Abstract
Evolution can occur on ecological time-scales, affecting community and ecosystem processes. However, the importance of evolutionary change relative to ecological processes remains largely unknown. Here, we analyse data from a long-term experiment in which we allowed plant populations to evolve for three generations in dry or wet soils and used a reciprocal transplant to compare the ecological effect of drought and the effect of plant evolutionary responses to drought on soil microbial communities and nutrient availability. Plants that evolved under drought tended to support higher bacterial and fungal richness, and increased fungal : bacterial ratios in the soil. Overall, the magnitudes of ecological and evolutionary effects on microbial communities were similar; however, the strength and direction of these effects depended on the context in which they were measured. For example, plants that evolved in dry environments increased bacterial abundance in dry contemporary environments, but decreased bacterial abundance in wet contemporary environments. Our results suggest that interactions between recent evolutionary history and ecological context affect both the direction and magnitude of plant effects on soil microbes. Consequently, an eco-evolutionary perspective is required to fully understand plant–microbe interactions.
Keywords: bacteria, Brassica rapa, drought, fungi, microbial communities, selection
1. Introduction
Despite the common assumption that evolutionary change occurs over very long time-scales, evolution often operates on ecologically relevant time-scales [1–3]. Rapid evolution happens when genetic changes occur quickly enough to have a measurable effect on ecological processes [2]. Several studies have documented the effects of rapid evolution on the outcome of species interactions [4,5], population dynamics [6,7] and ecosystem processes [8–10]. Although reports of rapid evolution are on the rise, these studies reveal little about the relative importance of evolutionary change compared with ecological processes. If evolutionary history only explains a trivial amount of variation in communities, relative to contemporary ecological conditions, then ecologists need not concern themselves with genetic changes [4].
Recently developed methods use the Price Equation to partition ecological from evolutionary effects on ecological variables, allowing comparisons of the relative strengths of ecological and evolutionary effects [11]. Evolutionary effects on ecological variables result from genetic changes that occur in response to historical environmental conditions. Ecological effects result from differential responses to contemporary environments. The limited datasets available to use this method suggest that evolutionary effects can be as important as ecological effects. For example, phenotypic variation of Trinidadian guppies that presumably arose from genetic changes in response to different predation regimes explained 85% of changes in community respiration [10,11]. In cases where genetic changes affect ecological processes, an understanding of evolutionary history is required to accurately interpret contemporary ecological effects.
The importance of evolutionary effects may depend on ecological context, a phenomenon which can be detected in the Price Equation [11] approach as an eco-evo interaction. For example, plant defences against herbivory (e.g. secondary compounds) may affect other ecological variables, such as litter decomposition rates [12]. Inducible plant defences that evolve in response to herbivory may decrease litter decomposition rates, but only when herbivores are present and the inducible defence trait is expressed. In this hypothetical example, we would observe a strong eco-evo interaction because evolutionary effects measured in one ecological context (herbivores present) would differ from evolutionary effects measured in another ecological context (herbivores absent). Failing to account for such interactions will under- or over-estimate both ecological and evolutionary effects and result in misinterpretations of treatment effects.
Rapid evolution and eco-evo interactions may be particularly important for above-ground–below-ground interactions between plants and microbes [13]. Plants benefit from rhizosphere microorganisms in various ways, including nutrient cycling and pathogen protection, while many microorganisms rely in turn on plant-derived organic matter (litter or root exudates) as a source of carbon and energy [14]. Changes in microbial communities can alter selection on plant traits [15]. Reciprocally, soil microbial community composition can be affected not only by plant species diversity [16,17], but also by genetic variation within plant species [18,19], suggesting that microbial communities may respond rapidly to genetic changes in their plant hosts. Such changes in microbial communities could feedback to influence plant distributions, productivity and global nutrient cycles [20]. Thus, plant–microbe interactions may be some of the most generally relevant and strongest eco-evo feedbacks, but these interactions remain largely unexplored in studies quantifying eco-evo interactions ([21], but see [22,23]).
Here, we used experimental evolution, followed by a reciprocal transplant experiment, to partition the ecological effects of drought and the effects of plant evolutionary response to drought on below-ground microbial (bacteria and fungi) communities and soil nutrient availability. We focus on drought because: (i) the frequency and duration of drought is expected to increase in the future for many parts of the world (IPCC 4th Assessment, 2007), (ii) below-ground microbial communities are sensitive to soil moisture conditions [24,25], and (iii) drought is a strong selective pressure that may drive rapid evolutionary responses in plant populations [26]. We use data from a multigenerational experiment that manipulated the soil moisture environment. Previously, data from this experiment were used to examine how changes in microbial communities in response to drought affected plant fitness responses in drought [27]. Here, we use different dependent variables from the same experiment to ask the converse question: does plant evolution in response to drought affect soil microbial communities and nutrient availability? Further, we apply the Price Equation approach [11] to partition and compare relative strengths of ecological and evolutionary responses to drought stress. We found that in many cases, the effects of plant evolution on below-ground microbial communities and nutrient concentrations were similar in magnitude to contemporary drought effects, but that the strength or direction of evolutionary effects depended on the ecological context in which they were measured.
