Abstract
Social behaviours such as mate choice require context-specific responses, often with evolutionary consequences. Increasing evidence indicates that the behavioural plasticity associated with mate choice involves learning. For example, poeciliids show age-dependent changes in female preference functions and express synaptic-plasticity-associated molecular markers during mate choice. Here, we test whether social cognition is necessary for female preference behaviour by blocking the central player in synaptic plasticity, NMDAR (N-methyl d-aspartate receptor), in a poeciliid fish, Xiphophorus nigrensis. After subchronic exposure to NMDAR antagonist MK-801, female preference behaviours towards males were dramatically reduced. Overall activity levels were unaffected, but there was a directional shift from ‘social’ behaviours towards neutral activity. Multivariate gene expression patterns significantly discriminated between females with normal versus disrupted plasticity processes and correlated with preference behaviours—not general activity. Furthermore, molecular patterns support a distinction between ‘preference’ (e.g. neuroserpin, neuroligin-3, NMDAR) and ‘sociality’ (isotocin and vasotocin) gene clusters, highlighting a possible conservation between NMDAR disruption and nonapeptides in modulating behaviour. Our results suggest that mate preference may involve greater social memory processing than overall sociality, and that poeciliid preference functions integrate synaptic-plasticity-oriented ‘preference’ pathways with overall sociality to invoke dynamic, context-specific responses towards favoured males and away from unfavoured males.
Keywords: synaptic plasticity, MK-801, mate choice, female preference, poeciliid, sexual selection
1. Introduction
Social behaviours require context-specific responses to other individuals. Female mate preference is a particularly intriguing social behaviour because females exhibit behavioural plasticity with profound evolutionary consequences, particularly in terms of sexual selection and speciation. While variation in female responses towards different male phenotypes can arise from numerous factors [1], increasing evidence indicates that the behavioural plasticity associated with vertebrate mate choice also involves learning [2]. For example, several poeciliids, including Xiphophorus nigrensis, show age-dependent changes in female preference functions [3–5] and express synaptic-plasticity-associated molecular markers during mate choice events [6–8].
Swordtail females, like many females in other taxa with alternative male mating strategies, generally prefer to associate with large, courting males and avoid smaller-size-class males using force-copulation mating strategies [9]. Our previous research identified dynamic expression patterns associated with preference behaviour during mate choice conditions in X. nigrensis females, including genes associated with synaptic plasticity, such as neuroserpin, neuroligin-3 and N-methyl d-aspartate receptor (NMDAR) [6,7,8,10]. Synaptic plasticity processes within the brain mediate dynamic learning and memory, and a critical component of the glutamatergic signalling underlying this plasticity is NMDAR [11]. Pharmacological disruption of NMDAR results in impaired learning, memory, social cognitive and perceptual processes [11–13]; however, NMDAR blockade has never before been used in a mate choice context.
Here, we test whether social cognition is necessary for female mate preference behaviour by exposing X. nigrensis females to a non-invasive, subchronic dose of the NMDAR antagonist MK-801, and then testing preference-associated behaviours and gene expression patterns. We compare behavioural response to NMDAR blockade under mate choice conditions to those under general social control (exposure to conspecific females) or with an asocial test of anxiety (scototaxis or light/dark preference). We predicted that disrupting the key molecular pathway for social cognition would alter female preference behaviour for appropriate male phenotypes (courters) relative to aversive male phenotypes (force-copulators). We further predicted that mate preference disruption subsequent to NMDAR blockade would have genomic consequences in behaviourally relevant gene expression modules. Therefore, we tested for disrupted brain expression profiles of genes known to have a rapid and preference-specific response in X. nigrensis females in control and MK-801 females exposed to identical male conditions. NMDAR antagonism disrupts multiple aspects of social learning and emotional processing [14–18], therefore we included genes linked to both preference behaviours (e.g. neuroserpin, neuroligin-3, NMDAR [6–8]) and general sociality (isotocin and vasotocin—fish homologues of oxytocin and vasopressin [19–21]) in fish to characterize the effects of disrupted social cognition on gene modules associated with these two critical aspects of mate choice behaviour.
2. Material and methods
(a). Behaviour and pharmacology
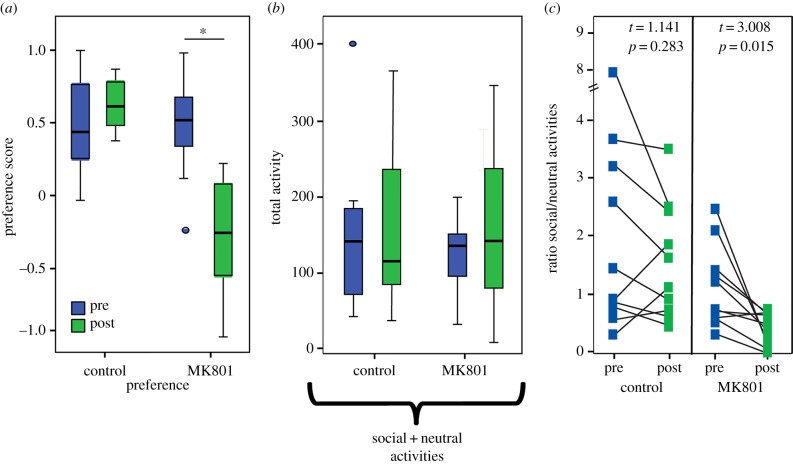
Xiphophorus nigrensis females were obtained from semi-wild populations (University of Texas Brackenridge Field Laboratories). Female behaviour was measured in non-contact dichotomous choice trials as in [6,8,10], with stimuli pairs consisting of a large and small male (LS; n = 20) or two size-matched conspecific females (FF; n = 15; see the electronic supplementary material). Male sizes ranged from 34.6 to 44.4 mm for large males and 20.7 to 25.7 mm for small males. Trials lasted 30 min, with the side of stimulus presentation switched mid-way through the trial to ensure preference measures assessed biases towards a specific stimulus rather than a particular side of the tank. We measured 11 aspects of female behaviours meant to characterize time and activity budgets incorporating horizontal and vertical movements within the tank, and whether these behaviours were oriented towards stimulus or neutral zones (table 1). Strength-of-preference measures are independent of stimulus phenotype, but we note that two control females preferred the small male in the pre- and post-tests, with one female consistently choosing the small male in both trials. For the ratio of general social to general neutral activities (figure 1c; electronic supplementary material, figure S3d and table S1), we divided the sum of general social behaviours (total glides + up-downs + laterals) by the sum of neutral behaviours (transits + circles + non-directed up-downs). All trials were scored live and videotaped. Final scoring was conducted by individuals blind to treatment. Two neutral measures (non-directed up-downs and circle swims) differed between the LS and FF behaviour experiments (non-directed up-downs p = 0.009; circles p = 4.26 × 10−6; see the electronic supplementary material); therefore, we present LS–FF comparative analyses with these two behaviours pooled as ‘other neutral swims’ in the main text. For supporting material comparative analyses, we also include versions with all behaviours.
