Abstract
Large herbivorous mammals play an important role in structuring African savannahs and are undergoing widespread population declines and local extinctions, with the largest species being the most vulnerable. The impact of these declines on key ecological processes hinges on the degree of functional redundancy within large-herbivore assemblages, a subject that has received little study. We experimentally quantified the effects of three browser species (elephant, impala and dik-dik) on individual- and population-level attributes of Solanum campylacanthum (Solanum incanum sensu lato), an encroaching woody shrub, using semi-permeable exclosures that selectively removed different-sized herbivores. After nearly 5 years, shrub abundance was lowest where all browser species were present and increased with each successive species deletion. Different browsers ate the same plant species in different ways, thereby exerting distinct suites of direct and indirect effects on plant performance and density. Not all of these effects were negative: elephants and impala also dispersed viable seeds and indirectly reduced seed predation by rodents and insects. We integrated these diffuse positive effects with the direct negative effects of folivory using a simple population model, which reinforced the conclusion that different browsers have complementary net effects on plant populations, and further suggested that under some conditions, these net effects may even differ in direction.
Keywords: biodiversity, bush encroachment, indirect effects, invasive species, megaherbivores, seed dispersal
1. Introduction
Diverse assemblages of large mammalian herbivores are a distinguishing feature of African savannah ecosystems. These assemblages often include species spanning several orders of magnitude in body mass, with corresponding variation in foraging mode [1]. Collectively, these consumers play important functional roles in determining the structure and composition of savannah vegetation [2–4]. However, with the possible exception of elephants [5,6], the contributions of different large-herbivore species to these effects remain poorly characterized. This is because observational data rarely enable the attribution of ecological impacts to individual species, while most experimental studies use non-selective ‘all-or-none’ exclosure designs (but see [7,8]).
Understanding species-specific effects of large herbivores in African savannahs is particularly urgent in light of continent-wide population declines and local extinctions [9,10]. Larger species are more vulnerable to overharvesting and displacement, and often disappear first, followed by successively smaller species [11]. The consequences of such faunal attrition for savannah vegetation depend on the degree of functional redundancy among sympatric large herbivores [12]: if different species play similar ecological roles, then their effects may be largely substitutable, and compensatory responses may buffer key system processes and properties following extinction [13].
Most prior studies of functional redundancy in herbivore assemblages come from aquatic systems, and the evidence for redundancy is mixed [14–18]. The scant data from terrestrial animal communities are similarly nuanced [19–21]. Although explicit investigations are lacking for terrestrial large herbivores, extensive data on resource partitioning among sympatric African ungulates [1,22,23] suggest that redundancy in the broadest sense is unlikely (as argued more generally by Loreau [24]). Yet, there is also considerable evidence of dietary overlap and competition among large herbivores (e.g. [7,25,26]), raising the prospect of redundancy in a more restricted sense—namely, the effect on shared forage species. Although this is a narrow conception of herbivores' functional role, it nonetheless has significant implications for how we understand and manage African savannahs: bush encroachment and plant invasions are major management concerns in these systems [27] and their drivers remain poorly known [28].
We investigated the effects of browsing mammals on Sodom apple (Solanum campylacanthum, henceforth ‘Solanum’) in semi-arid Kenyan savannah. Although native to East Africa, this shrub is considered among the 100 worst invasive plants in the region: a ‘bush encroacher’ that is ‘toxic to livestock’ and ‘a major threat to grazing’, which ‘has become invasive in some protected areas to the detriment of native vegetation’ [29]. It is therefore important to understand the extent to which native browsers regulate Solanum, and the degree to which that function is impaired by species loss.
We measured multiple components of Solanum performance in the presence of intact browser assemblages and in a graduated series of 1 ha exclosures that removed successively smaller bodied subsets of the fauna, enabling us to quantify direct and indirect effects of elephants (Loxodonta africana), impala (Aepyceros melampus) and dik-dik (Madoqua cavendishi). In addition, because we could not experimentally assess one important aspect of the interaction (long-distance seed dispersal), we developed a mathematical model of herbivores' net effects on plant populations and used it to explore the robustness of our conclusions under varying assumptions about the effects of dispersal on establishment. We hypothesized that although all three browser species eat Solanum, they have complementary (non-redundant) impacts on plant individuals and populations, because variation in body size and foraging mode leads to qualitatively distinct plant–herbivore interaction complexes.
2. Material and methods
(a). Study system and experimental design
Solanum campylacanthum Hochst. ex A. Rich. (Solanum incanum sensu lato) is a perennial woody shrub up to 2 m tall that occurs throughout much of eastern Africa. The fruits (yellow berries, green when immature) contain steroidal glycoalkaloids typical of the genus and are toxic to humans and cattle [30]. Ripe fruits at our site averaged 2.4 cm diameter and contained 210 seeds. The seeds are dormant, hard and small (3 × 2 mm, 5 mg).
Data were collected from 2008 to 2013 at the Mpala Research Centre in central Kenya (0°17′ N, 37°52′ E). The study area comprises semi-arid Acacia bushland on sandy loams, with a discontinuous understory [31]. Peak rainfall is April–May; the dry season is January–March. Experimental work was conducted within the Ungulate Herbivory Under Rainfall Uncertainty (UHURU) large-herbivore exclosures, established in 2008. Complete details of the design and baseline conditions are given by Goheen et al. [32]. Briefly, the experiment comprises the following four treatments, applied to 1 ha (100 × 100 m2) plots using different configurations of electric fencing: megaherbivore-exclusion (–Mega), which excludes species more than 1000 kg (elephant and giraffe only); mesoherbivore-exclusion (–Meso), which excludes herbivores more than 50 kg (eight mesoherbivore species, plus elephants and giraffe); total-exclusion (–All), which excludes herbivores more than 5 kg (dik-dik, plus all larger species); and control, which is unfenced. Three blocks, each containing one replicate of each treatment, are situated at each of three sites along a 20 km transect (36 total 1 ha plots, nine replicates per treatment). Mean annual precipitation increases approximately 45% from north to south along this transect [32]. Unless otherwise specified, the data described below were collected within all 36 plots. Raw data from the first 5 years of UHURU are published elsewhere [33].