2. Material and methods
(a). Experimental evolution treatments
We conducted a ‘selection in a controlled environment’ experiment [28] in the greenhouse at the W.K. Kellogg Biological Station (MI, USA) to allow replicate populations of rapid cycling Brassica rapa (standard stock lines, Wisconsin Fast Plants Program, University of Wisconsin, Madison, WI, USA) to evolve for three generations in response to wet (high soil moisture) and dry (low soil moisture) environments. Eight mesocosms (76 l pots; n = 4 per soil moisture treatment) were filled with sterilized potting media (one part Baccto High Porosity Mix (Michigan Peat Company), one part perlite and one part vermiculate, steam sterilized at 121°C for 16 h) and inoculated with the microbial community contained in soil collected from an early successional field near the Kellogg Biological Station. We sowed 128 B. rapa seeds into each mesocosm and manipulated soil moisture conditions by either providing no additional water after germination (dry treatment) or watering each mesocosm with 500–1500 ml of water every other day (wet treatment). Flowering plants were cross-pollinated with other plants in the same mesocosm, and a random set of the seeds produced in each mesocosm was used to initiate the next generation of selection. Each generation, new mesocosms were established by combining half of the soil from the old mesocosm with an equal amount of new sterile soil media. This strategy ensured that the soil microbial community experienced the same selection history as its associated plant population but also minimized any effects of nutrient drawdown resulting from differences in plant growth. We repeated this process for three generations of selection on B. rapa (and presumably many more generations of soil microbes, which may also differentiate during this time) and then sowed randomly selected seeds from each mesocosm individually into pots in a common garden environment to reduce maternal environmental effects. Plants in the common garden generation were watered as needed to ensure that all plants survived to reproduce and then were cross-pollinated with other individuals that originated from the same mesocosm. Soil moisture conditions in the common garden generation were intermediate between the dry and wet treatments in the experimental evolution trial, and plants were grown in potting media that was not inoculated with microbial communities. Seeds produced from each individual were collected for use in the reciprocal transplant experiment. Although there is potential for one generation of selection during this common garden generation, this means that any observed effects of evolutionary history are conservative estimates.
(b). Reciprocal transplant experiment
We conducted a reciprocal transplant experiment to examine how plant evolutionary history (genetic changes in plants following three generations of selection in wet or dry environments) affected soil microbial communities and soil nutrient availability. We compared this evolutionary effect to the contemporary ecological effect of soil moisture manipulations during the reciprocal transplant experiment. We grew B. rapa plants from populations that had evolved in wet or dry environments (‘plant evolutionary history’) with microbial communities that had experienced approximately 16 months of either wet or dry environments (‘microbe history’) in either wet or dry contemporary environmental conditions (‘contemporary environment’) in a 2 × 2 × 2 factorial design (n = 8 replicates per treatment, but because carbon, nitrogen and microbial communities were sampled from different subsets of these eight replicates, effectively n = 4). Pots (0.72 l) were filled with soil from the original mesocosms (eight original mesocosms with distinct microbe histories (four mesocosms had experienced approx. 16 months of wet environments; four mesocosms had experienced approx. 16 months of dry environments)). We planted four B. rapa seeds from the same evolutionary history treatment (wet or dry environments) into each pot, using one randomly selected seed from each of the four replicate mesocosms within that evolutionary history. Half of the pots from each plant evolutionary history × microbial history combination were kept consistently moist (wet contemporary environment), while the remaining pots were watered only when plants began to show signs of drought stress (dry contemporary environment). This strategy resulted in different percentage water contents between the two treatments (dry = 27% (±2.3); wet = 71% (±1.1), mean (±s.e.)). Following plant senescence, we sampled soil from approximately half of the replicates on the same day (n = 3 or 4 randomly selected pots in each treatment combination) and used qPCR to estimate fungal and bacterial abundance and DNA fingerprinting (T-RFLP) to estimate the richness and composition of microbial communities (detailed methods described in [27]). We also quantified available nitrate (NO3–) and ammonium (NH4+) with KCl extractions followed by analysis on a Flow Solution IV analyser (OI Analytical) and the percentage of carbon (C) and nitrogen (N) in the soil, and the C : N ratio with a Costech Model 4010 Elemental Combustion analyser (Costech Analytical Technologies Inc.).
(c). Data analysis
We tested for ecological effects (differences between contemporary soil moisture environments), evolutionary effects (differences between pots sown with plants from different evolutionary histories of drought) and microbial history effects (microbes from different drought histories) on three sets of dependent variables with MANOVA (performed using proc glm in SAS v. 9.2). The first MANOVA examined treatment effects on microbial variables (fungal and bacterial richness and abundance and fungal : bacterial ratio). A second MANOVA examined treatment effects on inorganic nitrogen (NO3− and NH4+) concentrations. A third MANOVA examined effects on %C, %N and C : N ratio (we analysed these last three variables separately from inorganic nitrogen concentrations because they were measured on a different subset of pots). In each analysis, we removed non-significant factors and interactions (p > 0.25; [29]) from the statistical model, but retained two-way interactions when the three-way interaction was significant. In the event of significant interactions, we used additional MANOVAs to estimate the effect of a factor at each level of the interacting factor.
These MANOVA analyses test whether or not ecological and evolutionary effects are significantly different from zero; however, we were primarily interested in estimating the magnitude of ecological and evolutionary effect sizes relative to each other. Therefore, we used equation 12 in [11] to estimate ecological and evolutionary effects on microbial community variables (fungal : bacterial ratio, fungal and bacterial abundance and richness, and fungal and bacterial species composition (previously calculated using the first principle axis score from PERMANOVA conducted on bacterial and fungal abundances [27])) and soil nutrient variables (NO3−, NH4+, %C, %N and C : N ratio). For microbial variables, we pooled replicates across microbial histories, as all interactions with microbial history had no significant effect in the MANOVA (p > 0.34). We did not pool replicates for abiotic soil variables, but rather partitioned ecological and evolutionary effects separately for each microbial history because microbial history was significant for MANOVA models including these variables.
