on the initial cost for the host and the prevalence of the symbiont in the host population.
Tolerance and resistance strategies are two ways by which a host can reduce the cost of being infected by a symbiont: resistance protects the host by reducing the load of symbionts, whereas tolerance reduces the cost of a given symbiotic load [50]. These two protection strategies have very different evolutionary consequences. When detrimental to the symbiont, a resistance strategy is expected to lead to the breakdown of symbiosis or to antagonistic coevolution, characterized by the arms race between evading strategies of the symbiont and resistance strategies of the host. By contrast, tolerance will secure the maintenance of the symbiosis and is expected to lead the evolution of a parasitic interaction towards commensalism [51] or even dependence. These different strategies will be now exemplified in the context of the control of the oxidative environment (figure 2).
Figure 2.

Influence of the oxidative stress in the maintenance and evolution of symbioses. ROS production is generally the first line of defence against the colonization by a parasite. As RONS are costly, their production will impact not only immunity, but also other life-history traits. It is thus important to take into account the overall effect of RONS production on host fitness (determined by the balance between the benefits associated with immune response and the cost associated with other life-history traits) to predict the evolution of the association. Indeed, if this balance is beneficial, a resistance strategy is more likely to be selected for, and the symbiotic association is expected to remain parasitic or to break down. On the opposite, if the balance is detrimental, a tolerance strategy is more likely to be selected for, and the symbiotic association is expected to evolve towards commensalism or even dependence.
(b). Arms race between host oxidative defence and parasite anti-oxidant response
In the case of a resistance strategy, oxidative stress may lead symbionts to develop a more efficient anti-oxidant response to overcome the immune-related RONS generated by the host. The ability of the snail Biomphalaria glabrata to produce reactive oxidative molecules by circulating immune cells and the one of its parasite Schistosoma mansoni to adjust its RONS anti-oxidant systems have been deeply studied [52–55]. The knock-down of anti-oxidant enzymes of the parasite increases the susceptibility of S. mansoni larvae to host-produced ROS, especially to H2O2 [54]. Considering the coevolutionary arms race between B. glabrata and S. mansoni, a close phenotypic concordance between the host oxidative capacity (OC) and the parasite anti-oxidant capacity (αOC) is expected and was confirmed using two geographically distinct populations [56]. Indeed, the high-OC snail population naturally interacts with the high-αOC parasite population, and similarly the low-OC snail population naturally interacts with the low-αOC parasite population [56]. In experimental crossed infections however, the low-αOC parasite population does not effectively infect high-OC snails, whereas the high-αOC parasites easily infects low-αOC snails [56], suggesting that the coevolutionary dynamic led to a reciprocal adaptation of the immune-related oxidative response of the host and its parasite.
(c). Evolution of tolerance in Wolbachia–insect symbioses
When a symbiont is vertically transmitted, its fitness depends upon its host fitness, thus reducing the likelihood of an antagonistic coevolution. Furthermore, when the symbiont is highly prevalent in the host population, tolerance strategies will be under selection to limit the cost of symbiosis at the holobiont level. The maternally transmitted intracellular bacteria Wolbachia pipientis, which can reach a high prevalence in host populations as a result of reproductive manipulations—even when they are physiologically costly—[57], are interesting model systems to understand the maintenance of symbiosis through the evolution of tolerance mechanisms. In cultured cells from the mosquito Aedes albopictus, the infection by Wolbachia is associated with a higher level of ROS (H2O2) and the synthesis of anti-oxidant proteins [58]. The artificial infection of Wolbachia in Ae. aegypti adults also increases H2O2 levels and the expression of Nox and Duox [59], suggesting that ROS were produced as an immune response. As this study focuses on an artificial infection, it is informative of the initial state of a Wolbachia-insect symbiosis: a state where the host immune response leads to a disruption of oxidative homeostasis. However, the evolution of tolerance through a reduction of ROS production may occur when the two partners coevolve [60]. Aedes polynesiensis is a naturally infected species in which Wolbachia infection is not associated with an increase in H2O2 levels. Indeed, ROS are equally produced in naturally infected and aposymbiotic (Wolbachia-free) mosquitoes [60]. By contrast, artificial injections of another Wolbachia strain in aposymbiotic mosquitoes increase H2O2 levels [60]. This finding suggests that the relatively low level of ROS generation in naturally infected individuals is the result of coevolutionary processes that occurred between the mosquito and the native strain. Presumably, tolerance is for the mosquito the optimal strategy to reduce the negative impact of Wolbachia on its life-history traits.