Elephant, impala and dik-dik are the dominant large herbivores at Mpala [34], and each is targeted by a different treatment in UHURU. Of the remaining browsers and mixed feeders, giraffe rarely forage in the understory [35], while gerenuk (Litocranius walleri) and eland (Taurotragus oryx) are uncommon. The remaining species known to occur in UHURU are grazers. Thus, experimental effects on Solanum are almost certainly driven by elephant, impala and/or dik-dik (an inference supported by more than 30 000 h of camera-trap data, described below).
(b). Population-level effects
We quantified Solanum abundance in two ways. First, we used data from 10 understory surveys conducted biannually from 2008 to 2013. Surveys used a 60 × 60 m2 grid in each plot with stakes at 10 m intervals (49 stakes per plot); we placed a 1 × 1 m quadrat at each stake and recorded presence/absence of Solanum. For analysis, we summed the occurrences within each plot for each survey, then averaged these sums across surveys to generate a single mean value for each plot. Second, in January 2012, we surveyed three parallel 60 × 5 m2 transects within each plot, recorded the total number of plants and scaled densities per hectare.
(c). Individual-level effects
Standing fruit crop was assessed in February 2011 for 10 randomly selected mature Solanum in each plot (n = 360). Because standing crop is an unreliable index of relative reproductive output across treatments owing to mammalian frugivory (see below), we surveyed an additional five plants per plot (n = 180) in September 2011, categorizing plants with more than or equal to 1 flower or bud as ‘reproductive’ and using the combined number of flowers and buds as a proxy for total reproductive output. In June 2012, we recorded height, canopy area (modelled as an ellipse) and mortality of 725 plants (approx. 20 per plot) that had been tagged in April of that year.
(d). Folivory and frugivory by browsers
In July 2011, we tagged three ripe, superficially undamaged fruits on each of five plants per plot (n = 180 plants, 540 fruits) by affixing a small plastic tie on the stem adjacent to each fruit. We re-surveyed these plants in September, recording the number of fruits missing (suggesting browser consumption), the number of insect attacks sustained by each fruit (indicated by exit holes in the fruit skin) and the number of fruits infected by fungus (indicated by brown discoloration; see [36] for use of similar criteria).
To determine the identity of herbivores feeding on Solanum and the frequency of herbivory events, we conducted a series of camera-trap assays (motion-triggered Bushnell Trophy Cam set to record 60 s videos). From April to November 2012, we logged 30 874 total trap-hours across 13 sites both inside and outside UHURU. We assessed baseline-browsing rates by camera-trapping 24 unmanipulated focal plants within UHURU (six per treatment, evenly distributed across the three sites). We similarly assessed frugivory by tying 10–20 fruits to 24 otherwise-fruitless focal plants with fishing line; this was done inside UHURU in April 2012 (same distribution of sampling effort as for the baseline-browsing assays above), and in several sites outside the experiment in August, September and November 2012.
(e). Seed dispersal by large herbivores
We quantified the frequency of Solanum seeds within impala dung by dissecting 93 fresh droppings. We opportunistically dissected elephant droppings to confirm that seeds were present, but did not attempt to quantify them. We assessed viability of seeds collected from impala dung, elephant dung and ripe fruits (n = 159, 90 and 170, respectively). Seeds were soaked for 3 h in 50% bleach (to deter fungal growth), dried, notched with a scalpel (to break dormancy) and placed on randomized agar plates that had been lightly coated with fungicide (metalaxyl and mancozeb). Germination was assessed after 14 days.
(f). Seed predation
To evaluate the impact of invertebrate seed predators on seed survival in fruits not consumed by ungulates, we collected 49 senescent fruits and counted the number of physically intact seeds remaining in each one. All of these fruits remained attached to the parent plant and had visible external insect and fungal damage; the pulp in these fruits was consumed or desiccated, suggesting that insect and fungal attack had largely run its course by the time fruits were collected. We collected only fruits whose original diameters could still be accurately measured from the intact shell (i.e. no shriveled or ruptured fruits). The number of seeds in undamaged fruits is a linearly increasing function of fruit diameter (r = 0.80, n = 36); we therefore estimated seed mortality by subtracting the number of intact seeds in damaged fruits from the number of seeds expected in undamaged fruits of equivalent size. In one case, this method yielded a negative seed-mortality estimate, which we rounded up to zero.
We have previously documented increases in small-mammal abundance within exclosures [32,33]. For this study, we quantified the intensity of rodent seed predation across treatments by placing six Petri dishes (three containing 100 seeds, three containing 200 seeds, all unwashed), beneath mature plants in each of the 12 experimental plots at the southern site (900 total seeds plot−1). We analysed the total number of seeds per plot removed after 48 h (the first interval in which some dishes were completely emptied). Several seed trays were camera-trapped to identify rodent seed predators.
(g). Data analysis
Unless otherwise specified, we averaged multiple measurements within each experimental plot to obtain a single mean value (thus, n = 36 for most analyses), then analysed these means using mixed-effects models with treatment, site and treatment × site as fixed effects, and block as a random effect (JMP 10.0). This model structure stems from the design of UHURU, in which sites were chosen to span multiple levels of rainfall [32]. However, we found few effects of site, and treatment effects were consistent in direction across sites; thus, we focus only on treatment effects, using Tukey's test for post hoc comparisons. Prior to analyses, non-normal data (Shapiro–Wilk W-test) were square-root, 4th-root or arcsine-square-root transformed as necessary. For mortality and probability of reproduction, which were highly skewed, we used a factorial binomial generalized linear model (GLM) without block effects and compared treatments using Bonferroni-corrected pairwise contrasts.
(h). Population model
Because the long-term effects of seed dispersal by browsers are exceedingly difficult to measure, we developed and parametrized a model of Solanum population dynamics to compare the net effects of different browsers on plant abundance under varying assumptions about how dispersal affects recruitment. Specifically, consistent with our experimental design and with the expectation of size-biased extinction in natural systems [11], we compared plant populations under three scenarios: the presence of both impala and elephants (corresponding to the control treatment); the presence of impala but not elephants (corresponding to the –Mega treatment) and the absence of both species (corresponding to the –Meso treatment). Dik-dik are implicitly present in all three scenarios, but did not disperse seeds (see Results), so we did not explicitly model their effects.
