Abstract
Predators influence prey populations not only through predation itself, but also indirectly through prompting changes in prey behaviour. The behavioural adjustments of prey to predation risk may carry nutritional costs, but this has seldom been studied in the wild in large mammals. Here, we studied the effects of an ambush predator, the African lion (Panthera leo), on the diet quality of plains zebras (Equus quagga) in Hwange National Park, Zimbabwe. We combined information on movements of both prey and predators, using GPS data, and measurements of faecal crude protein, an index of diet quality in the prey. Zebras which had been in close proximity to lions had a lower quality diet, showing that adjustments in behaviour when lions are within short distance carry nutritional costs. The ultimate fitness cost will depend on the frequency of predator–prey encounters and on whether bottom-up or top-down forces are more important in the prey population. Our finding is the first attempt to our knowledge to assess nutritionally mediated risk effects in a large mammalian prey species under the threat of an ambush predator, and brings support to the hypothesis that the behavioural effects of predation induce important risk effects on prey populations.
Keywords: anti-predator behaviour, African lion, Hwange National Park, risk effects, plains zebra
1. Introduction
Predators can affect their prey not only by killing them, but also by influencing their behaviour [1]. Predator-induced behavioural adjustments may carry costs for the prey through their influence on prey energetics and physiology. They can ultimately affect prey demography, in which case these effects are called ‘non-consumptive effects’ or ‘risk effects’ [2–4]. As revealed by a meta-analysis on predator–prey systems principally involving invertebrates, predators can have a greater effect on prey demography through risk effects than through direct consumption of individual prey [5]. In large mammalian systems, herbivores have been shown to use a wide array of behavioural adjustments to the presence of their predator (see [6,7] for habitat shift, [8] for temporal niche shifts and [9,10] for increase in vigilance levels).
Yet, very little is known on the risk effects associated with these behavioural adjustments. The few existing studies of risk effects of predation on large herbivores have come from the wolf (Canis lupus) and elk (Cervus elaphus) system of the Yellowstone National Park and have provided contrasted results (see [2,11] for evidence of significant risk effects and [12] for limited risk effects—keeping in mind that these contrasted results could arise from methodological differences [13]). New studies looking beyond behavioural adjustments in other natural systems are clearly needed to understand the risk effects of large mammalian predators on their prey populations.
Different factors may influence the occurrence and strength of risk effects. Microhabitat structure clearly influences predation risk and may thus ultimately affect diet selection [14]. Predator hunting mode is also likely to play an important role in risk effects: because ambush predators are relatively sedentary, the presence of their cues should be strongly indicative of predation risk and hence lead to strong behavioural adjustments by their prey. On the other hand, continuously moving active predators may saturate the habitat with cues of their presence and prey may be less responsive to these cues, as they provide less information regarding risk and associated risk effects are less important [15]. This has been supported by studies on invertebrates, with ambush predators producing stronger local risk effects than active predators [16,17]. This may also be the case in large mammalian systems as suggested by a recent behavioural study [18].
Here, we investigated whether the presence of an ambush predator, the African lion (Panthera leo), in the vicinity of plains zebra (Equus quagga) imposes nutritional costs in the latter. Nutritionally mediated risk effects are of particular interest as the quantity/quality of resources ingested is likely to directly influence prey body condition, survival and reproduction. The study was conducted in Hwange National Park, Zimbabwe, where plants grow on nutrient poor Kalahari sands and offer food with a low crude protein (CP) content to herbivores. CP content of the diet is important for herbivores, and especially for non-ruminants such as plains zebras, as they cannot synthesize some essential amino acids which can only be acquired through their diet [19]. Herbivores, and particularly non-ruminant herbivores, are therefore particularly constrained in these regions, and any factor influencing the ability of plains zebras to select the highest quality plants could have detrimental impacts on the fitness of individuals. Zebras are important prey for lions in this ecosystem [20], and behavioural studies have shown that, when chances of encountering lions are high, zebras increase their vigilance level [21] and select different habitats [7]. Here, we report on the possible nutritional costs of these behavioural adjustments.
2. Methods
(a). Study area and population
The study was carried out in Hwange National Park (HNP, ca 15 000 km² of semi-arid savannah) in western Zimbabwe (19°00′ S, 26°30′ E). The study population of plains zebra uses the Main Camp area (approx. 1000 km²) in the northern part of HNP, and the estimated zebra density in this area is 1.08 ± 0.53 individuals km−2 [22]. In this area, over 200 zebras have been individually identified by their unique stripe patterns.