(d). The evolution of dependence as an extreme case of tolerance
Dependence is the situation in which the partners cannot live independently. Because of drift occurring in populations of small-efficient sizes, the loss of redundant functions is frequently associated with dependence of a symbiont on its host. However, despite the hosts' large population sizes and the absence of provision of a new function by the symbiont, hosts can also become dependent upon their symbionts as a consequence of the evolution towards tolerance. As an example, the parasitoid wasp Asobara tabida depends on Wolbachia for its oogenesis: when the symbiont is removed, the host is no longer able to produce fertile eggs [61], and the ovaries of aposymbiotic females exhibit a strong apoptotic activity [62]. In insects, programmed cell death is known to occur at specific checkpoints to regulate oogenesis in response to various stresses [63]. As apoptosis can be triggered by oxidative stress [11], the dependence of A. tabida may be a side-effect of the evolution of tolerance following the disruption of the oxidative homeostasis. More precisely, the oxidative disturbance could have led to sub-optimal life-history traits, thus triggering the evolution of compensatory mechanisms by the host, consequently making it unable to regulate oxidative stress (and to produce fertile eggs) in the absence of Wolbachia [62,64,65]. This scenario is supported by the fact that the removal of Wolbachia alters the expression of oxidative stress related genes (in particular ferritin and transferrin) [65–67]. Moreover, the supplementation of diet with iron, which is known to increase oxidative stress, has the same effect on oogenesis as the removal of Wolbachia [65]. Therefore, the deregulation of redox homeostasis occurring in the ovaries of Wolbachia-free wasps may be a consequence of the tolerance of the host to its symbiont, associated with the oxidative cost of the infection.
The studies reviewed here show that RONS play a major role in the evolution of symbioses because of their role in the immune response and their physiological costs. The extent to which the oxidative environment influences host–symbiont coevolution remains nonetheless to be clarified; although a deregulation of the redox homeostasis is often viewed as a proximal response to symbiosis, the ultimate effects of this perturbation on trade-offs between host life-history traits have not been thoroughly studied yet.
4. Influence of an ecological perturbation of the oxidative environment on the breakdown of symbioses
Redox homeostasis plays a role in the selection of symbiotic communities and in the maintenance of the associations. As the oxidative balance results from a tight tuning from both partners, even a small perturbation can affect the oxidative balance and induce profound changes in the physiology of both partners, their interactions and the stability of the association.
(a). Influence of diet on the oxidative environment and ecosystem management of the gut-microbiota symbioses
We previously highlighted the key role of ROS in the control of the composition of the gut microbiota in Drosophila, through a fine-tuning of DUOX activity that favours the tolerance of the resident microbiota and the killing of pathogens. The gut microbiota is critical for the host health and a change in its composition can lead to metabolic disorders, such as obesity or diabetes, or behavioural modifications [2]. Interestingly, the composition of gut microbial communities is greatly influenced by diet [68,69]. In mice, a long-term high-fat diet increases the plasma ROS level and induces a shift in microbial composition towards an increase of bacteria that are tolerant to oxidative conditions, such as Escherichia coli and enterococcus [70]. Hence, oxidative stress could be, at least in part, the functional link between diet and alteration of gut microbiota leading to metabolic disorders [70].
