Abstract
Developmental stressors often have long-term fitness consequences, but linking offspring traits to fitness prospects has remained a challenge. Telomere length predicts mortality in adult birds, and may provide a link between developmental conditions and fitness prospects. Here, we examine the effects of manipulated brood size on growth, telomere dynamics and post-fledging survival in free-living jackdaws. Nestlings in enlarged broods achieved lower mass and lost 21% more telomere repeats relative to nestlings in reduced broods, showing that developmental stress accelerates telomere shortening. Adult telomere length was positively correlated with their telomere length as nestling (r = 0.83). Thus, an advantage of long telomeres in nestlings is carried through to adulthood. Nestling telomere shortening predicted post-fledging survival and recruitment independent of manipulation and fledgling mass. This effect was strong, with a threefold difference in recruitment probability over the telomere shortening range. By contrast, absolute telomere length was neither affected by brood size manipulation nor related to survival. We conclude that telomere loss, but not absolute telomere length, links developmental conditions to subsequent survival and suggest that telomere shortening may provide a key to unravelling the physiological causes of developmental effects on fitness.
Keywords: jackdaw, ageing, growth, biomarker
1. Introduction
Poor nutritional conditions during development impair subsequent fitness prospects in many species [1], including humans [2]. Stress research usually takes place in the laboratory, but fitness can only be measured in the wild, where some level of developmental stress is likely to be the rule rather than the exception. Little is known about the physiological mechanisms mediating effects of developmental conditions on fitness prospects and life histories. Here, we investigate whether telomere dynamics link developmental conditions to subsequent fitness prospects in free-living jackdaws, Corvus monedula.
Telomeres are DNA–protein complexes that protect the chromosome ends from fusing, but, when in shortened state, induce apoptosis or replicative senescence ([3] and references therein). In the absence of telomerase, telomeres shorten with each cell division due to incomplete end-replication [4], erosion of the single-strand overhang [5] and oxidative damage [6]. Accordingly, telomere length has been shown to shorten with age, and reflects remaining lifespan across species in nematodes [7], birds [8–13] and humans [14]. Furthermore, in captive zebra finches early-life telomere length predicted subsequent lifespan [11], but in free-living barn swallows no such relationship was found [15]. Moreover, the rate at which telomeres shorten is linked to various stressors experienced during life [16,17] such as, for example, smoking or rearing a chronically ill child [18], suggesting that environmental conditions and associated physiological stress may be reflected in telomere dynamics.
Telomeres shorten at much higher rate during development compared with adult life [10,19], which has been attributed to the rapid cell proliferation that accompanies growth [20–22]. Prenatal environmental conditions have been shown to be correlated with telomere length at young adulthood in humans [23,24]. Furthermore, poor growth is associated with increased oxidative stress and accelerated telomere shortening in wild birds [15,25,26]. However, these relationships are correlational and whether they are caused by the focal environmental conditions or an unidentified confound cannot be determined. A manipulation of environmental conditions largely solves the problem of unknown confounding variables. We are aware of only two experimental studies of rearing environment and telomere dynamics, and in these studies the manipulation had no effect on nestling telomere shortening rate [27,28]. Thus, whether early-life telomere dynamics links developmental conditions to subsequent fitness prospects remains an unresolved issue.
We manipulated the developmental conditions of C. monedula nestlings by modification of natal brood size, to experimentally study the effects of rearing environment on growth and telomere dynamics. We measured telomere length at the ages of 5 and 30 days, and at the adult age in recruits (nestlings that returned as breeding birds). Thus, we were able to investigate the effects of manipulated brood size on both telomere length and shortening rate, and relate these to survival until adulthood. We also investigated whether individuals with long telomeres as nestlings also had long telomeres as adults. This is of interest because we have previously shown that adult telomere length is a predictor of survival in our study species [10], and thus it is possible that nestlings with long telomeres carry this advantage through to adulthood.
2. Material and methods
(a). Study system
We studied jackdaw life histories in seven different nest-box colonies in the vicinity of Groningen, The Netherlands (53.1708° N, 6.6064° E) in the period 2005–2013. Regular nest checks were performed to determine laying date, clutch size and hatch date as previously described [29]. Nestling blood samples were collected (±50 μl from the inner metatarsal vein at day 5 and brachial vein at day 30) for telomere measurement between 2005 and 2010 at ages 5 and 30 days old. Nestlings were ringed when 30 days old with a combination of colour bands and a metal numbered ring that was unique for each individual bird, enabling follow-up measurements of survival through observation (until 2013) without the necessity of recapture.