Table 1.
Behaviour ethogram for X. nigrensis females exposed to dichotomous choice conditions. We measured 11 behaviours characterizing all commonly performed horizontal and vertical movements within the tank, and whether these behaviours were oriented towards stimulus or neutral zones. Behaviours were categorized into preference, general social or neutral. Preference behaviours are defined as time/activities directed towards one stimulus zone over another and are expressed as a bias measure between stimulus individuals. General social behaviours were defined as overall measures of time or activity directed towards both stimulus zones (glides, up-down swims and lateral swims); and general neutral activities are non-socially directed (transits, circle swims and non-directed up-down swims). Total activity is the sum of all general behaviours (three general social + three general activity behaviours).
| behaviour | category | description |
|---|---|---|
| association bias | preference | time spent in the association zone with stimulus a/(time with stimulus a + stimulus b), where time with stimulus a is greater than time with stimulus b |
| preference score | preference | association bias + log ((1 + no. glides to stimulus a)/total transits) |
| glide bias | preference | number of glides performed towards stimulus a/(no. glides with stimulus a + stimulus b) |
| up-down bias | preference | number of up-down swims performed towards stimulus a/(no. up-down swims with stimulus a + stimulus b) |
| total association | general sociality (social) | time spent in both association zones/total time of the trial |
| glide swims | general sociality (social) | circular swim wherein the female orients towards a stimulus, then swims away from him, turns and then returns to the barrier |
| up-down swims | general sociality (social) | female orients to a stimulus barrier and makes a vertical swim at least one body length in distance, then returns to the same relative location oriented towards the stimulus barrier |
| lateral swims | general sociality (social) | horizontal swims along the stimulus barrier |
| circle swims | general activity (neutral) | circular swims in the tank not directed towards a particular stimulus/barrier |
| non-directed up-down swims | general activity (neutral) | vertical swims not directed towards a particular stimulus/barrier; rarely performed in the LS experiment so not included in the main text DFA analysis (see the electronic supplementary material) |
| transits | general activity (neutral) | total swims out of either association zone into the middle of the tank |
Figure 1.
Behavioural disruptions vary across preference, general social and neutral activity measures. (a) MK-801 females (n = 10) had lower post-treatment preference than controls (n = 10), but (b) overall activity levels (sum of all three general social and three neutral behaviours; table 1) were not altered. However, the character of activity changed, with a shift from social (general approach behaviours directed towards either stimulus zone regardless of stimulus ID) towards neutral (behaviours not directed towards either stimulus zone). (c) The ratio of social to neutral behaviours ((sum of three social behaviours)/(sum of three neutral behaviours)) was lower in post-trials for MK-801 females but not controls. ‘Preference’ represents biased measures towards one stimulus over another (see table 1 for behavioural definitions). Box plots indicate first and third quartiles, the line indicates the median and whiskers indicate high and low values. Circles denote outliers. (Online version in colour.)
The experimental paradigm consisted of a 6-day procedure. On day 1, each female was pre-tested with either LS or FF stimuli. Females were then randomly assigned to treatment groups (LS: n = 10 control, n = 10 MK-801; FF: n = 7 control, n = 8 MK-801). Days 2–5 consisted of four daily sessions of non-invasive control (home tank water) or pharmacological exposure (20 μM MK-801 in home tank water). Females were placed into a beaker containing 150 ml of water or MK-801 for 1 hour and then returned to their home tanks. On day 6, each female was post-tested. LS-exposed females were euthanized via rapid decapitation immediately after the post-trial for whole-brain total RNA extraction.
MK-801 is a non-competitive antagonist to NMDAR previously used in teleosts [17,18]. We chose a short-term repeated, or subchronic, drug exposure protocol as in [18]. We measured post-treatment behaviour 24 h after final MK-801 exposure in an attempt to reduce acute drug effects on our gene and behaviour measures [22]. We adapted an immersion-based subchronic MK-801 concentration used by Swain et al. [18] that does not increase locomotion behaviour in zebrafish and is similar to other studies looking at subchronic NMDAR antagonists in rats [22–24]. To ensure MK-801 effects in the LS-exposed females were not driven by changes in serum oestradiol, we measured a proxy for circulating oestradiol via a water-borne assay previously validated for this species [25] immediately prior to behaviour testing. There were no differences in pre- versus post-treatment (control: t = 0.60, p = 0.57; MK-801: t = 1.72, p = 0.12).
As a control for generalized (non-social) anxiolytic effects of NMDAR blockade, we also tested a group of females in a supplemental scototaxis [26] assay. Fish were placed into a tank evenly divided into black and white compartments for 15 min. We measured spatial preference for the dark half plus number of entries into the white half. Pharmacological exposure paradigm for the scototaxis experiment (pre-test followed by 4 days of non-invasive pharmacological treatment and then post-test on day 6) was exactly as described for the dichotomous choice assays. See the electronic supplementary material for detailed scototaxis methods.
