Abstract
Domestic dogs are particularly skilled at using human visual signals to locate hidden food. This is, to our knowledge, the first series of studies that investigates the ability of dogs to use only auditory communicative acts to locate hidden food. In a first study, from behind a barrier, a human expressed excitement towards a baited box on either the right or left side, while sitting closer to the unbaited box. Dogs were successful in following the human's voice direction and locating the food. In the two following control studies, we excluded the possibility that dogs could locate the box containing food just by relying on smell, and we showed that they would interpret a human's voice direction in a referential manner only when they could locate a possible referent (i.e. one of the boxes) in the environment. Finally, in a fourth study, we tested 8–14-week-old puppies in the main experimental test and found that those with a reasonable amount of human experience performed overall even better than the adult dogs. These results suggest that domestic dogs’ skills in comprehending human communication are not based on visual cues alone, but are instead multi-modal and highly flexible. Moreover, the similarity between young and adult dogs’ performances has important implications for the domestication hypothesis.
Keywords: Canis familiaris, communication, dog, domestication, object choice, voice following
1. Introduction
Recent research has shown that domestic dogs (Canis familiaris) possess highly specialized social skills that allow them to read human communicative behaviour [1–3]. They can use human communicative acts such as pointing and gazing in flexible ways especially to find hidden food, see for review [4,5]. Dogs appear to be more skilled than great apes in solving problems in cooperative contexts, and one current hypothesis is that their ability to comprehend human communicative acts developed through convergent evolution as a result of domestication [2,6]. While there is general agreement that domestication has been functional in selecting for dogs’ special cognitive skill and special attachment to and caring for human beings [7–10], there is currently an open debate concerning both the actual differences between the cognitive skills of dogs and wolves and the role of phylogeny and ontogeny in the development of those skills. The ‘domestication hypothesis’ [2,11,12] suggests that the ability of dogs to pay special attention to humans has been selected for through domestication and is probably part of the current genetic repertoire with which every dog is endowed. In general, the domestication hypothesis provides two main types of evidence for this claim:
(1) phylogenetically, wolves appear unable to use human communicative cues in the same way ([12–14], but see [15]) and dogs often outperform great apes in their understanding of these cues [11,16,17]; and
(2) ontogenetically, dogs appear to be able to use these cues even as puppies [11,14,18–20]. Moreover, dogs’ high performance in communication tasks does not appear to require extensive exposure to humans [11,18,21,22] (but see [15,23]), and the conditions in which they are reared and the extent of their training seem to exert only little influence [14,22,24].
An alternative approach has on the other hand criticized the focus on the role that genetic selection and therefore biological predisposition plays in the capacity of dogs to interpret human communicative cues, by emphasizing the role that ontogenetic development and socialization might play in the emergence of these skills. In other words, the claim is that most of these skills are the simple product of learning from extended exposure to humans [15,25].
The debate between supporters of these different hypotheses is open for a number of reasons: current evidence on dogs’ skills in using human communicative cues relies almost exclusively on visual cues such as pointing or gaze following, some studies lack conditions controlling for alternative low-level explanations for dogs’ performance, and little work has been conducted on young puppies. Moreover, while new investigations are trying to tease apart the role of testing environments and socialization in dogs’ performance, it appears that minor methodological decisions such as the exact implementation of the gesture or the occurrence of reinforcement could have a major impact on the ultimate performance of the dogs [24].
In this paper, we further extend the scope of prior investigations of dogs’ understanding of human communicative cues and the role of domestication and ontogeny in such skills. We do so by investigating for the first time, to our knowledge, not a visual communicative cue but rather an auditory one: voice direction. Moreover, we run a series of controls to rule out alternative explanations for the dogs’ capacity to solve the task and most importantly we test puppies in the same main experimental condition.
A recent study [26] investigated the ability of children and chimpanzees to follow human voice direction without any visual cues. Specifically, an experimenter squatted behind a large barrier and vocalized excitement towards one of the two containers at each end of the barrier. One-year-old human infants were able to follow this auditory cue—the adult's voice direction—to a target, that is, the box containing a toy. By contrast, chimpanzees tested with the same experimental set-up but with food as a reward were unable to follow a human's voice direction to a target (i.e. the box containing food).
Most contemporary hypotheses about the most important ostensive cues for dogs’ understanding of human communication only consider visual processes, including, for example, eye contact, following gaze direction and so forth [12,20,27,28]. According to these hypotheses, dogs should not be able to follow human voice direction to locate hidden food without being able to see the human's eyes or other visible cues such as a pointing gesture. However, we know very little about dogs’ capacity to use auditory information in communicative contexts. Moreover, the fact that 1-year-old human infants can follow voice direction suggests that it is possibly a very basic skill, one that is particularly useful for referential communication when the communicator is not visible [26]. This is, to our knowledge, the first series of studies to test whether adult dogs and puppies can use a human's voice direction to locate hidden food. These studies rely on the same apparatus and an almost identical procedure to the previous study with children and chimpanzees [26]. Moreover, by investigating how puppies with different rearing history perform in the same task, this study sheds some new light on the role that socialization might play in the capacity to attend to communicative cues produced through a modality mostly uninvestigated: the auditory one.
