Abstract
The singing of song birds can form complex signal systems comprised of numerous subunits sung with distinct combinatorial properties that have been described as syntax-like. This complexity has inspired inquiries into similarities of bird song to human language; but the quantitative analysis and description of song sequences is a challenging task. In this study, we analysed song sequences of common nightingales (Luscinia megarhynchos) by means of a network analysis. We translated long nocturnal song sequences into networks of song types with song transitions as connectors. As network measures, we calculated shortest path length and transitivity and identified the ‘small-world’ character of nightingale song networks. Besides comparing network measures with conventional measures of song complexity, we also found a correlation between network measures and age of birds. Furthermore, we determined the numbers of in-coming and out-going edges of each song type, characterizing transition patterns. These transition patterns were shared across males for certain song types. Playbacks with different transition patterns provided first evidence that these patterns are responded to differently and thus play a role in singing interactions. We discuss potential functions of the network properties of song sequences in the framework of vocal leadership. Network approaches provide biologically meaningful parameters to describe the song structure of species with extremely large repertoires and complex rules of song retrieval.
Keywords: bird song sequence, network analysis, syntax, nightingale, Luscinia megarhynchos
1. Introduction
Bird song belongs to the most complex animal communication systems. Though species differ considerably in their song organization, in all cases investigated so far, information (e.g. on the constitution or motivation of the signaller) can be encoded on several, potentially independently operating, levels [1].
To date, the majority of research on the organization and functions of song has been conducted on the inventory of the behaviour, i.e. repertoire size and repertoire composition (reviewed in, for example, [2]). Recently, however, it has been shown that individual differences in the production of certain song elements convey information about the singer and are used in communication (reviewed in [3]). The same holds true for rules of song or element delivery in long singing sequences. The sequencing of song types has been shown to relate for example to individuality [4], geographical origin [5,6], contextual variables [7–9] and learning [10,11]. Sequential rules of song type delivery have attracted the attention of biologists, psychologists and linguists, in particular, because these rules might shed light on learning, memorization and retrieval processes of large amounts of acoustic information [12].
The ‘syntactical’ similarities between the organization of complex bird song and human language have been stressed repeatedly, reviewed in [13–16], though on the other hand, the complexity of human syntax has been described as the central difference between human and non-human animal communication systems [17].
A crucial issue in the study of complex communication systems, such as bird song, is the detection of structural rules by methods of pattern detection or other mathematical algorithms. For example, traditionally the sequential order of song types or their constituents have been illustrated by flow charts and transition probabilities in the context of Markov chain analyses were calculated [18–21]. Alternatively, transitions of songs or within-song components were analysed by means of permutational approaches [22,23] or entropy calculations [4,20]. Taken together, the global finding of conventional inquiries into the orderliness of song performances is that sequencing is neither at random nor fully deterministic in most species [16]. Rather stochastic regularities in a songster's performance point to decisional processes during vocal interactions, endogenous periodicities in song control, learned associations of song compounds or even memory constraints during acquisition or retrieval [11,24].
A promising alternative to investigate the organization of song sequences might be the use of network analysis. Network analysis is based on graph theory and is widely used in several scientific areas as for example physics, computer science, linguistics and social sciences. In biology, network analysis was applied for example to food webs, social organization and, more recently, to molecular networks (reviewed in [25]). A pioneering attempt to apply network analysis to birdsong analysed the sequence of phrases in a 20 min song recording of a California thrasher [26]. This exemplary analysis was very promising: establishing network analysis as an approach to study bird song might have manifold implications, for example, in uncovering functions of sequential ‘syntax-like’ patterns for communication, for providing data for comparative studies of complex communication systems, including human language, where network analysis is a well-established tool in the analysis of syntactical rules (reviewed in [27]).
Finally, the approach may be useful in contributing to aspects of central processing and decision making in the singing bird or its addressees—that is males or females listening.
