Abstract
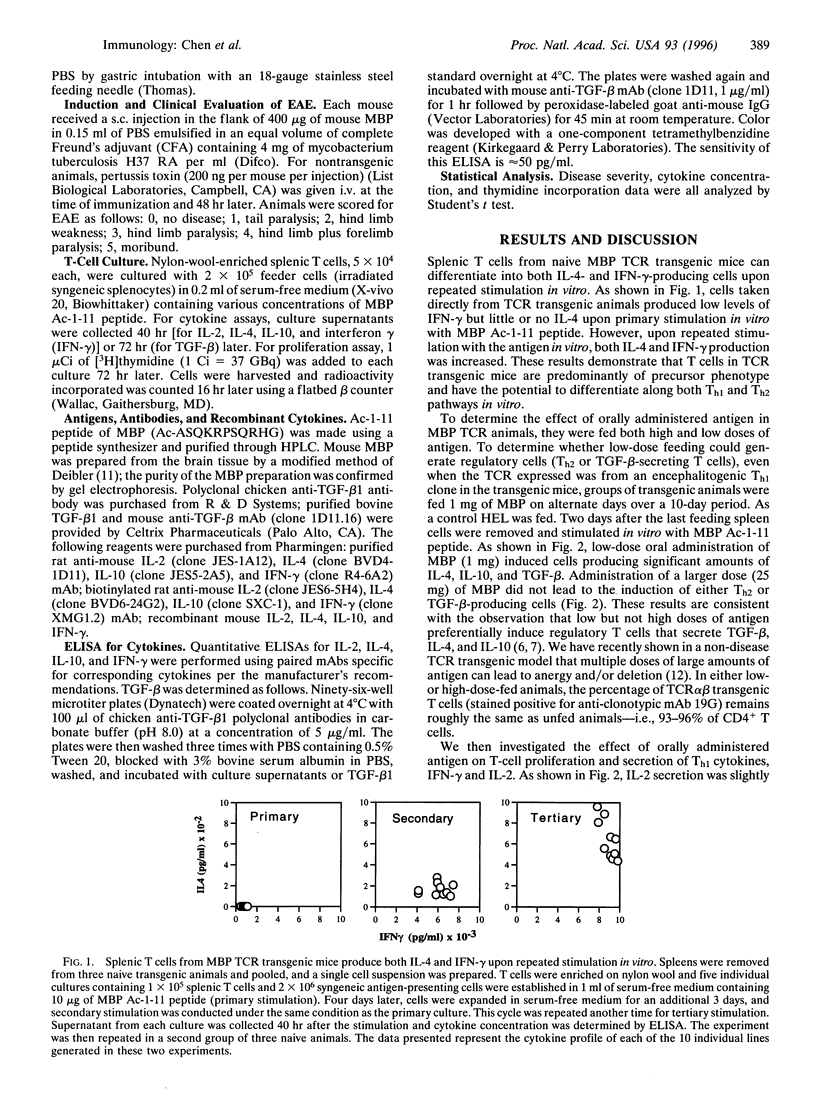
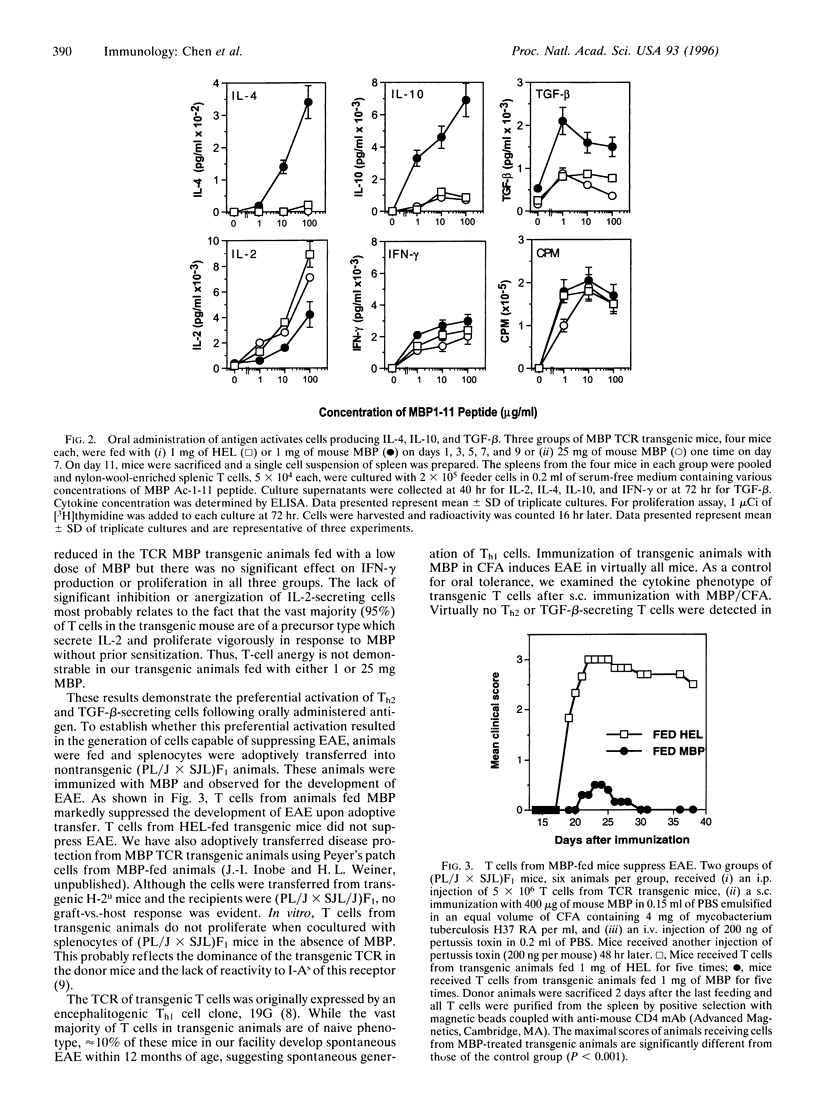
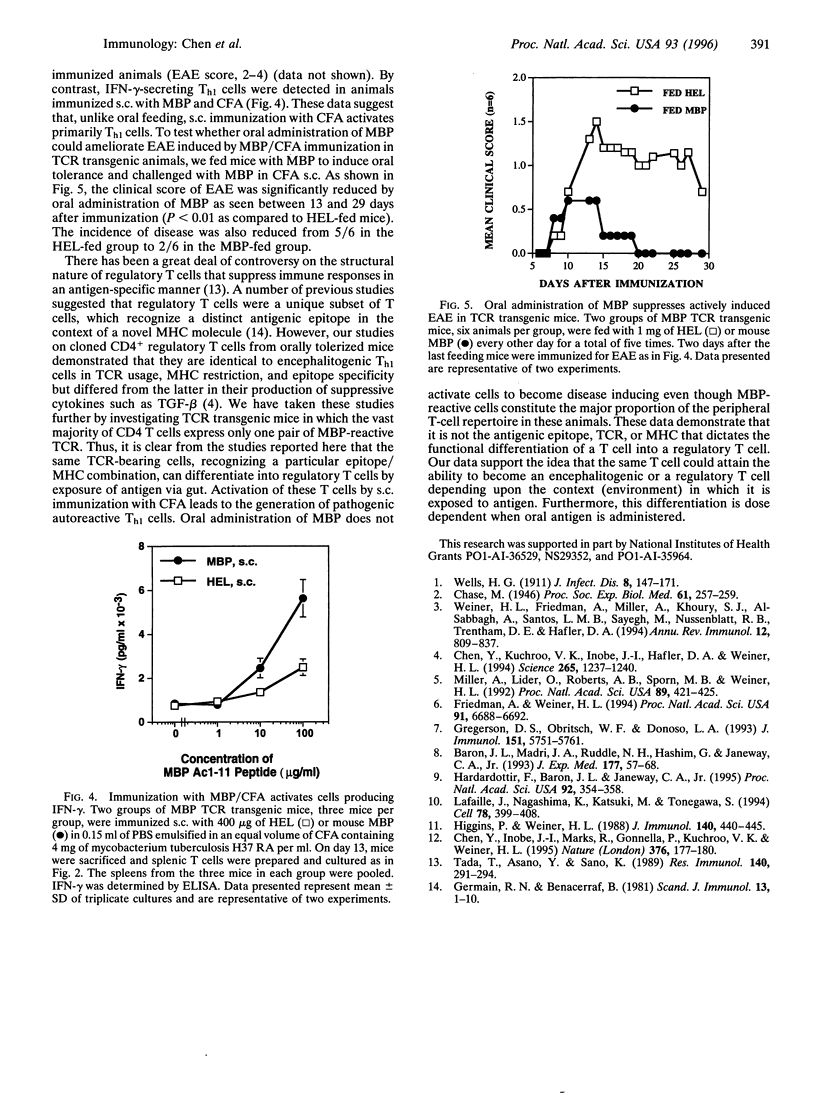
Orally administered antigens induce a state of immunologic hyporesponsiveness termed oral tolerance. Different mechanisms are involved in mediating oral tolerance depending on the dose fed. Low doses of antigen generate cytokine-secreting regulatory cells, whereas high doses induce anergy or deletion. We used mice transgenic for a T-cell receptor (TCR) derived from an encephalitogenic T-cell clone specific for the acetylated N-terminal peptide of myelin basic protein (MBP) Ac-1-11 plus I-Au to test whether a regulatory T cell could be generated from the same precursor cell as that of an encephalitogenic Th1 cell and whether the induction was dose dependent. The MBP TCR transgenic mice primarily have T cells of a precursor phenotype that produce interleukin 2 (IL-2) with little interferon gamma (IFN-gamma), IL-4, or transforming growth factor beta (TGF-beta). We fed transgenic animals a low-dose (1 mg x 5) or high-dose (25 mg x 1) regimen of mouse MBP and without further immunization spleen cells were tested for cytokine production. Low-dose feeding induced prominent secretion of IL-4, IL-10, and TGF-beta, whereas minimal secretion of these cytokines was observed with high-dose feeding. Little or no change was seen in proliferation or IL-2/IFN-gamma secretion in fed animals irrespective of the dose. To demonstrate in vivo functional activity of the cytokine-secreting cells generated by oral antigen, spleen cells from low-dose-fed animals were adoptively transferred into naive (PLJ x SJL)F1 mice that were then immunized for the development of experimental autoimmune encephalomyelitis (EAE). Marked suppression of EAE was observed when T cells were