2. Study 1
First, we tested adult dogs using a procedure identical to the one used in the study with human infants [26].
(a). Material and methods
(i). Subjects
Twenty-four dogs, 12 females and 12 males, participated in this experiment. One additional dog had to be excluded, because he was uncomfortable in the testing situation. No breed was excluded (table A in the electronic supplementary material provides more information about the dogs breed and age). Only dogs older than 1 year (mean age ±s.d. = 5.5 ± 3.2 years) were tested in this experiment. They were selected from a database of dogs volunteered by owners who were willing to have their dogs participate in this type of study. The dogs had mixed experience with experimental testing with some of them being completely naive and others quite experienced (range 0–19, average seven studies). In any case, none had ever participated in a study relying only on auditory cues. The experiment was conducted in a room dedicated to dog studies and the subjects’ owners were not present during the procedure.
(ii). Set-up and materials
For this study, we used almost the same set-up as in Rossano et al. [26]. A large wooden barrier (160 × 0.9 × 122 cm) was placed in a room, equidistant from both sidewalls of that room (figure 1). Two identical containers (39 × 17 × 14) were used as hiding locations. A handle was attached to each container so that the experimenter could place both containers on the ground at the same time. Both containers had a covered bottom with food under it in order to control for olfactory cues. An assistant placed the dog 2 m away, perpendicular to the centre of the barrier. A curtain was set in front of the subject (figure 1).
Figure 1.
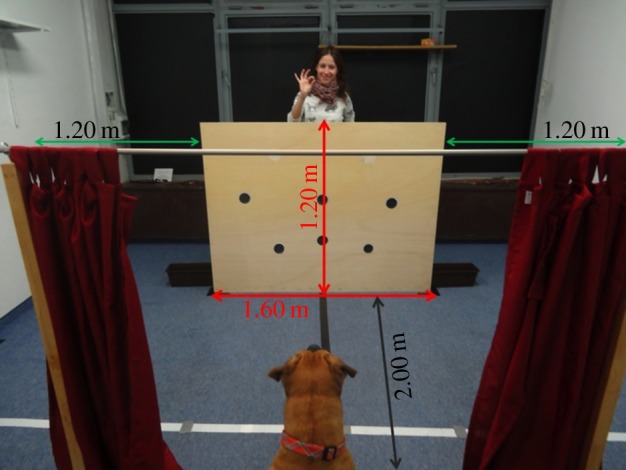
General set-up. (Online version in colour.)
(iii). Procedure
Pretest. The assistant led the dog around the room (including behind the barrier) to familiarize the subject with the testing situation and ensure the subject could see what was behind the barrier. The dog was then led to the starting point and the first part of a two-part pretest began. First, the experimenter stood behind the barrier and showed a piece of food to the subject above the centre of the barrier and called the subject's name. Then she placed the food in one of the containers in full view of the dog (i.e. the hand was always visible so that the subject could track the food visually). The location of the container with the food was semi-randomized with no more than two consecutive trials on the same side. The criterion for passing this pretest phase was successful retrieval of the food in a block of four consecutive trials (two on each side). All participants passed this first pretest except for one dog, which needed another block of four trials to pass.
The second part of the pretest resembled the first part but in this phase the subject could not track the food visually when it was placed in the container. Instead, after showing the dog the food, the experimenter squatted down behind the barrier and placed it in one of the containers so that the dog only saw the experimenters’ hand during the food placement. The criterion for passing this second phase of the pretest was identical to the first. All subjects passed this pretest in the first block of four trials.
Experimental condition. The test proceeded as follows: the assistant placed the dog at the starting position and oriented her/him towards the barrier. The experimenter stood behind the centre of the barrier, showed the dog the food, called her/his name and said ‘Pass auf’ (German for ‘Watch!/Pay attention!’) to attract the dog's attention. Then the assistant closed the curtain. The experimenter then squatted down behind the barrier and placed the food in one of the containers. She then placed both containers on the ground, simultaneously, on each side of the barrier. The experimenter then stood up and the assistant opened the curtain, while holding the dog by the collar. Both containers were now visible to the dog. The experimenter again called for the dog's attention by calling the dog's name and saying ‘Pass auf’. Then she squatted down behind the barrier again and oriented her face and body towards the baited container, while being physically closer to the unbaited container (figure 2). In this position, if the dog went to the baited container, then it would indicate that the dog is following the directional information gathered from the experimenter's voice, whereas if the dog went to the unbaited container, it would indicate that the dog is going towards the source of sound. Remaining in the same position behind the barrier, the experimenter then expressed excitement by saying ‘Oh look, look there, this is great!’ twice. After the first time, the assistant released the dog so s/he could choose one of the containers. The dog was rewarded with the food in the container only if s/he approached the baited container first. After each trial, the assistant led the dog around the room and the experimenter pulled both containers behind the barrier before starting the next trial. Each subject participated in 12 consecutive trials in total. The placement of the target food was counterbalanced across trials with no more than two times in a row on the same side.
Figure 2.
