Abstract
Female mate choice and male–male competition are the typical mechanisms of sexual selection. However, these two mechanisms do not always favour the same males. Furthermore, it has recently become clear that female choice can sometimes benefit males that reduce female fitness. So whether male–male competition and female choice favour the same or different males, and whether or not females benefit from mate choice, remain open questions. In the horned beetle, Gnatocerus cornutus, males have enlarged mandibles used to fight rivals, and larger mandibles provide a mating advantage when there is direct male–male competition for mates. However, it is not clear whether females prefer these highly competitive males. Here, we show that female choice targets male courtship rather than mandible size, and these two characters are not phenotypically or genetically correlated. Mating with attractive, highly courting males provided indirect benefits to females but only via the heritability of male attractiveness. However, mating with attractive males avoids the indirect costs to daughters that are generated by mating with competitive males. Our results suggest that male–male competition may constrain female mate choice, possibly reducing female fitness and generating sexual conflict over mating.
Keywords: sexual selection, direct benefits, indirect benefits, heritability
1. Introduction
Sexual selection is responsible for the evolution of many exaggerated morphologies and behaviours [1], and the two main mechanisms of sexual selection are male–male competition and female mate choice, although these rarely act independently (reviewed in [2]). However, while the two mechanisms often act simultaneously, and can favour the same phenotypes [3], there is no logical necessity that they act in a reinforcing manner [4,5]. Indeed, the two do sometimes target different males or characters [6–8], and thus can oppose one another. This has potential implications for the fitness consequences of sexual selection, and shows that it is necessary to investigate both mate choice and competition to fully understand it [9].
Male–male competition was historically the least contentious mechanism of sexual selection, in part, because it is often so obvious [10]. It has even been suggested that most secondary sexual traits evolve primarily as weapons used in sexual competition [11]. What seems certain is that males with larger weapons are typically more competitive and better fighters, and because of this are able to increase their mating opportunities by excluding rivals (reviewed in [12,13]). It is therefore clear that by being competitively superior, males gain fitness, but what is in it for females?
Classical concepts of sexual selection basically assume that mating with competitively superior/attractive males provides fitness benefits to females too [1,14,15]. For example, competitively superior males with large weapons and superior fighting ability can provide females access to better territories [1], and it has been suggested that weapons should become the target of female preference precisely because fighting success is an honest indicator of male quality [11]. Females could also benefit indirectly if their offspring inherit the sire's genetic quality (good genes) [16,17] or sons inherit their father's attractiveness or competitiveness [18,19]. It is not clear how these arguments hold when females prefer less competitive males as mates, but do not get to mate with them [20], and traditional concepts of mate-choice benefits are also complicated by both inter- and intralocus sexual conflict [21,22].
It is possible that male sexual traits do not provide females with information about potential mate-choice benefits, but are instead used to coerce or manipulate females [21,23]. If so, females can incur fitness costs by mating with ‘attractive’ males, but they can also evolve resistance to harmful sexual coercion [21,23,24]. If this, in turn, reduces the efficacy of male sexual traits, then sexually antagonistic coevolution may occur [25,26]. There is compelling evidence for sexual manipulation in a range of taxa and for female fitness loss when mating with more ‘attractive’ males [27–32].
Females could also be exposed to indirect costs by mating with more attractive or competitive males [33]. This can occur when males and females express shared traits that are regulated by the same genes, but optimal trait values differ between the sexes [33,34]. This means the genes that make good males do not always make good females, generating negative sire–daughter (and dam–son) fitness associations and indirect fitness costs [35], although these can be reduced by offspring sex-ratio adjustment [36]. Again, evidence consistent with sexually antagonistic selection on shared traits is widespread [37–40], which seems to restrict the likelihood and/or magnitude of good genes benefits of sexual selection.
Male–male competition has been well studied in the horned beetle G. cornutus. Males fight using their enlarged mandibles, a character females lack [41,42], with weapon size determining fight outcomes—males with larger mandibles are better fighters. Mandible size is also positively genetically associated with competitive mating success [39,43,44], but daughters sired by large-weaponed males have lower fitness owing to intralocus sexual conflict [39]. However, there is little known about male attractiveness and the fitness consequences of female mate choice in these beetles, and these issues are the focus of this study. First, we assessed the target of female preference and then the direct female fitness effects of mating with preferred, attractive males. Next, we investigated fitness components for both sons and daughters sired by attractive males. Finally, the genetic variances and covariances for a range of male traits were assessed in a half-sib breeding design. Our study also allowed us to assess some direct and indirect effects of mating with highly competitive males.
2. Material and methods
The stock population of G. cornutus used in this study originated from adults collected in Miyazaki City (31° 54′ N, 131° 25′ E), Japan in June 1957. This population has since been maintained on wholemeal flour enriched with yeast (see [41,42] for details). These beetles are stored product pests therefore, laboratory conditions were tailored to closely mimic natural conditions. Stocks were maintained in constant temperature chambers at 25°C, 60% relative humidity, and with a photoperiod cycle of 14 L : 10 D. G. cornutus larvae do not pupate at high densities, therefore, following Okada & Miyatake [41], final-instar larvae were individually placed in each well of a 24-well tissue culture plate with 1 g of food to obtain adults (Cellstar; Greiner Bio-One, Frickenhausen, Germany). This protocol ensures that the emerging adults do not interact with conspecifics. As G. cornutus males can take up to 7 days to attain sexual maturity ([45] and see [36]), we allowed individuals from both sexes to mature for 14 days before being used in experiments. All experiments within this study follow this maintenance protocol, unless stated otherwise.
To assess female mate preference, a random subset of third-instar larvae were collected from the stock population and allowed to develop in individual cells of a 24-well plate (as above). Adult (virgin) males and females were randomly picked from this subset, weighed on an electronic balance (Mettler-Toledo AG, Laboratory and Weighing Technologies, Greifensee, Switzerland) to the nearest 0.01 mg (body mass) and then paired for observations.