In haematophagous insects, the gut microbiota experiences oxidative challenges during a blood meal. In the mosquito Anopheles gambiae, the catabolism of a blood meal releases large amounts of haem and leads to the generation of ROS [71]. Interestingly, the gut bacterial community is strongly reshaped after a blood meal, with a reduction of diversity and an increase in Enterobacteriaceae. These dominant bacteria have an anti-oxidant capacity that enables them to maintain gut redox homeostasis [68].
The gut is constantly exposed to new diets that can affect among other physiological parameters, the oxidative environment. The examples presented above highlight how oxidative changes associated with diet can profoundly reshape the gut community and stabilize the ‘ecosystem’. The understanding of the ecosystem management of gut-microbiota symbioses is an ongoing challenge to develop new therapies against metabolic disorders or measures to control vector-borne diseases, often transmitted by haematophagous insects.
(b). Deregulation of oxidative processes in response to climate changes
Environmental changes can also disrupt the redox homeostasis in symbiotic interactions, leading to the breakdown of the association, as observed in corals in response to the global climate change. Coral reefs are complex ecosystems based on the mutualistic interaction between scleractinian cnidarians and unicellular dinoflagellate algae of the genus Symbiodinium (‘zooxanthellae’). Environmental stresses, such as light or high temperature, can strongly impact corals, leading to a phenomenon called ‘coral bleaching’, which consists of the loss of the zooxanthellae from the coral tissues [72].
Coral bleaching under stressed conditions is likely to be caused by a combination of several mechanisms, among which are the induction of host cell death [73] and the production of ROS after damage of the photosynthetic apparatus of the zooxanthellae [74]. This increase in ROS concentration overcomes the symbiont anti-oxidant capacity and damages both symbiont and host tissues [74]. If it is largely accepted that ROS mainly trigger the cnidarian/dinoflagellate breakdown through their toxic effect, the downstream events linked to apoptosis-mediated disruption of the symbiosis are poorly understood. ROS and •NO are suggested to play a pivotal role during the bleaching response, triggering the NF-κB signalling pathway that results in the induction of the NOS enzyme in host cells [75]. One mechanism leading to symbiont loss is the induction of apoptosis by •NO and ONOO–, but their respective involvement in bleaching remains unclear. While ONOO– has a bleaching-inducing capacity linked to the release of pro-apoptotic molecules by damaged mitochondrial membranes [76], levels of ONOO– generated in vivo may be insufficient to influence symbiont loss, suggesting a key role for •NO through the mediation of apoptotic pathways [77].
Oxidative stress also affects the Ca2+ homeostasis, leading to the induction of cell death via apoptosis and necrosis, the change in cytoskeletal and cell adhesion, and the decrease in calcification [78]. The intense oxidative stress that results in the disruption of various cellular processes could lead to the ‘perception’ of the symbiont as a toxic partner for the host [72]. Recently, it was shown that thermal stress induces the degradation of the cnidarian host mitochondria independently of symbiont cellular deterioration [79], suggesting that the oxidative stress altering the host tissues could also trigger the bleaching.
All together, these results suggest that the disturbance of the redox homeostasis by environmental stresses is one mechanism at the basis of the coral-symbioses breakdown. In the context of global warming and accelerated environmental changes, the understanding of the mechanisms leading to the disruption of symbiosis will be relevant to develop conservative measures.
6. Conclusion and perspectives
The regulation of the oxidative environment plays a pivotal role in the molecular ‘conversation’ between hosts and symbionts. Indeed, RONS are key molecules during symbiosis, as mediators during the interplay between organisms, and as toxic molecules during the induction of the immune-mediated oxidative burst. It is interesting to notice that RONS have been initially characterized within cells for this dual nature as signalling molecules and effectors, emphasizing the ubiquity of redox processes and their associated effects. Because of the influence of RONS on various life-history traits, the molecular interplay will shape the interactions and affect the extended phenotype, but will not necessarily canalize the output. As each partner can adjust the redox balance towards its own interest during the coevolutionary history of the symbiosis, variations of the balance will generate a great diversity of symbiotic interactions (figure 3).