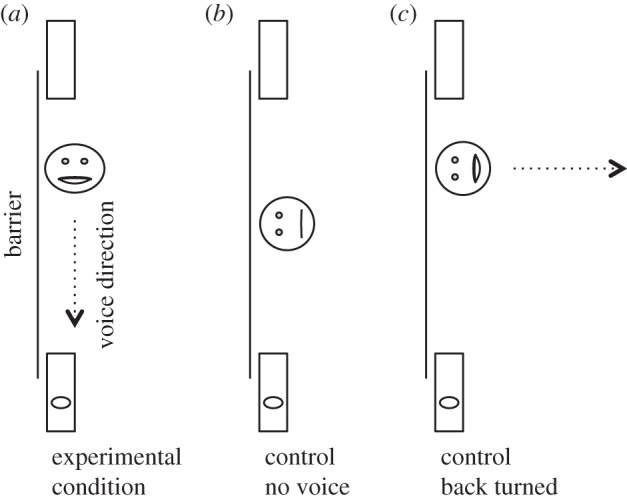
Position of the experimenter and voice direction in the different conditions.
(iv). Coding and analysis
The dogs’ choices were coded as correct if the dog chose the container where the food had been placed first (by touching it), or if the dog went around the barrier on the correct side (i.e. approached from the side of the baited container). A choice was coded as incorrect if the dog chose the unbaited container first (by touching it), or if s/he went around the barrier on that side. We additionally coded whether the dog went towards the ‘box first’ (i.e. interpreted the call in a referential way) or towards the ‘experimenter first’ (i.e. interpreted the call as an invitation to approach). We did not pre-specify trial length. A trial ended as soon as the dog made a choice or if the dog went in the opposite direction (away from the barrier). In the latter case, the helper would return the dog to the starting point and the trial was repeated. This never happened in study 1.
A second coder, unaware of the purpose of the study, coded 20% of the video material. There was perfect agreement between coders over ‘correct choices’ (Cohen's κ = 1) and a good level of agreement over ‘box first’ (Cohen's κ = 0.847). Data were analysed with non-parametric test statistics. All tests were two tailed and the α-level was set to 0.5.
(b). Results
The dogs successfully followed the experimenter's voice direction to find the hidden food (mean correct: 7.6 trials, Wilcoxon signed-ranks test: T+= 186.0, n = 19 (five ties), p < 0.001). They found this cue straightforward from the beginning (first four trials: mean = 2.8, T+= 130.0, n = 16 (eight ties), p < 0.001) and maintained a significant performance over all trials (middle four trials: mean = 2.5; T+= 61.5, n = 11 (13 ties), p = 0.009; last four trials: mean = 2.3; T+= 21.0, n = 6 (18 ties), p = 0.031). However, performance declined when comparing the first four trials with the last four trials (T+= 18.0, n = 14 (10 ties), p = 0.027). We found no effect of sex (Mann–Whitney U: U = 44.0, nm = 12, nf = 12, p = 0.101).
In an analysis of first trials, we checked to see whether dogs first went to the box or whether they went around the barrier to the experimenter. They had no preference (box first: n = 14, experimenter first: n = 10); but importantly, dogs that chose the experimenter first did not choose the correct container (correct: n = 4, incorrect: n = 6, p = 0.754), whereas dogs that chose the container first tended to be correct (correct: n = 11, incorrect: n = 3, p = 0.057). This additional result shows that some dogs may not have comprehended the adult's vocalization referentially, but those who did, comprehended it correctly from the first trial over three-quarters of the time.
Finally, we correlated the number of previous studies the dogs had participated in with the correct choices of each subject in study 1. The correlation did not show any significant effect of experience with testing on the performance in this task (n = 24, Spearman's ρ = –0.312, p = 0.138). If anything, the negative direction of the correlation suggests that more experience with testing might have a hindering effect (possibly because prior testing might have led them to pay particular attention to visual cues, rather than auditory ones).
3. Study 2: control no voice
A possible alternative explanation for the results observed in study 1 would be that dogs are not really relying on auditory cues to locate the hidden food. Rather, they could simply locate the hidden food via smell. Recent work has suggested that dogs could use odour cues in similar tasks when the experimenter was not present in the room but were at chance when the experimenter remained in the room [29], and other works have recently criticized the lack of controls on olfactory cues in some prior studies using communicative cues to locate hidden food [30]. To control for this possibility, we ran a second study using the same procedure as in study 1 but this time the experimenter did not produce any excited vocalization from behind the barrier.
(a). Material and methods
(i). Subjects
Sixteen new dogs, eight males and eight females, participated in this experiment. One additional dog had to be excluded because she was uncomfortable in the testing situation. See Table A in the electronic supplementary material for more detailed information about the subjects’ breed and age. Only dogs older than 1 year (mean age ±s.d. = 6.1 ± 3.5 years) participated in this experiment. The dogs had mixed experience with experimental testing with some of them being completely naive and others quite experienced (range 0–27, average seven studies).
(ii). Set-up and materials
The set-up and the materials were exactly the same as in the experimental condition.