We manipulated brood size by net +2 or −2 nestlings as follows: three nestlings were moved to the brood that was enlarged, and one of the original nestlings from this enlarged brood was relocated to the matched reduced brood. We chose either to reduce or enlarge all broods, and thus have no unmanipulated broods, to increase statistical power with respect to the manipulation effects. Pairs of broods were matched by hatch date and the manipulation was carried out when the oldest nestling was 5 days old (day of hatching was day 1). Translocated nestlings were randomly chosen using random numbers generated by a computer or smartphone. When a brood that was to be reduced contained only two nestlings, we reduced brood size with one nestling. As a consequence, some broods were enlarged with only one nestling in cases where the matched reduced brood contained only two nestlings (mean brood sizes ± s.e. after manipulation were 2.66 ± 0.06 and 4.59 ± 0.14 for reduced and enlarged broods, respectively). Date that the first egg of a clutch was laid, clutch size, brood size, nestling mass and day 5 telomere length did not differ significantly between manipulation groups before the experimental exchange of the nestlings (electronic supplementary material, table S1).
Our telomere assays are labour intensive, and we therefore made a selection of the samples to be analysed that included all nestlings from manipulation dyads from which at least one fledgling survived beyond 1 March of the next year. This yields higher statistical power than randomly selecting nestlings, because it provides a better control for rearing and genetic background. Recruitment rate will by definition be higher in our sample than in the population at large, but the estimated effects of nestling traits on recruitment will not be biased. In total, 54 broods were selected (26 reduced and 28 enlarged), with 50 and 107 fledglings from reduced and enlarged broods, respectively. With respect to the survival analysis, we omitted offspring from two colonies (n = 3 broods) because survival was not recorded in these very small colonies (we removed the nest-boxes because of low occupation). Hence for the survival analysis, the sample size was 152 experimental fledglings from 51 broods.
Some individuals—those that fledged in 2010 in particular (the last year for which we measured telomere length)—may yet recruit while being falsely coded as ‘not returned’ in this analysis, causing bias in the estimate of survival and recruitment. However, based on the observed distribution of age at recruitment of earlier years, the expected frequency of such cases is less than 5% and therefore unlikely to bias our analysis. Furthermore, including the year 2010 as additional factor in the analysis did not change the model fit (data not shown), suggesting that the potential problem of censored cases biasing our analysis is negligible.
(b). Telomere length assay
Telomere length was determined in erythrocytes using pulsed-field gel electrophoresis as previously described [10]. In short, DNA was extracted from erythrocyte nuclei using the CHEF Genomic DNA Plug kit (Bio-Rad, Hercules, CA). DNA was digested overnight with proteinase K at 50°C. About half of the digested DNA was simultaneously digested with Hind III (60U), Hinf I (30U) and Msp I (60U) for 18 h at 37°C in NEB2 buffer. Digested DNA from each sample was separated by pulsed-field gel electrophoresis at 14°C for 24 h (3 V cm−1, initial switch time 0.5 s, final switch time 7.0 s). Dried gels (Bio-Rad model 538) were hybridized overnight using a 32P-end-labelled oligo (5′-CCCTAA-3′)4 that binds to the 3′ end-cap telomere overhang. Note that because of the latter no oligos bind to the interstitial telomeric repeats, because these are double stranded, and hence interstitial telomeric repeats are not included in the signal (this contrasts with telomere measurements using e.g. qPCR, which technique does not distinguish between different telomere types). Subsequently, the radioactive signal was determined (Cyclone Storage Phosphor System, PerkinElmer), resulting in a gel picture with a distribution of grey values (smear) reflecting the distribution of telomere lengths in a sample. Individual telomere length size distributions were quantified through densitometry using ImageJ v. 1.38x as previously described [10]. In short, this method determines the lower and upper boundaries of the measurement window over which telomere length is determined, obtaining a telomere length distribution per lane/sample. For each lane, the lower boundary was taken to be the point where the signal was lowest in the short-end telomere range (i.e. the background intensity). The upper boundary was set to be at the point where the signal first dropped below Y, where Y is the sum of the background intensity at the side representing long telomeres, plus 10 per cent of the difference between the peak intensity and the background intensity [10]. We used the mean values of the individual telomere length size distributions for further analyses. The coefficient of variation calculated over the day 5 and 30 samples per individual, which estimate provides an upper limit to the coefficient of variation due to measurement error since it includes the variable telomere shortening in this period, was 2.9% on average.