We estimated the ecological effect size of contemporary soil moisture and the effect size of plant evolutionary history, and interactions between these. We estimated the ecological effect of drought on each microbial and soil variable (X) separately in each evolutionary environment (plants from dry versus wet evolutionary histories), where Xij represents the mean of variable X in the ith plant evolutionary history treatment growing in the jth contemporary soil moisture treatment. For pots with plants that evolved in dry environments, we estimated the ecological effect of drought as the difference between dry and wet contemporary soil environments:
| 2.1a |
For pots with plants that evolved in wet environments, we also estimated the ecological effect of drought as the difference between dry and wet contemporary soil environments:
| 2.1b |
Similarly, we estimated the effect of plant evolutionary history on each microbial and soil variable separately for each contemporary soil moisture environment. For pots from dry contemporary environments, we estimated the evolutionary effect as the difference between pots with plants from dry and wet evolutionary histories:
| 2.2a |
For pots from wet contemporary environments, we estimated the evolutionary effect similarly:
| 2.2b |
As an overall ecological effect of drought, we also estimated the difference between wet and dry environments, averaged across both plant evolutionary histories:
 |
2.3 |
Similarly, we estimated the effect of plant evolutionary history as the difference between pots with plants from wet and dry evolutionary histories, averaged across both contemporary soil moisture environments:
 |
2.4 |
Further, we estimated interactions between plant evolutionary history and contemporary soil moisture, based on eqn 11 in [11]. The interactive effect estimates the extent to which an evolutionary effect in one contemporary soil moisture environment differs from an evolutionary effect in the other contemporary soil moisture environment:
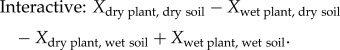 |
2.5 |
We determined standard deviations of effect sizes from the distribution of 10 000 bootstrapped estimates. Positive ecological effect sizes indicate that contemporary drought treatments increased the magnitude of a given variable. Positive evolutionary effect sizes indicate that plant populations which had evolved under drought conditions increased the magnitude of the variable compared with plant populations which had evolved in wet soil moisture environments. Positive interactive effects indicate that the observed variable is greater than expected from the additive effect of ecological and evolutionary effects. For example, if the ecological effect of drought increases a microbial variable by 100 units, and plant evolution in dry environments increases that microbial variable by 50 units, then an observed effect greater than 150 units in dry contemporary soils with plants from a dry evolutionary history would yield a positive interactive effect.
3. Results
On average, the magnitude of ecological effects (effects of contemporary drought environment) and evolutionary effects (effects of plant evolutionary responses to drought) were similar (figure 1); however, the relative contributions of each of these factors (i.e. ecological and evolutionary) were dependent on the particular dependent variable. Moreover, strong interactions between ecological and evolutionary effects were also detected, indicating that the effects of plant evolutionary history depended on the contemporary soil moisture environment, and reciprocally, that the effects of contemporary drought depended on the evolutionary history of the plants (figure 1).
Figure 1.
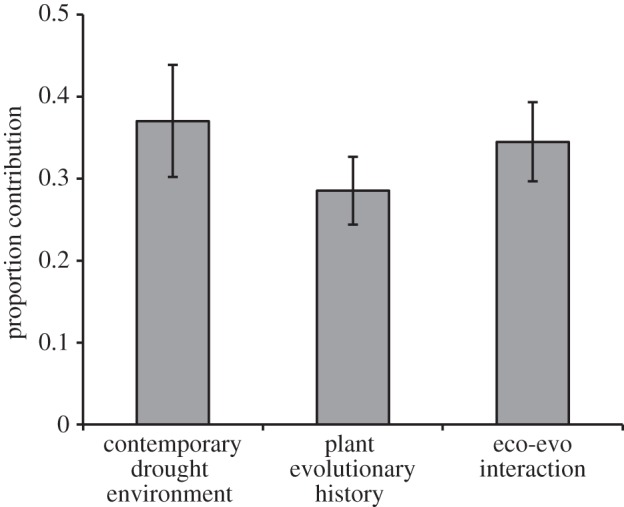
Mean contributions of contemporary drought environment (ecological effect), plant evolutionary history (evolutionary effect) and their interaction to all microbial and nutrient variables measured. Bars represent means (±s.e.) of the absolute values of each effect size.
When averaged across both plant evolutionary histories, contemporary drought treatments had no significant ecological effect on the microbial community (table 1), but tended to support higher bacterial richness, lower fungal abundance, increased F : B ratios and different fungal community composition (higher scores on the first PCoA axis) (figure 2a and the electronic supplementary material, table S1). When averaged across both contemporary soil moisture treatments, plants that evolved in dry environments had no significant evolutionary effect on the microbial community (table 1), but tended to support increased bacterial and fungal richness, increased bacterial abundance, lower fungal abundance, increased F : B ratios and different fungal and bacterial community compositions (figure 2a).
Table 1.
MANOVA results testing the effects of contemporary drought environment, plant evolutionary history and microbe history on (a) fungal and bacterial abundance and richness and fungal : bacterial ratio, (b) nitrate and ammonium concentrations and (c) per cent carbon, per cent nitrogen and carbon : nitrogen ratio. (Non-significant interactions (p > 0.25) were removed from the model; in (c), the model retained significant two-way interactions, because the three-way interaction was part of the model.)
| effect | (a) microbial community |
(b) nitrogen availability |
(c) %C, %N, C : N |
|||
|---|---|---|---|---|---|---|
| F1,26 | p | F1,25 | p | F1,22 | p | |
| contemporary drought | <0.000 | 0.987 | 90.0 | <0.001 | 1.15 | 0.296 |
| plant evolutionary history | 1.47 | 0.236 | 2.84 | 0.105 | 3.59 | 0.071 |
| microbe history | — | — | 11.1 | 0.003 | 3.22 | 0.086 |
| contemporary × evolution | 4.25 | 0.049 | — | — | 0.817 | 0.376 |
| contemporary × microbe | — | — | — | — | 0.488 | 0.492 |
| evolution × microbe | — | — | — | — | 0.071 | 0.793 |
| contemporary × evo × microbe | — | — | — | — | 2.09 | 0.162 |
Figure 2.