(b). Gene expression assay
Whole-brain tissue was dissected and placed in RNAlater (Ambion) overnight at 4°C, and stored at −80°C. Total RNA extraction, cDNA synthesis and qPCR measures have been previously described [6,8]. See the electronic supplementary material for detailed gene inclusion and expression assay parameters. Gene expression levels were normalized by deriving the residuals from a linear regression of the measured gene concentration by input cDNA concentration (as in [7,8]). Input cDNA was measured using Quanti-IT RiboGreen RNA reagent (Molecular Probes). Previous validation experiments established that RiboGreen RNA reagent measures RNA and cDNA with equal effectiveness [6], and therefore, as in [6–8], we normalize via cDNA quantification as a better reflection of actual input target into each well.
(c). Statistics
Unless otherwise noted, statistical analyses used log-transformed values for activity measures (i) because individual behaviours were not normally distributed and (ii) to keep all behavioural measurements on a similar scale for multivariate calculations. Tests using raw or log-transformed values yielded identical patterns. Groupwise comparisons were measured with t-tests followed by Benjamini–Hochberg correction [27]. Multivariate analyses used MANOVA. Discriminant function analysis (DFA) was used for data reduction and to classify response patterns. CanR2 is the correlation between DFA scores and treatment. We used Pearson correlations for input variables onto DFA axes, and glm for gene and behaviour DFA score comparison. See the electronic supplementary material for expanded statistical details including supplemental FF and scototaxis assays. All analyses were conducted using R v. 2.13.1.
3. Results
(a). N-methyl d-aspartate receptor blockade and behaviour
NMDAR blockade resulted in context-specific behavioural disruptions in females exposed to dichotomous choice conditions. Total association time trended lower in treated but not controls for both LS- and FF-exposed females (LS-MK-801: p = 0.06; control: p = 0.87; table 2; FF-MK-801: p = 0.07; control: p = 0.31; electronic supplementary material, table S4). In LS females, preference behaviours were significantly reduced following MK-801 (table 2; electronic supplementary material, figure S1), including association bias (p = 0.01), preference score (figure 1a; p = 0.01) and up-down bias (p = 0.01), while control females showed no decline (table 2). By contrast, there were no reductions in preference measures for FF-exposed MK-801 females pre- versus post-exposure (electronic supplementary material, figure S2 and table S4). For general behaviours, both LS- and FF-exposed MK-801 females showed a pattern of increased transits compared with pre-exposure levels (LS-MK-801: p = 0.06; FF-MK-801: p = 0.03), and lateral swims were reduced in LS females regardless of treatment (MK-801: p = 0.04; control: p = 0.01; table 2; electronic supplementary material, figures S1 and S2).
Table 2.
Female X. nigrensis behaviours when exposed to mate choice conditions pre- versus post-NMDAR blockade. Italicized values remained significant after FDR correction.
| behaviour | category | control pre versus post |
MK801 pre versus post |
||||
|---|---|---|---|---|---|---|---|
| t | p-value | FDR p-value | t | p-value | FDR p-value | ||
| association bias | preference | 0.36 | 0.73 | 0.87 | 4.71 | 0.001 | 0.01 |
| preference score | preference | −1.28 | 0.23 | 0.64 | 3.93 | 0.003 | 0.01 |
| glide bias | preference | −0.71 | 0.50 | 0.87 | 1.94 | 0.09 | 0.11 |
| up-down bias | preference | −0.36 | 0.73 | 0.87 | 4.23 | 0.002 | 0.01 |
| total association | social | −0.24 | 0.82 | 0.87 | 2.51 | 0.03 | 0.06 |
| glides | social | −2.92 | 0.02 | 0.09 | 0.70 | 0.50 | 0.50 |
| up-downs | social | 0.47 | 0.65 | 0.87 | 2.20 | 0.06 | 0.08 |
| laterals | social | 5.09 | 6.54 × 10−4 | 0.007 | 3.02 | 0.01 | 0.04 |
| circles | neutral | 0.19 | 0.86 | 0.87 | 2.32 | 0.05 | 0.07 |
| n.d. up-down swims | neutral | −0.17 | 0.87 | 0.87 | 1.34 | 0.21 | 0.23 |
| transits | neutral | −1.58 | 0.15 | 0.54 | −2.68 | 0.03 | 0.06 |
In LS-exposed females, total activity levels were unchanged by treatment (figure 1b), but there was a significant shift in the proportion of social to neutral behaviours in MK-801 females (figure 1c; pre versus post: MK-801: p = 0.02; control: p = 0.28; electronic supplementary material, table S1). By contrast, the ratio of social to neutral behaviours was not altered following MK-801 treatment in FF females (electronic supplementary material, figure S3) although overall activity did increase (MK-801: p = 0.02; control: p = 0.33), driven by increased neutral behaviours (MK-801: p = 0.01; control: p = 0.19).
Given the anxiolytic effects of NMDAR blockade, the generalized pattern of increased transits across the tank may be due to increased exploration. Therefore, we conducted a control scototaxis assay. Looking within each trial, swordtails spent significantly more time in the black side of the testing tank (electronic supplementary material, figure S4a,b; control pre time black versus white: p = 7.07 × 10−3; control post: p = 4.26 × 10−4; MK-801 pre: p = 1.59 × 10−4), but this was eliminated after MK-801 exposure (MK-801 post time black versus white: p = 0.72). Looking across trials, MK-801 exposure significantly reduced proportion of time in black compared with pre-treatment measures (electronic supplementary material, figure S4c; pre versus post: MK-801: p = 0.02; control: p = 0.54), and treated individuals increased entries to white (electronic supplementary material, figure S4d; pre versus post: MK-801: p = 0.02; control: p = 0.49).