A graphical depiction of the model is shown in the electronic supplementary material, figure S1, and table S1 contains a full list of parameters and their empirical estimates. In the absence of elephant and impala, mature plants (x0) produce viable seeds at rate r0 and die at rate d0. A fraction s0 of seeds escape predation by insects and fungus and fall beneath the parent plant; of these, a fraction p0 escape predation by rodents, and a fraction e0 of the survivors establish and mature. We let K be the maximum number of plants that can establish in the available space; the more plants there are, the less space is available for new recruits. Because plants at our site reproduce almost continuously (in all but the driest conditions), we capture the dynamics in the ordinary differential equation
| 2.1 |
This system has two equilibria. One occurs when there are no plants 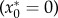 and is unstable, so any recolonization will lead to population expansion. The other is given by
and is unstable, so any recolonization will lead to population expansion. The other is given by
| 2.2 |
The population persists (and the equilibrium is stable) as long as r0s0p0e0 ≥ d0.
In the presence of impala (but not elephants), plants (x1) reproduce and die at modified rates r1 and d1. A fraction f1 of the seeds are ingested by impala; the remaining 1 − f1 uningested seeds have the same insect/fungus predation and establishment rates as when browsers are absent (s0 and e0, respectively). However, because seed predation by rodents was reduced in the presence of impala (see Results), we use p1 to denote the new fraction of seeds that escape rodent predation. We assume that seeds ingested by impala escape predation by insects and rodents, but may be killed during gut passage; the fraction of ingested seeds that are ultimately dispersed in viable condition is v. A fraction e of these v dispersed seeds establish and mature. We assume that v and e are equivalent for different browsers; thus, these two parameters are not indexed to the number of browser species present. We further assume that the maximum plant load K that can exist in the available space is unaffected by the number of browsers present. Finally, we assume that the browsers themselves are unaffected by Solanum abundance. Then,
| 2.3 |
Again, there are two possible equilibria, one of which 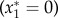 is unstable. The other is given by
is unstable. The other is given by
| 2.4 |
We assume (informed by our data, see Results) that the system with all browsers present (control) differs from the elephants-absent (–Mega) system in the fraction of seeds ingested by browsers, in plant reproductive and death rates, and in the intensity of seed predation (but not, as noted above, in the viability v and establishment rates e of ingested seeds). Thus, if r1, d1, f1, p1, e and v describe the system in the absence of elephants, with equilibrium plant abundance given by equation (2.4), then the intact system with all browsers present is described by r2, d2, f2, p2, e and v, with equilibrium abundance given by the modified version of equation (2.4)
| 2.5 |
We can estimate most of these parameters from our data (see the electronic supplementary material, table S1). Plant mortality (d0, d1, d2), seed escape from rodent predation (p0, p1, p2) and fractions of seeds ingested by browsers (f1, f2) were measured directly in—Meso,–Mega and control plots (subscripts 0, 1 and 2, respectively). Seed production (r0, r1, r2) could not be computed directly from standing fruit crop because fruits in some treatments are consumed before they can be counted. We therefore calculated rainfall-adjusted estimates of seed production by: (i) quantifying mean flower production in each treatment in the dry (January–April) and wet (April–June) seasons, (ii) using these two extremes to estimate flower production during the remainder of the year (assuming a linear relationship between rainfall and flower production), (iii) re-scaling by the observed flower–fruit conversion rate (which did not differ among treatments), and (iv) multiplying estimated fruit set by 210, the mean number of seeds per fruit (which is a function of fruit diameter and did not differ among treatments). We estimated seed escape from insects/fungus (s0) by first multiplying the fraction of non-ingested fruits damaged by insects/fungus by the mean survivorship of seeds in damaged fruits, then adding the fraction of fruits that were not attacked. Because K is assumed to be set by space and equivalent in all scenarios, its value does not influence the relative comparison of the scenarios. We lack data to estimate the establishment rates (e0, e) and the fraction of ingested seeds that are dispersed in viable condition (v); we therefore use our model to explore the effects of varying these parameters.
3. Results
(a). Population-level responses
Overall mean frequency of Solanum presence within 1 m2 quadrats from 2008 to 2013 increased with each successive reduction in the browser assemblage (8.7% in control; 11.1% in –Mega; 12.1% in –Meso; 16.8% in –All) (F3,18 = 4.33, p = 0.018). Absolute abundance varied through time (reflecting, among other things, a severe drought in 2009), but the rank-ordering of treatments remained constant from March 2011 through to the most recent survey in March 2013 (figure 1a). Total Solanum densities in January 2012 were nearly three-times greater in –All than in control plots (figure 1b; F3,18 = 6.20, p = 0.004).
Figure 1.
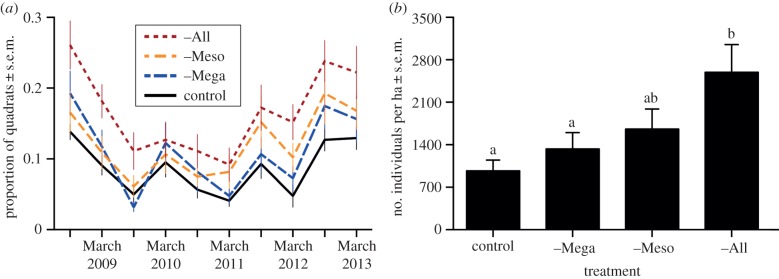
Population-level responses to experimental browser exclusion. (a) Proportion of 1 m2 quadrats containing at least one Solanum plant from October 2008 to March 2013. (b) Solanum densities in January 2012. Letters show significant differences. (Online version in colour.)