Briefly, each virgin female was individually aspirated into in a plastic dish (17 mm diameter, 20 mm high) lined with a filter paper (17 mm diameter) for traction. After 30 min, one male was added to each dish, and then the pair was continuously observed until the end of copulation. Pre-copulatory courtship display in this species is similar to that of several other beetles [7,46,47]. A male orients behind the female, mounts her and then repeatedly taps her back along with simultaneous rubbing using his tarsi and body. Females accepting this courtship respond by ovipositor extension, which is followed by genital coupling and a short copulation. Males were removed immediately after copulation to avoid rematings.
Attractiveness is frequently positively associated with courtship in insects, including flour beetles [7,47–50]. Thus, we measured courtship rate (CR: number of courtship bouts per second, during a single successful courtship period) as a measure of courtship quality [7,46]. In G. cornutus, females determine whether copulation occurs or not, so copulation latency (CL: the time from male introduction to commencement of copulation) was measured as an indicator of male attractiveness (see [19,47,50,51]) and female preference (cf. [7,46]). Both these measures were highly repeatable (regression of independent measures of the traits (measure one on measure two): CR, r = 0.550, p < 0.0001, n = 51; CL, r = 0.660, p < 0.0001, n = 51). The no-choice approach was employed to remove potentially confounding male–male competition which we know favours males with large mandibles [39,43,44]. Furthermore, CL in no-choice trials is widely used as a measure of attractiveness/preference in mate choice studies [19,47,50–57] and is consistent with definitions of preference—female propensity to mate with a male [58]. Note that the no-choice protocol may not mimic natural conditions, but it is needed to isolate female preference from the confounds of male–male competition.
(a). Direct effects
To assess the direct benefits of mating with attractive males, adult (virgin) males and females were chosen randomly from the stock population (as above) to establish mating pairs. Male attractiveness (measured via CL) was recorded for 51 mating pairs (as above) and females were discarded. The next day, each male was paired with a new virgin female, and the pairs observed until end of copulation (this enabled us to regress two independent measures of CR (and CL) against each other to assess trait repeatability: see above). Females were then transferred into an egg-laying vial (70 mm diameter, 25 mm high) containing excess food (20 g) for two months. Lifetime reproductive success (LRS) of each female was scored as the total number of adult offspring emerging from these vials. This provides a good proxy for female LRS (see [36,45,59]), and such proxies of LRS have been used to good effect in other studies [60]. Females were then moved into new vials (40 mm high, 15 mm diameter) containing an excess of the culture medium (4 g) and assessed for survival weekly until death.
(b). Indirect effects
To assess the indirect benefits of mating with attractive males, a random assortment of 200 virgin males and females was collected from the stock population (as above), and male attractiveness was again assessed (as above). The 50 most attractive (fastest to mate) and 50 least attractive (slowest to mate) males were then identified from extremes of the CL distribution for subsequent use. On the following day, each attractive and unattractive male (sire) was individually paired with a virgin female (dam) chosen randomly from the stock population (family: n = 50 in each treatment). After copulation, each female was maintained in an egg-laying vial (70 mm diameter, 25 mm high) containing an excess of food (20 g) for four weeks. We collected 10 (+5) eggs per parental pair (at random) to assess offspring performance (five eggs per parent were collected as reserves). One offspring of each sex (per family) was randomly chosen for a longevity assay. These individuals were allowed to remain in their developmental vials, provided with fresh food every month and assessed for survival on a weekly basis. One male offspring per family was randomly chosen for measurement of mandible length and testes volume. These individuals were allowed to remain in their developmental vials with fresh food (4 g) for 14 days post-eclosion, allowing sufficient time for males to reach full sexual maturity (see above). Subsequently, the testes were removed from the body using a fine forceps and carefully separated from the surrounding tissue in deionized water following Yamane et al. [44]. The length (L) and width (W) of both testes were measured to the nearest 0.01 mm with the dissecting microscope monitoring system (VM-60, Olympus, Tokyo, Japan), and testis volume (mm3) was calculated using the formula V = (πLW2)/6. This volume was used as a proxy for testis size. Mandible length of each male (±0.01 mm) was measured as described in Katsuki et al. [36]. Another son per family was randomly chosen for measurement of CL (male attractiveness) and CR (courtship quality). Virgin females were used for these assays and were chosen randomly from the stock culture. We also took one daughter per family (chosen at random) and paired them with a stock virgin male (picked at random) following the same mating protocol as previously. Immediately after copulation, each female was transferred into an egg-laying vial (70 mm diameter, 25 mm high) containing excess medium (20 g) and scored for LRS (as described above for the direct benefits assays).
(c). Genetic analysis
We used a paternal half-sib breeding design [61] to estimate the narrow sense heritability of male attractiveness (CL), male courtship quality (CR), mandible length (secondary sexual character) and body mass (an index of body size) as well as their genetic variances and covariances. Briefly, virgin males and females were chosen from the stock population at random (as described earlier) and each male (sire: n = 38) was randomly assigned to four virgin females (dams). Each male was paired with a single female in a mating chamber (17 mm diameter, 20 mm high) lined with filter paper (17 mm diameter) for traction. Pairs were observed until end of copulation and then immediately after copulation, females were transferred to egg-laying vials with an excess of culture medium. This was done successively for 4 days (four dams per sire), and each female was allowed to oviposit for two months. Offspring from each female were reared to the final-instar larval stage (approx. eight weeks) under laboratory conditions identical to the parental generation. A subset of these final-instar larvae (per dam per sire combination) were chosen at random and reared individually to adulthood in 24-well plates with 1 g of food. From these, one son per dam for each sire was randomly selected for measurement of CL, CR, mandible length and body size at two weeks after eclosion (n = 152).