(b). Multivariate measures of genes and behaviour
Blocking NMDAR altered multiple aspects of female behaviour, therefore we used multivariate approaches to directly compare post-treatment suites of behaviour and gene expression between MK-801 and control females. Post-treatment LS-exposed female behaviours differed by treatment (MANOVA 10 input behaviours, F = 11.55, p = 5.48 × 10−4; see the electronic supplementary material, table S2, for univariate comparisons). DFA on these same behaviours using treatment as the categorical-dependent variable correctly categorized females with 100% accuracy, and jack-knife resampling resulted in an overall rate of 90% correct classification (80% controls; 100% MK-801). MK-801 and control females were significantly different in multivariate space (figure 2a; F = 230.89, p = 1.04 × 10−11), and behaviour DFA scores were correlated with treatment category (CanR2 = 0.93). Preference and general sociality measures loaded positively towards controls, whereas only transits loaded towards the MK-801 females (figure 2a). All input preference measures were significantly correlated with behaviour axis scores (association bias: r = 0.84, p = 1.44 × 10−5; preference score: r = 0.86, p = 9.66 × 10−6; glide bias: r = 0.59, p = 0.01; up-down bias: r = 0.79, p = 9.93 × 10−5), as were two of four overall sociality measures (total association: r = 0.52, p = 0.03; total up-down swims: r = 0.65, p = 0.01), but not neutral activity measures (table 3). Importantly, the multivariate behavioural response was context-specific, with LS- and FF-exposed females exhibiting divergent patterns of behavioural response following treatment (figure 2b; input MANOVA: F = 3.87, p = 0.04; DFA output: F = 88.40, p = 6.43 × 10−8; see also electronic supplementary material, figure S5). See the electronic supplementary material, tables S5–S8 for supplemental and context-specific DFA input variable loading statistics.
Figure 2.
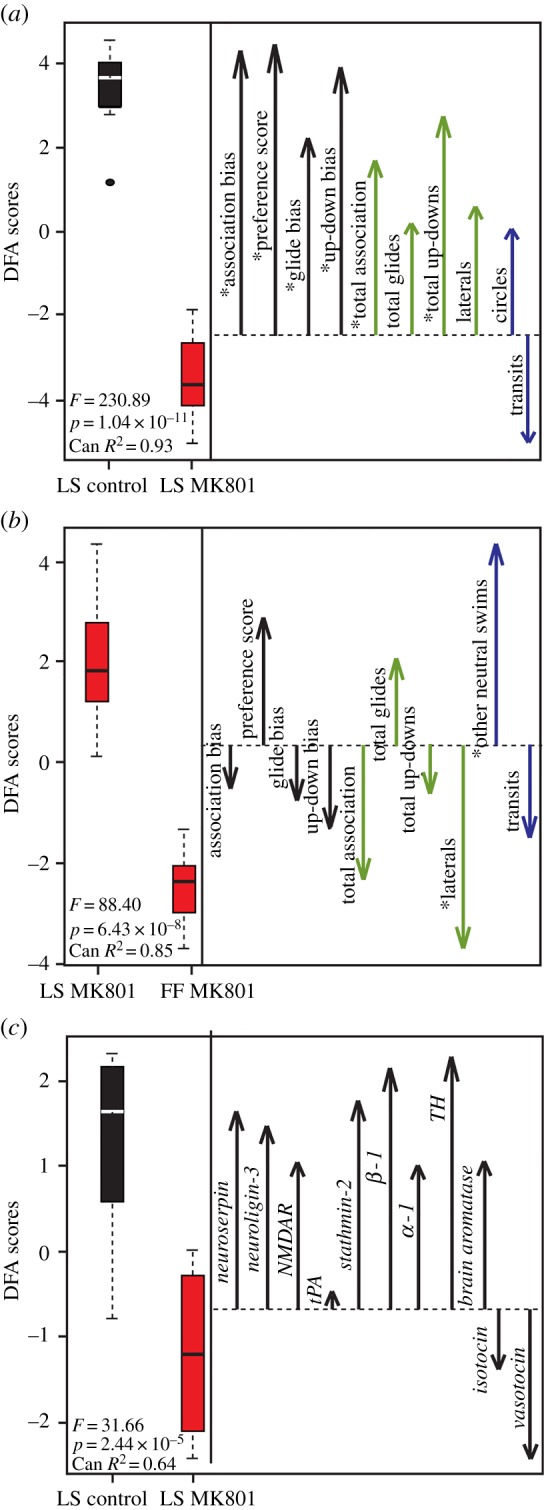
NMDAR antagonist disrupts behaviour and gene expression patterns in multivariate space. Box plots represent the DFA scores from 10 input behaviours under mate choice conditions (a; LS behaviour DFA), from MK-801-treated females by social context (b; MK-801(LS)–MK-801(FF) (pooled ‘other neutral swims’)) and from DFA on 11 input gene variables (c; LS gene DFA). Box plot parameters are described in figure 1 legend. For each DFA, vectors represent the orientation of input variables along the (a,b) behaviour or (c) gene DFA axis. Preference behaviour vectors are black (first four vectors), social behaviours are light grey (online: green; next four vectors) and neutral behaviours are dark grey (online: blue; last two vectors) and point towards the group with higher mean values. Vector length indicates the potency of each variable to discriminate between control and MK-801 females. Input behaviours marked with asterisk (*) are significantly correlated with DFA scores (table 3; electronic supplementary material, table S8). Gene nomenclature for (c): neuroserpin (serpin1), neuroligin-3 (nlgn3), NMDAR (grin1), tPA (plat), stathmin-2 (stmn2), β-1 adrenergic receptor (Adrb1), α-1 adrenergic receptor (Adra1), tyrosine hydroxylase (TH), brain aromatase (Cyp191b), vasotocin (AVT), isotocin (IT). (Online version in colour.)
Table 3.