(b). Individual-level responses
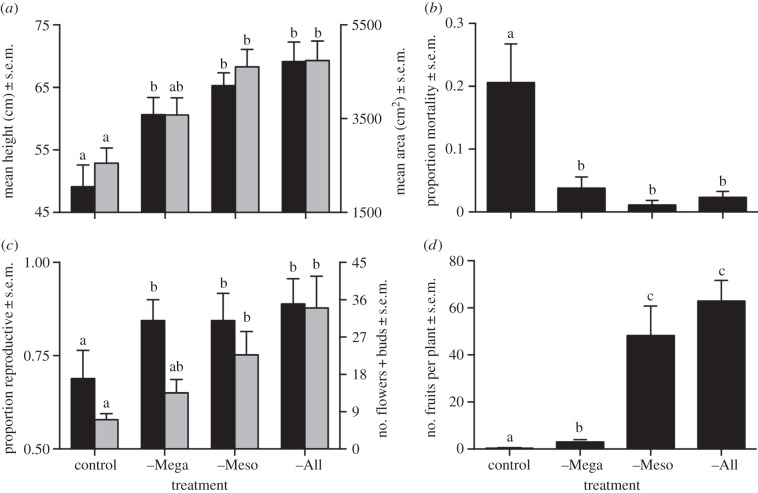
Elephants alone affected mean plant height, which was 20–40% greater in all exclusion treatments than in control plots (figure 2a; F3,18 = 10.53, p = 0.0003). Mean individual canopy area increased 40% with elephant exclusion and another 30% with impala exclusion, but did not change with dik-dik exclusion (figure 2a; F3,18 = 8.08, p = 0.001).
Figure 2.
Individual-level responses to browser exclusion. (a) Mean height (black bars) and canopy area (grey bars), June 2012. (b) Proportional mortality, April–June 2012. (c) Probability of reproduction (black bars) and estimated reproductive output (grey bars), September 2011. (d) Standing fruit crop, February 2011. Letters show significant differences.
Elephants also had unique effects on plant mortality, which was more than fivefold greater in control plots than in any herbivore-exclusion treatment (figure 2b; binomial GLM, logit link, 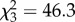 , p < 0.0001). Smaller plants were more likely to die (logistic regression of height on mortality, n = 716 plants,
, p < 0.0001). Smaller plants were more likely to die (logistic regression of height on mortality, n = 716 plants, 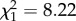 , p = 0.004), suggesting a cryptic cost of size suppression by browsers.
, p = 0.004), suggesting a cryptic cost of size suppression by browsers.
Finally, elephants alone reduced probability of reproduction, which was 20–30% greater in the three exclusion treatments than in control plots (figure 2c; binomial GLM, logit link, 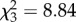 , p = 0.03). However, total reproductive output increased monotonically with each successive level of browser exclusion (figure 2c; F3,18 = 6.2, p = 0.004) and was positively correlated with plant size across treatments (r = 0.15, F1,168 = 28.6, p < 0.0001 for height; r = 0.47, F1,171 = 49.0, p < 0.0001 for canopy area), suggesting another cost of size suppression. Standing fruit crop increased eightfold (from 0.39 to 3.04 fruits per plant) when elephants were excluded, and by another order of magnitude when impala were excluded, but was unaffected by dik-dik exclusion (figure 2d; F3,18 = 83.6, p < 0.0001).
, p = 0.03). However, total reproductive output increased monotonically with each successive level of browser exclusion (figure 2c; F3,18 = 6.2, p = 0.004) and was positively correlated with plant size across treatments (r = 0.15, F1,168 = 28.6, p < 0.0001 for height; r = 0.47, F1,171 = 49.0, p < 0.0001 for canopy area), suggesting another cost of size suppression. Standing fruit crop increased eightfold (from 0.39 to 3.04 fruits per plant) when elephants were excluded, and by another order of magnitude when impala were excluded, but was unaffected by dik-dik exclusion (figure 2d; F3,18 = 83.6, p < 0.0001).
(c). Frugivory and folivory by browsers
Across all treatments, 267 of the 540 fruits tagged in July 2011 had disappeared by September. The proportion of tagged fruits missing was nearly three-times greater in control and –Mega plots than in –Meso and –All plots (figure 3a; F3,18 = 14.03, p < 0.0001), implicating impala.
Figure 3.
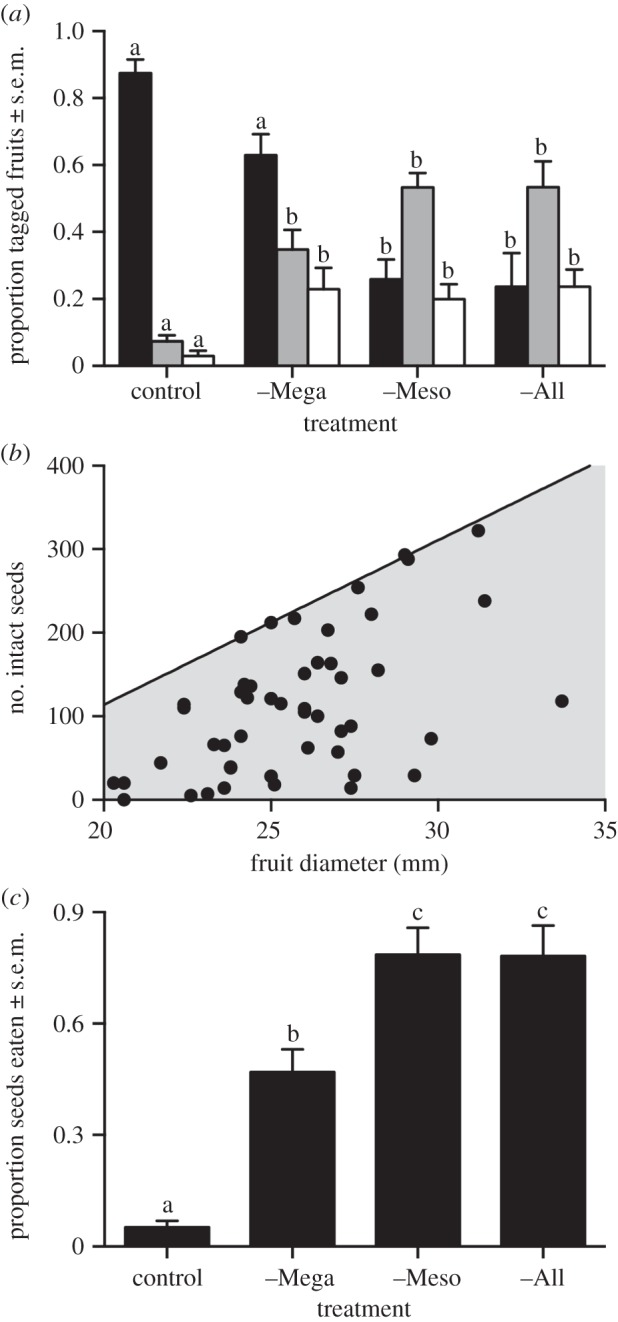
Frugivory and seed predation. (a) Proportion of tagged fruits that were consumed by browsers (black bars), attacked by insects (grey bars) and attacked by fungus (white bars), September 2011. (b) Fruit size versus seed number for fruits damaged by insects and fungi. Black line shows regression for undamaged fruits (data not shown). (c) Proportion of seeds removed from Petri dishes by rodents, June 2013. Letters show significant differences.