transferred from MBP-fed transgenic animals but not from animals that were not fed. In contrast to oral tolerization, s.c. immunization of transgenic animals with MBP in complete Freund's adjuvant induced IFN-gamma-secreting Th1 cells in vitro and experimental encephalomyelitis in vivo. Despite the large number of cells reactive to MBP in the transgenic animals, EAE was also suppressed by low-dose feeding of MBP prior to immunization. These results demonstrate that MBP-specific T cells can differentiate in vivo into encephalitogenic or regulatory T cells depending upon the context by which they are exposed to antigen.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber J. M., de Boer E., Garner C. D., Hasnain S. S., Wever R. Vanadium K-edge X-ray absorption spectroscopy of bromoperoxidase from Ascophyllum nodosum. Biochemistry. 1989 Sep 19;28(19):7968–7973. doi: 10.1021/bi00445a062. [DOI] [PubMed] [Google Scholar]
- Baron J. L., Madri J. A., Ruddle N. H., Hashim G., Janeway C. A., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993 Jan 1;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B., Germain R. N. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand J Immunol. 1981;13(1):1–10. doi: 10.1111/j.1365-3083.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Chen Y., Inobe J., Marks R., Gonnella P., Kuchroo V. K., Weiner H. L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995 Jul 13;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Friedman A., Weiner H. L. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson D. S., Obritsch W. F., Donoso L. A. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993 Nov 15;151(10):5751–5761. [PubMed] [Google Scholar]