We applied a basic network analysis to the song of the common nightingale (Luscinia megarhynchos) by assigning song types as the nodes (also called vertices) and first-order transitions between song types as edges of the network (for an introduction to networks, see e.g. [25]). Nightingales seem particularly well suited to inquire into song complexity. Males learn large song type repertoires of about 180 different song types per individual male [4,28]. The repertoire size has been suggested to be an honest indicator of male quality: male nightingales with large repertoire sizes arrive earlier at the breeding site, have longer wings, and are heavier and in a better condition than males with smaller repertoires [29]. In sequences of song, renditions of same song types are spaced out and tend to reoccur after a particular interval ‘filled’ with renditions of other song types [4].
We addressed the following questions: do network approaches and measures to quantify network properties provide biologically meaningful data in describing the song structure of nightingale individuals? How would these measures relate to conventional song measures such as the repertoire size or methods to describe sequential patterns and to other male qualities potentially related to fitness as the age of the singer?
In addition, would a comparison of song types and their ‘roles’ in networks across males allow us to uncover shared rules for the use of these types in the singing of males?
Based on the results of the descriptive network analysis, we developed a playback experiment. Here, we asked whether male birds that hear song types with certain transition patterns would adjust their singing according to the transition patterns of the song types heard in the playback.
2. Material and methods
All analyses presented in this study are based on recordings of male nightingales in Berlin Treptower Park, a municipal city park (for details, see e.g. [29,30]). As part of a long-term project on song and breeding behaviour, males of the population were individually marked by coloured leg rings (for details, see e.g. [31]). The age class (yearling or older) was determined by subtle, though characteristic feather features [30,32,33]. We analysed nocturnal song of 19 males (2008: three males, 2009: three males, 2010: 12 males, 2011: one male, each male contributed only once). The males were selected randomly based solely on the quality of the song recording. Given that all recordings were obtained during the first days of the breeding season and males still sang at night (which they mostly stop after pair formation [34]), it is most probable that these males were (still) unmated and represent a good cross section of the population under study.
Nocturnal song was recorded using a Sennheiser ME66/K6 directional microphone connected to a portable Marantz PMD-600 solid-state recorder (sampling frequency 44 100 Hz, 16 bit). All sound analyses were conducted with the software Avisoft SASlab Pro v. 4.52 (R. Specht, Berlin, Germany). For a visual comparison of songs, we calculated and printed spectrograms of recordings with a resolution of 22 050 Hz, 16 bit. Songs were assigned to song types using a semi-automated method to identify nightingale song types using a combination of spectrogram cross correlation and visual comparison developed by M.W. (for details, see [35]).
The comparison based on a song type catalogue developed in our group (at the time of the study containing 623 different song types of 96 different males recorded from the years 2002 to 2011). Nightingales copy song types very accurately, and accordingly, each song in a given song sequence can either be assigned to a type already sung earlier by the same or another individual and thus is part of the catalogue, or establishes a new type. As a formal definition, we characterized songs as belonging to the same song type when they differed in not more than three of approximately 10 element types in the first two sections of the song and included the same repetitive sections. This did not only allow reliable comparisons within one recording, but also comparisons of the singing of different birds ([4,28]; see the electronic supplementary material, figure S1, for illustrations). The majority of song types could be assigned to catalogue songs, new song types were indexed and added to the catalogue. As a result of this assignment, each song sequence was translated into a sequence of song type names. Same song types in the singing of all males were indexed by the same song type name. These sequences formed the base for all following analyses.
As ‘conventional’ measures to be compared to data from the network analysis, we determined the birds' repertoire size and patterns of song-type transitions. Repertoire size was determined as the number of different song types in 533 consecutive songs (equalling approx. 1 h spontaneous nocturnal singing; see the electronic supplementary material, figure S2, for an example of a song sequence). This sample size has been shown to be sufficient to approach the asymptote of repertoire curves in the species [36]. To analyse whether some song types were sung in transition more often than would be expected by chance, we determined a ‘distance to chance χ²’ value for each bird as follows: we generated a song type transition matrix with current song type and following song type in rows and columns and the number of how often the respective transitions were sung as cell values for each individual. From this we calculated χ² values. We calculated the distance between these observed values and expected χ² values calculated from 2000 randomized versions of the birds' song sequence defining the chance level. Sequence randomization was performed by using the function sample() in R v. 2.14.1 [37]. We compared these two ‘conventional’ measures (repertoire size, χ² differences) to describe the song composition of nightingales with measures from quantifying network structure.