(iii). Procedure
Prior to testing, the dogs needed to pass the same two pretests as in the experimental condition. The procedure of the test trials resembles the one of the experimental condition with the following differences: after squatting down behind the barrier the second time (i.e. after having placed the boxes beside the barrier and shown the food reward to the subject), the experimenter did not orient her face/body towards one container, instead she turned around at the middle of the barrier, oriented her face away from the barrier and remained silent. After 5 s, the assistant released the dog so that s/he could choose one of the containers. Each subject received 12 consecutive trials. The placement of the target food was semi-randomized across trials with no more than two times in a row at the same side.
(iv). Coding and analysis
We coded the same measurements as in study 1. We repeated one trial for one dog after he walked away from the barrier/boxes rather than directly towards them.
A second coder, unaware of the purpose of the study, coded 20% of the video material. Inter-rater reliability was perfect for both measures, ‘correct choices’ and ‘box first’ (Cohen's κ = 1). Data were analysed with non-parametric test statistics. All tests were two tailed and the α-level was set to 0.5.
(b). Results
Without the auditory cue produced by the experimenter, dogs were not able to find the hidden food (mean correct: 6.0 trials, Wilcoxon signed-ranks test: T+= 9.0, n = 6 (10 ties), p = 1.0; figure 3). We did not find any change of performance across trials (first, middle and last four trials; comparing first four with last four, all p > 0.3) and we did not detect any effect of sex (Mann–Whitney U: U = 32.0, nm = 8, nf = 8, p = 1.0).
Figure 3.
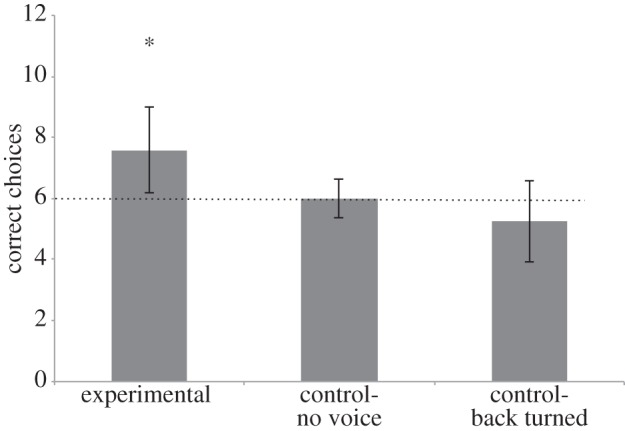
Average of correct choices in the different conditions. The dashed line depicts the chance level. The asterisk (*) indicates significance (p < 0.05).
In the first trial, dogs preferred to choose the box first ((box first: n = 13, experimenter first: n = 3, p = 0.021). However, they did not choose the correct/baited container more often than the unbaited one (box first: correct: n = 5, incorrect: n = 8, p = 0.581; experimenter first: correct: n = 2, incorrect: n = 1, p = 1.0; in total: correct: n = 7, incorrect: n = 9, p = 0.804).
4. Study 3: control back turned
Two alternative explanations for the results observed in study 1 would be that: (i) dogs are not using voice direction to solve the task but some other auditory cue; and that (ii) dogs do not really comprehend voice direction in a referential manner (i.e. as being about the food and one of the boxes). Concern (i) would suggest that they are responding to some other possible auditory cue such as the noise produced by the experimenter squatting down behind the barrier, before producing the vocalization. Concern (ii) would suggest that a change in the directionality of the vocalization should not affect the dogs’ performance. To address both concerns, we ran a control study in which all the experimenter's movements behind the barrier were identical to what was done in study 1, but this time the excited vocalization was not addressed towards either of the boxes but rather towards the wall behind the experimenter.
(a). Material and methods
(i). Subjects
Sixteen new dogs, eight males and eight females, participated in this experiment. For more detailed information about the subjects’ breed and age, see table A in the electronic supplemental material. Two additional dogs were excluded from the test (one was uncomfortable in the testing situation and a second one did not pass the pretest). Only dogs older than 1 year (mean age ± s.d. = 5.6 ± 2.9 years) were selected from the database. The dogs had mixed experience with experimental testing with some of them being completely naive and others quite experienced (range 0–11, average three studies).
(ii). Set-up and materials
The set-up and the materials were the same as in the experimental condition.
(iii). Procedure
As in the two other studies, subjects needed to pass the pretest before they could participate in the test.
The procedure of the test trials resembled the one in the experimental condition with the following difference: after squatting down behind the barrier, the experimenter positioned herself closer to the empty box but she oriented her head away from the barrier, towards the wall behind it (figure 2) before producing the same excited vocalizations as in study 1. Just like in all prior studies, the boxes were always visible beside the barrier by the time the curtain would be open in front of the dog and before the experimenter would squat down for the second time. Each subject received 12 consecutive trials. The placement of the target food was semi-randomized across trials with no more than two times in a row at the same side.
(iv). Coding and analysis
We coded the same measurements as in study 1.
We repeated one trial for two dogs and four trials for a third dog, because they had not moved directly towards the barrier/boxes, but rather away from them.
Reliability coding (20% of the video material) revealed a good inter-rater reliability (‘correct choices’: Cohen's κ = 0.875; ‘box first’: Cohen's κ = 0.874). Data were analysed with non-parametric test statistics. All tests were two tailed and the α-level was set to 0.5.