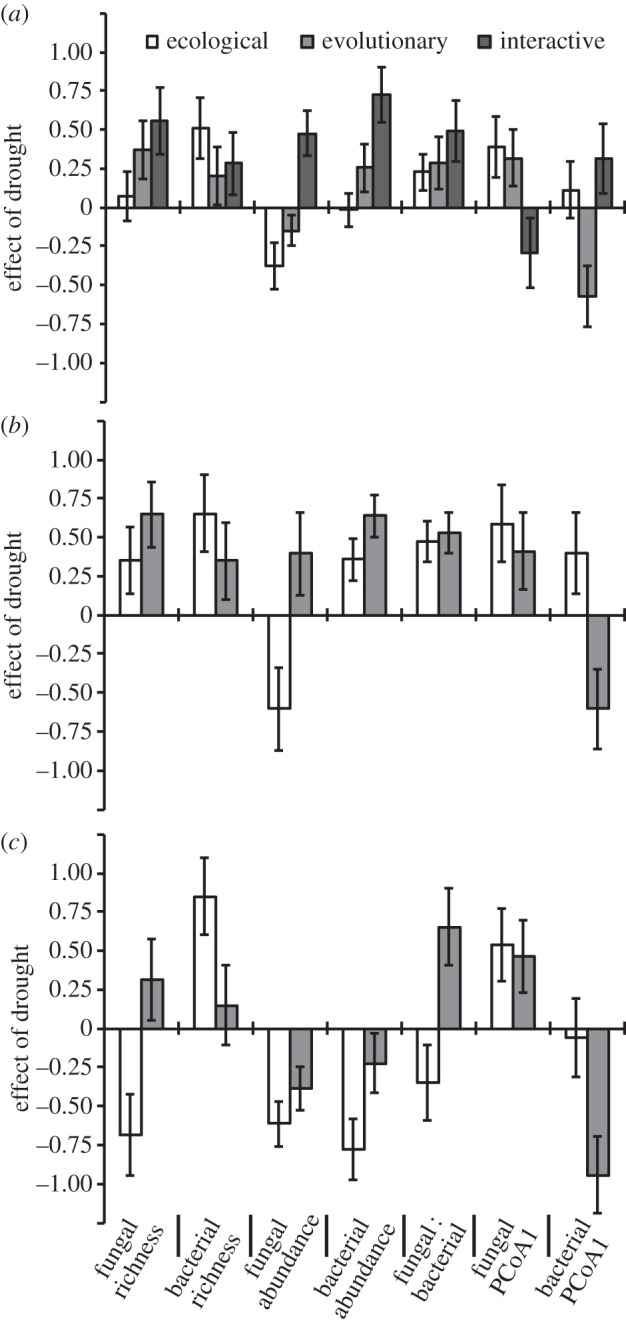
The proportional contribution of contemporary drought (ecological), and plant drought history (evolutionary) and their interactive effects on soil microbial communities. Positive contributions indicate that drought increased the magnitude of the variable (e.g. fungal richness was higher when grown with plants that evolved in dry environments). In (a), ecological and evolutionary effects averaged across environments and the strength of their interaction. In (b), ecological effects were estimated using only plants from dry evolutionary histories; evolutionary effects were estimated only in dry contemporary environments. In (c), ecological effects were estimated using only plants from wet evolutionary histories; evolutionary effects were estimated only in wet contemporary environments. Error bars represent the standard deviation of 10 000 bootstrap estimates.
However, a significant interaction between plant evolutionary history and contemporary soil moisture on microbial communities (i.e. eco-evo interaction; table 1) obscured the main ecological and evolutionary effects. Averaged ecological and evolutionary effects were misleading because the relative strength of these effects on microbial communities depended on the context in which they were measured; evolutionary effects were dependent on ecological context, and ecological effects were dependent on evolutionary context (figure 2b versus 2c; table 1; p = 0.049 for the plant evolutionary history × contemporary soil moisture interaction). In fact, the magnitudes of the interactions between ecological and evolutionary processes were often similar to or larger than the main effects of ecological or evolutionary processes in explaining the dynamics of soil microbial communities (figure 2a). For example, in contemporary dry environments, plant evolutionary history had strong effects on fungal and bacterial richness and abundance and tended to alter microbial community composition (figure 2b; MANOVA: F1,12 = 5.5, p = 0.04). Yet, in contemporary wet environments, the evolutionary effects of plant history were weak or opposite in direction (MANOVA: F1,14 = 0.36, p = 0.56) and tended to reduce fungal and bacterial abundances (figure 2c).