Figure 3.

Influence of the regulation of the oxidative environment on the evolution of the holobiont. As common signalling molecules, RONS affect various biological functions of the host and the symbiont, thus influencing many aspects of their interaction. The resulting selective pressures, acting on one partner or at the holobiont as a whole, drive the evolution of major characteristics of the symbiosis. In particular, they can influence the position of the association along the continuum between parasitism and mutualism, the level of dependence between partners and the stability of the symbiotic association.
It is now necessary to also integrate to this interplay the constantly changing environment in which symbiotic associations evolve, and the associated selective pressures. On the one hand, the ability to regulate RONS can play a crucial role in the adaptive potential of organisms and the selection for evolutionary novelties; the best example being probably the evolution of mitochondria. Also, the increased mutation rate associated with the presence of radicals can eventually promote new genotypes whose extended phenotype is fitter in a new environment, and thus influence the evolution of the holobiont. On the other hand, the rupture of symbiosis in response to environmental changes can affect the structure of the whole ecosystem. For instance, the bleaching of corals in response to global warming, mediated by RONS, affects coral reefs in terms of species richness and diversity that directly disturb the food chain.
Taking into account the coevolutionary processes underlying the persistence of symbiotic associations also appear to be critical to an understanding of the response of partners to change. If we take the example of mammalian hosts and environmental microbes, the coevolutionary processes allowing the tolerance of such microbes by the host lead to a state of ‘evolved’-dependence where the host immune system is now dependent on the presence of these microbes to be efficiently regulated [80]. Interestingly, the mechanisms selected to tolerate these ‘old friends’ can now explain the emergence of autoimmune diseases such as allergies, diabetes or inflammatory bowel disease in aseptic environments where these microbes are depleted [80]. Similarly, the mechanisms involved in limiting oxidative perturbations induced by environmental microbes—with which humans have evolved—could now explain the prevalence of several diseases such as diabetes or neurodegenerative diseases, for which an ROS deregulation is observed [11].
To summarize, using RONS and the oxidative environment as perhaps the most ancient example, we highlighted that mediators of interspecies communication play a central role in the interactions between partners and can promote evolutionary novelties and coevolution between partners, which in turn can shape ecosystems and affect health and disease.
Acknowledgements
We thank R. Augustin, C. Finet, M. McFall-Ngai and F. Vavre for helpful comments on the manuscript.
Funding statement
Y.M. and D.M. are supported by the Agence Nationale de la Recherche (ANR-2010-BLAN-170101/ImmunSymbArt) and N.K. by the Marie Curie Actions FP7-PEOPLE-2010-IOF/272684/SymbiOx.