As the copulation ritual in this species is very quick, with several ‘drumming’ displays leading to a copulation and most copulations lasting only 3–4 s, CL and CR data were measured on different days to ensure accuracy. Briefly, on the first day of mating, each male was paired with a stock virgin female and CL was recorded (as described earlier). On the next day, each male was paired with another virgin female and measures of CR, mandible length and body size were recorded (as above).
(d). Statistical analysis
GLM was used to assess potential relationships between attractiveness (CL) and CR and mandible size and to assess associations between CR and mandible size. Direct benefits of female mate choice were assessed using regression where female longevity and LRS were used as dependent variables and male attractiveness (CL), male mandible length and female body size were used as predictors. For the indirect benefits-related analysis, survival rate from egg to adult and traits measured in sons (developmental period, longevity, body size, mandible length, testis size, CL and CR) and daughters (developmental period, longevity, body size and LRS) were analysed using a GLM, with attractiveness (attractive and unattractive) as the predictor. Initial full models were simplified by removal of non-significant terms to achieve the final models [62]. Results were revalidated using bootstrapping (1000 iterations); however, as our findings remained unchanged, we present only the standard analyses. All analyses were carried out using JMP v10 (SAS), unless stated otherwise, and all model assumptions were tested and met (either with log transformation or as raw data).
Variance components for our genetic analysis were initially estimated from a nested ANOVA [61]. Heritability estimates and their standard errors were calculated following Falconer & Mackay [63] and Lynch & Walsh [61]. Z-scores were used to test whether h2 were significantly different from zero [64,65]. While heritability estimates can be obtained with good accuracy with the traditional methods because of our balanced design [66], estimation of accurate genetic correlations between traits requires a more powerful approach. Therefore, we used a multivariate mixed animal-model approach [67]. Briefly, an animal model with courtship latency, CR, mandible length and male body weight as dependent variables was fitted against the mean for each trait as a fixed effect and the additive genetic effect for each trait as random (sire effect). Note that our experimental design does not allow separation of the maternal effects from the residual variance. Variance and covariance components were extracted using post-processing options within ASREML (see [67] for details), and genetic correlations between traits estimated along with their standard errors. Different approaches can often yield varied values of genetic correlations. So, in order to validate our results, we also calculated means for each sire as a family mean, controlling for the individual effects of offspring and then used these family means for another estimation of genetic correlations with Pearson product–moment correlation coefficient [61]. Heritabilities obtained with the ANOVA and animal model were of the same sign and magnitude but we only present the former.
3. Results
Male attractiveness (CL) was found to be solely determined by CR with mandible size having no significant bearing on attractiveness (overall model: r2 = 0.38, F2,48 = 13.96, p < 0.001. CR: F1,48 = 27.90, p < 0.001. Mandible size: F1,48 = 0.02, p = 0.896). This indicates females prefer to mate with males that court more vigorously rather than males that are more competitive (superior in male–male competition). Additionally, it should be noted that CR was unrelated to mandible size (F1,49 = 0.0001, p = 0.99), so the most competitive males did not court the most (this is also reflected in the genetic analyses below).
(a). Direct benefits of mate choice
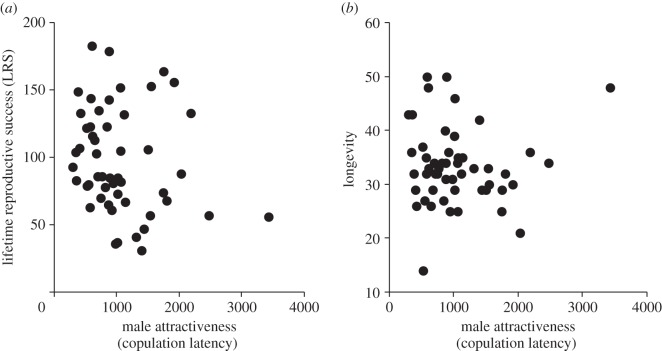
For female LRS and longevity, there was a multivariate effect of female body size (Wilks lambda = 0.61, F2,45 = 14.77, p < 0.001) and post hoc tests showed this was only via LRS which was positively associated with female body size (β = 0.63, F1,45 = 28.55, p < 0.001). There were no other significant effects and neither male attractiveness (CL; figure 1a,b) nor competitiveness (mandible size) were associated with either measure of female fitness either in multivariate or univariate space (all p > 3.32). Note that these findings remained unaltered even with the inclusion of CR rather than attractiveness (CL). Thus, direct female fitness seems unaffected by male attractiveness (CL) and male competitive ability (mandible size).
Figure 1.
Male attractiveness (copulation latency, seconds) was not associated with (a) female lifetime reproductive success nor (b) female longevity (weeks). Note increases on the x-axis represent decreased attractiveness.
(b). Indirect benefits of mate choice
Offspring of attractive sires had significantly shorter developmental periods compared with the offspring of unattractive sires (see tables 1 and 2 for details). Multivariate GLM of sons' fitness found sire attractiveness was significantly associated with the multivariate combination of fitness measures (Wilks' lambda = 0.68, F7,92 = 6.11, p < 0.001). Univariate post hoc tests showed that sons of attractive males were more attractive (i.e. had shorter CL) and had significantly higher CRs than sons of unattractive sires (tables 1 and 2). Sire attractiveness had no significant effects on any other sons' characters other than development time. The multivariate effect of sire on daughters' fitness (Wilks' lambda = 0.85, F4,95 = 4.20, p = 0.004) was purely driven by effects on development time (table 1), and the other daughter fitness measures (longevity, body size and LRS) were unaffected by sire attractiveness (tables 1 and 2).
Table 1.