All analyses were performed using the software R v. 2.14.1 [37]. For the depiction of song sequences in networks, we used the plot function of the R package ‘network’ [38]. In these graphs, the positioning of nodes followed the ‘Fruchterman–Reingold algorithm’. The purpose of this algorithm is to position the nodes of a network in two-dimensional space in a way that all the edges are of more or less equal length and there are as few crossing edges as possible [39].
Network measures were calculated by using the respective functions in the R package ‘igraph’ [40]. All measures in this first part of network analysis were calculated based on undirected networks (regardless of the direction of song transition). First, we calculated the ‘shortest path’ (or geodesic distances) between each possible pairs of nodes. This is the path connecting both nodes with the minimal number of nodes in between. From that we calculated the mean of the shortest path length of all possible pairs of nodes per individual bird [41]. Second, we calculated the ‘transitivity’ by using the following equation: transitivity = (number of triangles × 3)/number of connected triples. This measure represents the probability that the adjacent nodes of a given node were connected as well. In other words, the more neighbours of a given node were connected with each other, the higher was the transitivity value (see figure 1a,b for an illustration of both measures). Transitivity measures were adjusted for repertoire size by calculating the respective residuals of the original transitivity values to a regression line obtained by linear regression of transitivity on repertoire size in random graphs with systematically variation of graph sizes.
Figure 1.
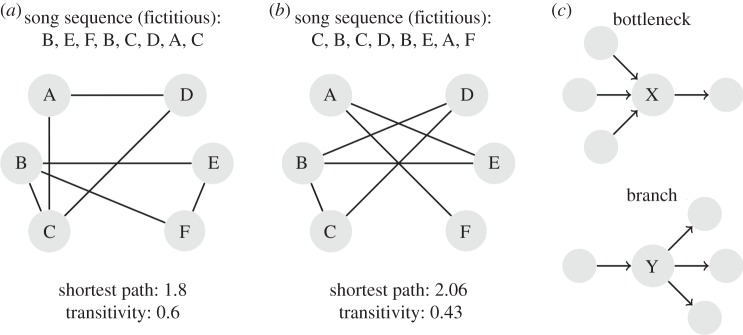
(a,b) Illustration of network measures. The exemplarily sequences depicted in (a,b) result in different values of transitivity (connected nodes) and shortest paths (minimal number of nodes between any two song types). (a) High transitivity value due to a high proportion of triangular-shaped connections, for example A,D,C and B,E,F. (b) Only one triangle (→ lower transitivity), but high shortest path value due to the long ways needed to connect C and D with F. (c) Transition properties of particular song types: bottleneck (X) and branch (Y).
We used average shortest path and transitivity measures in combination to decide whether nightingale song networks fulfil the properties of small-world networks. Networks with small average shortest path lengths (Sp) and large transitivity (Tr) values relative to corresponding random networks are termed small-world networks [42,43]. We used the ‘erdos.renyi.game’ function in igraph to construct random networks (each n = 2000) with the same number of nodes and edges and average number of edges per node as the respective observed network of nightingale songs. Small-world-ness was calculated by Swn = (Trobserved/mean Trrandom)/(SPobserved/mean SPrandom), if Swn > 1, the network was regarded as small-world following the suggestions in [43].
Networks with small-world properties are often found in social, technological and biological systems and were used to describe the sequence of words in human language [44]. Shared properties of these small-world networks have been studied extensively [42,45,46].