References
- 1.De Bary A. 1879. Die Erscheinung der Symbiose. Strasburg, France: Verlag Karl J. Trübner. [Google Scholar]
- 2.McFall-Ngai M, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margulis L. 1970. Origin of eukaryotic cells. New Haven, CT: Yale University Press. [Google Scholar]
- 4.Hentschel U, Steinert M, Hacker J. 2000. Common molecular mechanisms of symbiosis and pathogenesis. Trends Microbiol. 8, 226–231. ( 10.1016/S0966-842X(00)01758-3) [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg E, Zilber-Rosenberg I. 2011. Symbiosis and development: the hologenome concept. Birth Defects Res. C Embryo. Today 93, 56–66. ( 10.1002/bdrc.20196) [DOI] [PubMed] [Google Scholar]
- 6.Dawkins R. 1982. The extended phenotype: the long reach of the gene. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2, 820–832. ( 10.1038/nrmicro1004) [DOI] [PubMed] [Google Scholar]
- 8.Nathan C, Cunningham-Bussel A. 2013. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361. ( 10.1038/nri3423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez MC, Andriantsitohaina R. 2009. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 11, 669–702. ( 10.1089/ars.2007.1993) [DOI] [PubMed] [Google Scholar]
- 10.Imlay J. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454. ( 10.1038/nrmicro3032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. ( 10.1152/physrev.00018.2001) [DOI] [PubMed] [Google Scholar]
- 12.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. ( 10.1016/j.tplants.2011.03.007) [DOI] [PubMed] [Google Scholar]
- 13.Schippers JHM, Nguyen HM, Lu D, Schmidt R, Mueller-Roeber B. 2012. ROS homeostasis during development: an evolutionary conserved strategy. Cell. Mol. Life Sci. 69, 3245–3257. ( 10.1007/s00018-012-1092-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feelisch M, Martin JF. 1995. The early role of nitric oxide in evolution. Trends Ecol. Evol. 10, 496–499. ( 10.1016/S0169-5347(00)89206-X) [DOI] [PubMed] [Google Scholar]
- 15.Stuart-Smith K. 2002. Demystified. Nitric oxide. Mol. Pathol. 55, 360–366. ( 10.1136/mp.55.6.360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown GC. 2010. Nitric oxide and neuronal death. Nitric oxide 23, 153–165. ( 10.1016/j.niox.2010.06.001) [DOI] [PubMed] [Google Scholar]
- 17.Ray PD, Huang B-W, Tsuji Y. 2012. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990. ( 10.1016/j.cellsig.2012.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cáp M, Váchová L, Palková Z. 2012. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxid. Med. Cell. Longev. 2012, 976753.. ( 10.1155/2012/976753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackstone N. 2009. Mitochondria and the redox control of development in cnidarians. Semin. Cell Dev. Biol. 20, 330–336. ( 10.1016/j.semcdb.2008.12.006) [DOI] [PubMed] [Google Scholar]
- 20.Engel P, Moran N. 2013. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. ( 10.1111/1574-6976.12025) [DOI] [PubMed] [Google Scholar]
- 21.Ha E-M, Oh C-T, Bae YS, Lee W-J. 2005. A direct role for dual oxidase in Drosophila gut immunity. Science 310, 847–850. ( 10.1126/science.1117311) [DOI] [PubMed] [Google Scholar]
- 22.Ha E-M, Oh C-T, Ryu J-H, Bae Y-S, Kang S-W, Jang I-H, Brey PT, Lee W-J. 2005. An antioxidant system required for host protection against gut infection in Drosophila. Dev. Cell 8, 125–132. ( 10.1016/j.devcel.2004.11.007) [DOI] [PubMed] [Google Scholar]
- 23.Ha E-M, Lee K-A, Seo YY, Kim S-H, Lim J-H, Oh B-H, Kim J, Lee W-J. 2009. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat. Immunol. 10, 949–957. ( 10.1038/ni.1765) [DOI] [PubMed] [Google Scholar]
- 24.Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid–vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642. ( 10.1038/nrmicro957) [DOI] [PubMed] [Google Scholar]
- 25.Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ. 2004. NO means “yes” in the squid–vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cell. Microbiol. 6, 1139–1151. ( 10.1111/j.1462-5822.2004.00429.x) [DOI] [PubMed] [Google Scholar]