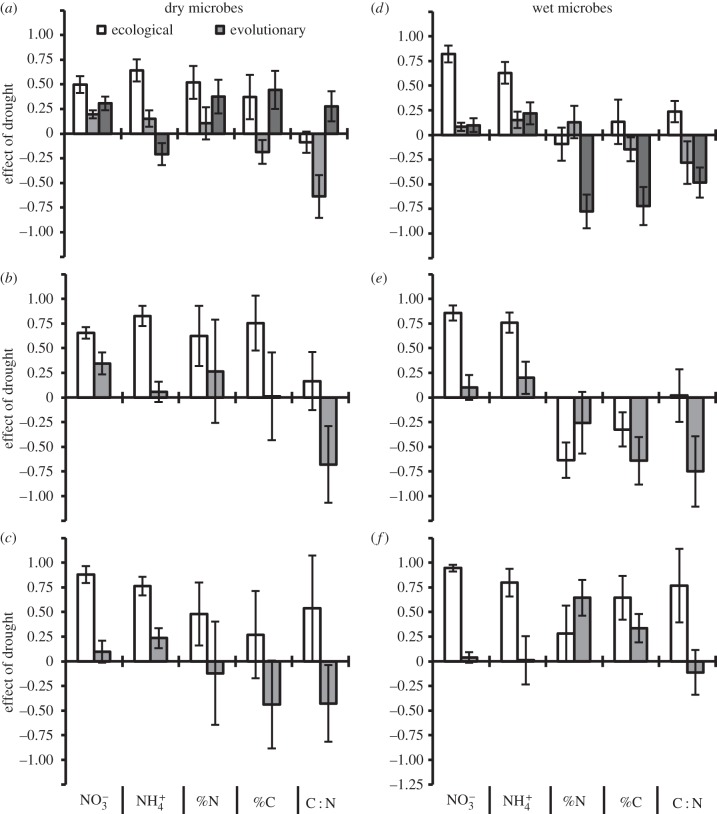
Concentrations of soil nutrients were primarily driven by the contemporary soil moisture environment—nitrogen concentrations were higher in dry soils (table 1 and figure 3a,d). Plants that evolved in dry soil environments also tended to increase nitrogen concentration, but this evolutionary effect was considerably weaker than the ecological effect of soil moisture (figure 3) and the evolutionary effect in the MANOVA was only marginally significant (p = 0.1; table 1). Plants that evolved in dry environments also had a marginally significant effect in reducing the carbon content and the C : N ratio of soils (p = 0.07; table 1). This effect was stronger than the ecological effect of soil moisture when measured in dry environments (figure 2b,e), but not when measured in wet environments (figure 2c,f), although this interaction was not statistically significant in the MANOVA (table 1).
Figure 3.
The proportional contribution of contemporary drought (ecological), and plant drought history (evolutionary) and their interactive effects on soil nutrients. Positive contributions indicate that drought increased the variable (e.g. NO3− concentrations were higher in dry environments). (a–c) Effects with microbes from dry environments. (d–f) Effects with microbes from wet environments. In (a,d), ecological and evolutionary effects averaged across environments and the strength of their interaction. In (b,e), ecological effects were estimated using only plants from dry evolutionary histories; evolutionary effects were estimated only in dry contemporary environments. In (c,f), ecological effects were estimated using only plants from wet evolutionary histories; evolutionary effects were estimated only in wet contemporary environments. Error bars represent the standard deviations of 10 000 bootstrap estimates.
Surprisingly, microbe history did not significantly affect the microbial community (table 1). This result contrasts with previously published work on this system that showed effects of microbial history on bacterial richness and community composition [27]. These contrasting results probably result from the decreased power associated with the MANOVA approach used here. Microbes from historical drought treatments significantly decreased nitrogen availability and tended to increase C and N concentrations (table 1).
4. Discussion
In the past decade, evolutionary ecology studies have documented many cases of evolution occurring on ecological time-scales [1–3], while community genetics research has demonstrated that the genetic identity of a focal species can affect associated community members and ecosystem processes [18,30,31]. Here, we integrate concepts from these two fields and show that rapid evolutionary responses to a novel stress associated with climate change have community-wide consequences for associated species. Specifically, plant evolutionary responses to drought affected the structure of below-ground microbial communities, setting the stage for eco-evo feedbacks between plants and soil microbes. After only three generations of selection, plants that evolved in dry environments affected the abundance and diversity of bacterial and fungal communities in the soil. Importantly, the magnitudes and direction of these evolutionary effects were largely dependent on the ecological context in which they were measured.
Although recent studies have demonstrated that evolution can contribute to the outcome of ecological processes, few have quantified the importance of evolutionary processes relative to ecological processes [11]. We compared the relative importance of an ecological factor (soil moisture) and an evolutionary factor (plant evolution in response to soil moisture) on soil microbial communities and soil nutrient availability (figure 1). Our results suggest that rapid evolutionary responses of plant populations to an abiotic stressor are as important as the direct ecological effects of that stressor for below-ground microbial communities, even exceeding the importance of ecological processes in some cases (figure 2). These results are consistent with the few previous studies that have estimated ecological and evolutionary effect sizes, which also found that evolutionary effects can be as important as ecological effects in explaining an ecological variable [2,11]. The strength of these evolutionary effects suggests that ecologists should consider the evolutionary history of their focal species because strong evolutionary effects may either mask or enhance effects measured in ecological experiments [4,5,32].