(b). Zebra and lion GPS data
In seven different zebra harems, we equipped one of the females with a GPS radio-collar recording locations every hour. Plains zebras are a gregarious species that form non-territorial harems, and individuals of a harem move together and are seldom separated. Zebras feed mostly during the day, but feeding also occurs at night [23]. If the presence of a predator is detected during a night, then the foraging behaviour of zebras is likely to be influenced that night but also during the following day, because zebras will be aware of the presence of their predator in their vicinity and lion kills during daytime hours are not rare (unpublished data 2005–2007). As part of the long-term monitoring of the lion population in HNP, lions were also equipped with GPS radio-collars, with locations available hourly from 18.00 to 07.00. We used data from 10 GPS radio-collared lions (seven males and three females) that were in the same study area as the seven zebra harems at the time of this study. In HNP, male lions form coalitions and female lions form prides that can be considered rather independent groups as they are seldom sighted together (less than 10% of the sightings), and both male and female lions are successful hunters [20]. We cannot rule out the possibility that undetected lions were present during our study, but most lion groups were collared in the study area (90% of the lion groups seen in the study area included one GPS radio-collared individual). Additionally, lion group sizes are not very large in HNP and lions from the same group stay together most of the time, with females from a pride sighted together in 89.2 ± 7.4% of sightings. Hence, we are confident that the movements of the majority of lions were known in the study area and that GPS fixes from collared lions provide a reliable description of the risk of predation by lions. Mean straight-line distance moved between two GPS fixes (i.e. over a period of 1 h) was 521 m (10, 25, 75 and 90% percentiles were 0, 11, 835 and 2071 m, respectively) for female lions and 746 m (10, 25, 75 and 90% percentiles were 0, 17, 1166 and 2889 m, respectively) for male lions.
(c). Faecal sample collection and analysis of diet quality
The CP content of faeces is a good index of diet quality in horses, and probably in equids generally, as it provides information on the digestibility of the diet [24], and on the availability of CP which can be limiting for growth and reproduction in horses [25]. Faecal samples were collected during February–March 2010 from known zebras (97 samples from 42 individuals in 14 harems) after observation of defecation. All samples were dried within 48 h, either by air-drying or in a low heat (40°C) field oven and stored dry at room temperature until assayed. Chemical analyses of faecal samples were performed in the chemistry laboratory of INRA-URP3F (Lusignan). All samples were ground (1 mm grid) and analysed by near-infrared spectroscopy (NIRS). A NIR calibration was carried out on a larger sample pool and was used to predict the N content for all our samples (calibration properties: mean = 4.7, range = 1.95–7.4, s.d. = 0.91, R² = 0.94, SE of cross validation = 0.34, SD/SECV = 2.7). CP content was calculated by multiplying the N content by 6.25. All values of CP used for statistical analyses correspond to predicted values from NIRS.
(d). Index of predation risk
As lions are mainly active during the night [26,27], we used the available lion GPS-collar data representative of lion whereabouts in the landscape, and calculated an index of predation risk during the night (from 18.00 to 07.00). As stated before, this index is likely to influence the foraging behaviour of zebras not only at night but also during the day since zebras aware of their predator presence in their vicinity are likely to adjust their behaviour to this risk. Mean retention time of food in equids is around 44.5 h [28], so faecal samples represent an integrative measure of the quality of the diet of the previous 1–3 days. All GPS data from the nights between 20 h and 68 h prior to defecation were thus used. We calculated the minimum distance at which zebras had been from lions during those nights and used it as an index of predation risk. When there were no GPS data available for the zebra harem, we used field observations of zebras to know the location of the zebra harem early in the morning during the days before faecal sampling. We calculated the minimum distance between these locations and lion positions between 05.00 and 07.00 those days and used this distance as an index of predation risk. For these cases, we overlooked possible encounters earlier in the night and consequently underestimated the frequency and strength of anti-predator responses, a conservative approach as it will work against detection of a clear pattern.