Input variable loading onto behaviour and gene DFA axes in X. nigrensis females exposed to mate choice conditions. Italicized values remained significant after FDR correction.
| input behaviours to behaviour DFA |
input genes to gene DFA |
||||||
|---|---|---|---|---|---|---|---|
| behaviour | category | r-value | p-value | FDR p-value | r-value | p-value | |
| association bias | preference | 0.84 | 2.88 × 10−6 | 1.44 × 10−5 | neuroserpin | 0.31 | 0.19 |
| preference score | preference | 0.86 | 9.66 × 10−7 | 9.66 × 10−6 | neuroligin-3 | 0.29 | 0.22 |
| glide bias | preference | 0.59 | 0.007 | 0.01 | NMDAR | 0.23 | 0.33 |
| up-down bias | preference | 0.79 | 2.98 × 10−5 | 9.93 × 10−5 | tPA | 0.02 | 0.93 |
| total association | social | 0.52 | 0.02 | 0.03 | stathmin-2 | 0.32 | 0.17 |
| glides | social | 0.33 | 0.16 | 0.19 | β-1 adrenergic | 0.37 | 0.10 |
| up-downs | social | 0.65 | 0.002 | 0.005 | α-1 adrenergic | 0.22 | 0.35 |
| laterals | social | 0.38 | 0.10 | 0.14 | TH | 0.39 | 0.09 |
| circles | neutral | 0.31 | 0.19 | 0.19 | brain aromatase | 0.23 | 0.33 |
| transits | neutral | −0.32 | 0.17 | 0.19 | isotocin | −0.09 | 0.71 |
| vasotocin | −0.23 | 0.32 | |||||
We measured whole-brain gene expression patterns in control versus MK-801 females to compare dynamic transcriptional responses following LS exposure. When assessed individually, post-trial patterns were not altered by MK-801 (electronic supplementary material, table S3), and MANOVA on all 11 genes reflected these univariate trends (F = 1.28, p = 0.37). However, at the multivariate level, LS gene DFA significantly discriminated between control and MK-801 females (figure 2c; F = 31.66, p = 2.44 × 10−5, CanR2 = 0.64). Overall classification rate was 85% (80% correct for controls, 90% correct for MK-801 females). Jackknife resampling classification was 55% correct overall (60% controls and 50% MK-801 females). All genes previously associated with female preference and receptivity/sexual behaviours (including NMDAR) loaded towards controls, whereas isotocin and vasotocin loaded instead towards MK-801 females (figure 2c). No single gene was significantly correlated with gene axis scores (table 3).
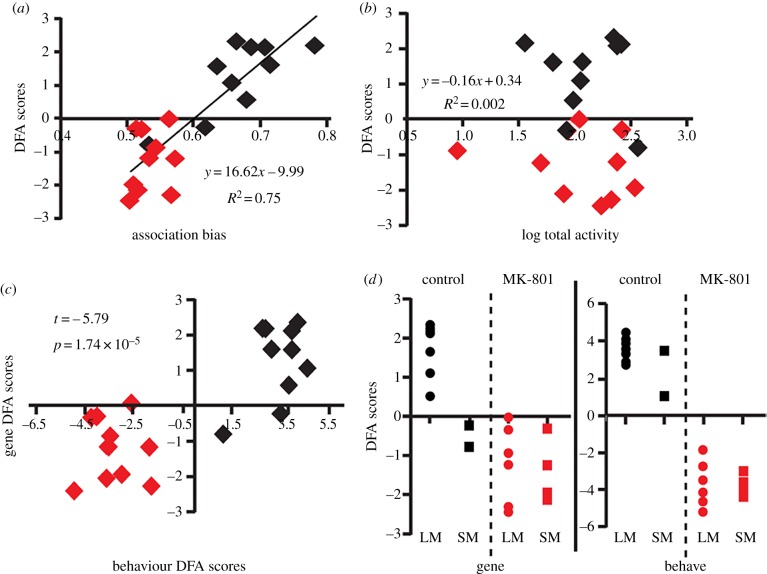
Gene DFA scores also reflected individual variation in behaviour and were correlated with three of four preference measures (table 4 and figure 3a; association bias: r = 0.86, p = 9.49 × 10−6; preference score: r = 0.69, p = 0.004; up-down bias: r = 0.58, p = 0.03). By contrast, gene DFA scores were not correlated with any general time or activity measures (table 4), including total activity (figure 3b; r = −0.04, p = 0.87). Behaviour DFA scores significantly predicted gene DFA scores (figure 3c; t = −5.79; p = 1.74 × 10−5).
Table 4.
Gene DFA score correlations with behaviours in female X. nigrensis exposed to mate choice conditions. Italicized values remained significant after FDR correction.
| behaviour | category | r | p-value | FDR p-value |
|---|---|---|---|---|
| association bias | preference | 0.86 | 9.49 × 10−7 | 9.49 × 10−6 |
| preference score | preference | 0.69 | 0.001 | 0.004 |
| glide bias | preference | 0.44 | 0.05 | 0.11 |
| up-down bias | preference | 0.58 | 0.008 | 0.03 |
| total association | social | 0.48 | 0.03 | 0.09 |
| glides | social | 0.16 | 0.50 | 0.50 |
| up-downs | social | 0.34 | 0.14 | 0.20 |
| laterals | social | 0.34 | 0.14 | 0.20 |
| circles | neutral | 0.24 | 0.32 | 0.35 |
| transits | neutral | −0.32 | 0.16 | 0.21 |
Figure 3.
Blocking NMDAR results in multivariate gene expression correlations with preference but not total activity. Gene DFA scores were correlated with (a) association bias but not (b) total activity. (c) Behaviour DFA scores significantly predict gene DFA scores. Black diamonds, controls; grey (online: red) diamonds, MK-801 females. (d) Controls preferring the small male occupy similar multivariate space to MK-801 females in the gene DFA (left y-axis), but not in the behaviour DFA (right y-axis). ‘Preferred’ status was assigned by calculating the association bias score for each stimulus class (large or small male). Circles indicate females preferring the large male and squares indicate females preferring the small male. (Online version in colour.)