(b). Results
Dogs were not able to find the hidden food when the vocalization was not directed towards any salient object in the room but rather towards the wall. Instead we found a tendency to move towards the unbaited box, which means that they were going towards the source of the sound (mean correct: 5.3 trials, Wilcoxon signed-ranks test: T+= 11.5, n = 11 (five ties), p = 0.063; figure 3). We did not find any change of performance across trials (comparing first four with last four, all p = 0.494) and we did not detect any effect of sex (Mann–Whitney U: U = 27.5, nm = 8, nf = 8, p = 0.647).
First trial analysis revealed a clear preference for the side with the unbaited box (binomial test: correct: n = 13, incorrect: n = 3, p = 0.021). Dogs did not preferentially choose the experimenter (n = 10) or the box first (n = 6, p = 0.122).
5. Study 4: puppies
Once we had established that adult dogs could reliably use voice direction for referential purposes, we wanted to assess how early in development the capacity to follow voice direction emerges. In particular, we wanted to assess whether this skill emerges after long exposure to humans or rather can be found very early in development. For this purpose, we decided to test young puppies in the main experimental condition used for the adult dogs (study 1). Moreover, we had the opportunity to assess whether socialization processes, in particular the amount of exposure to humans, would potentially affect the emergence of this cognitive skill. The opportunity came from a fortunate combination that allowed for a sort of natural experiment. Besides testing a group of puppies who lived with their owners as pets, a breeder offered to bring to the laboratory eight puppies with the same genetic pool (part of the same litter). Even more fortunately, two of these eight puppies interacted with the breeder on a regular basis, while the remaining six lived with their littermates with little human contact. Therefore, in addition to a comparison between the performance of puppies living with humans as pets versus living with their littermates, we had a natural, minimal control for the specific effect of socialization once breed, age and genes were kept the same.
(a). Material and methods
(i). Subjects
Sixteen dog puppies, eight males and eight females, participated in this experiment. Two additional female puppies were excluded, one (belonging to the less socialized group, and nine weeks old) did not pass the pretest and the other (belonging to normal socialized group, and eight weeks old) was not comfortable in the test room. The age ranged from eight to 14 weeks, with an average age of 10 weeks (±s.d. = ±1.7 weeks).
None of the puppies had previously participated in any experiment. The exact age, breed and sex of each dog is listed in Table A of the electronic supplementary material. The puppies were recruited directly from breeders, from puppy courses in different dog schools or from a database with volunteer owners.
(ii). Set-up and materials
The set-up and the materials were the same as in the experimental condition.
(iii). Procedure
The procedure was exactly the same as in the experimental condition (figure 2).
The only difference with the adult dogs’ test was the inclusion of a warm-up phase before the pretest started. During this phase (lasting approx. 10 min), the experimenter played with the subject, cuddled him/her and talked to him/her. In a pilot phase, we had noticed that the puppies were initially distressed by the new environment and not attentive to the experimenter. We therefore included this warm-up to accustom the puppies to the test room and to increase the general attention towards the experimenter.
(iv). Coding and analysis
We coded the same measurements as in the experimental condition.
We repeated one trial for four dogs and three trials for one dog, because they had not moved directly towards the barrier/boxes, but rather away from them.
Reliability coding (20% of the video material) revealed a perfect agreement (‘correct choices’: Cohen's κ = 1; ‘box first’: Cohen's κ = 1). Data were analysed with non-parametric test statistics. All tests were two tailed and the α-level was set to 0.5.
(b). Results
The puppies were able to use the human's voice direction to find hidden food (mean correct: 8.1 trials, Wilcoxon: T+= 120.0, n = 16 (0 ties), p = 0.005). The performance of the puppies did not differ from that of the adults in the experimental condition (Mann–Whitney U: U = 154.0, nadult = 24, npuppy = 16, p = 0.293).
Contrary to what was observed for the adult subjects, the puppies did not reliably use the cue right from the beginning (first four trials: mean = 2.6, T+= 78.0, n = 14 (two ties), p = 0.127) but reached a significant performance over the following trials (middle four trials: mean = 2.9; T+= 55.0, n = 10 (six ties), p = 0.002; last four trials: mean = 2.7; T+= 58.0, n = 11 (five ties), p = 0.014). However, performance did not increase when comparing the first four trials with the last four trials (T+= 24.0, n = 10 (six ties), p = 0.828). We did not find an effect of sex (Mann–Whitney U: U = 20.5, nm = 8, nf = 8, p = 0.242).
We did not find a preference for any container in the first trial (binomial test: correct: n = 10, incorrect: n = 6, p = 0.454) and puppies preferentially went to the box first in the first trial (n = 15, p = 0.001).
If we then consider the two groups of puppies with different rearing histories, we found that the less socialized puppies performed at chance level (all trials: mean = 5.4; T+= 4.0, n = 5, p = 0.437; first four trials: mean = 1.2; T+= 2.5, n = 5, p = 0.312), whereas the human socialized group significantly chose the correct container (all trials: mean = 9.4; T+= 66.0, n = 11, p = 0.001; first four trials: mean = 3.2; T+= 45.0, n = 9 (two ties), p = 0.004; figure 4). The two puppies that received normal human socialization from the breeder performed better than their less socialized littermates overall (mean = 8 versus 5.4) and had done so already in the first four trials (mean = 3.5 versus 1.2).