- Hardardottir F., Baron J. L., Janeway C. A., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht H. J., Sobek H., Haag T., Pfeifer O., van Pée K. H. The metal-ion-free oxidoreductase from Streptomyces aureofaciens has an alpha/beta hydrolase fold. Nat Struct Biol. 1994 Aug;1(8):532–537. doi: 10.1038/nsb0894-532. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., Nagashima K., Katsuki M., Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994 Aug 12;78(3):399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Machius M., Wiegand G., Huber R. Crystal structure of calcium-depleted Bacillus licheniformis alpha-amylase at 2.2 A resolution. J Mol Biol. 1995 Mar 3;246(4):545–559. doi: 10.1006/jmbi.1994.0106. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Structural and genetic analysis of protein stability. Annu Rev Biochem. 1993;62:139–160. doi: 10.1146/annurev.bi.62.070193.001035. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Ladenstein R., Huber R., Bolognesi M., Avigliano L., Petruzzelli R., Rossi A., Finazzi-Agró A. Refined crystal structure of ascorbate oxidase at 1.9 A resolution. J Mol Biol. 1992 Mar 5;224(1):179–205. doi: 10.1016/0022-2836(92)90583-6. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund P., Sjöberg B. M., Eklund H. Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature. 1990 Jun 14;345(6276):593–598. doi: 10.1038/345593a0. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980 Sep 10;255(17):8199–8205. [PubMed] [Google Scholar]
- Rosenzweig A. C., Frederick C. A., Lippard S. J., Nordlund P. Crystal structure of a bacterial non-haem iron hydroxylase that catalyses the biological oxidation of methane. Nature. 1993 Dec 9;366(6455):537–543. doi: 10.1038/366537a0. [DOI] [PubMed] [Google Scholar]