Indeed, impala accounted for 100% of fruit consumption recorded on camera traps within UHURU (electronic supplementary material, video S1). In control and –Mega plots, impala consumed more than 75% of experimentally added fruits within two weeks, at rates averaging 4.2 and 7.2 fruits plant−1 day−1, respectively. No fruits were removed from camera-trapped plants in –Meso or –All plots. Camera-trapping conducted outside UHURU confirmed that the vast majority of frugivory (88%) was by impala, with an additional 7% by elephants. Elephants killed 12 focal plants (16%) (electronic supplementary material, video S2).
Dik-dik, impala and elephant collectively accounted for 92% of browsing events on the foliage of unmanipulated focal plants camera-trapped within UHURU (8% were by hares and guinea fowl, which are not manipulated in UHURU). On average, plants in control plots were browsed 0.6 times per day, with dik-dik accounting for 47% of folivory events.
(d). Seed predation
Browser exclusion increased both the proportion of tagged fruits that sustained insect and fungus damage (figure 3a; F3,18 = 11.4, p = 0.0002 for insects; F3,18 = 8.3, p = 0.001 for fungus) and the mean number of insect exit scars per fruit (from 0.5 in control to more than 2 in –Meso and –All) (F3,18 = 6.94, p = 0.002). These differences were entirely explained by differential fruit consumption across treatments: of the 273 tagged fruits that were not consumed, 74% were damaged, regardless of herbivory treatment. Estimated seed mortality in fruits damaged by insects and fungus ranged from 0 to 100%, averaging 52% (figure 3b).
Seed predation by rodents was greatest in –All and –Meso, intermediate in –Mega and least in control plots (figure 3c; one-way ANOVA, F3,8 = 30.4, p = 0.0001). Seed predators included grass rats (Arvicanthis niloticus), rock rats (Aethomys hindei) and pouched mice (Saccostomus mearnsi: electronic supplementary material, video S3).
(e). Seed dispersal
Solanum seeds were found in 37% of impala defecations (2.6 ± 0.7 seeds per defecation), as well as in elephant dung. We confirmed viability for both uningested and ingested seeds. Germination was successful for 171 of 190 seeds taken from ripe fruits (90.0%), for 142 of 159 seeds from fresh impala dung (89%) and for 26 of 90 seeds from elephant dung (29%). We interpret these results conservatively owing to the artificial conditions necessary to germinate seeds in the laboratory, concluding only that gut passage does not necessarily reduce seed viability. We do not know what fraction of ingested seeds is digested and thus never dispersed, nor to what extent gut passage affects establishment rates in nature (the v and e terms in our model).
(f). Model analysis
As noted above, we estimated all parameters except for maximum plant load (K), establishment rates (e0, e) and the fraction of ingested seeds dispersed (v). Because we assumed K to be the same for all herbivory regimes, its value does not affect comparisons of equilibrium plant abundance, and although all three remaining parameters affect absolute abundance, the relevant quantity for assessing relative abundance is the ratio ve/e0, the combined effect of ingestion and dispersal on establishment (which encompasses the various processes known to affect recruitment, such as effects of gut passage on viability and germination, distance from parent plant and other aspects of microsite quality, fertilization by dung, etc.).
Figure 4a shows three possible regions that specify the net effects of the different herbivory scenarios on equilibrium plant abundance, depending on the value of ve/e0. For low values of ve/e0 (region I), plant abundance is greatest in the absence of both elephants and impala (–Meso), followed by the scenario in which impala are present and elephants absent (–Mega), and lastly by the intact assemblage with both elephants and impala present (control) 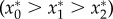 . Here, impala reduce plant abundance and adding elephants intensifies this effect, yet the plant population persists. For intermediate values of ve/e0 (region II), plant abundance is greatest when impala are present and elephants are absent (i.e. impala have a net positive effect), intermediate when both impala and elephants are absent, and lowest when all browsers are present
. Here, impala reduce plant abundance and adding elephants intensifies this effect, yet the plant population persists. For intermediate values of ve/e0 (region II), plant abundance is greatest when impala are present and elephants are absent (i.e. impala have a net positive effect), intermediate when both impala and elephants are absent, and lowest when all browsers are present 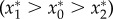 . Finally, for very large values of ve/e0 (region III), plant abundance is greater in the presence of impala—both with and without elephants—than it is when neither browser species is present
. Finally, for very large values of ve/e0 (region III), plant abundance is greater in the presence of impala—both with and without elephants—than it is when neither browser species is present 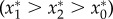 . In this region, elephants still have a suppressive net effect, but this is outweighed by the strong positive effect of impala.
. In this region, elephants still have a suppressive net effect, but this is outweighed by the strong positive effect of impala.
Figure 4.
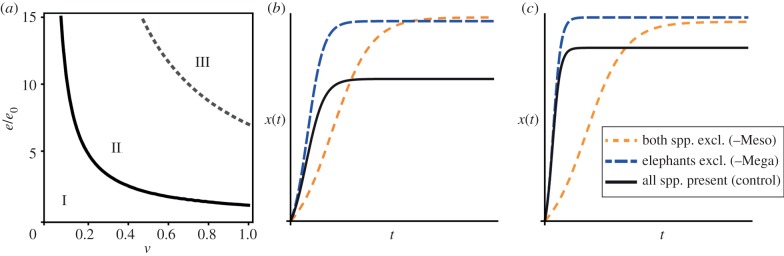
Model analysis. (a) Contour plot showing outcomes of the three browsing scenarios as a function of the dispersal effect ve/e0. Roman numerals correspond to the three regions described in the text. (b,c) Temporal dynamics of plant abundance, x(t), showcasing regions I (b) and II (c). For illustration, we fixed v = 0.6 (60% of ingested seeds are dispersed) and e0 = 0.005 (0.5% of all seeds establish in the absence of browsers); we then varied e to obtain e/e0 = 1 (neutral effect of dispersal on establishment (b)) and e/e0 = 3 (threefold greater establishment rate for dispersed seeds (c)). All other parameters are as specified in the electronic supplementary material, table S1. (Online version in colour.)