We also found strong interactions between ecological and evolutionary effects (figure 2a). In most cases, our estimates of the strengths of ecological and evolutionary factors that were averaged across environments (figure 2a) were not representative of the strength or direction of these forces in either of the environmental contexts (figure 2b,c). For example, strong evolutionary effects in dry environments were sometimes offset by evolutionary effects in the opposite direction in wet environments (e.g. fungal and bacterial abundance, figure 2). These strong interactions suggest that the effects of plant evolution on microbial communities are context-dependent. In other words, prominent evolutionary effects under one set of environmental conditions do not necessarily translate to another set of environmental conditions. Reciprocally, although microbial communities responded to the ecological effect of drought stress, the direction or extent to which they responded depended on the evolutionary history of the plants in the community. Interestingly, interactive effects were typically positive, indicating that the effects of ecological and evolutionary processes were greater than additive. In other words, the ecological effects of drought on a number of microbial community metrics (e.g. fungal richness, fungal abundance and bacteria abundance) were greater than expected when the associated plants had also evolved under drought conditions (figure 2). These results suggest that evolutionary effects exacerbate ecological effects and also indicate that the ecological effect of drought on soil microbes cannot be accurately assessed without considering the evolutionary history of the plants in those soils.
Such interactions between ecological and evolutionary processes may be common, or even necessary, to generate eco-evo feedbacks. In a study of eco-evo feedbacks, the aphid genotypes that were favoured by natural selection depended on population density, but aphid evolution affects population density [33]. Similarly, in predator–prey interactions between rotifers and algae, the evolutionary response of algae depends on the densities of both rotifers and algae in the community, but algal evolution stabilizes abundances in the community [6,34]. In both cases, although ecological factors (e.g. density) drive evolutionary responses, the evolution of traits also affects the ecological factor, resulting in an eco-evo feedback. Future research should examine whether such eco-evo interactions are common in natural communities, and whether such interactions are required to generate eco-evo feedbacks.
Plants that evolved in different soil moisture environments appear to have rapidly evolved changes in traits that affect soil microbial communities. As all plants experienced a common garden generation to minimize maternal environmental effects, the observed differences between plant histories are most likely owing to genetic changes in the populations [28]. The mechanism by which plant trait evolution affects microbial communities is not entirely clear, however. Previous studies on this system showed that selection gradients on B. rapa flowering time, specific leaf area and above-ground biomass were similar in both wet and dry soils [15], and minimal evolutionary responses of growth and phenological traits were observed in response to the three generations of selection in wet and dry soil moisture environments [27], suggesting that these traits were not responsible for the observed differences in microbial communities. However, we have not yet estimated selection on below-ground traits, such as root biomass or architecture, nitrogen uptake, or litter and exudate production, which may have greater effects on soil properties and microbial communities. It is possible that plant adaptation to dry environments results in greater below-ground biomass that provides more resources for soil microbial communities. Alternately, the evolutionary effect of B. rapa on microbial communities may not be owing to a single trait, but rather a multi-trait drought-tolerant phenotype that evolves in response to dry environmental conditions [35].
Relative to the effects of plant evolution on microbial abundance and richness, nitrogen concentrations in the soil were determined largely by the contemporary soil moisture environment. Species of fungi and bacteria that reduce nitrate and oxidize ammonium may be more susceptible to drought stress, leaving greater concentrations of available nitrogen in dry soils [36]. Alternatively, plant uptake of nitrogen may be water-limited in dry environments, leaving higher concentrations of nitrogen in dry soils. Although the magnitude of the effect of plant evolutionary history was smaller than the ecological effect of soil moisture, this evolutionary effect (figure 2) was greater than many ecologists might expect in most analyses of ecosystem function, which largely ignore the effects of evolutionary history (but see [10,37]). By contrast, we found a larger, but non-significant, effect of plant evolutionary history on soil carbon content and C : N ratios (figure 2). Plants that evolved under drought conditions were associated with reduced C : N ratios (figure 2) and thus may have experienced a relatively higher nitrogen environment. Because plants have a more difficult time accessing nitrogen resources in dry environments [38,39], such an evolutionary effect that increases nitrogen availability could facilitate plant growth in dry soils and lead to positive eco-evo feedbacks between plants and soil microbes. Alternatively, plants that evolved under drought conditions may produce less litter or delay senescence relative to plants that evolved in wet conditions and provision the soil with less carbon. This is further evidence that eco-evo dynamics between plants and soil microbes may be important not only for microbial community structure, but also for regulating microbial-mediated ecosystem processes.
Previous work demonstrated that changes in microbial communities in response to drought stress contributed to plant adaptation to novel drought environments [27]. Our results here demonstrate that plant evolution had reciprocal effects and increased microbial abundance and diversity under drought conditions. These reciprocal ecological and evolutionary effects between plants and their associated soil communities set the stage for eco-evo feedbacks and co-adaptation among plants and microbes [3,40]. Plant evolution can drive ecological changes in microbial communities (figure 2), and ecological changes in microbial communities affect plant growth, phenology and fitness [27], which may drive further evolutionary and ecological changes above-ground. Such eco-evo feedbacks may be common in plant–microbe interactions for several reasons. First, plant genotypes are known to influence microbial communities [18,19]. Second, plant–soil feedbacks can contribute to plant–plant competitive interactions and coexistence [41–43], and recent studies suggest that plant–soil feedbacks can contribute to fitness differences among plant genotypes within a species [18,44]. Finally, eco-evo dynamics are expected to occur when organisms have large effects on their environment and as a result can alter the selective environment for themselves and other species in the community (i.e. niche construction) [45]. Given that plants supply organic carbon to heterotrophic microorganisms in soils, and that microbes are responsible for nutrient cycling and decomposition, plants and microbes can be key drivers of environmental quality for both above-ground and below-ground populations. Other microbial systems have served as excellent models for the importance of rapid evolution for ecological processes (Escherichia coli [46], Daphnia [47], rotifers and algae [6,34]), but studies of eco-evo dynamics in plant–microbe interactions are historically lacking [21]. Interactions between plants and soil microbes have the potential to be among the strongest and most common eco-evo feedbacks. As our results demonstrate, rapid evolutionary changes in plants (this study) and rapid changes to below-ground communities [27] can be important to mediating drought responses of both above-ground and below-ground partners and illustrate the need for an evolutionary perspective in the study of plant–soil interactions.