(e). Analyses
In total, 65 faecal samples from 30 individuals for which information on zebra and lion position were simultaneously available were used in the analyses. We used linear-mixed models (function lmer of package lme4 [29], using R software [30]) with CP content of the faeces as the dependant variable and distance to lions (two classes: lions closer or further than 2 km) and sex of the individual as fixed effects. There is no agreement about the distance at which zebras can detect the presence of lions, but several studies have shown that zebras may adjust their behaviour to the presence of lions within a 2 km radius [7,8,21]. Lions are territorial, and home ranges of the monitored lions clearly encompassed the monitored area. We controlled for the sex of the individual zebra in the model, as males and females might differ in the quality of the diet they are ingesting (as observed in Camargue horses [31]). Because individuals from different sexes may differ in their response to predation risk [32], we made a preliminary check for such differential responses. Because the interaction between sex and distance to lions had no effect, it is not presented hereafter owing to the sample size. We included a random effect on the intercept for data collected on the same day in the same group, thus accounting for the fact that samples coming from members of the same harem on the same date were correlated. We also included a random effect on the intercept for individuals nested in harems, to take account of repeated measures on individuals and groups. A random effect varying among individuals nested in harems was included on the coefficient relating distance to lions to CP in the faeces, to allow for different reactions to risk of predation between individuals in their groups, and also to have better estimates of the effects and reduce type I error (see [33]). As there are some controversies on the use of likelihood ratio test in mixed models [34], we computed 95% confidence intervals on the parameter estimate using parametric bootstrap (10 000 replications).
3. Results
The faeces of male zebras had lower CP content than did those of females (estimate = −0.35; 95% CI: −0.68 to −0.01, taking females as reference). CP content in the faeces of zebras was lower when lions had been close during the nights prior to defecating than when lions had been farther away (figure 1, estimate = −0.66; 95% CI: −1.21 to −0.09, taking the class distance more than 2 km as reference). CP content ranged from 3.85% to 5.65% when lions were within 2 km of the zebra, and from 4.40% to 8.17% when lions were further than 2 km from the zebra.
Figure 1.
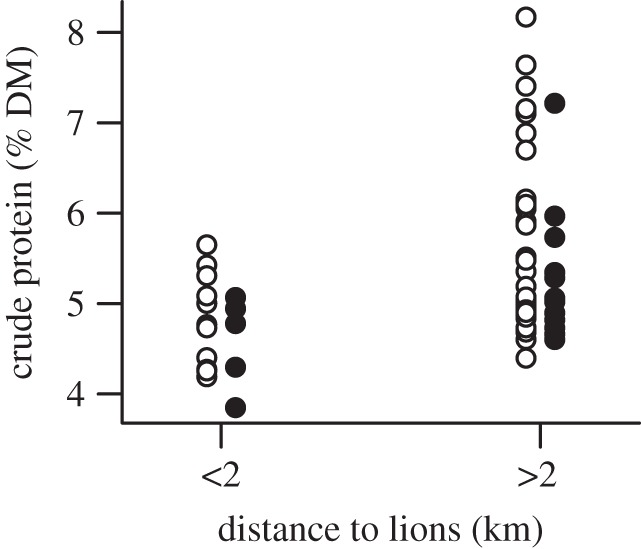
Effects of the distance to lions in the previous nights (see Methods) on the crude protein content of the faeces (a good index of diet quality) of plains zebras in Hwange National Park, Zimbabwe. Empty and filled circles represent females and males, respectively. DM, dry matter.
4. Discussion
Large apex predators are threatened worldwide and there is an urgent need to fully understand their role in the ecosystems [35]. This study is one of the first attempts to assess the nutritionally mediated risk effects of predation in large mammals in a system different from the North American Yellowstone wolf–elk system. Our finding provides support for the hypothesis that behavioural adjustments to the risk of predation carry costs for large mammalian prey [3]. The CP content of zebra faeces was 14% lower when zebras had been in the vicinity of lions in the preceding nights, suggesting that the behavioural adjustments previously observed [7,21] may also entail nutritional costs.
The CP content of faeces in HNP is low for zebras (approx. 5% during the wet season) compared with other sites (e.g. 7% in Mkuzi Game Reserve, South Africa, [36] or 7.5% in the Kruger National Park, South Africa [37], both during the wet season), and obtaining forage with the highest CP content may be crucial to maintaining a positive protein balance. The variance of CP contents declined when lions where nearby, and high CP levels (more than 6%) were obtained only when zebras had been foraging far from lions. The decline in diet quality when lions are close could thus be critical in this low nutrient environment.