For impala to have a positive net effect on Solanum abundance (i.e. regions II and III), e must be greater than e0 regardless of the value of v (provided v > 0). In other words, dispersal must enhance establishment—indirect suppression of rodent seed predation cannot by itself generate a facilitative net effect. Temporal dynamics are shown for representative scenarios corresponding to region I (figure 4b) and region II (figure 4c); we consider region III unlikely because it requires extremely strong beneficial effects of dispersal.
4. Discussion
(a). Population-level responses to browser loss: no evidence for functional compensation
After 4.5 years, Solanum abundance was lowest in the presence of all browsers and increased with each successive species deletion. This rank-ordering of treatments was established after roughly 2.5 years and has remained constant since (figure 1a), indicating that dik-dik, impala and elephants have complementary (non-redundant) effects at the population level. Correspondingly, we found no evidence that smaller browsers compensate for the loss of larger species. The obvious caveat to this conclusion is that our 1 ha experimental plots are too small to induce a numerical response of smaller browsers (although the likelihood of such density compensation even in genuine extinction scenarios is questionable [37]).
However, we should have been able to detect behavioural or functional compensation—for example, if increased food availability in plots without larger herbivores caused smaller species to spend more time or consume more biomass in those plots. Along these lines, Young et al. [7] used similar exclosures to document compensatory habitat use by zebras in plots from which cattle and elephants were independently excluded. The lack of such effects in this study accords with data [32] showing no compensatory increases in dung density of impala in –Mega plots or dik-dik in –Meso plots. We acknowledge, however, that extrapolating from exclosures to the landscape can be misleading; explicit evaluation of the behavioural and functional responses (and their spatial grain) of small browsers to the removal of larger ones would increase our confidence in the interpretation proposed here. We do see compensatory increases in rodent density in exclosures [32], leading to increased seed predation (figure 3c), but this alone was insufficient to equalize Solanum densities across treatments (as predicted by our model).
This role of native browsers in suppressing an encroaching shrub that is toxic to cattle suggests an incentive for wildlife conservation in rangelands. This is a tangible management issue in our study region of Laikipia, Kenya, which comprises a ‘mosaic of wildlife-friendly and wildlife-intolerant’ private properties, many of which are used for cattle production [38, p. 53]. Ranchers associate Solanum with overgrazing (M. Littlewood, Mpala Ranch 2013, personal communication), yet our results show that it thrives in plots that are both under-grazed and under-browsed.
(b). Effects of different browsers on individual plants differ quantitatively and qualitatively
Elephants interacted strongly with Solanum: their exclusion influenced nearly every response variable measured and was the only treatment with a significant impact on survivorship, probability of reproduction and height (which in turn was a correlate of mortality risk and reproductive output) (figure 2a–c). Elephants also dispersed viable seeds and reduced seed predation by rodents (figure 3c), and thus their effects are not uniformly negative. However, elephants' effect on fruit removal and standing crop was weak relative to that of impala (figure 2d), suggesting that impala are the dominant seed dispersers. This interpretation is supported by camera-trap data, which showed impala foraging specifically on Solanum fruit (electronic supplementary material, video S1); we did not observe such selectivity by elephants, which ate plants whole (electronic supplementary material video S2), suggesting that seed dispersal by elephants comes at great risk of mortality to the parent plant.
The effects of elephants on tree cover in savannahs has received much attention [5,39], and their impacts on particular tree species have been documented (e.g. [40]). By contrast, remarkably few studies have addressed elephants' impacts on small shrubs and understory plants such as Solanum [7]. Yet, strong effects of elephants on smaller plants may be common: for example, Young et al. [7] showed that elephant exclusion reduced forb cover by 33%.
Like elephants, impala reduced canopy area and reproductive output, dispersed seeds and reduced seed predation (figures 2 and 3); unlike elephants, they did not directly reduce survivorship or likelihood of reproduction (figure 2). Dik-dik were unique among the three browsers in that they did not consume fruits, whether owing to gape limitation or fruit chemistry. Similarly, relative to plots from which elephants and impala were excluded, the additional exclusion of dik-dik had little impact on plant size, reproduction, adult mortality and seed predation (figures 2 and 3). Yet, dik-dik were the most frequent browsers of Solanum foliage and were the only species whose effects on Solanum were entirely negative: their detrimental effects via browsing were not offset by potentially beneficial effects of seed dispersal or reduced seed predation.
We hypothesize that this last observation helps to explain an apparent discrepancy between our results at the individual and population levels, namely that dik-dik exclusion caused a pronounced increase in Solanum abundance despite their weak individual-level effects, whereas elephants' population-level effects were surprisingly weak given their strong negative effects on individual performance (figures 1 and 2). In contrast to dik-dik, the negative effects of browsing by elephants and impala may be mitigated by beneficial effects on seed survival and recruitment, thus dampening the population-level response of Solanum.
(c). Model results indicate contrasting effects of different seed-dispersing browsers
Using empirical estimates of reproduction, fruit ingestion, seed predation and mortality (electronic supplementary material, table S1), we found plausible combinations of v (fraction of ingested seeds that are dispersed) and the ratio e/e0 (relative establishment rate of dispersed versus non-dispersed seeds) for which impala facilitated plants. Regions I and II in figure 4a encompass what we consider the most realistic values of these parameters. For example, if 60% of ingested seeds are dispersed, but dispersal has no effect on establishment (region I), then abundance will be greatest in the absence of browsers (figure 4b); if the same fraction of seeds are dispersed and those seeds are three times more likely to establish (region II), then plant abundance will be greater in the presence of impala (figure 4c).
In contrast to impala, elephants always have negative net effects on Solanum populations, and plant abundance is unlikely to be greater in the presence of elephants than in their absence (region III would require, e.g. that 80% of ingested seeds are dispersed and that dispersal increases establishment rates 10-fold). Thus, although an admittedly phenomenological description of the dynamics, the model is useful in two ways. First, it captures how different direct and indirect effects of elephants and impala on individual plants and seeds translate into non-redundant population-level impacts. Second, it suggests that there are realistic (perhaps even likely) scenarios in which the signs of those impacts differ, although our experimental results to date are more consistent with region I and figure 4b.