Figure 4.
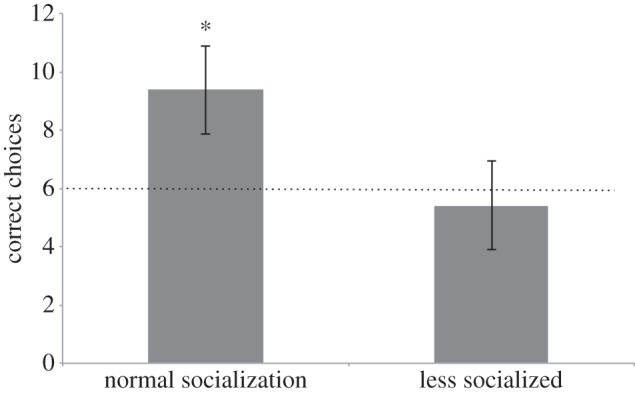
Average of correct choices of the puppies in study 4, divided by the rearing background (normal socialization, n = 11; less socialized, n = 5). The dashed line depicts the chance level. The asterisk (*) indicates significance (p < 0.05).
Interestingly, the group of normally socialized puppies even outperformed the adult subjects (Mann–Whitney U: U = 52.5, npuppy = 11, nadult = 24, p = 0.003). Note that we did not find a correlation of age and performance in the group with the normal socialized puppies (n = 11, Spearman's ρ = 0.386, p = 0.241).
6. Discussion
The series of studies here reported show that dogs can locate hidden food relying only on auditory information. Dogs can do so without the aid of any visual information such as observing pointing gestures or being able to detect the experimenter's gaze during the test. They do not rely on olfactory cues to successfully solve the task and appear to use the directionality of the human vocalization in a referential manner (i.e. if the vocalization was not directed towards one of the boxes, they went towards the source of sound). This is, to our knowledge, the first study showing this skill and the first study that detached ostensive auditory cues from visual communicative gestures. These results specifically challenge previous claims concerning the crucial role of human eye contact in the understanding of visual communicative cues [12,20,31]. It is important to note that in the first four trials dogs are overall as good as (if not better than) human infants [26]. The finding that the ones who go to the box first appear to go to the correct container, while the ones who go to the experimenter first do not, suggests that at least some dogs interpret human vocalizations in a referential manner, and that they can do this independently of the visual modality, and study 3 provides additional evidence for this claim.
The fact that dogs can easily use this kind of information while chimpanzees cannot [26], suggests that this skill might have developed as a function of domestication and close contact with human beings. However, our fourth study further refines this claim. Puppies perform similar to adult dogs in the experimental condition and can follow voice direction and use this signal in a referential manner to locate hidden food right from the first few trials. Note, however, that this is true only for the puppies that lived with humans as pets. The ones that had little exposure to humans in their first few weeks of life performed at chance level. This could be an indication that dogs generally need a certain amount of exposure to humans in order to develop this skill. On the other hand, it might also be expected that ‘socially deprived’ dogs exhibit deficits in their cognitive development compared to more normally socialized puppies, and that this deprivation might explain their inability to use this cue ([32], see [33]). It is likely that the skill reported in this study is learned, though very quickly and through a minimal amount of exposure to humans.
The puppies who lived with humans as pets showed a high performance independent of their age and they performed significantly better than the adult dogs in the same experimental task. We believe that this is the first study to show an ontogenetic decrease in the capacity to use a communicative cue to locate the food. One possible explanation for the better performance of puppies over adult dogs would be the addition of 10 min of warm-up in the puppy study. This time might have made the puppies more familiar with the experimenter's voice and therefore more capable of tracking the directionality of the initial vocalization. An alternative explanation would be that adult dogs’ hearing gets worse with time. A third explanation could be that the salience of only auditory cues declines as they occur fairly rarely in isolation in normal dog–human interactions and adult dogs might end up relying more on visual cues.
Overall, our study expands the range of human communicative cues previously investigated, controls for alternative explanations, and provides important new data to the debate about the role of domestication in the development of these skills. There is some ontogenetic change, yet in the opposite direction of what one might expect, and amount of exposure to humans does matter for their performance. However, since puppies of 8–14 weeks old are capable of performing remarkably well in this task, our study also shows that the learning must have been extremely rapid during ontogeny. In other words, our results align with previous literature that proposes that dogs seem to have a predisposition to care about humans and their communicative cues. So much so that they can easily learn how to use auditory cues to find hidden food in an object choice paradigm.
The expansion of the range of communicative cues investigated would allow for a more accurate and more finely grained picture of what might be genetically predetermined, what is clearly learned through development and socialization, and the role that the special nature of human–dog relationships play in the emergence of this cognitive skill. In order to draw more accurate conclusions about the impact of domestication on this skill, it would be necessary to test wolves in this paradigm.