4. Discussion
NMDAR is the central player underlying synaptic plasticity [11]. Pharmacological disruption of NMDAR signalling impairs multiple aspects of learning and memory [11–13], including reductions in working memory and social behaviours [23,28,29]. In this study, we tested the functional consequences of subchronic blockage of NMDAR transmission using a classic taxon in female mate choice and sexual selection, the northern swordtail fish (X. nigrensis). We observed behavioural disruptions that varied by social context, as behaviour associated with NMDAR blockade in females interacting with males (LS) was distinct from that of females interacting with other females. Specifically, we found severe reduction in female preference behaviour coupled with more moderate changes in social and general activities for females exposed to mate choice conditions (figure 1), whereas females exposed to general social conditions exhibited disruptions only in neutral activities and total association (electronic supplementary material, figures S2 and S3). Overall, there was no change in total activity exhibited by females exposed to males; however, the nature of activity was altered, with a significant shift from social to non-social behaviours, suggesting that cognitive processing is critical for normal social responses. Our results suggest that mate choice is part of a dynamic learning process in these poeciliids, and female preference response in particular requires working social memory to discriminate among potential mates.
NMDAR blockade disrupts the social cognitive processes necessary to manifest a mate choice response in X. nigrensis females. This may result from overall reductions in social behaviour, drug-induced decline in specific behaviours or sensory processing associated with NMDAR antagonism and/or difficulty of the social task. While we observed the greatest decline in preference behaviours, we also observed a shift away from social-type behaviours towards neutral activities, namely transits across the tank. We found a similar pattern in females exposed to other females (and increased locomotion in our scototaxis assay), indicating that reduced sociality coupled with increased locomotion may be a generalized response to NMDAR blockade. However, behavioural responses clearly diverged by social context, and the possibility that the reduction in preference was solely a by-product of social withdrawal appears unlikely given that our preference measures reflect biases in relative behavioural measures independent of total association time with males. This suggests that the observed pattern reflects an inability to respond appropriately to social stimuli. NMDAR blockade can increase locomotor and stereotyped activities [14,30]; and in fish, acute treatment can induce alterations of stereotyped movements [15,17,18]. We observed some evidence for trace residual drug effects in our subchronic dosage paradigm; however, even removing stereotypic behaviours from any preference analysis we still see a reduction in response (e.g. reduction in association time bias). Because NMDAR signalling contributes to so many aspects of neural functioning, future experiments will attempt to differentiate between deficits in social cognition versus sensory processing.
Females altered biased attention towards one male over the other following NMDAR blockade. Controls generally displayed strong biases for their favoured male, while treatment females failed to discriminate between the large courting male and the small coercive male with either time or behavioural measures. NMDAR deficits appear to scale with the level of difficulty in cognitive tasks [31], therefore the particular sensitivity of preference behaviours to NMDAR blockade may indicate that preference entails greater social memory processing than overall sociality. We propose that our subchronic paradigm (24 h gap between drug exposure and behaviour testing) altered preference behaviour by disrupting social cognition via altered NMDAR-mediated glutamatergic pathways while minimizing more immediate effects of drug uptake (stereotyped or erratic movements, perceptual disruptions).
NMDAR antagonists generally act at the functional level of the synapse [11,13], although there is some evidence for downstream transcription effects [16,24]. In the current experiment, we predicted that disrupted mate preference responses would be reflected in gene modules linked to dynamic preference behaviours if blocked glutamatergic signalling altered normal female responses to males. We measured expression for 11 genes previously associated with social behaviours in X. nigrensis females [6–8], including five synaptic-plasticity-linked genes associated with female preference (neuroserpin, neuroligin-3, NMDA-receptor, tPA and stathmin-2), four associated with female receptivity or socio-sexual behaviours (β-1 adrenergic receptor, α-1 adrenergic receptor, tyrosine hydroxylase and brain aromatase) and two associated with general social and reproductive behaviours in teleosts (vasotocin and isotocin). Preference/receptivity markers (including genes linked with synaptic plasticity) were associated with controls. Unexpectedly, the vasotocin/isotocin cluster loaded instead towards MK-801 females (figure 2).
The distinction in multivariate patterns between vasotocin/isotocin transcripts and the preference/receptivity cluster following NMDAR blockade is intriguing. Vasotocin and isotocin are the fish homologues of vasopressin and oxytocin, two nonapeptides closely associated with sociality in mammals (including affiliative and sexual behaviours [32]), plus social/emotional processing, stress response modulation, learning and memory [33–36]. Oxytocin has antipsychotic properties [28,37,38] and has been shown to restore deficits in sensorimotor gating induced by MK-801 in rats [39]. The association between MK-801 exposure and isotocin/vasotocin may indicate an unexpected conservation with mammalian patterns in modulating aspects of glutamatergic signalling in fish, and may also reflect molecular stress and coping responses to cognitive disruption [33,34]. The roles of vasotocin and isotocin in female teleost social behaviour—let alone preference—are not particularly well understood, but their importance in male social and reproductive behaviours is clearly conserved [20,21,40,41], and both modulate general sociality and anxiety-related behaviours in fish [19]. Future experiments exploring the functional role of these two peptides in female preference behaviours will help to elucidate this relationship, and may highlight a conserved influence of mood and emotional processing in teleost social decision-making.
The distinction between plasticity-preference and sociality-affiliation gene clusters for the 11 genes assayed in the current experiment is consistent with previous brain expression profiles of untreated female swordtails experiencing different social contexts (including large and small male, and female-only social treatments [8]). This earlier work identified context-specific expression patterns splitting into two main axes of social discrimination: a major axis scaling with mate choice complexity (preference-associated genes) and a secondary general social affiliation/aversion axis characterized by isotocin/vasotocin [8]. Here, we recapitulate this distinction while keeping social context constant, indicating NMDAR disruption may have altered female perception of social context.
We found that individual preference or multivariate behavioural disruptions induced by NMDAR blockade were correlated with differential multivariate gene expression patterns following male exposure (figure 3 and table 4). By contrast, there were no correlations with any general activities, indicating these patterns were not driven by neutral locomotion. The behavioural and gene expression patterns associated with NMDAR blockade relative to controls suggest a potential role for synaptic-plasticity-associated processes in mediating appropriate mate preference responses.