(c). Statistical analyses
We analysed the effects of the brood size manipulation (reduced versus enlarged) on nestling growth and telomere length using mixed-effects models. We included birth nest as random term to account for the dependence of nestlings that shared the same genetic ancestry and pre-manipulation environment. Rearing nest was not included as a random term in the models, due to limited degrees of freedom, but this term also had a negligible effect on the estimated fixed effects. Instead of selecting the best model from a large set of possible models, we built a specific model for each question/hypothesis. Final models were achieved by omitting non-significant terms from these specific models, similar to model selection based on the lowest Akaike information criterion (AIC). p-values were calculated using log-likelihood ratio tests.
We used logistic regression to study the effects of fledgling mass, brood size manipulation and telomere dynamics on survival and recruitment. We tested the effect of birth or rearing nest as random terms, but since most survivors originated from different broods (34 survivors out of 27 broods), birth and/or rearing nest had a negligible effect on the fixed-effects estimates and significance levels, and were therefore excluded from the model. To account for possible spatial and temporal variation in telomere length independent of the manipulation, we included birth year and colony as fixed factors. All analyses were performed in R using the lme4 and MASS packages.
3. Results
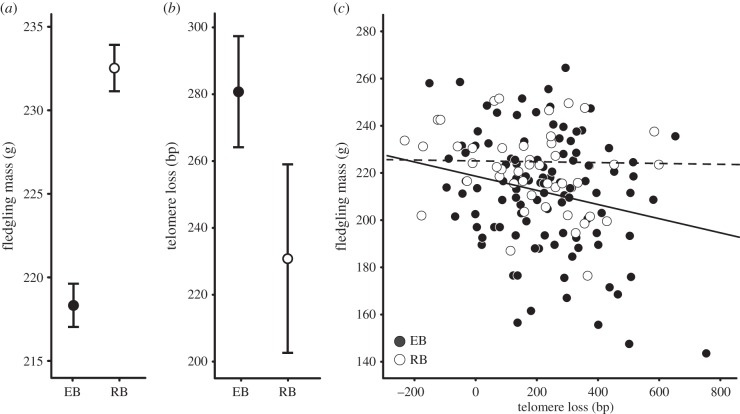
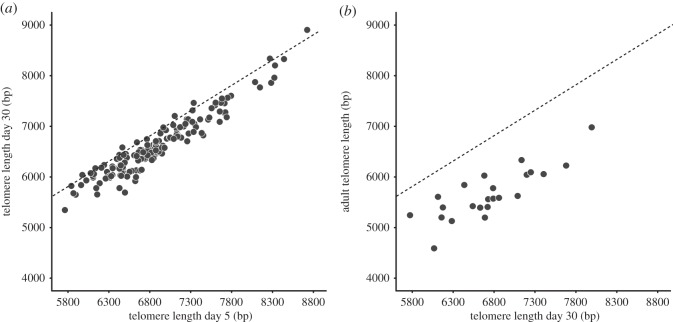
Fledgling mass was lower in birds reared in enlarged broods (table 1, model 1; figure 1a). Telomere length at ages 5 and 30 days were strongly correlated (r = 0.95; p < 0.001; figure 2a) and average telomere loss over this 25-day period was 266 bp (s.e. = 14.44). Telomere length at the end of the rearing period (age 30 days) was shorter in nestlings in enlarged broods, but this difference was not statistically significant (table 1, model 2). However, this analysis does not control for the large variation in initial telomere length (figure 2a), and when we include the initial telomere length (day 5) into the model, we find a significantly lower day 30 telomere length in enlarged broods compared with reduced broods (table 1, model 3; figure 1b). Thus, adverse rearing conditions accelerated telomere attrition.
Table 1.
Effects of brood size manipulation on fledgling mass and telomere shortening. Birth nest was included as random term in each model. Family/residual σ denotes the variance in the birth nest random term and the residual variance. The estimates of the fixed effects were calculated after rejection of non-significant terms. The covariate ‘D5 brood size’ denotes the brood size at day 5 before the manipulation. n = 157 nestlings in 54 broods. p-values were determined with log-likelihood ratio tests and significant values (less than 0.05) are shown in italic.