- 26.Kremer N, Schwartzman J, Augustin R, Zhou L, Ruby EG, Hourdez S, McFall-Ngai MJ. 2014. The dual nature of haemocyanin in the establishment and persistence of the squid–vibrio symbiosis. Proc. R. Soc. B 281, 20140504 ( 10.1098/rspb.2014.0504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Dunn AK, Wilneff J, McFall-Ngai MJ, Spiro S, Ruby EG. 2010. Vibrio fischeri flavohaemoglobin protects against nitric oxide during initiation of the squid–Vibrio symbiosis. Mol. Microbiol. 78, 903–915. ( 10.1111/j.1365-2958.2010.07376.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Dufour YS, Carlson HK, Donohue TJ, Marletta M, Ruby EG. 2010. H-NOX-mediated nitric oxide sensing modulates symbiotic colonization by Vibrio fischeri. Proc. Natl Acad. Sci. USA 107, 8375–8380. ( 10.1073/pnas.1003571107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldroyd GED, Downie JA. 2008. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 59, 519–546. ( 10.1146/annurev.arplant.59.032607.092839) [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Yang S, Tang F, Zhu H. 2012. Symbiosis specificity in the legume: rhizobial mutualism. Cell. Microbiol. 14, 334–342. ( 10.1111/j.1462-5822.2011.01736.x) [DOI] [PubMed] [Google Scholar]
- 31.Puppo A, Pauly N, Boscari A, Mandon K, Brouquisse R. 2013. Hydrogen peroxide and nitric oxide: key regulators of the legume–Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 18, 2202–2219. ( 10.1089/ars.2012.5136) [DOI] [PubMed] [Google Scholar]
- 32.Cárdenas L, Martínez A, Sánchez F, Quinto C. 2008. Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J. 56, 802–813. ( 10.1111/j.1365-313X.2008.03644.x) [DOI] [PubMed] [Google Scholar]
- 33.Shaw SL, Long SR. 2003. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 132, 2196–2204. ( 10.1104/pp.103.021113.Grant) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K, Suzuki A, Abe M, Higashi S, Uchiumi T. 2008. Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol. Plant. Microbe Interact. 21, 1175–1183. ( 10.1094/MPMI-21-9-1175) [DOI] [PubMed] [Google Scholar]
- 35.Ramu SK, Peng H-M, Cook DR. 2002. Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol. Plant. Microbe Interact. 15, 522–528. ( 10.1094/MPMI.2002.15.6.522) [DOI] [PubMed] [Google Scholar]
- 36.Peleg-Grossman S, Melamed-Book N, Levine A. 2012. ROS production during symbiotic infection suppresses pathogenesis-related gene expression. Plant Signal. Behav. 7, 409–415. ( 10.4161/psb.19217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Giudice J, Cam Y, Damiani I, Fung-Chat F, Meilhoc E, Bruand C, Brouquisse R, Puppo A, Boscari A. 2011. Nitric oxide is required for an optimal establishment of the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol. 191, 405–417. ( 10.1111/j.1469-8137.2011.03693.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchon N, Broderick N, Chakrabarti S, Lemaitre B. 2009. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344. ( 10.1101/gad.1827009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altura MA, Stabb E, Goldman W, Apicella M, McFall-Ngai MJ. 2011. Attenuation of host NO production by MAMPs potentiates development of the host in the squid–vibrio symbiosis. Cell. Microbiol. 13, 527–537. ( 10.1111/j.1462-5822.2010.01552.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohar DP, Haridas S, Gantt JS, VandenBosch K. 2007. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume–rhizobia symbiosis. New Phytol. 173, 39–49. ( 10.1111/j.1469-8137.2006.01901.x) [DOI] [PubMed] [Google Scholar]
- 41.Peleg-Grossman S, Volpin H, Levine A. 2007. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J. Exp. Bot. 58, 1637–1649. ( 10.1093/jxb/erm013) [DOI] [PubMed] [Google Scholar]