In X. nigrensis, preference behaviour is predominantly characterized through biased attention towards ornamented, courting males over alternative phenotypes that lack ornamentation and employ a force-copulation strategy [9]. This particular social task involves discrimination between attractive and aversive stimuli, and is likely to require processes that mediate both social cognition and fear modulation. Comparing the natural variation in behaviour and gene expression in unaltered females (controls) relative to our MK-801 group suggests that NMDAR blockade modulates the perception of valence in an aversive–attractive choice test. In our current study, two controls preferred the small, coercive phenotype and exhibited gene DFA classifications identical to MK-801 females (figure 3d). NMDAR antagonists disrupt normal fear processing and can induce anxiolytic behavioural responses [11,42], and these properties are conserved in fish [15]. The two control females exhibiting a preference for small males may have different fear responses than the population average. This idea is supported by our non-social scototaxis assay results where MK-801 exposure increased willingness to enter and spend time in a tank environment that is typically aversive, and suggests NMDAR blockade reduced anxiety/fear response to potentially threatening conditions. In a mate choice context, MK-801 may have alleviated aversive responses to small male stimuli, and future experiments should test the importance of fear of small males in driving female preferences for large males.
5. Conclusion
The full repertoire of behavioural responses to paired male and female stimuli was altered following MK-801 exposure; however, we found that preference behaviours were most severely disrupted for females exposed to males, but not other females. These male-exposed females altered their activity budget away from social behaviours towards neutral activities. Blocking NMDAR altered preference even 24 h after exposure, suggesting that our results were caused by altered glutamatergic transmission rather than a direct psychomotor response to MK-801. Multivariate expression patterns of genes previously associated with female preference/receptivity and general sociality were similarly disrupted, and transcriptional profiles discriminated between treated and control females exposed to the same social conditions. Our results suggest that disrupting NMDAR interferes with a female's ability to socially discriminate in a mate choice context, potentially through alterations of social cognition and fear modulation processes. Poeciliid preference functions incorporate synaptic-plasticity-oriented ‘preference’ gene modules with general sociality to produce context-appropriate responses to male exposure. Our results implicate NMDAR and synaptic plasticity processes as a key component in modulating female preference responses towards favoured males and away from unfavoured males.
Acknowledgements
We thank Katherine Ruddick, Sarah Ziari, Ian Etheredge and Korey Conley for assistance with behaviour trials, Chad Brock and Eben Gering for assistance with statistical analyses, Cummings laboratory members for helpful discussions and Kathleen Lynch, Ryan Wong and Hans Hofmann for comments on earlier versions of this manuscript. We thank the University of Texas Brackenridge Field Laboratory for animal care facilities. The authors declare no conflict of interests.
All experimental procedures were approved by IACUC (#AUP-2010-00148) at the University of Texas at Austin.
Funding statement
This work was supported by an Undergraduate Research Fellowship to W.V. and NSF SGER IOS-0813742 and NSF IOS-0843000 to M.E.C.
References
- 1.Ryan MJ, Cummings ME. 2013. Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 44, 437–459. ( 10.1146/annurev-ecolsys-110512-135901) [DOI] [Google Scholar]
- 2.Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, Svensson EI. 2012. The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 27, 511–519. ( 10.1016/j.tree.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 3.Kodric-Brown A, Nicoletto PF. 2001. Age and experience affect female choice in the guppy (Poecilia reticulata). Am. Nat. 157, 316–323. ( 10.1086/319191) [DOI] [PubMed] [Google Scholar]
- 4.Morris MR, Rios-Cardenas O, Brewer J. 2010. Variation in mating preference within a wild population influences the mating success of alternative mating strategies. Anim. Behav. 79, 673–678. ( 10.1016/j.anbehav.2009.12.018) [DOI] [Google Scholar]
- 5.Wong RY, So P, Cummings ME. 2011. How female size and male displays influence mate preference in a swordtail. Anim. Behav. 82, 691–697. ( 10.1016/j.anbehav.2011.06.024) [DOI] [Google Scholar]
- 6.Cummings ME, Larkins-Ford J, Reilly CRL, Wong RY, Ramsey M, Hofmann HA. 2008. Sexual and social stimuli elicit rapid and contrasting genomic responses. Proc. R. Soc. B 275, 393–402. ( 10.1098/rspb.2007.1454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch KS, Ramsey ME, Cummings ME. 2012. The mate choice brain: comparing gene profiles between female mate choice and male coercive poecillids. Genes Brain Behav. 11, 222–229. ( 10.1111/j.1601-183X.2011.00742.x) [DOI] [PubMed] [Google Scholar]
- 8.Ramsey ME, Maginnis TL, Wong RY, Brock C, Cummings ME. 2012. Identifying context-specific gene profiles of social, reproductive and mate preference behavior in a fish species with female mate choice. Front. Neurosci. 6, 62 ( 10.3389/fnins.2012.00062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan MJ, Rosenthal GG. 2001. Variation and selection in swordtails. In Model systems in behavioral ecology (ed. Dugatkin LA.), pp. 133–148. Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Wong RY, Ramsey ME, Cummings ME. 2012. Localizing brain regions associated with female mate preference behavior in a swordtail. PLoS ONE 7, e50355 ( 10.1371/journal.pone.0050355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reidel G, Platt B, Micheau J. 2003. Glutamate receptor function in learning and memory. Behav. Brain Res. 140, 1–47. ( 10.1016/S0166-4328(02)00272-3) [DOI] [PubMed] [Google Scholar]