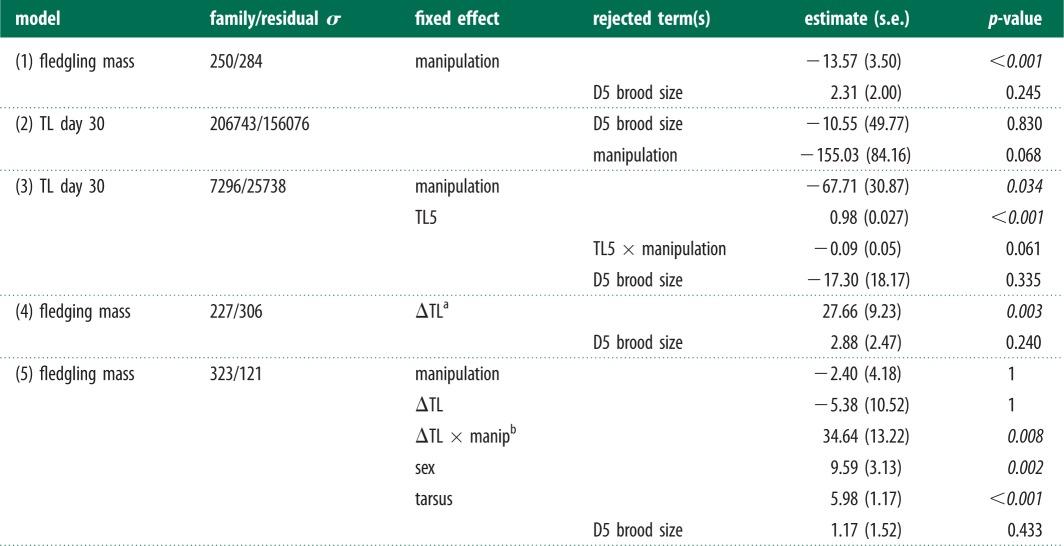 |
aΔTL is telomere length difference between day 5 minus day 30.
bThe slopes of the relation between ΔTL and fledgling mass per manipulation category were 0.008 ± 0.009 (reduced) and 0.025 ± 0.008 (enlarged) g bp−1.
Figure 1.
Fledgling mass and telomere shortening (±s.e.) in relation to brood size manipulation (EB, enlarged brood; RB, reduced brood). (a) Fledgling mass. (b) Telomere loss. (c) Telomere loss in relation to fledging mass (the solid line represents EB broods, the stroke line RB broods). See table 1 for statistical details.
Figure 2.
Correlations within individuals between telomere lengths measured at different ages. (a) Telomere length at day 30 against telomere length at day 5, and (b) telomere length in adulthood against telomere length at fledging (day 30). The dashed line describes equal values of x and y, thus the vertical distance to this line indicates the amount of telomere shortening. Note that we succeeded in sampling only 23 out of 30 recruits for telomere length.
We further examined the association between growth and telomere attrition to investigate whether telomere attrition yielded information on development over and above the information contained in fledgling mass. When pooling all broods, fledgling mass was negatively correlated with telomere attrition (table 1, model 4). We chose to use mass as dependent variable because it allows us to control for other factors affecting mass such as offspring sex. Further analysis showed that this correlation was present among nestlings in enlarged broods only (table 1, model 5, ΔTL × manip interaction term: p = 0.008; figure 1c). Thus, variation in growth was not related with telomere shortening per se, but only when experimental rearing conditions are poor.
Adult telomere length (average age at sampling ± s.e.: 2.77 ± 0.16) was highly correlated with telomere length of that same individual when it was a fledgling (r = 0.83; p < 0.001; figure 2b). This correlation opens the possibility that any advantageous effect of telomere length is carried for life.
In total, 34 out of 152 fledglings were observed to have survived beyond 1 March of the year after fledging, and 30 of these 34 birds recruited into our nest-box colonies. The effects of the manipulation and telomere dynamics on survival were indistinguishable between survivors and recruits (table 2); and here we only discuss the results on fledgling survival. Reduced and enlarged broods produced eight and 26 survivors, respectively (18% and 24% n.s.; table 2, model 2). Nestling telomere length at either day 5 or day 30 was not associated with survival (table 2, models 3 and 4), but telomere attrition between days 5 and 30 was significantly lower in survivors compared with non-survivors (table 2, model 5; figure 3). We tested for a quadratic effect of telomere shortening on survival, but this term was not significant (p = 0.72), indicating that the relationship between telomere shortening rate and survival is approximately linear (figure 4).
Table 2.