The mechanisms leading to the lower diet quality under increased predation risk were not studied in detail here, as the data collected did not allow a rigorous investigation of these mechanisms. Identifying these mechanisms is, however, clearly a research priority for the future. Observations of predator–prey behavioural interactions in the Hwange ecosystem (independent of the faecal sample collection from this study) suggest that the decrease in diet quality detected here could arise from either a habitat shift [7], possibly to safer habitats of lower quality, or from an increase in the intense vigilance level of zebras when lions are in their vicinity [21], leaving less time to select patches or bites of higher quality.
Ultimately, the fitness costs associated with the lower diet quality demonstrated here will depend on the frequency of possible encounters between zebras and lions, i.e. when zebras are in the vicinity of lions and able to detect their presence (see also [12] for similar considerations). Further, the population consequences of the lower diet quality under the risk of predation will depend on whether the population is more limited by bottom-up or top-down factors and on how these effects interact. Across the landscape, the relative amounts of safe versus risky habitat seem to strongly affect which process dominates, with bottom-up forces more important in a landscape that is predominately safe and top-down forces more important when risky habitat predominates [38,39]. The Hwange zebra population is likely to be limited by predation (unpublished data), and hence individuals might make a trade-off between diet quality and the risk of predation with lower fitness costs.
Quantifying predation risk solely from GPS-collared predator locations can substantially underestimate the frequency and strength of anti-predator responses, because you cannot detect encounters between predators and prey that occur in the movements between GPS fixes, and because encounters between prey and un-collared predators can go undetected as well [13]. We believe this underestimation bias is relatively minor in our study for three reasons: (i) lions are not cursorial predators and do not move very far in 1 h; (ii) almost all lion groups had one collared individual and individuals from the same group tend to stay together most of the time and (iii) our study revealed a strong response from zebras.
In a recent study of elk, body reserves and reproduction were little affected by encounters with wolves, suggesting that the behavioural adjustments to the risk of predation were not high enough to affect the fitness of this prey [12]. Because this study quantified risk from GPS locations taken at intervals of 3 h and wolf is a cursorial predator, it is likely that the encounter rate between elk and wolves was underestimated, which could partly explain the contrasted results compared with other studies on the same system [2,11]. However, this result is consistent with predictions based on the predator hunting mode [16,17], as wolves are active predators and hence not expected to induce strong risk effects. Our study is the first attempt to our knowledge to assess nutritionally mediated risk effects in a large herbivore in response to an ambush predator, and is consistent with the expectation that ambush predators are likely to induce strong risk effects. This is also consistent with the results from a recent behavioural study [18] where herbivores responded behaviourally to the presence of lion, an ambush predator, but not to the presence of wild dog (Lycaon pictus), an active predator.
Our study adds to the current debate on the risk effects of predation in large mammalian systems [2,11,12] and highlights the need to investigate the fitness costs associated with the behavioural effects of predation. It further draws attention to the risk that such effects on the nutrition of prey—prompted by the mere presence of predators—could easily be misinterpreted as bottom-up phenomena (as pointed by Christianson & Creel [11]). The link between the lower diet quality demonstrated here and the fitness of individuals, and ultimately their population dynamics, is a clear research priority.
Acknowledgements
The Director General of the Zimbabwe Parks and Wildlife Management Authority is acknowledged for providing the opportunity to carry out this research and for permission to publish this manuscript. Relevant animal care protocols were followed, and approval received from the appropriate agencies. This work was funded by the Centre National de la Recherche Scientifique (Programme Zones Ateliers) and the Agence Nationale de la Recherche (FEAR project ANR-08-BLAN-0022). The lion fieldwork was made possible with grants from the Darwin Initiative for Biodiversity Grant 162/09/015, the Eppley Foundation, Disney Foundation, Rufford Maurice-Laing Foundation, Marwell Preservation Trust, Regina B. Frankenburg Foundation, Panthera Foundation, Boesak and Kruger Foundation, SATIB Trust and the generosity of the late Rivington and Joan Winant, and the Recanati-Kaplan Foundation. We thank all the people who participated to the lion fieldwork, particularly Jane Hunt, Nic Elliot and Brent Stapelkamp. We thank Veronique Menanteau and Corinne Melin for their help during technical analyses. This manuscript greatly benefited from helpful comments from Burt P. Kotler and Scott Creel.