- 42.Jamet A, Mandon K, Puppo A, Hérouart D. 2007. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J. Bacteriol. 189, 8741–8745. ( 10.1128/JB.01130-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Correa-Aragunde N, Graziano M, Lamattina L. 2004. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218, 900–905. ( 10.1007/s00425-003-1172-7) [DOI] [PubMed] [Google Scholar]
- 44.Jamet A, Sigaud S, Van de Sype G, Puppo A, Hérouart D. 2003. Expression of the bacterial catalase genes during Sinorhizobium meliloti–Medicago sativa symbiosis and their crucial role during the infection process. Mol. Plant. Microbe Interact. 16, 217–225. ( 10.1094/MPMI.2003.16.3.217) [DOI] [PubMed] [Google Scholar]
- 45.Stearns S. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. [Google Scholar]
- 46.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. ( 10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 47.Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745. ( 10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. 2003. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc. Natl Acad. Sci. USA 100, 14 139–14 144. ( 10.1073/pnas.2036262100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeJong RJ, Miller LM, Molina-Cruz A, Gupta L, Kumar S, Barillas-Mury C. 2007. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae . Proc. Natl Acad. Sci. USA 104, 2121–2126. ( 10.1073/pnas.0608407104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svensson EI, Råberg L. 2010. Resistance and tolerance in animal enemy–victim coevolution. Trends Ecol. Evol. 25, 267–274. ( 10.1016/j.tree.2009.12.005) [DOI] [PubMed] [Google Scholar]
- 51.Roy B, Kirchner JW. 2000. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54, 51–63. ( 10.1111/j.0014-3820.2000.tb00007.x) [DOI] [PubMed] [Google Scholar]
- 52.Hahn UK, Bender RC, Bayne CJ. 2001. Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: role of reactive oxygen species. J. Parasitol. 87, 292–299. ( 10.1645/0022-3395(2001)087[0292:KOSMSB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 53.Guillou F, Roger E, Moné Y, Rognon A, Grunau C, Théron A, Mitta G, Coustau C, Gourbal BEF. 2007. Excretory-secretory proteome of larval Schistosoma mansoni and Echinostoma caproni, two parasites of Biomphalaria glabrata . Mol. Biochem. Parasitol. 155, 45–56. ( 10.1016/j.molbiopara.2007.05.009) [DOI] [PubMed] [Google Scholar]
- 54.Mourão MDM, Dinguirard N, Franco GR, Yoshino TP. 2009. Role of the endogenous antioxidant system in the protection of Schistosoma mansoni primary sporocysts against exogenous oxidative stress. PLoS Negl. Trop. Dis. 3, e550 ( 10.1371/journal.pntd.0000550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X-J, Sabat G, Brown JF, Zhang M, Taft A, Peterson N, Harms A, Yoshino TP. 2009. Proteomic analysis of Schistosoma mansoni proteins released during in vitro miracidium-to-sporocyst transformation. Mol. Biochem. Parasitol. 164, 32–44. ( 10.1016/j.molbiopara.2008.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moné Y, Ribou A-C, Cosseau C, Duval D, Théron A, Mitta G, Gourbal B. 2011. An example of molecular co-evolution: reactive oxygen species (ROS) and ROS scavenger levels in Schistosoma mansoni/Biomphalaria glabrata interactions. Int. J. Parasitol. 41, 721–730. ( 10.1016/j.ijpara.2011.01.007) [DOI] [PubMed] [Google Scholar]
- 57.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 58.Brennan LJ, Keddie BA, Braig HR, Harris HL. 2008. The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS ONE 3, e2083 ( 10.1371/journal.pone.0002083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. 2011. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti . Proc. Natl Acad. Sci. USA 109, E23–E31. ( 10.1073/pnas.1116932108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andrews ES, Crain PR, Fu Y, Howe DK, Dobson SL. 2012. Reactive oxygen species production and Brugia pahangi survivorship in Aedes polynesiensis with artificial Wolbachia infection types. PLoS Pathog. 8, e1003075 ( 10.1371/journal.ppat.1003075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl Acad. Sci. USA 98, 6247–6252. ( 10.1073/pnas.101304298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pannebakker BA, Loppin B, Elemans CPH, Humblot L, Vavre F. 2007. Parasitic inhibition of cell death facilitates symbiosis. Proc. Natl Acad. Sci. USA 104, 213–215. ( 10.1073/pnas.0607845104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCall K. 2004. Eggs over easy: cell death in the Drosophila ovary. Dev. Biol. 274, 3–14. ( 10.1016/j.ydbio.2004.07.017) [DOI] [PubMed] [Google Scholar]