Acknowledgements
We thank Sandy Erwin, Mark Hammond, Brent Lehmkuhl and Robert Snyder for greenhouse and laboratory assistance. Dylan Weese and two anonymous reviewers provided valuable feedback on an earlier version of the manuscript.
Data accessibility
This is Kellogg Biological Station publication no. 1717. Data are publicly available on Dryad, doi:10.5061/dryad.m172g.
Funding statement
Financial support was provided by the Rackham Research Endowment and Michigan AgBioResearch. This project was also supported by grants from the National Science Foundation to J.A.L. (DEB 0918963) and C.P.t. (DMS1312490), and National Research Initiative grant nos. 2008-35107-04481 and 2011-67019-30225 (both to J.T.L.) from the US Department of Agriculture National Institute of Food and Agriculture.
References
- 1.Thompson JN. 1998. Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332. ( 10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- 2.Hairston NG, Jr, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127. ( 10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 3.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 4.Strauss SY, Lau JA, Schoener TW, Tiffin P. 2008. Evolution in ecological field experiments: implications for effect size. Ecol. Lett. 11, 199–207. ( 10.1111/j.1461-0248.2007.01128.x) [DOI] [PubMed] [Google Scholar]
- 5.terHorst CP, Miller TE, Levitan DR. 2010. Evolution of prey in ecological time reduces the effect size of predators in experimental microcosms. Ecology 91, 629–636. ( 10.1890/09-1481.1) [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Jones LE, Ellner SP, Fussman GF, Hairston NG., Jr 2003. Rapid evolution drives ecological dynamics in a predator-prey system. Nature 424, 303–306. ( 10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 7.Becks L, Ellner SP, Jones LE, Hairston NG., Jr 2011. Reduction of adaptive genetic diversity radically alters eco-evolutionary community dynamics. Ecol. Lett. 13, 989–997. [DOI] [PubMed] [Google Scholar]
- 8.Lennon JT, Martiny JBH. 2008. Rapid evolution buffers ecosystem impacts of viruses in a microbial food web. Ecol. Lett. 11, 1178–1188. [DOI] [PubMed] [Google Scholar]
- 9.Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. 2009. Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458, 1167–1170. ( 10.1038/nature07974) [DOI] [PubMed] [Google Scholar]
- 10.Bassar RD, et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621. ( 10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellner SP, Geber MA, Hairston NG., Jr 2011. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603–614. ( 10.1111/j.1461-0248.2011.01616.x) [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer JA, Bailey JK, Hart SC, Wimp GM, Chapman SK, Whitham TG. 2005. The interactions of plant genotype and herbivory decelerate leaf litter decomposition and alter nutrient dynamics. Oikos 110, 133–145. ( 10.1111/j.0030-1299.2005.13650.x) [DOI] [Google Scholar]
- 13.Schweitzer JA, Juric I, van de Voorde TFJ, Clay K, van der Putten WH, Bailey JK. 2014. Are there evolutionary consequences of plant-soil feedbacks along soil gradients? Funct. Ecol. 28, 55–64. ( 10.1111/1365-2435.12201) [DOI] [Google Scholar]
- 14.Bever JD, Platt TG, Morton ER. 2012. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbio. 66, 265–283. ( 10.1146/annurev-micro-092611-150107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau JA, Lennon JT. 2011. Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection on plant trait. New Phytol. 193, 215–224. ( 10.1111/j.1469-8137.2011.03790.x) [DOI] [PubMed] [Google Scholar]
- 16.Zak DR, Holmes WE, White DC, Peacock AD, Tilman D. 2003. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84, 2042–2050. ( 10.1890/02-0433) [DOI] [Google Scholar]
- 17.Bartelt-Ryser J, Joshi J, Schmid B, Brandl H, Balser T. 2005. Soil feedbacks of plant diversity on soil microbial communities and subsequent plant growth. Perspect. Plant Ecol. 7, 27–49. ( 10.1016/j.ppees.2004.11.002) [DOI] [Google Scholar]
- 18.Schweitzer JA, Bailey JK, Fischer DG, Leroy CJ, Lonsdorf EV, Whitham TG, Hart SC. 2008. Plant–soil–microorgansism interactions: heritable relationship between plant genotype and associated soil microorganisms. Ecology 89, 773–781. ( 10.1890/07-0337.1) [DOI] [PubMed] [Google Scholar]
- 19.Stultz CM, Whitham TG, Kennedy KJ, Deckert R, Gehring CA. 2009. Genetically-based susceptibility to herbivory influences the ectomycorrhizal fungal communities of a foundation tree species. New Phtyol. 184, 657–667. ( 10.1111/j.1469-8137.2009.03016.x) [DOI] [PubMed] [Google Scholar]
- 20.Bever JD, Westover KM, Antonovics J. 1997. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J. Ecol. 85, 561–573. ( 10.2307/2960528) [DOI] [Google Scholar]
- 21.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions beteween the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640. ( 10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith DS, Schweitzer JA, Turk P, Bailey JK, Hart SC, Shuster SM, Whitham TG. 2012. Soil-mediated local adaptation alters seedling survival and performance. Plant Soil 352, 243–251. ( 10.1007/s11104-011-0992-7) [DOI] [Google Scholar]