(a). Transition patterns of song types
Beyond these measures describing general properties of the networks, we looked for nodes (representing song types) with specific structural functions. Here, we used directed networks for analysis (with regard to direction of song transition). Each node (song type) specified in a directed network can be characterized by the number of different nodes following it, named out degree, and the number of different nodes (song types) preceding it, named in degree. The proportion of both degrees is a good estimate of specific transition patterns. Following the definitions in [26], we assigned song types into four categories depending on their respective transition patterns. (1) Bottlenecks = song types with many different preceding song types (high in degree) and few following song types (low out degree). Bottlenecks are narrowing the song bout to specific songs in transition. (2) Branches = song types with few preceding song types (low in degree) and many different following song types (high out degree), so that these song types are ‘opening’ the song sequence to more variability (see figure 1c for an illustration). (3) One-way patterns = one preceding song type and one following song type. These song types built linear transitions (songs that occur only once in the analysed song sequences were excluded). (4) Hourglasses = many different preceding and following song types. These song types might serve as hubs, i.e. highly connected nodes.
(b). Playback experiment
After determining the proportion of in degree and out degree for each song type and each male, we investigated whether different males used the same song types to realize the two transition patterns bottleneck and branch, i.e. whether the same song types are used across individuals to ‘open’ or ‘close’ a song sequence to more or less variability. For this, we identified the 20 song types with highest bottleneck values and highest branch values for each bird in our analysis (n = 19) and determined how often same song types occurred across the individuals. Assuming that other males in our population would share these song types and their transition patterns, too, we conducted a playback experiment with songs of these two transition patterns: playbacks contained songs with either bottleneck or branch transition pattern song types in order to address whether nightingales select response songs of same or different transition patterns.
The song types for the playback were selected based on the transition feature analysis described above. Each of 12 target birds was tested with two playbacks: a ‘bottleneck playback’ (12 song types with the respective transition pattern in the 19 analysed birds) and a ‘branch playback’ (again, 12 song types with branch transition pattern). As sources for the playback stimuli, we selected the respective song types from recordings of 12 different nightingales unknown to the focus birds. Each target bird received its two playback treatments consisting of the song of one source bird with at least 1 h interval between playbacks. The sequence of treatments was randomized across birds.
The playback files were broadcast with a portable MP X10i, ODYS player in .wav-format. The player was connected to a custom-build speaker (DKA Daniel Kiefer Audio, Heidelberg, Germany). Twelve spontaneously singing male nightingales in and around Berlin Treptower Park were tested at night with interactive playbacks (each playback song was started after the focus bird had finished its own song) in spring 2011 (28 April–05 May, 00.00–02.00). Songs were broadcast from at least 15 m away from the singing bird. Playback volume was standardized to peak amplitude of 86 dB SPL at 1 m distance (as measured with a CEL 314 precision sound level metre, integration time 125 ms). This corresponds to natural amplitude peaks measured in singing males [47].
Numerous studies have shown that nightingales readily respond to nocturnal playbacks and adjust their singing depending on the playback stimuli (e.g. [30,31]). To test whether the birds in our study responded differently to the two playback treatments, we analysed the target birds' reactions to the playbacks by comparing the following response measure between the two playback treatments: for each song type a bird sang during a playback trial, we assigned the respective out degree/in degree value calculated based on the song of 19 birds (see above).