Acknowledgements
We thank the dog owners. Without their support this work would not be possible. We also thank Katrin Schumann, Ulrike Kachel, Sandra Quintero, Suska Nolte, Katja Waldherr, Viktoria Walther, Cora Rüdt von Collenberg, Vera Ehrich and Katja Weber for help with data collection, coding and reliability coding. Finally, we thank Malinda Carpenter for helpful feedback on the design of the studies, and Richard Moore and Karen Bradley for helpful feedback on an earlier draft of the manuscript.
The studies here reported were performed in full accordance with German legal regulations and the guidelines for the treatment of animals in behavioural research and teaching of the Association for the Study of Animal Behaviour (ASAB).
Data accessibility
All data used in these analyses are available in the Dryad repository at: doi:10.5061/dryad.c241p.
References
- 1.Cooper JJ, Ashton C, Bishop S, West R, Mills DS, Young RJ. 2003. Clever hounds: social cognition in the domestic dog (Canis familiaris). Appl. Anim. Behav. Sci. 81, 229–244. ( 10.1016/S0168-1591(02)00284-8) [DOI] [Google Scholar]
- 2.Hare B, Tomasello M. 2005. Human-like social skills in dogs? Trends Cogn. Sci. 9, 439–444. ( 10.1016/j.tics.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 3.Miklósi Á, Topál J, Csányi V. 2004. Comparative social cognition: what can dogs teach us? Anim. Behav. 67, 995–1004. ( 10.1016/j.anbehav.2003.10.008) [DOI] [Google Scholar]
- 4.Miklósi Á, Soproni K. 2006. A comparative analysis of the animals’ understanding of the human pointing gesture. Anim. Cogn. 9, 81–93. ( 10.1007/s10071-005-0008-1) [DOI] [PubMed] [Google Scholar]
- 5.Kaminski J, Nitzschner M. 2013. Do dogs get the point? A review of dog–human communication ability. Learn. Motiv. 44, 294–302. ( 10.1016/j.lmot.2013.05.001) [DOI] [Google Scholar]
- 6.Wobber V, Hare B. 2009. Testing the social dog hypothesis: are dogs also more skilled than chimpanzees in non-communicative social tasks? Behav. Process. 81, 423–428. ( 10.1016/j.beproc.2009.04.003) [DOI] [PubMed] [Google Scholar]
- 7.Topál J, Miklósi Á, Csányi V, Dóka A. 1998. Attachment behavior in dogs (Canis familiaris): a new application of Ainsworth's (1969) strange situation test. J. Comp. Psychol. 112, 219–229. ( 10.1037/0735-7036.112.3.219) [DOI] [PubMed] [Google Scholar]
- 8.Topál J, Gácsi M, Miklósi Á, Virányi Z, Kubinyi E, Csányi V. 2005. Attachment to humans: a comparative study on hand-reared wolves and differently socialized dog puppies. Anim. Behav. 70, 1367–1375. ( 10.1016/j.anbehav.2005.03.025) [DOI] [Google Scholar]
- 9.Palmer R, Custance D. 2008. A counterbalanced version of Ainsworth's strange situation procedure reveals secure-base effects in dog–human relationships. Appl. Anim. Behav. Sci. 109, 306–319. ( 10.1016/j.applanim.2007.04.002) [DOI] [Google Scholar]
- 10.Merola I, Prato-Previde E, Marshall-Pescini S. 2012. Social referencing in dog-owner dyads? Anim. Cogn. 15, 175–185. ( 10.1007/s10071-011-0443-0) [DOI] [PubMed] [Google Scholar]
- 11.Hare B, Brown M, Williamson C, Tomasello M. 2002. The domestication of social cognition in dogs. Science 298, 1634–1636. ( 10.1126/science.1072702) [DOI] [PubMed] [Google Scholar]
- 12.Miklósi Á, Kubinyi E, Topál J, Gácsi M, Virányi Z, Csányi V. 2003. A simple reason for a big difference: wolves do not look back at humans, but dogs do. Curr. Biol. 13, 763–766. ( 10.1016/S0960-9822(03)00263-X) [DOI] [PubMed] [Google Scholar]
- 13.Gácsi M, Gyoöri B, Virányi Z, Kubinyi E, Range F, Belényi B, Miklósi Á. 2009. Explaining dog wolf differences in utilizing human pointing gestures: selection for synergistic shifts in the development of some social skills. PLoS ONE 4, e6584 ( 10.1371/journal.pone.0006584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virányi Z, Gácsi M, Kubinyi E, Topál J, Belényi B, Ujfalussy D, Miklósi Á. 2008. Comprehension of human pointing gestures in young human-reared wolve (Canis lupus) and dogs (Canis familiaris). Anim. Cogn. 11, 373–387. ( 10.1007/s10071-007-0127-y) [DOI] [PubMed] [Google Scholar]
- 15.Udell MAR, Dorey NR, Wynne CDL. 2008. Wolves outperform dogs in following human social cues. Anim. Behav. 76, 1767–1773. ( 10.1016/j.anbehav.2008.07.028) [DOI] [Google Scholar]