- Simons B. H., Barnett P., Vollenbroek E. G., Dekker H. L., Muijsers A. O., Messerschmidt A., Wever R. Primary structure and characterization of the vanadium chloroperoxidase from the fungus Curvularia inaequalis. Eur J Biochem. 1995 Apr 15;229(2):566–574. doi: 10.1111/j.1432-1033.1995.tb20499.x. [DOI] [PubMed] [Google Scholar]
- Tada T., Asano Y., Sano K. Present understanding of suppressor T cells. Res Immunol. 1989 Mar-Apr;140(3):291–345. doi: 10.1016/0923-2494(89)90064-3. [DOI] [PubMed] [Google Scholar]
- Van Schijndel J. W., Barnett P., Roelse J., Vollenbroek E. G., Wever R. The stability and steady-state kinetics of vanadium chloroperoxidase from the fungus Curvularia inaequalis. Eur J Biochem. 1994 Oct 1;225(1):151–157. doi: 10.1111/j.1432-1033.1994.00151.x. [DOI] [PubMed] [Google Scholar]
- Verschueren K. H., Kingma J., Rozeboom H. J., Kalk K. H., Janssen D. B., Dijkstra B. W. Crystallographic and fluorescence studies of the interaction of haloalkane dehalogenase with halide ions. Studies with halide compounds reveal a halide binding site in the active site. Biochemistry. 1993 Sep 7;32(35):9031–9037. doi: 10.1021/bi00086a008. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Friedman A., Miller A., Khoury S. J., al-Sabbagh A., Santos L., Sayegh M., Nussenblatt R. B., Trentham D. E., Hafler D. A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- van Schijndel J. W., Simons L. H., Vollenbroek E. G., Wever R. The vanadium chloroperoxidase from the fungus, Curvularia inaequalis. Evidence for the involvement of a histidine residue in the binding of vanadate. FEBS Lett. 1993 Dec 27;336(2):239–242. doi: 10.1016/0014-5793(93)80811-8. [DOI] [PubMed] [Google Scholar]
- van Schijndel J. W., Vollenbroek E. G., Wever R. The chloroperoxidase from the fungus Curvularia inaequalis; a novel vanadium enzyme. Biochim Biophys Acta. 1993 Feb 13;1161(2-3):249–256. doi: 10.1016/0167-4838(93)90221-c. [DOI] [PubMed] [Google Scholar]