Post hoc analysis of variance for traits measured in sons and daughters of attractive and unattractive sires. As larval sex determination is not possible in this species, survival data collected from the egg to adult stage has not been split by sex.
| traits | effect | d.f. | mean square | F | p |
|---|---|---|---|---|---|
| survival rate (egg to adult) | attractiveness | 1 | 0.010 | 0.640 | 0.4255 |
| error | 98 | 0.016 | |||
| son | |||||
| developmental period | attractiveness | 1 | 96.27 | 16.185 | 0.0001 |
| error | 98 | 5.95 | |||
| longevity | attractiveness | 1 | 2.89 | 0.134 | 0.7154 |
| error | 98 | 21.61 | |||
| body size | attractiveness | 1 | 0.0001 | 0.004 | 0.9490 |
| error | 98 | 0.0196 | |||
| mandible length | attractiveness | 1 | 0.0002 | 0.077 | 0.7819 |
| error | 98 | 0.0025 | |||
| testes size | attractiveness | 1 | 0.001 | 0.009 | 0.9262 |
| error | 98 | 0.154 | |||
| attractiveness (copulation latency) | attractiveness | 1 | 2940539 | 12.761 | 0.0006 |
| error | 98 | 23043 | |||
| courtship rate | attractiveness | 1 | 0.0357 | 19.836 | <0.0001 |
| error | 98 | 0.0018 | |||
| daughter | |||||
| developmental period | attractiveness | 1 | 70.56 | 14.375 | 0.0003 |
| error | 98 | 4.91 | |||
| longevity | attractiveness | 1 | 7.84 | 0.192 | 0.6621 |
| error | 98 | 40.81 | |||
| body size | attractiveness | 1 | 0.002 | 0.092 | 0.7622 |
| error | 98 | 0.023 | |||
| lifetime reproductive success: LRS | attractiveness | 1 | 1953.64 | 2.091 | 0.1513 |
| error | 98 | 934.23 | |||
Table 2.
Means ± standard error of traits measured in sons and daughters within each treatment. N = 50 in each treatment. As sex determination at larval stages is impossible, egg to pupa data have been shown independent of sex.
| attractive | unattractive | |
|---|---|---|
| survival rate (egg to adult) | 0.824 ± 0.018 | 0.804 ± 0.018 |
| sons | ||
| developmental period (days) | 44.11 ± 0.36 | 46.07 ± 0.33 |
| longevity (weeks) | 29.76 ± 0.65 | 30.10 ± 0.67 |
| body size (mg) | 2.743 ± 0.019 | 2.745 ± 0.021 |
| mandible length (mm) | 0.397 ± 0.007 | 0.400 ± 0.007 |
| testes size × 10−2 (mm3) | 2.809 ± 0.059 | 2.816 ± 0.052 |
| copulation latency: attractiveness (seconds) | 917.0 ± 59.4 | 1259.9 ± 75.4 |
| courtship rate (per second) | 0.092 ± 0.007 | 0.054 ± 0.004 |
| daughters | ||
| developmental period (days) | 42.80 ± 0.30 | 44.48 ± 0.33 |
| longevity (weeks) | 34.54 ± 0.88 | 33.98 ± 0.93 |
| body size (mg) | 2.733 ± 0.022 | 2.724 ± 0.021 |
| lifetime reproductive success: LRS | 106.10 ± 4.56 | 97.26 ± 4.07 |
(c). Genetic associations
All traits measured were significantly and highly heritable (table 3). There was a significant negative genetic correlation between CL and CR (genotypes that court more are more attractive (mate faster)) and a positive genetic correlation between mandible length and body size (table 3). Thus, attractive males tend to have a higher courtship display frequency, whereas males with larger mandibles have larger bodies (mass). Genetic correlations for other trait pairs were non-significant (figure 2a,b and table 3).
Table 3.
Heritabilities (h2) and genetic correlations ± standard error in male traits. Heritabilities (h2) are given on the diagonal and additive genetic correlations below the diagonal (LogL G-test = 22.87, p < 0.0001). Italicized values in parentheses are Pearson product–moment correlation coefficients that were calculated from family means. Estimates significantly different from zero (p < 0.05) are shown in bold.
| copulation latency | courtship rate | mandible length | body size | |
|---|---|---|---|---|
| copulation latency | 0.783 ± 0.338 | |||
| courtship rate |
−0.983 ± 0.254 (−0.660) |
0.697 ± 0.333 | ||
| mandible length | 0.285 ± 0.391 (0.211) |
−0.159 ± 0.428 (−0.119) |
0.673 ± 0.332 | |
| body size | 0.254 ± 0.398 (0.198) |
−0.449 ± 0.442 (−0.207) |
0.674 ± 0.244 (0.628) |
0.734 ± 0.335 |
Figure 2.
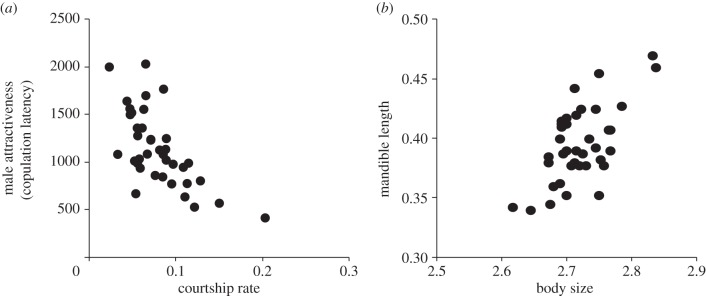
The significant genetic associations between (a) courtship rate (per second) and male attractiveness (copulation latency: seconds) and (b) between body size (mg) and mandible length (mm). Each circle shows a family mean for each sire. Note, an increase in copulation latency represents decreased attractiveness, so more attractive males have lower y-axis values.