- 23.Pregitzer CC, Bailey JK, Hart SC, Schweitzer JA. 2010. Soils as agents of selection: feedbacks between plants and soils alter seedling survival and performance. Evol. Ecol. 24, 1045–1059. ( 10.1007/s10682-010-9363-8) [DOI] [Google Scholar]
- 24.Fierer N, Schimel JP, Holden PA. 2003. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 45, 63–71. ( 10.1007/s00248-002-1007-2) [DOI] [PubMed] [Google Scholar]
- 25.Chen MM, Zhu YG, Su YH, Chen BD, Fu BJ, Marschner P. 2007. Effects of soil moisture and plant interactions on the soil microbial community structure. Eur. J. Soil Biol. 43, 31–38. ( 10.1016/j.ejsobi.2006.05.001) [DOI] [Google Scholar]
- 26.Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA 104, 1278–1282. ( 10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau JA, Lennon JT. 2012. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl Acad. Sci. USA 109, 14 058–14 062. ( 10.1073/pnas.1202319109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conner JK. 2003. Artificial selection: a powerful tool for ecologists. Ecology 84, 1650–1660. ( 10.1890/0012-9658(2003)084[1650:ASAPTF]2.0.CO;2) [DOI] [Google Scholar]
- 29.Underwood AJ. 1997. Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Whitham TG, et al. 2003. Community and ecosystem genetics: a consequence of the extended phenotype. Ecology 84, 559–573. ( 10.1890/0012-9658(2003)084[0559:CAEGAC]2.0.CO;2) [DOI] [Google Scholar]
- 31.Hersch-Green EI, Turley NE, Johnson MTJ. 2011. Community genetics: what have we accomplished and where should we be going? Phil. Trans. R. Soc. B 366, 1453–1460. ( 10.1098/rstb.2010.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connell JH. 1980. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35, 131–138. ( 10.2307/3544421) [DOI] [Google Scholar]
- 33.Turcotte MM, Reznick DN, Hare JD. 2013. Experimental test of an eco-evolutionary dynamic feedback loop between evolution and population density in the green peach aphid. Am. Nat. 181, S46–S57. ( 10.1086/668078) [DOI] [PubMed] [Google Scholar]
- 34.Becks L, Ellner SP, Jones LE, Hairston NG., Jr 2010. Reduction of adaptive genetic diversity radically alters eco-evolutionary community dynamics. Ecol. Lett. 13, 989–997. [DOI] [PubMed] [Google Scholar]
- 35.Franks SJ. 2011. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 190, 249–257. ( 10.1111/j.1469-8137.2010.03603.x) [DOI] [PubMed] [Google Scholar]
- 36.Gleeson DB, Herrmann AM, Livesley SJ, Murphy DV. 2008. Influence of water potential on nitrification and structure of nitrifying bacterial communities in semiarid soils. Appl. Soil Ecol. 40, 189–194. ( 10.1016/j.apsoil.2008.02.005) [DOI] [Google Scholar]
- 37.Walsh MR, DeLong JP, Hanley TC, Post DM. 2012. A cascade of evolutionary change alters consumer-resource dynamics and ecosystem function. Proc. R. Soc. B 279, 3184–3192. ( 10.1098/rspb.2012.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanguilig VC, Yambao EB, O'toole JC, Dedatta SK. 1987. Water stress effects on leaf elongation, leaf water potential, transpiration, and nutrient uptake of rice, maize, and soybean. Plant Soil 103, 155–168. ( 10.1007/BF02370385) [DOI] [Google Scholar]
- 39.Matzner SL, Richards JH. 1996. Sagebrush (Artemisia tridentate Nutt.) roots maintain nutrient uptake capacity under water stress. J. Exp. Bot. 47, 1045–1056. ( 10.1093/jxb/47.8.1045) [DOI] [Google Scholar]
- 40.Pelletier F, Garant D, Hendry AP. 2009. Eco-evolutionary dynamics. Phil. Trans. R. Soc. B 364, 1483–1489. ( 10.1098/rstb.2009.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bever JD. 2003. Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol. 157, 465–473. ( 10.1046/j.1469-8137.2003.00714.x) [DOI] [PubMed] [Google Scholar]
- 42.Bonanomi G, Giannino F, Mazzoleni S. 2005. Negative plant–soil feecback and species coexistence. Oikos 111, 311–321. ( 10.1111/j.0030-1299.2005.13975.x) [DOI] [Google Scholar]
- 43.Casper BB, Castelli JP. 2007. Evaluating plant–soil feedback together with competition in a serpentine grassland. Ecol. Lett. 10, 394–400. ( 10.1111/j.1461-0248.2007.01030.x) [DOI] [PubMed] [Google Scholar]
- 44.Lankau RA, Wheeler E, Bennett AE, Strauss SY. 2011. Plant–soil feedbacks contribute to an intransitive competitive network that promotes both genetic and species diversity. J. Ecol. 99, 176–185. ( 10.1111/j.1365-2745.2010.01736.x) [DOI] [Google Scholar]
- 45.Odling-Smee J, Odling-Smee FJ, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution, vol 37 Monographs in Population Biology Princeton, NJ: Princeton University Press. [Google Scholar]
- 46.Bohannan BJM, Lenski RE. 2000. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 3, 362–377. ( 10.1046/j.1461-0248.2000.00161.x) [DOI] [Google Scholar]
- 47.Duffy MA, Hall SR. 2008. Selective predation and rapid evolution can jointly dampen effects of virulent parasites on Daphnia populations. Am. Nat. 171, 499–510. ( 10.1086/528998) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is Kellogg Biological Station publication no. 1717. Data are publicly available on Dryad, doi:10.5061/dryad.m172g.