- 16.Bräuer J, Kaminski J, Riedel J, Call J, Tomasello M. 2006. Making inferences about the location of hidden food: social dog, causal ape. J. Comp. Psychol. 120, 38–47. ( 10.1037/0735-7036.120.1.38) [DOI] [PubMed] [Google Scholar]
- 17.Kirchhofer KC, Zimmermann F, Kaminski J, Tomasello M. 2012. Dogs (Canis familiaris), but not chimpanzees (Pan troglodytes), understand imperative pointing. PLoS ONE 7, e30913 ( 10.1371/journal.pone.0030913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riedel J, Schumann K, Kaminski J, Call J, Tomasello M. 2008. The early ontogeny of human–dog communication. Anim. Behav. 75, 1003–1014. ( 10.1016/j.anbehav.2007.08.010) [DOI] [Google Scholar]
- 19.Agnetta B, Hare B, Tomasello M. 2000. Cues to food location that domestic dogs (Canis familiaris) of different ages do and do not use. Anim. Cogn. 3, 107–112. ( 10.1007/s100710000070) [DOI] [Google Scholar]
- 20.Kaminski J, Schulz L, Tomasello M. 2012. How dogs know when communication is intended for them. Dev. Sci. 15, 222–232. ( 10.1111/j.1467-7687.2011.01120.x) [DOI] [PubMed] [Google Scholar]
- 21.Hare B, Rosati A, Kaminski J, Bräuer J, Call J, Tomasello M. 2010. The domestication hypothesis for dogs’ skills with human communication: a response to Udell et al. (2008) and Wynne et al. (2008). Anim. Behav. 79, e1–e6. ( 10.1016/j.anbehav.2009.06.031) [DOI] [Google Scholar]
- 22.Gácsi M, Kara E, Belényi B, Topál J, Miklósi Á. 2009. The effect of development and individual differences in pointing comprehension of dogs. Anim. Cogn. 12, 471–479. ( 10.1007/s10071-008-0208-6) [DOI] [PubMed] [Google Scholar]
- 23.Udell MAR, Dorey NR, Wynne CDL. 2010. The performance of stray dogs (Canis familiaris) living in a shelter on human-guided object-choice tasks. Anim. Behav. 79, 717–725. ( 10.1016/j.anbehav.2009.12.027) [DOI] [Google Scholar]
- 24.Pongracz P, Gacsi M, Hegedus D, Peter A, Miklosi A. 2013. Test sensitivity is important for detecting variability in pointing comprehension in canines. Anim. Cogn. 16, 721–735. ( 10.1007/s10071-013-0607-1) [DOI] [PubMed] [Google Scholar]
- 25.Udell MAR, Dorey NR, Wynne CDL. 2010. What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol. Rev. 85, 327–345. ( 10.1111/j.1469-185X.2009.00104.x) [DOI] [PubMed] [Google Scholar]
- 26.Rossano F, Carpenter M, Tomasello M. 2012. One-year-old infants follow others’ voice direction. Psychol. Sci. 23, 1298–1302. ( 10.1177/0956797612450032) [DOI] [PubMed] [Google Scholar]
- 27.Virányi Z, Topál J, Gácsi M, Miklosi Á, Csányi V. 2004. Dogs respond appropriately to cues of humans’ attentional focus. Behav. Process. 66, 161–172. ( 10.1016/j.beproc.2004.01.012) [DOI] [PubMed] [Google Scholar]
- 28.Topál J, Gergely G, Erdőhegyi Á, Csibra G, Miklósi Á. 2009. Differential sensitivity to human communication in dogs, wolves, and human infants. Science 325, 1269–1272. ( 10.1126/science.1176960) [DOI] [PubMed] [Google Scholar]
- 29.Szetei V, Miklósi Á, Topál J, Csányi V. 2003. When dogs seem to lose their nose: an investigation on the use of visual and olfactory cues in communicative context between dog and owner. Appl. Anim. Behav. Sci. 83, 141–152. ( 10.1016/S0168-1591(03)00114-X) [DOI] [Google Scholar]
- 30.Udell MAR, Wynne CDL. 2010. Ontogeny and phylogeny: both are essential to human-sensitive behaviour in the genus Canis. Anim. Behav. 79, e9–e14. ( 10.1016/j.anbehav.2009.11.033) [DOI] [Google Scholar]
- 31.Miklosi A, Topal J. 2013. What does it take to become ‘best friends’? Evolutionary changes in canine social competence. Trends Cogn. Sci. 17, 287–294. ( 10.1016/j.tics.2013.04.005) [DOI] [PubMed] [Google Scholar]
- 32.Miklosi A, Topal J. 2011. On the hunt for the gene of perspective taking: pitfalls in methodology. Learn. Behav. 39, 310–313. ( 10.3758/s13420-011-0038-2) [DOI] [PubMed] [Google Scholar]
- 33.Rutter M, Kreppner J, Croft C, Murin M, Colvert E, Beckett C, Castle J, Sonuga-Barke E. 2007. Early adolescent outcomes of institutionally deprived and non-deprived adoptees. III. Quasi-autism. J. Child Psychol. Psychiatry 48, 1200–1207. ( 10.1111/j.1469-7610.2007.01792.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in these analyses are available in the Dryad repository at: doi:10.5061/dryad.c241p.