4. Discussion
A major finding of this study was that the most attractive males, those most preferred by females, were not the highly competitive males with large mandibles. This is despite the fact these fighter males enjoy significant mating advantages when in direct competition for females [39,43,44]. Instead, females prefer to mate with males that court more, but as noted, in competitive mating situations, females mate with the more competitive phenotypes. So it seems that male–male competition can constrain female choice in horned beetles, and similar findings have been reported in other taxa [6,20]. In some regards, our findings most closely resemble those reported for cockroaches, where the most competitive male phenotypes were not preferred by females [6], and similarly, male attractiveness and competitiveness were not phenotypically or genetically correlated in the beetles, suggesting these are independent routes to male fitness.
Nonetheless, there was no evidence of direct benefits to female mate choice as neither male attractiveness nor competitive ability (mandible size) were associated with female longevity or LRS. As with all negative results this could be a sampling issue, but indirect benefits, which should be harder to detect [68], were found (see below) suggesting this may not be a type II error. Additionally, previous work has also failed to find direct benefits for females mating with males bearing large mandibles [59]. In any case, failure to find direct benefits of mate choice is not without precedent [69]. So it appears that even if females are able to mate with the males they prefer, they do not gain or lose any fecundity or longevity (from single matings). Male attractiveness often signals direct benefits to females [47,70,71], but mating with preferred males can also negatively impact female fitness [48,72]. In the beetles, neither of these situations applied, but we also restricted mating opportunities and it is possible that either phenotype (attractive/competitive) could coerce females into mating more than is optimal with additional mating opportunities [73]. This may be especially true for the beetles as the competitive males are highly aggressive towards conspecifics [43,74], and females could therefore suffer direct costs from attacks by these males (cf. [75,76]). In such situations, male–male competition and female choice are not expected to be reinforcing [21]. Nevertheless, determining whether repeated interactions with more competitive males reduces female direct-fitness in G. cornutus requires further work.
When direct-fitness effects are small or absent, indirect benefits can be enough to maintain mate preference [77], and there was evidence of indirect benefits of female mate choice. This is true of matings with more attractive and more competitive males, as in both instances females' sons would inherit their father attractiveness or competitiveness. Mandible size has previously been shown to be heritable and respond to selection, [39,43], although realized estimates were lower than here, and we additionally show here that overall attractiveness and CR are both heritable and significantly genetically correlated—estimates are also high for these characters. High heritability estimates may be a reflection of the apparent opposing female preference and male–male competition generating balancing selection on these traits (cf. [6]), which is analogous to a genotype by environment interaction (G × E). G × Es are able to maintain significant genetic variation [78] (reviewed in [79]), which is broadly consistent with the heritability estimates we report here. Be that as it may, mating with attractive or competitive males will lead females to produce more attractive or competitive sons and indirect fitness is enhanced via this route.
Similar to findings from other insects [50,60,69,80,81], we found no indirect benefits through daughters, as neither their longevity or fecundity were enhanced by having attractive fathers. Thus, mating with attractive males does not enhance daughters' fitness. However, females mating with attractive males do not suffer the reductions in daughters' fitness that are seen in matings with the highly competitive male phenotype [39]. This reduction in fitness occurs because the body-shape changes associated with developing large mandibles—the competitive phenotype—are transmitted to daughters, and the masculinized female phenotype has reduced egg space in the abdomen [39]. So, although mating with attractive males does not enhance elements of daughters' fitness, it does mean that fitness costs associated with masculinization are avoided. Furthermore, because of trade-offs between male-pupal viability and investment in the mandibles [82], indirect benefits realized from highly competitive mates could be additionally reduced (although this reduction was not detected in this study). Thus, mating with attractive males should be more beneficial for female G. cornutus, which could explain female preference in the first place.
The offspring of attractive sires also developed faster. It is not clear if this is beneficial or not, however, as there can be costs to rapid growth [83,84]. It is also not entirely consistent with good gene formulations of sexual selection, which are predicated on heritable enhancement of survivorship/longevity [85], and we found no evidence of attractive males siring offspring that survived better or lived longer. Thus, our findings of indirect benefits of mate choice are probably best viewed as Fisherian [86].
In summary, we found that frequency of courtship determines male attractiveness and is the focus of female choice rather than mandible size, which signals male competitiveness. Thus, the most attractive males are not those that win the most fights, and hence female choice and male–male competition do not favour the same phenotypes in G. cornutus. This is one of an expanding number of species where choice and competition favour different traits/males. Additionally, while mating with attractive males does not provide direct benefits to female, attractiveness was heritable, and by mating with attractive males, females avoid the cost of producing the masculinized, low-fitness daughters sired by highly competitive males. Nonetheless, male–male competition may constrain females, potentially generating sexual conflict over mating. Thus, it appears that complex bouts of selection generated by female choice, male–male competition and sexual conflict all act on male mandibles and courtship, and the net outcome of selection remains to be established.
Acknowledgements
We thank the Royal Society for financial support and the Editors and anonymous referees for comments that greatly improved the manuscript.