Survival and recruitment (in parentheses) rates by logistic regression. The factors ‘birth year’ and ‘rearing colony’ were included as fixed effects in each model, to account for temporal and spatial variation in survival and recruitment. Sample sizes are 34 survivors (30 recruits) from 152 fledglings. p-values less than 0.05 are shown in italic.
| model | fixed effect | estimate | s.e. | p-value |
|---|---|---|---|---|
| 1 | fledgling mass | 0.01 (0.02) | 0.01 (0.01) | 0.241 (0.087) |
| 2 | manipulation | 0.54 (0.72) | 0.48 (0.54) | 0.393 (0.179) |
| 3 | TL day 5 | –0.16 (0.006) | 0.35 (0.36) | 0.639 (0.986) |
| 4 | TL day 30 | 0.05 (0.25) | 0.33 (0.34) | 0.970 (0.515) |
| 5 | ΔTL | 2.49 (2.73) | 1.20 (1.28) | 0.037 (0.033) |
| 6 | ΔTL fledgling mass | 2.28 (2.30) 0.006 (0.01) | 1.24 (1.32) 0.01 (0.01) | 0.066 (0.080) 0.530 (0.223) |
Figure 3.
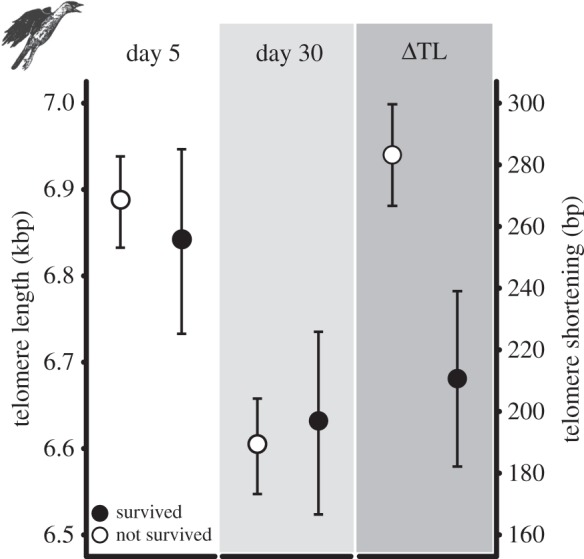
Telomere length and telomere shortening (±s.e.) in relation to survival. Panels from left to right show telomere length at ages 5 days and 30 days, and the change in telomere length in the nestling phase (day 5–day 30) for survivors and non-survivors separately. See table 2 for statistical details.
Figure 4.
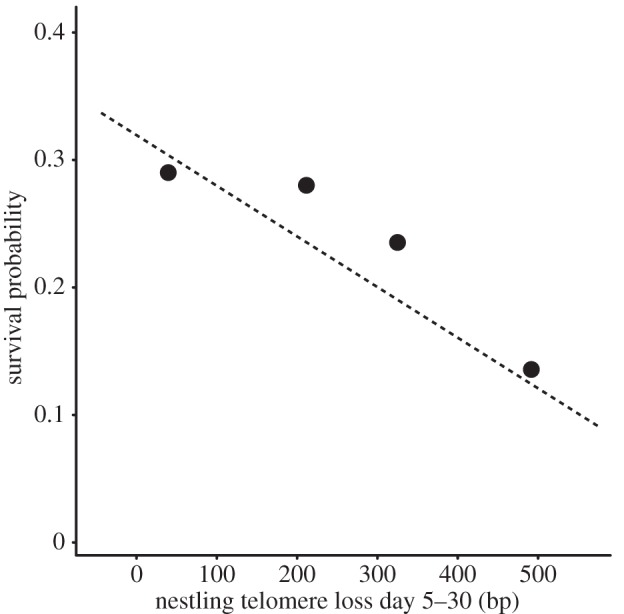
Telomere loss and survival probability. The four markers show the average survival probabilities per telomere loss quartile, which were made for graphical representation only (statistical analyses were based on individual values). The line shows the logistic regression based on model 5 in table 2.
Some fledglings may disperse outside our study area, and hence not be recorded as survivors, which would confound our analysis when the likelihood of dispersal depends on telomere attrition. To test this hypothesis, we compared telomere attrition between dispersers and non-dispersers, making use of the fact that we studied multiple colonies, and hence our sample included known dispersers (n = 5 out of 30 recruits). Telomere attrition did not differ between dispersing and philopatric fledglings (difference = −4.56 ± 85.80 bp; p = 0.99). We recognize that a larger dataset is required to detect subtle associations between telomere attrition and dispersal, but for now we conclude that a potential bias caused by dispersal is negligible.
Mean age at recruitment was 2.77 (s.e. = 0.16) years, ranged from 1 to 4 years old and did not differ between fledglings from reduced and enlarged broods. Telomere length and attrition were not significantly associated with age of recruitment, or reproductive success of recruits (results not shown), but we note that sample sizes for these tests were small (n = 23 sampled recruits).