- 64.Aanen DK, Hoekstra RF. 2007. The evolution of obligate mutualism: if you can't beat 'em, join 'em. Trends Ecol. Evol. 22, 506–509. ( 10.1016/j.tree.2007.08.007) [DOI] [PubMed] [Google Scholar]
- 65.Kremer N, Voronin D, Charif D, Mavingui P, Mollereau B, Vavre F. 2009. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 5, e1000630 ( 10.1371/journal.ppat.1000630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kremer N, Dedeine F, Charif D, Finet C, Allemand R, Vavre F. 2010. Do variable compensatory mechanisms explain the polymorphism of the dependence phenotype in the Asobara tabida–Wolbachia association? Evolution 64, 2969–2979. ( 10.1111/j.1558-5646.2010.01034.x) [DOI] [PubMed] [Google Scholar]
- 67.Kremer N, Charif D, Henri H, Gavory F, Wincker P, Mavingui P, Vavre F. 2012. Influence of Wolbachia on host gene expression in an obligatory symbiosis. BMC Microbiol. 12(Suppl. 1), S7 ( 10.1186/1471-2180-12-S1-S7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Gilbreath TM, Kukutla P, Yan G, Xu J. 2011. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 6, e24767 ( 10.1371/journal.pone.0024767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu GD, et al. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. ( 10.1126/science.1208344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao Y, Sun J, Ding Y, Le G, Shi Y. 2013. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 97, 1689–1697. ( 10.1007/s00253-012-4323-6) [DOI] [PubMed] [Google Scholar]
- 71.Oliveira JHM, et al. 2011. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 7, e1001320 ( 10.1371/journal.ppat.1001320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vidal-Dupiol J, et al. 2009. Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiol. 9, 14 ( 10.1186/1472-6793-9-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paxton CW, Davy SK, Weis VM. 2013. Stress and death of cnidarian host cells play a role in cnidarian bleaching. J. Exp. Biol. 216, 2813–2820. ( 10.1242/jeb.087858) [DOI] [PubMed] [Google Scholar]
- 74.Weis VM. 2008. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J. Exp. Biol. 211, 3059–3066. ( 10.1242/jeb.009597) [DOI] [PubMed] [Google Scholar]
- 75.Perez S, Weis V. 2006. Nitric oxide and cnidarian bleaching: an eviction notice mediates breakdown of a symbiosis. J. Exp. Biol. 209, 2804–2810. ( 10.1242/jeb.02309) [DOI] [PubMed] [Google Scholar]
- 76.Snyder CM, Shroff EH, Liu J, Chandel NS. 2009. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS ONE 4, e7059 ( 10.1371/journal.pone.0007059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hawkins TD, Davy SK. 2013. Nitric oxide and coral bleaching: is peroxynitrite generation required for symbiosis collapse? J. Exp. Biol. 216, 3185–3188. ( 10.1242/jeb.087510) [DOI] [PubMed] [Google Scholar]
- 78.DeSalvo MK, Voolstra CR, Sunagawa S, Schwarz JA, Stillman JH, Coffroth MA, Szmant AM, Medina M. 2008. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol. Ecol. 17, 3952–3971. ( 10.1111/j.1365-294X.2008.03879.x) [DOI] [PubMed] [Google Scholar]
- 79.Dunn SR, Pernice M, Green K, Hoegh-Guldberg O, Dove SG. 2012. Thermal stress promotes host mitochondrial degradation in symbiotic cnidarians: are the batteries of the reef going to run out? PLoS ONE 7, e39024 ( 10.1371/journal.pone.0039024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rook GAW. 2012. Hygiene hypothesis and autoimmune diseases. Clin. Rev. Allergy Immunol. 42, 5–15. ( 10.1007/s12016-011-8285-8) [DOI] [PubMed] [Google Scholar]