Data accessibility
Crude protein content: Dryad: doi:10.5061/dryad.g2m0j.
References
- 1.Lima SL. 1998. Non lethal effects in the ecology of predator–prey interactions. Bioscience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 2.Creel S, Christianson D, Liley S, Winnie JA. 2007. Predation risk affects reproductive physiology and demography of elk. Science 315, 960 ( 10.1126/science.1135918) [DOI] [PubMed] [Google Scholar]
- 3.Creel S, Christianson D. 2008. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201. ( 10.1016/j.tree.2007.12.004) [DOI] [PubMed] [Google Scholar]
- 4.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334, 1398–401. ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 5.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 6.Creel S, Winnie JAJ, Maxwell B, Hamlin K, Creel M. 2005. Elk alter habitat selection as an antipredator response to wolves. Ecology 86, 3387–3397. ( 10.1890/05-0032) [DOI] [Google Scholar]
- 7.Valeix M, Loveridge AJ, Chamaillé-Jammes S, Davidson Z, Murindagomo F, Fritz H, Macdonald DW. 2009. Behavioral adjustments of African herbivores to predation risk by lions: spatiotemporal variations influence habitat use. Ecology 90, 23–30. ( 10.1890/08-0606.1) [DOI] [PubMed] [Google Scholar]
- 8.Valeix M, Fritz H, Loveridge AJ, Davidson Z, Hunt JE, Murindagomo F, Macdonald DW. 2009. Does the risk of encountering lions influence African herbivore behaviour at waterholes? Behav. Ecol. Sociobiol. 63, 1483–1494. ( 10.1007/s00265-009-0760-3) [DOI] [Google Scholar]
- 9.Laundré JW, Hernandez L, Altendorf KB. 2001. Wolves, elk, and bison: reestablishing the ‘landscape of fear’ in Yellowstone National Park U.S.A. Can. J. Zool. 79, 1401–1409. ( 10.1139/cjz-79-8-1401) [DOI] [Google Scholar]
- 10.Périquet S, Valeix M, Loveridge AJ, Madzikanda H, Macdonald DW, Fritz H. 2010. Individual vigilance of African herbivores while drinking: the role of immediate predation risk and context. Anim. Behav. 79, 665–671. ( 10.1016/j.anbehav.2009.12.016) [DOI] [Google Scholar]
- 11.Christianson D, Creel S. 2010. A nutritionally mediated risk effect of wolves on elk. Ecology 91, 1184–1191. ( 10.1890/09-0221.1) [DOI] [PubMed] [Google Scholar]
- 12.Middleton AD, Kauffman MJ, McWhirter DE, Jimenez MD, Cook RC, Cook JG, Albeke SE, Sawyer H, White PJ. 2013. Linking anti-predator behaviour to prey demography reveals limited risk effects of an actively hunting large carnivore. Ecol. Lett. 16, 1023–1030. ( 10.1111/ele.12133) [DOI] [PubMed] [Google Scholar]
- 13.Creel S, Winnie JA, Christianson D. 2013. Underestimating the frequency, strength and cost of anti-predator responses with data from GPS collars: an example with wolves and elk. Ecol. Evol. 3, 5189–5200. ( 10.1002/ece3.896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay ME, Fuller PJ. 1981. Seed escape from heteromyid rodents: the importance of microhabitat and seed preference. Ecology 62, 1395–1399. ( 10.2307/1937304) [DOI] [Google Scholar]
- 15.Schmitz OJ. 2005. Behavior of predators and prey and links with population-level processes. In Ecology of predator–prey interactions (eds Barbosa P, Castellanos I.), pp. 256–278. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Preisser EL, Orrock JL, Schmitz OJ. 2007. Predator hunting mode and habitat domain alter nonconsumptive effects in predator-prey interactions. Ecology 88, 2744–2751. ( 10.1890/07-0260.1) [DOI] [PubMed] [Google Scholar]
- 17.Miller JRB, Ament JM, Schmitz OJ. 2013. Fear on the move: predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J. Anim. Ecol. 83, 214–222. ( 10.1111/1365-2656.12111) [DOI] [PubMed] [Google Scholar]