This simple model can be refined and extended as additional data become available. Particularly useful would be data on seed passage (v) and retention times from feeding trials, which could be combined with GPS-collar data to calculate seed shadows and evaluate the effects of differences between impala and elephants in dispersal distance and risk-sensitive habitat use. Information on S. campylacanthum recruitment dynamics (e), and its dependency on disperser identity and dung characteristics, would be similarly valuable.
(d). The complexity of large herbivore–plant interactions makes redundancy unlikely
Collectively, our results show that the interactions between Solanum plants and mammalian browsers are complex: browsers of different sizes, foraging modes and selectivity exert distinct suites of direct and indirect effects on plants, of which some are detrimental and others beneficial. Although it is well known that large herbivores can be important seed dispersers and exert cryptic indirect effects on plants, there is a persistent tendency to treat large-mammal herbivory as a simple pairwise interaction: surprisingly few studies have integrated the positive effects of seed dispersal and indirect interactions with the negative direct effects of folivory [41,42].
We suggest that the diversity of direct and indirect mechanisms that determine the impact of large-herbivore populations on their food plants makes functional redundancy, even in the narrowest sense, extremely unlikely [20,24]. Many studies have assessed redundancy based on functional groupings or diet overlap, but this approach is unreliable owing to ‘poor correlations between consumers’ diet and their direct and indirect impacts on a given ecosystem function' [18]. Our study highlights one reason why such correlations are poor: there are many different ways to eat the same plant.
Acknowledgements
We thank the Government of Kenya for permission to conduct this research, S. Kurukura, M. Mohamed and A. Hassan for field assistance, and S. Levin and S. Pacala for discussion, and anonymous reviewers for helpful comments.
Data accessibility
Datasets supporting this article have been published as a Data Paper [33].
Funding statement
Financial support was provided by the US National Science Foundation (OISE-0852961), the Princeton Environmental Institute's Grand Challenges Programme, Canada's National Sciences and Engineering Research Council, the Sherwood Family Foundation, a Templeton Foundation FQEB grant and National Geographic's Committee for Research and Exploration (CRE-9291-13).
References
- 1.McNaughton SJ, Georgiadis NJ. 1986. Ecology of African grazing and browsing mammals. Annu. Rev. Ecol. Syst. 17, 39–65. ( 10.1146/annurev.es.17.110186.000351) [DOI] [Google Scholar]
- 2.van Langevelde F, et al. 2003. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84, 337–350. ( 10.1890/0012-9658(2003)084[0337:EOFAHO]2.0.CO;2) [DOI] [Google Scholar]
- 3.Augustine DJ, McNaughton SJ. 2004. Regulation of shrub dynamics by native browsing ungulates on East African rangeland. J. Appl. Ecol. 41, 45–58. ( 10.1111/j.1365-2664.2004.00864.x) [DOI] [Google Scholar]
- 4.Sankaran M, Augustine DJ, Ratnam J. 2013. Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna. J. Ecol. 101, 1389–1399. ( 10.1111/1365-2745.12147) [DOI] [Google Scholar]
- 5.Laws RM. 1970. Elephants as agents of habitat and landscape change in East Africa. Oikos 21, 1–15. ( 10.2307/3543832) [DOI] [Google Scholar]
- 6.Bond WJ. 1994. Keystone species. In Biodiversity and ecosystem function (eds Schulze ED, Mooney HA.), pp. 237–253. Berlin, Germany: Springer. [Google Scholar]
- 7.Young TP, Palmer TM, Gadd ME. 2005. Competition and compensation among cattle, zebras, and elephants in a semi-arid savanna in Laikipia, Kenya. Biol. Conserv. 122, 351–359. ( 10.1016/j.biocon.2004.08.007) [DOI] [Google Scholar]
- 8.Staver AC, Bond WJ, Stock WD, van Rensburg SJ, Waldram MS. 2009. Browsing and fire interact to suppress tree density in an African savanna. Ecol. Appl. 19, 1909–1919. ( 10.1890/08-1907.1) [DOI] [PubMed] [Google Scholar]
- 9.Newmark WD. 1996. Insularization of Tanzanian parks and the local extinction of large mammals. Conserv. Biol. 10, 1549–1556. ( 10.1046/j.1523-1739.1996.10061549.x) [DOI] [Google Scholar]
- 10.Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, Green RE, Hutton JM. 2010. Large mammal population declines in Africa's protected areas. Biol. Conserv. 143, 2221–2228. ( 10.1016/j.biocon.2010.06.007) [DOI] [Google Scholar]
- 11.Cardillo M, et al. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241. ( 10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 12.Walker BH. 1992. Biodiversity and ecological redundancy. Conserv. Biol. 6, 18–23. ( 10.1046/j.1523-1739.1992.610018.x) [DOI] [Google Scholar]
- 13.Naeem S, Li S. 1997. Biodiversity enhances ecosystem reliability. Nature 390, 507–509. ( 10.1038/37348) [DOI] [Google Scholar]
- 14.Duffy JE, MacDonald KS, Rhode JM, Parker JD. 2001. Grazer diversity, functional redundancy, and productivity in seagrass beds: an experimental test. Ecology 82, 2417–2434. ( 10.1890/0012-9658(2001)082[2417:GDFRAP]2.0.CO;2) [DOI] [Google Scholar]
- 15.Micheli F, Halpern BS. 2005. Low functional redundancy in coastal marine assemblages. Ecol. Lett. 8, 391–400. ( 10.1111/j.1461-0248.2005.00731.x) [DOI] [Google Scholar]