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Wong BBM, Candolin U. 2005. How is female mate choice affected by male competition? Biol. Rev. 80, 559–571. ( 10.1017/S1464793105006809) [DOI] [PubMed] [Google Scholar]
- 3.Pizzari T, Birkhead TR. 2000. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789. ( 10.1038/35015558) [DOI] [PubMed] [Google Scholar]
- 4.Arnold SJ. 1983. Sexual selection: the interface of theory and empiricism. In Mate choice (ed. Bateson P.), pp. 67–108. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Moore AJ. 1990. The evolution of sexual dimorphism by sexual selection: the separate effects of intrasexual selection and intersexual selection. Evolution 44, 315–331. ( 10.2307/2409410) [DOI] [PubMed] [Google Scholar]
- 6.Moore AJ, Moore PJ. 1999. Balancing sexual selection through opposing mate choice and male competition. Proc. R. Soc. Lond. B 266, 711–716. ( 10.1098/rspb.1999.0694) [DOI] [Google Scholar]
- 7.Kotiaho JS. 2002. Sexual selection and condition dependence of courtship display in three species of horned dung beetles. Behav. Ecol. 13, 791–799. ( 10.1093/beheco/13.6.791) [DOI] [Google Scholar]
- 8.Candolin U. 2004. Opposing selection on a sexually dimorphic trait through female choice and male competition in a water boatman. Evolution 58, 1861–1864. ( 10.1111/j.0014-3820.2004.tb00470.x) [DOI] [PubMed] [Google Scholar]
- 9.Hunt J, Breuker CJ, Sadowski JA, Moore AJ. 2009. Male–male competition, female mate choice and their interaction: determining total sexual selection. J. Evol. Biol. 22, 13–26. ( 10.1111/j.1420-9101.2008.01633.x) [DOI] [PubMed] [Google Scholar]
- 10.Hosken DJ, House CM. 2011. Sexual selection. Curr. Biol. 21, R62–R65. ( 10.1016/j.cub.2010.11.053) [DOI] [PubMed] [Google Scholar]
- 11.Berglund A, Bisazza A, Pilastro A. 1996. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399. ( 10.1111/j.1095-8312.1996.tb01442.x) [DOI] [Google Scholar]
- 12.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 13.Emlen DJ. 2008. The evolution of animal weapons. Ann. Rev. Ecol. Evol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 14.Cordero C, Eberhard WG. 2003. Female choice of sexually antagonistic male adaptations: a critical review of some current research. J. Evol. Biol. 16, 1–6. ( 10.1046/j.1420-9101.2003.00506.x) [DOI] [PubMed] [Google Scholar]
- 15.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 16.Welch AM, Semlitsch RD, Gerhardt HC. 1998. Call duration as an indicator of genetic quality in male gray tree frogs. Science 280, 1928–1930. ( 10.1126/science.280.5371.1928) [DOI] [PubMed] [Google Scholar]
- 17.Lesna I, Sabelis MW. 1999. Diet dependent female choice for males with ‘good genes’ in a soil predatory mite. Nature 401, 581–584. ( 10.1038/44125) [DOI] [Google Scholar]
- 18.Wedell N, Tregenza T. 1999. Successful fathers sire successful sons. Evolution 53, 620–625. ( 10.2307/2640798) [DOI] [PubMed] [Google Scholar]
- 19.Taylor ML, Wedell N, Hosken DJ. 2007. The heritability of attractiveness. Curr. Biol. 17, R959–R960. ( 10.1016/j.cub.2007.09.054) [DOI] [PubMed] [Google Scholar]
- 20.Bluhm CK, Gowaty PA. 2004. Social constraints on female mate preference in mallards, Anas platyrhynchos, decrease offspring viability and mother productivity. Anim. Behav. 68, 977–983. ( 10.1016/j.anbehav.2004.01.013) [DOI] [Google Scholar]
- 21.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 22.Hosken DJ, Stockley P, Tregenza T, Wedell N. 2009. Monogamy and the battle of the sexes. Ann. Rev. Entomol. 54, 361–378. ( 10.1146/annurev.ento.54.110807.090608) [DOI] [PubMed] [Google Scholar]
- 23.Holland B, Rice WR. 1998. Perspective: chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7. ( 10.2307/2410914) [DOI] [PubMed] [Google Scholar]
- 24.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol. 18, 41–47. ( 10.1016/S0169-5347(02)00004-6) [DOI] [Google Scholar]
- 25.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA.), pp. 123–166. London, UK: Academic Press. [Google Scholar]
- 26.Rice WR, Holland B. 1997. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific red queen. Behav. Ecol. Sociobiol. 41, 1–10. ( 10.1007/s002650050357) [DOI] [Google Scholar]
- 27.Rice WR. 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 361, 232–234. ( 10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- 28.Rice WR. 1998. Male fitness increases when females are eliminated from the gene pool: implications for the Y-chromosome. Proc. Natl Acad. Sci. USA 95, 6217–6221. ( 10.1073/pnas.95.11.6217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook PA, Wedell N. 1999. Non-fertile sperm delay female remating. Nature 397, 486 ( 10.1038/17257) [DOI] [Google Scholar]
- 30.Martin OY, Hosken DJ. 2004. The reproductive consequences of population divergence through sexual conflict. Curr. Biol. 14, 906–910. ( 10.1016/j.cub.2004.04.043) [DOI] [PubMed] [Google Scholar]
- 31.Montrose VT, Harris WE, Moore PJ. 2004. Sexual conflict and cooperation under naturally occurring male enforced monogamy. J. Evol. Biol. 17, 443–452. ( 10.1046/j.1420-9101.2003.00654.x) [DOI] [PubMed] [Google Scholar]
- 32.Hotzy C, Arnqvist G. 2009. Sperm competition favours harmful males in seed beetles. Curr. Biol. 19, 1–4. ( 10.1016/j.cub.2009.01.045) [DOI] [PubMed] [Google Scholar]