Fledgling mass has previously been shown to be a predictor of survival and recruitment in wild birds [30], including jackdaws [31]. However, we found only a weak association between fledging mass and survival in the present dataset (table 2, model 1). Jackdaws are sexually dimorphic in size, but correcting mass for sex and size did not significantly improve the model fit. More importantly, the change in the estimate of the effect of telomere attrition on survival after including fledgling mass in the model was negligible (table 2, model 6), indicating that these effects are additive rather than that one variable can be substituted by the other.
4. Discussion
Developmental conditions can have strong effects on fitness prospects in humans and other species, but in free-living animals in particular little is known of the underlying mechanisms. We manipulated developmental conditions in jackdaw nestlings and found that adverse rearing conditions accelerate telomere shortening (figure 1). This experimental result is in agreement with observational studies that reported positive correlations between body size and telomere length [15,26]. A low rate of telomere shortening in the nestling phase resulted in high survival until adulthood (figure 3), independent of brood size manipulation and fledgling mass. The effect on survival is substantial because survival probability was threefold higher for fledglings that lost the fewest base pairs compared with fledglings at the opposite end of the telomere loss range (figure 4). Our findings together suggest that the relationship between developmental conditions and fitness prospects is linked by telomere shortening rate.
While our manipulation of developmental conditions has the advantage of generating a natural stressor (many siblings), unravelling the mechanism underlying the manipulation effects is difficult because the manipulation changes developmental conditions in multiple dimensions. For example, nestlings in larger broods are likely to receive less food, face more competition when the parents bring food and require less energy for thermoregulation, and these factors will each trigger a physiological response ([32–34] and references therein). Of interest in this context is that the association between growth and telomere shortening was apparent in enlarged broods, with high telomere loss being associated with low growth, but not in reduced broods (figure 1c). This finding mirrors a previous finding in starlings showing that nestlings which had heavier competitors lost more telomere base pairs than nestlings at the top end of the weight rank within the brood [28]. These findings suggest that low growth rate per se does not accelerate telomere attrition, but that it depends on the context whether telomeres are also affected. Individuals that grew less well presumably obtained less food, but the effect of low per capita provisioning rate may be restricted to low growth when brood size is small and competition is therefore low, while low provisioning rate in combination with high competition may cause high telomere shortening in addition to low growth.
We previously showed that long telomeres provide adult jackdaws with a survival advantage [10], as in other avian species where this was investigated [8,9,12,13,35,36]. It is of relevance therefore that fledglings with long telomeres also had long telomeres in adulthood (figure 2b), because this suggests that advantageous effects of long telomeres, in part due to benign developmental conditions, are carried for life. However, more study years are required to verify whether individuals that fledge with long telomeres do indeed enjoy higher survival rates in adulthood.
We found that telomere shortening rather than telomere length predicted survival until adulthood (figure 3), and likewise the brood size manipulation affected telomere shortening without significantly affecting fledgling telomere length. In principle, when there is an association of a factor with telomere shortening, one would also expect an association of that factor with absolute telomere length. However, absolute telomere length at a given age is the outcome of initial telomere length and subsequent attrition, and hence variation in initial telomere length induced by genetic and other parental factors may reduce the accuracy of telomere length as a biomarker later in life. In particular, genetic effects are likely to be important in this context, because reported heritabilities of telomere length are high [37–39] (but see [27]). When variation in initial telomere length is large relative to the telomere shortening, as is the case in our study (figure 2a), the association between telomere shortening and final length may be weak (in our study, R2 = 0.02 for the correlation between telomere shortening and telomere length at day 30; R2 was corrected for regression to the mean following Verhulst et al. [40]). In a similar vein, when telomere shortening rather than telomere length is the primary variable that contains information, it is not surprising for absolute telomere length to predict survival (e.g. [11]); we interpret this as an indication that telomere attrition was a relatively important source of variation in absolute telomere length when compared with the contribution of initial telomere length. We do however predict that in such a situation the telomere loss would be an even better predictor of survival.