- 16.Hoey AS, Bellwood DR. 2009. Limited functional redundancy in a high-diversity system: single species dominates key ecological process on coral reefs. Ecosystems 12, 1316–1328. ( 10.1007/s10021-009-9291-z) [DOI] [Google Scholar]
- 17.Burkepile DE, Hay ME. 2011. Feeding complementarity versus redundancy among herbivorous fishes on a Caribbean reef. Coral Reefs 30, 351–362. ( 10.1007/s00338-011-0726-6) [DOI] [Google Scholar]
- 18.Aguilera MA, Navarrette SA. 2012. Functional identity and functional structure change through succession in a rocky intertidal marine herbivore assemblage. Ecology 93, 75–89. ( 10.1890/11-0434.1) [DOI] [PubMed] [Google Scholar]
- 19.Ostfeld RS, Manson RH, Canham CD. 1997. Effects of rodents on survival of tree seeds and seedlings invading old fields. Ecology 78, 1531–1542. ( 10.1890/0012-9658(1997)078[1531:EOROSO]2.0.CO;2) [DOI] [Google Scholar]
- 20.Thibault KM, Ernest SKM, Brown JH. 2010. Redundant or complementary? Impact of a colonizing species on community structure and function. Oikos 119, 1719–1726. ( 10.1111/j.1600-0706.2010.18378.x) [DOI] [Google Scholar]
- 21.Philpott SM, Pardee GL, Gonthier DJ. 2012. Cryptic biodiversity effects: importance of functional redundancy revealed through addition of food web complexity. Ecology 93, 992–1001. ( 10.1890/11-1431.1) [DOI] [PubMed] [Google Scholar]
- 22.Jarman PJ. 1974. Social organization of antelope in relation to their ecology. Behaviour 48, 215–267. ( 10.1163/156853974X00345) [DOI] [Google Scholar]
- 23.du Toit JT. 1990. Feeding-height stratification among African browsing ruminants. Afr. J. Ecol. 28, 55–61. ( 10.1111/j.1365-2028.1990.tb01136.x) [DOI] [Google Scholar]
- 24.Loreau M. 2004. Does functional redundancy exist? Oikos 104, 508–611. ( 10.1111/j.0030-1299.2004.12685.x) [DOI] [Google Scholar]
- 25.Arsenault R, Owen-Smith N. 2002. Facilitation versus competition in grazing herbivore assemblages. Oikos 97, 313–318. ( 10.1034/j.1600-0706.2002.970301.x) [DOI] [Google Scholar]
- 26.Prins HHT, de Boer WF, van Oeveren H, Correia A, Mafuca J, Olff H. 2006. Coexistence and niche segregation of three small bovid species in southern Mozambique. Afr. J. Ecol. 44, 186–198. ( 10.1111/j.1365-2028.2006.00619.x) [DOI] [Google Scholar]
- 27.Beale CM, et al. 2013. Ten lessons for the conservation of African savannah ecosystems. Biol. Cons. 167, 224–232. ( 10.1016/j.biocon.2013.08.025) [DOI] [Google Scholar]
- 28.Ward D. 2005. Do we understand the causes of bush encroachment in African savannas? Afr. J. Range Forage Sci. 22, 101–105. ( 10.2989/10220110509485867) [DOI] [Google Scholar]
- 29.Lusweti A, Wabuyele E, Ssegawa P, Mauremootoo JR. 2011. Invasive plants of East Africa, Lucid v. 3.5 key and fact sheets. keys.lucidcentral.org/keys/v3/EAFRINET.
- 30.van Wyk B, van Heerden F, van Oudtshoorn B. 2002. Poisonous plants of South Africa. Pretoria, South Africa: Briza Publications. [Google Scholar]
- 31.Augustine DJ. 2003. Spatial heterogeneity in the herbaceous layer of a semi-arid savanna ecosystem. Plant Ecol. 167, 319–332. ( 10.1023/A:1023927512590) [DOI] [Google Scholar]
- 32.Goheen JR, et al. 2013. Piecewise disassembly of a large-herbivore community across a rainfall gradient: the UHURU experiment. PLoS ONE 8, e55192 ( 10.1371/journal.pone.0055192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kartzinel TR, Goheen JR, Charles GK, DeFranco E, Maclean JE, Otieno TO, Palmer TM, Pringle RM. 2014. Plant and small-mammal reponses to large-herbivore exclusion in an African savanna: five years of the UHURU experiment. Ecology 95, 787 ( 10.1890/13-1023R.1) [DOI] [Google Scholar]
- 34.Augustine DJ. 2010. Response of native ungulates to drought in semi-arid Kenyan rangeland. Afr. J. Ecol. 48, 1009–1020. ( 10.1111/j.1365-2028.2010.01207.x) [DOI] [Google Scholar]
- 35.Young TP, Isbell LA. 1991. Sex differences in giraffe feeding ecology: energetic and social constraints. Ethology 87, 79–89. ( 10.1111/j.1439-0310.1991.tb01190.x) [DOI] [Google Scholar]
- 36.Whitehead SR, Bowers MD. 2013. Evidence for the adaptive significance of secondary compounds in vertebrate-dispersed fruits. Am. Nat. 182, 563–577. ( 10.1086/673258) [DOI] [PubMed] [Google Scholar]
- 37.Houlahan JE. 2007. Compensatory dynamics are rare in natural ecological communities. Proc. Natl Acad. Sci. USA 104, 3273–3277. ( 10.1073/pnas.0603798104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadd ME. 2005. Conservation outside of parks: attitudes of local people in Laikipia, Kenya. Environ. Conserv. 32, 50–63. ( 10.1017/S0376892905001918) [DOI] [Google Scholar]
- 39.Asner GP, Levick SR. 2012. Landscape-scale effects of herbivores on treefall in African savannas. Ecol. Lett. 15, 1211–1217. ( 10.1111/j.1461-0248.2012.01842.x) [DOI] [PubMed] [Google Scholar]
- 40.MacGregor SD, O'Connor TG. 2004. Response of Acacia tortilis to utilization by elephants in a semi-arid African savanna. South Afr. J. Wildlife Res. 34, 55–66. [Google Scholar]
- 41.Vellend M, Knight TM, Drake JM. 2006. Antagonistic effects of seed dispersal and herbivory on plant migration. Ecol. Lett. 9, 319–326. ( 10.1111/j.1461-0248.2005.00878.x) [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Pérez J, Wiegand K, Ward D. 2011. Interaction between ungulates and bruchid beetles and its effect on Acacia trees: modeling the costs and benefits of seed dispersal to plant demography. Oecologia 167, 97–105. ( 10.1007/s00442-011-1964-6) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets supporting this article have been published as a Data Paper [33].