- 33.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288. ( 10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 34.Rice WR, Chippindale AK. 2001. Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693. ( 10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 35.Pischedda A, Chippindale AK. 2006. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 4, 2099–2103. ( 10.1371/journal.pbio.0040356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsuki M, Harano T, Miyatake T, Okada K, Hosken DJ. 2012. Intralocus sexual conflict and offspring sex ratio . Ecol. Lett. 15, 193–197. ( 10.1111/j.1461-0248.2011.01725.x) [DOI] [PubMed] [Google Scholar]
- 37.Chippindale AK, Gibson JR, Rice WR. 2001. Negative genetic correlation for adult fitness between the sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675. ( 10.1073/pnas.98.4.1671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foerster K, Coulson T, Sheldon BC, Pemberton JM, Clutton-Brock TH, Kruuk LEB. 2007. Sexually antagonisitic genetic variation for fitness in red deer. Nature 447, 65–67. ( 10.1038/nature05912) [DOI] [PubMed] [Google Scholar]
- 39.Harano T, Okada K, Nakayama S, Miyatake T, Hosken DJ. 2010. Intralocus sexual conflict unresolved by sex-limited trait expression. Curr. Biol. 20, 2036–2039. ( 10.1016/j.cub.2010.10.023) [DOI] [PubMed] [Google Scholar]
- 40.Innocenti P, Morrow EH. 2010. The sexually antagonistic genes of Drosophila melanogaster. PLoS Biol. 8, 1000335 ( 10.1371/journal.pbio.1000335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada K, Miyatake T. 2010. Effect of losing on male fights of broad-horned flour beetle, Gnatocerus cornutus . Behav. Ecol. Sociobiol. 64, 361–369. ( 10.1007/s00265-009-0852-0) [DOI] [Google Scholar]
- 42.Okada K, Miyatake T. 2010. Plasticity of size and allometry in multiple sexually selected traits in an armed beetle Gnatocerus cornutus. Evol. Ecol. 24, 1–13. ( 10.1007/s10682-010-9370-9) [DOI] [Google Scholar]
- 43.Okada K, Miyatake T. 2009. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Anim. Behav. 77, 1057–1065. ( 10.1016/j.anbehav.2009.01.008) [DOI] [Google Scholar]
- 44.Yamane T, Okada K, Nakayama S, Miyatake T. 2010. Dispersal and ejaculatory strategies associated with exaggeration of weapon in an armed beetle. Proc. R. Soc. Lond. B 277, 1705–1710. ( 10.1098/rspb.2009.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuda Y, Yoshida T. 1984. Population biology of the broad-horned flour beetle, Gnathocerus cornutus (F) (Coleoptera, Tenebrionidae). 1. Life table and population parameters. Appl. Entomol. Zool. 19, 129–131. [Google Scholar]
- 46.Kotiaho JS, Simmons LW, Tomkins JL. 2001. Towards a resolution of the lek paradox. Nature 410, 684–686. ( 10.1038/35070557) [DOI] [PubMed] [Google Scholar]
- 47.Okada K, Fuchikawa T, Omae Y, Katsuki M. 2013. Pre-copulatory sexual selection in the cigarette beetle Lasioderma serricorne. Behav. Ecol. Sociobiol. 67, 53–59. ( 10.1007/s00265-012-1424-2) [DOI] [Google Scholar]
- 48.Partridge L, Fowler K. 1990. Non-mating costs of exposure to males in female Drosophila melanogaster. J. Insect Physiol. 36, 419–425. ( 10.1016/0022-1910(90)90059-O) [DOI] [Google Scholar]
- 49.Fedina TY, Lewis SM. 2008. An integrative view of sexual selection in Tribolium flour beetles. Biol. Rev. 83, 151–171. ( 10.1111/j.1469-185X.2008.00037.x) [DOI] [PubMed] [Google Scholar]
- 50.Simmons LW, Holley R. 2011. Offspring viability benefits but no apparent costs of mating with high quality males. Biol. Lett. 7, 419–421. ( 10.1098/rsbl.2010.0976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shakelton MA, Jennions MD, Hunt J. 2005. Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice trials. Behav. Ecol. Sociobiol. 58, 1–8. ( 10.1007/s00265-004-0907-1) [DOI] [Google Scholar]
- 52.Ritchie MG, Halsey EJ, Gleason JM. 1999. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou and Hall cycles in D. melanogaster song. Anim. Behav. 58, 649–657. ( 10.1006/anbe.1999.1167) [DOI] [PubMed] [Google Scholar]
- 53.Bussière L, Hunt J, Jennions MD, Brooks R. 2006. Sexual conflict and cryptic female choice in the black field cricket Teleogryllus oceanicus. Evolution 60, 792–800. ( 10.1554/05-378.1) [DOI] [PubMed] [Google Scholar]
- 54.Narraway C, Hunt J, Wedell N, Hosken DJ. 2010. Genotype-by-environment interactions for female preference. J. Evol. Biol. 23, 2550–2557. ( 10.1111/j.1420-9101.2010.02113.x) [DOI] [PubMed] [Google Scholar]
- 55.Michalczyk L, Millard AL, Martin OY, Lumley AJ, Emerson BC, Gage MJG. 2011. Experimental evolution exposes female and male responses to sexual selection and conflict in Tribolium castaneum. Evolution 65, 713–724. ( 10.1111/j.1558-5646.2010.01174.x) [DOI] [PubMed] [Google Scholar]
- 56.Okada K, Blount JD, Sharma MD, Snook RR, Hosken DJ. 2011. Male attractiveness, fertility and susceptibility to oxidative stress are influenced by inbreeding in Drosophila simulans. J. Evol. Biol. 24, 363–371. ( 10.1111/j.1420-9101.2010.02170.x) [DOI] [PubMed] [Google Scholar]
- 57.Ingleby FC, Hunt J, Hosken DJ. 2013. Heritability of male attractiveness persists despite evidence for unreliable signals in Drosophila simulans. J. Evol. Biol. 26, 311–324. ( 10.1111/jeb.12045) [DOI] [PubMed] [Google Scholar]
- 58.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327. ( 10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 59.Katsuki M, Okada Y, Okada K. 2012. Impacts of diet quality on life-history and reproductive traits in male and female armed beetle, Gnatocerus cornutus . Ecol. Entomol. 37, 463–470. ( 10.1111/j.1365-2311.2012.01390.x) [DOI] [Google Scholar]