- 12.Gao X-M, Elmer GI, Adams-Huet B, Tamminga CA. 2009. Social memory in mice: disruption with an NMDA antagonist and attenuation with antipsychotic drugs. Pharmacol. Biochem. Behav. 92, 236–242. ( 10.1016/j.pbb.2008.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunduz-Bruce H. 2009. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res. Rev. 60, 279–286. ( 10.1016/j.brainresrev.2008.07.006) [DOI] [PubMed] [Google Scholar]
- 14.Andine P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E, Sandberg M. 1999. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J. Pharmacol. Exp. Ther. 290, 1393–1408. [PubMed] [Google Scholar]
- 15.Riehl R, et al. 2011. Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicol. Teratol. 33, 658–667. ( 10.1016/j.ntt.2011.05.011) [DOI] [PubMed] [Google Scholar]
- 16.Rujescu D, et al. 2006. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol. Psych. 59, 721–729. ( 10.1016/j.biopsych.2005.08.029) [DOI] [PubMed] [Google Scholar]
- 17.Sison M, Gerlai R. 2011. Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio). Behav. Brain Res. 220, 331–337. ( 10.1016/j.bbr.2011.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain HA, Sigstad C, Scalzo FM. 2004. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio). Neurotoxicol. Teratol. 26, 725–729. ( 10.1016/j.ntt.2004.06.009) [DOI] [PubMed] [Google Scholar]
- 19.Braida D, Donzelli A, Martucci R, Capurro V, Busnelli M, Chini B, Sala M. 2012. Neurohypophyseal hormones manipulation modulate social and anxiety-related behavior in zebrafish. Psychopharmacology 220, 319–330. ( 10.1007/s00213-011-2482-2) [DOI] [PubMed] [Google Scholar]
- 20.Goodson JL, Bass AH. 2000. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature 403, 769–772. ( 10.1038/35001581) [DOI] [PubMed] [Google Scholar]
- 21.Thompson RR, Walton JC. 2004. Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav. Neurosci. 118, 620–626. ( 10.1037/0735-7044.118.3.620) [DOI] [PubMed] [Google Scholar]
- 22.Mandillo S, Rinaldi A, Oliverio A, Mele A. 2003. Repeated administration of phencyclidine, amphetamine and MK-801 selectively impairs spatial learning in mice: a possible model of psychotomimetic drug-induced cognitive deficits. Behav. Pharmacol. 14, 533–544. ( 10.1097/00008877-200311000-00006) [DOI] [PubMed] [Google Scholar]
- 23.Wang H-X, Gao W-J. 2012. Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex. Neuropharmacology 62, 1808–1822. ( 10.1016/j.neuropharm.2011.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xi D, Zhang W, Wang H-X, Stradtman GG, III, Gao W-J. 2009. Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int. J. Neuropsychopharmacol. 12, 1395–1408. ( 10.1017/S146114570900042X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey ME, Wong RY, Cummings ME. 2011. Estradiol, reproductive cycle and preference behavior in a northern swordtail. Gen. Comp. Endocrinol. 170, 381–390. ( 10.1016/j.ygcen.2010.10.012) [DOI] [PubMed] [Google Scholar]
- 26.Maximo C, Marques de Brito T, Dias CA, Gouveia A, Jr, Morato S. 2010. Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 5, 221–228. ( 10.1038/nprot.2009.225) [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. 2001. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284. ( 10.1016/S0166-4328(01)00297-2) [DOI] [PubMed] [Google Scholar]
- 28.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. 2005. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychoharmacology 30, 1883–1894. ( 10.1038/sj.npp.1300722) [DOI] [PubMed] [Google Scholar]
- 29.Qiao H, et al. 2001. Clozapine, but not haloperidol reverses social behavior deficit in mice during withdrawal from chronic phencyclidine treatment. Neuroreport 12, 11–15. ( 10.1097/00001756-200101220-00010) [DOI] [PubMed] [Google Scholar]
- 30.Carey R, Dai H, Gui J. 1998. Effects of dizocilpine (MK-801) on motor activity and memory. Psychopharmacology 137, 241–246. ( 10.1007/s002130050616) [DOI] [PubMed] [Google Scholar]
- 31.Bolton MM, Heaney CF, Sabbagh JJ, Murtishaw AS, Magcalas CM, Kinney JW. 2012. Deficits in emotional learning and memory in an animal model of schizophrenia. Behav. Brain Res. 233, 35–44. ( 10.1016/j.bbr.2012.04.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donaldson ZR, Young LJ. 2008. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904. ( 10.1126/science.1158668) [DOI] [PubMed] [Google Scholar]
- 33.Balment RJ, Lu W, Weybourne E, Warne JM. 2006. Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. Gen. Comp. Endocrinol. 147, 9–16. ( 10.1016/j.ygcen.2005.12.022) [DOI] [PubMed] [Google Scholar]
- 34.Lee H-J, Macbeth AH, Pagani JH, Young WS. 2009. Oxytocin: the great facilitator of life. Prog. Neurobiol. 88, 127–151. ( 10.1016/j.pneurobio.2009.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Windle RJ, Shanks N, Lightman SL, Ingram CD. 1997. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138, 2829–2834. [DOI] [PubMed] [Google Scholar]
- 36.Yagi K, Onaka T, Yoshida A. 1998. Role of NMDA receptors in the emotional memory associated with neuroendocrine responses to conditioned fear stimuli in the rat. Neurosci. Res. 30, 279–286. ( 10.1016/S0168-0102(98)00008-X) [DOI] [PubMed] [Google Scholar]
- 37.Caldwell HK, Stephens SL, Young WS., III 2009. Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol. Psych. 14, 190–196. ( 10.1038/sj.mp.4002150) [DOI] [PubMed] [Google Scholar]
- 38.MacDonald K, Feifel D. 2012. Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatr. 24, 130–148. ( 10.1111/j.1601-5215.2011.00634.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feifel D, Reza T. 1999. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology 141, 93–98. ( 10.1007/s002130050811) [DOI] [PubMed] [Google Scholar]
- 40.Black MP, Reavis RH, Grober MS. 2004. Socially induced sex change regulates forebrain isotocin in Lythrypnus dalli. Neuroreport 15, 185–189. ( 10.1097/00001756-200401190-00036) [DOI] [PubMed] [Google Scholar]
- 41.Oldfield RG, Hofmann HH. 2011. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol. Behav. 102, 296–303. ( 10.1016/j.physbeh.2010.11.022) [DOI] [PubMed] [Google Scholar]
- 42.Pietersen CY, et al. 2007. An animal model of emotional blunting in schizophrenia. PLoS ONE 2, e1360 ( 10.1371/journal.pone.0001360) [DOI] [PMC free article] [PubMed] [Google Scholar]