- 18.Thaker M, Vanak AT, Owen CR, Ogden MB, Niemann SM, Slotow R. 2011. Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology 92, 398–407. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair ARE, Fryxell JM, Caughley G. 2006. Wildlife ecology, conservation, and management, 2nd edn Oxford: Blackwell Publishing. [Google Scholar]
- 20.Davidson Z, Valeix M, Van Kesteren F, Loveridge AJ, Hunt JE, Murindagomo F, Macdonald DW. 2013. Seasonal diet and prey preference of the African lion in a waterhole-driven semi-arid savanna. PLoS ONE 8, e55182 ( 10.1371/journal.pone.0055182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Périquet S, et al. 2012. Influence of immediate predation risk by lions on the vigilance of prey of different body size. Behav. Ecol. 23, 970–976. ( 10.1093/beheco/ars060) [DOI] [Google Scholar]
- 22.Chamaillé-Jammes S, Valeix M, Bourgarel M, Murindagomo F, Fritz H. 2009. Seasonal density estimates of common large herbivores in Hwange National Park, Zimbabwe. Afr. J. Ecol. 47, 804–808. ( 10.1111/j.1365-2028.2009.01077.x) [DOI] [Google Scholar]
- 23.Klingel H. 1967. Soziale Organisation und Verhalten freilebender Steppenzebras. Z. Tierpsychol. 24, 580–624. [PubMed] [Google Scholar]
- 24.Mesochina P, Martin-Rosset W, Peyraud J-L, Duncan P, Micol D, Boulot S. 1998. Prediction of the digestibility of the diet of horses: evaluation of faecal indices. Grass Forage Sci. 53, 189–196. ( 10.1046/j.1365-2494.1998.5320189.x) [DOI] [Google Scholar]
- 25.Martin-Rosset W. 1990. L'alimentation des chevaux. Paris, France: INRA. [Google Scholar]
- 26.Schaller GB. 1972. The Serengeti lion. Chicago, IL: University of Chicago Press. [Google Scholar]
- 27.Cozzi G, Broekhuis F, Mcnutt JW, Turnbull LA, Macdonald DW, Schmid B. 2012. Fear of the dark or dinner by moonlight? Reduced temporal partitioning among Africa's large carnivores. Ecology 93, 2590–2599. ( 10.1890/12-0017.1) [DOI] [PubMed] [Google Scholar]
- 28.Duncan P, Foose T, Gordon I, Gakahu C. 1990. Comparative nutrient extraction from forages by grazing bovids and equids: a test of the nutritional model of equid/bovid competition and coexistence. Oecologia 84, 411–418. ( 10.1007/BF00329768) [DOI] [PubMed] [Google Scholar]
- 29.Bates D, Maechler M, Bolker B. 2013. Linear mixed-effects models. R package version 0.999999-2; http://www.CRAN.R-project.org/. [Google Scholar]
- 30.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- 31.Duncan P. 1992. Horses and grasses. The nutritional ecology of equids and their impact on the camargue. Berlin, Austria: Springer. [Google Scholar]
- 32.Winnie J, Creel S. 2007. Sex-specific behavioural responses of elk to spatial and temporal variation in the threat of wolf predation. Anim. Behav. 71, 215–225. ( 10.1016/j.anbehav.2006.07.007) [DOI] [Google Scholar]
- 33.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. ( 10.1093/beheco/arn145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-PLUS. New York, NY: Springer. [Google Scholar]
- 35.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 36.Edwards PB. 1991. Seasonal variation in the dung of African grazing mammals, and its consequences for coprophagous insects. Funct. Ecol. 5, 617–628. ( 10.2307/2389480) [DOI] [Google Scholar]
- 37.Codron D, Lee-Thorp JA, Sponheimer M, Codron J, De Ruiter D, Brink JS. 2007. Significance of diet type and diet quality for ecological diversity of African ungulates. J. Anim. Ecol. 76, 526–537. ( 10.1111/j.1365-2656.2007.01222.x) [DOI] [PubMed] [Google Scholar]
- 38.Laundré JW. 2010. Behavioral response races, predator–prey shell games, ecology of fear, and patch use of pumas and their ungulate prey. Ecology 91, 2995–3007. ( 10.1890/08-2345.1) [DOI] [PubMed] [Google Scholar]
- 39.Laundré JW, et al. In press The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology. ( 10.1890/13.1083.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Crude protein content: Dryad: doi:10.5061/dryad.g2m0j.