- 60.Sharma MD, Griffin RM, Hollis J, Tregenza T, Hosken DJ. 2012. Reinvestigating good genes benefits of mate choice in Drosophila simulans. Biol. J. Linn. Soc. 106, 295–306. ( 10.1111/j.1095-8312.2012.01883.x) [DOI] [Google Scholar]
- 61.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 62.Grafen A, Hails R. 2002. Modern statistics for the life sciences. Oxford, UK: Oxford University Press. [Google Scholar]
- 63.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics, 4th edn New York, NY: Longman. [Google Scholar]
- 64.Rønning B, Jensen H, Moe B, Bech C. 2007. Basal metabolic rate: heritability and genetic correlations with morphological traits in the zebra finch. J. Evol. Biol. 20, 1815–1822. ( 10.1111/j.1420-9101.2007.01384.x) [DOI] [PubMed] [Google Scholar]
- 65.Åkesson M, Bensch S, Hasselquist D, Tarka M, Hansson B. 2008. Estimating heritabilities and genetic correlations: comparing the ‘animal model’ with parent–offspring regression using data from a natural population. PLoS ONE 3, e1739 ( 10.1371/journal.pone.0001739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanckenhorn WU, Hosken DJ. 2003. Heritability of three condition surrogates in the yellow dung fly. Behav. Ecol. 14, 612–618. ( 10.1093/beheco/arg052) [DOI] [Google Scholar]
- 67.Wilson AJ, Reale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 68.Kirkpatrick M, Barton NH. 1997. The strength of indiect selection on female mating preferences. Proc. Natl Acad. Sci. USA 94, 1282–1286. ( 10.1073/pnas.94.4.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor ML, Wedell N, Hosken DJ. 2010. Attractive males do not sire superior daughters. Evol. Ecol. 24, 195–205. ( 10.1007/s10682-009-9298-0) [DOI] [Google Scholar]
- 70.Forsgren E, Karlsson A, Kvarnemo C. 1996. Female sand gobies gain direct benefits by choosing males with eggs in their nests. Behav. Ecol. Sociobiol. 39, 91–96. ( 10.1007/s002650050270) [DOI] [Google Scholar]
- 71.Östlund S, Ahnesjö I. 1998. Female fifteen-spined sticklebacks prefer better fathers. Anim. Behav. 56, 1177–1183. ( 10.1006/anbe.1998.0878) [DOI] [PubMed] [Google Scholar]
- 72.Sakurai G, Kasuya E. 2008. The costs of harassment in the adzuki bean beetle. Anim. Behav. 75, 1367–1373. ( 10.1016/j.anbehav.2007.09.010) [DOI] [Google Scholar]
- 73.Hosken DJ, Martin OY, Born J, Huber F. 2003. Sexual conflict in Sepsis cynipsea: female reluctance, fertility and mate choice. J. Evol. Biol. 16, 485–490. ( 10.1046/j.1420-9101.2003.00537.x) [DOI] [PubMed] [Google Scholar]
- 74.Hongo Y. 2012. Mating interaction of the Japanese horned beetle Trypoxylus dichotomus septentrionalis: does male-excluding behavior induce female resistance? Acta Ethol. 15, 195–201. ( 10.1007/s10211-012-0128-y) [DOI] [Google Scholar]
- 75.Moore AJ, Gowaty PA, Wallin WG, Moore PJ. 2001. Sexual conflict and the evolution of female mate choice and male social dominance. Proc. R. Soc. Lond. B 268, 517–523. ( 10.1098/rspb.2000.1399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore AJ, Gowaty PA, Moore PJ. 2003. Females avoid manipulative males and live longer. J. Evol. Biol. 16, 523–530. ( 10.1046/j.1420-9101.2003.00527.x) [DOI] [PubMed] [Google Scholar]
- 77.Kirkpatrick M. 1996. Good genes and direct selection in the evolution of mating preferences. Evolution 50, 2125–2140. ( 10.2307/2410684) [DOI] [PubMed] [Google Scholar]
- 78.Via S, Lande R. 1985. Genotype–environment interactions and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 79.Ingleby FC, Hunt J, Hosken DJ. 2010. The role of genotype-by-environment interactions in sexual selection. J. Evol. Biol. 23, 2031–2045. ( 10.1111/j.1420-9101.2010.02080.x) [DOI] [PubMed] [Google Scholar]
- 80.Kotiaho JS, Simmons LW. 2003. Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. J. Insect Physiol. 49, 817–822. ( 10.1016/S0022-1910(03)00117-3) [DOI] [PubMed] [Google Scholar]
- 81.Simmons LW, Kotiaho JS. 2007. The effects of reproduction on courtship, fertility and longevity within and between alternative male mating tactics of the horned beetle, Onthophagus binodis. J. Evol. Biol. 20, 488–495. ( 10.1111/j.1420-9101.2006.01274.x) [DOI] [PubMed] [Google Scholar]
- 82.Okada K, Katsuki M, Okada Y, Miyatake T. 2011. Immature performance linked with exaggeration of a sexually selected trait in an armed beetle. J. Evol. Biol. 24, 1737–1743. ( 10.1111/j.1420-9101.2011.02303.x) [DOI] [PubMed] [Google Scholar]
- 83.Lankford TE, Billerbeck JM, Conover DO. 2001. Evolution of intrinsic growth rates. II. Trade-offs with vulnerability to predation in Menidia menidia. Evolution 55, 873–1881. ( 10.1111/j.0014-3820.2001.tb00836.x) [DOI] [PubMed] [Google Scholar]
- 84.Fischer K, Zeilstra I, Hetz SK, Fiedler K. 2004. Physiological costs of growing fast: does accelarated growth reduce pay-off in adult fitness? Evol. Ecol. 18, 343–353. ( 10.1007/s10682-004-2004-3) [DOI] [Google Scholar]
- 85.Wade M. In press. Genotype by environment interactions and sexual selection: female choice in a complex world. In Genotype by environment interactions and sexual selection (eds Hunt J, Hosken DJ.). Oxford, UK: Oxford University Press. [Google Scholar]
- 86.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725. ( 10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]