Associations between telomere length and fitness proxies may be due to a direct (causal) effect of telomere length on, for example, survival, when animals die because their telomeres have reached a critical length. Alternatively, telomere length is a biomarker that reflects various forms of cumulative (DNA) damage and for that reason alone is a predictor of fitness proxies. Critically, short telomere lengths can have direct detrimental effects, as illustrated by the human disease dyskeratosis in which very short telomeres result in an early death [41], or telomerase-deficient mice, which have a very short lifespan only when the effect of telomerase deficiency on telomere length is accumulated over a number of generations [42]. However, telomeres in these two examples are substantially shorter than those found in the general population, which by itself suggests that observed associations between telomere length and fitness proxies that are observed in the general population do not reflect direct effects. We see our result that telomere shortening predicted survival, while absolute length did not, as support for the hypothesis that telomeres predict fitness proxies in the general population because they are a biomarker for phenotypic state, as opposed to the hypothesis that telomere length directly affects fitness. This view is further supported by the observation that the shortest telomere lengths in (old) adult jackdaws [10] were substantially longer (more than 4100 bp) than the critical limit that causes cell senescence in human cells in vitro, which is 78 bp [43]. At the same time, we are aware that we may not be able to measure telomeres that are less than a few hundred base pairs long when they occur on just one or a few chromosomes, because in our measurements many cells and chromosomes are pooled. Thus, we cannot yet rule out the possibility that individuals with high rates of telomere shortening that did not survive their first winter also had one or more telomeres of a critical length, causing their death directly.
Acknowledgements
The comments of two anonymous referees improved the manuscript.
This research project was approved by the animal experimentation committee of the University of Groningen under licence numbers 4071 and 5871.
Data accessibility
Our dataset is available online in the electronic supplementary material (dataset1).
Funding statement
H.M.S. was supported by an NWO Vici grant to S.V.
References
- 1.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 2.Lummaa V, Clutton-Brock T. 2002. Early development, survival and reproduction in humans. Trends Ecol. Evol. 17, 141–147. ( 10.1016/S0169-5347(01)02414-4) [DOI] [Google Scholar]
- 3.Armanios M, Blackburn EH. 2012. The telomere syndromes. Nat. Rev. Genet. 13, 693–704. ( 10.1038/nrg3246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olovnikov AM. 1973. A theory of marginotomy: the incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41, 181–190. ( 10.1016/0022-5193(73)90198-7) [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Ben-Porath I, Carey V, O'Connor BF, Hahn WC, Weinberg RA. 2003. Erosion of the telomeric single-strand overhang at replicative senescence. Nat. Genet. 33, 492–496. ( 10.1038/ng1127) [DOI] [PubMed] [Google Scholar]
- 6.Zglinicki von T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344. ( 10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- 7.Joeng KS, Song EJ, Lee K-J, Lee J. 2004. Long lifespan in worms with long telomeric DNA. Nat. Genet. 36, 607–611. ( 10.1038/ng1356) [DOI] [PubMed] [Google Scholar]
- 8.Haussmann MF, Winkler D, Vleck CM. 2005. Longer telomeres associated with higher survival in birds. Biol. Lett. 1, 212–214. ( 10.1098/rsbl.2005.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683. ( 10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salomons HM, Mulder E, van de Zande L, Haussmann MF, Linskens M, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelier F, Vleck CM, Holberton RL, Marra PP. 2013. Telomere length, non-breeding habitat and return rate in male American redstarts. Funct. Ecol. 27, 342–350. ( 10.1111/1365-2435.12041) [DOI] [Google Scholar]
- 13.Barrett EL, Burke TA, Hammers M, Komdeur J, Richardson DS. 2013. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol. Ecol. 22, 249–259. ( 10.1111/mec.12110) [DOI] [PubMed] [Google Scholar]
- 14.Boonekamp JJ, Simons MJP, Hemerik L, Verhulst S. 2013. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12, 330–332. ( 10.1111/acel.12050) [DOI] [PubMed] [Google Scholar]
- 15.Caprioli M, Romano M, Romano A, Rubolini D, Motta R, Folini M, Saino N. 2013. Nestling telomere length does not predict longevity, but covaries with adult body size in wild barn swallows. Biol. Lett. 9, 20130340 ( 10.1098/rsbl.2013.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monaghan PP, Haussmann MFM. 2005. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 17.Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. 2007. Telomere length predicts survival independent of genetic influences. Aging Cell 6, 769–774. ( 10.1111/j.1474-9726.2007.00340.x) [DOI] [PubMed] [Google Scholar]
- 18.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315. ( 10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauliny AA, Larsson KK, Blomqvist DD. 2011. Telomere dynamics in a long-lived bird, the barnacle goose. BMC Evol. Biol. 12, 257 ( 10.1186/1471-2148-12-257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frenck RW, Blackburn EH, Shannon KM. 1998. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl Acad. Sci. USA 95, 5607–5610. ( 10.1073/pnas.95.10.5607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rufer N, Brümmendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. 1999. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 190, 157–167. ( 10.1084/jem.190.2.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeichner SLS, Palumbo PP, Feng YY, Xiao XX, Gee DD, Sleasman JJ, Goodenow MM, Biggar RR, Dimitrov DD. 1999. Rapid telomere shortening in children. Blood 93, 2824–2830. [PubMed] [Google Scholar]
- 23.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wüst S, Wadhwa PD. 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl Acad. Sci. USA 108, E513–E518. ( 10.1073/pnas.1107759108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haussmann MFM, Longenecker ASA, Marchetto NMN, Juliano SAS, Bowden RMR. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger S, le Vaillant M, Lebard T, Reichert S, Stier A, Maho YL, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 26.Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P. 2004. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. Lond. B 271, 1571–1576. ( 10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voillemot M, Hine K, Zahn S, Criscuolo FO, Gustafsson L, Doligez B, Bize P. 2012. Effects of brood size manipulation and common origin on phenotype and telomere length in nestling collared flycatchers. BMC Ecol. 12, 1–8. ( 10.1186/1472-6785-12-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nettle D, Monaghan P, Boner W, Gillespie R, Bateson M. 2013. Bottom of the heap: having heavier competitors accelerates early-life telomere loss in the European starling, Sturnus vulgaris. PLoS ONE 8, e83617 ( 10.1371/journal.pone.0083617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomons HM, Dijkstra C, Verhulst S. 2008. Strong but variable associations between social dominance and clutch sex ratio in a colonial corvid. Behav. Ecol. 19, 417–424. ( 10.1093/beheco/arm149) [DOI] [Google Scholar]
- 30.Perrins CM. 1965. Population fluctuations and clutch-size in the great tit, Parus major L. J. Anim. Ecol. 34, 601–647. ( 10.2307/2453) [DOI] [Google Scholar]
- 31.Verhulst S, Salomons HM. 2004. Why fight? Socially dominant jackdaws, Corvus monedula, have low fitness. Anim. Behav. 68, 777–783. ( 10.1016/j.anbehav.2003.12.020) [DOI] [Google Scholar]
- 32.Naguib M, Riebel K, Marzal A, Gil D. 2004. Nestling immunocompetence and testosterone covary with brood size in a songbird. Proc. R. Soc. Lond. B 271, 833–838. ( 10.1098/rspb.2003.2673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhulst S, Holveck M-J, Riebel K. 2006. Long-term effects of manipulated natal brood size on metabolic rate in zebra finches. Biol. Lett. 2, 478–480. ( 10.1098/rsbl.2006.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 35.Bauch C, Becker PH, Verhulst S. 2014. Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol. Ecol . 23, 300–310. ( 10.1111/mec.12602) [DOI] [PubMed] [Google Scholar]
- 36.Foote CG, Daunt F, González-Solís J, Nasir L, Phillips RA, Monaghan P. 2011. Individual state and survival prospects: age, sex, and telomere length in a long-lived seabird. Behav. Ecol. 22, 156–161. ( 10.1093/beheco/arq178) [DOI] [Google Scholar]
- 37.Broer L, et al. 2013. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 21, 1163–1168. ( 10.1038/ejhg.2012.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsson M, Pauliny A, Wapstra E, Uller T, Schwartz T, Blomqvist D. 2011. Sex differences in sand lizard telomere inheritance: paternal epigenetic effects increases telomere heritability and offspring survival. PLoS ONE 6, e17473 ( 10.1371/journal.pone.0017473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn T, Robertson BC, Will M, Eason DK, Elliott GP, Gemmell NJ. 2011. Inheritance of telomere length in a bird. PLoS ONE 6, e17199 ( 10.1371/journal.pone.0017199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur. J. Epidemiol. 28, 859–866. ( 10.1007/s10654-013-9845-4) [DOI] [PubMed] [Google Scholar]
- 41.Batista LFZ, et al. 2011. Telomere shortening and loss of self-renewal in Dyskeratosis congenita induced pluripotent stem cells. Nature 474, 399–402. ( 10.1038/nature10084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choudhury AR, et al. 2006. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 39, 99–105. ( 10.1038/ng1937) [DOI] [PubMed] [Google Scholar]
- 43.Capper R, Britt-Compton B, Tankimanova M, Rowson J, Letsolo B, Man S, Haughton M, Baird DM. 2007. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 21, 2495–2508. ( 10.1101/gad.439107) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our dataset is available online in the electronic supplementary material (dataset1).