3. Results
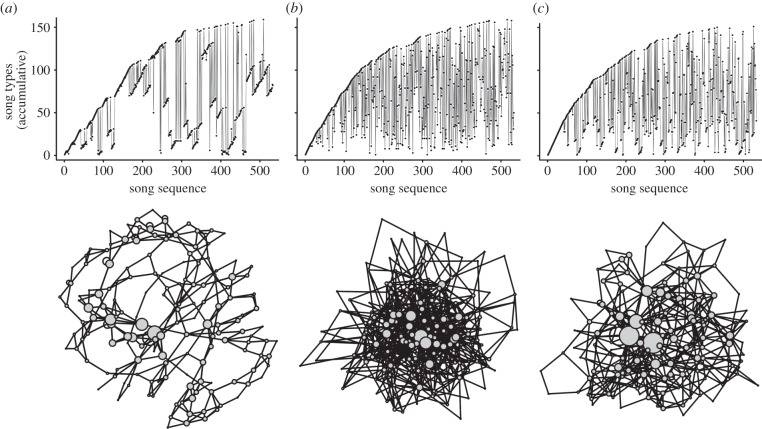
We performed a network analysis on song sequences of 19 nightingales and compared results with conventional song measures (table 1). Figure 2a–c gives a graphical presentation of song sequences. This figure contains song repertoire curves and song sequence networks for a male nightingale (bird id = 7, table 1) singing rather ‘ordered’ (figure 2a), a randomized sequence, based on the song types and their frequency of occurrence of this bird (bird id = 7) (figure 2b), and a bird (bird id = 5, table 1) singing in rather ‘disorderly’ sequences (figure 2c). Hereby, we refer to ‘orderliness’ not as binary measure but instead as a gradient measure, with the distance to chance χ2 serving as the estimate of orderliness. Note that both birds have about the same repertoire size (as can be inferred from similar maxima of repertoire curves in figure 2).
Table 1.
Conventional and network measures used in this study to describe the properties of song sequences sung by 19 male nightingales (ordered by repertoire size). All measures refer to song sequences of 533 successive songs. For details on measures, see text.
| conventional measures |
network measures |
||||
|---|---|---|---|---|---|
| bird id | repertoire size | distance χ² | transitivity | shortest paths | small-world-ness |
| 9 | 92 | 7317 | 0.34 | 3.27 | 3.32 |
| 2 | 118 | 8305 | 0.31 | 4.23 | 4.12 |
| 15 | 135 | 8292 | 0.28 | 4.13 | 4.95 |
| 12 | 143 | 4276 | 0.15 | 3.04 | 3.04 |
| 5 | 151 | 7249 | 0.17 | 3.50 | 4.29 |
| 18 | 157 | 11 022 | 0.31 | 4.81 | 6.39 |
| 7 | 159 | 15 001 | 0.30 | 6.28 | 5.71 |
| 19 | 161 | 9447 | 0.21 | 3.97 | 5.43 |
| 1 | 162 | 11 183 | 0.20 | 4.06 | 5.59 |
| 10 | 175 | 6300 | 0.17 | 3.75 | 5.08 |
| 17 | 180 | 10 349 | 0.18 | 4.15 | 5.70 |
| 4 | 181 | 11 537 | 0.22 | 4.86 | 6.52 |
| 13 | 182 | 12 437 | 0.19 | 4.25 | 6.14 |
| 16 | 183 | 14 493 | 0.19 | 5.18 | 6.10 |
| 3 | 188 | 11 184 | 0.14 | 4.28 | 4.98 |
| 14 | 192 | 9078 | 0.16 | 4.36 | 5.53 |
| 11 | 196 | 15 416 | 0.20 | 5.60 | 6.70 |
| 6 | 197 | 4726 | 0.12 | 3.65 | 4.63 |
| 8 | 208 | 8863 | 0.15 | 4.46 | 6.07 |
Figure 2.
(Top) Cumulative repertoire curves. Five hundred and thirty-three subsequent songs (about 1 h) of nocturnal nightingale song. X-axis: position of the song in sequence. Y-axis: different song types. (a) Bird ‘id 7’ (table 1), singing ordered. (b) Song sequence of bird ‘id 7’ (table 1) in an order randomized version. (c) Bird ‘id 5’ (table 1) singing ‘disorderly’. (Bottom) The same song sequences in network presentation (layout ‘Fruchterman–Reingold’): circles (nodes) = song types (size refers to song type frequency), lines (edges) = transitions of song types. Bird ‘id 5’ 151 nodes, 533 edges; bird ‘id 7’ 159 nodes, 533 edges.
A second ‘conventional’ measure, distance to chance χ², cannot directly be derived from repertoire curves. However, an impression of orderliness occurs due to the distribution of dots in the curve: orderly singing birds possess sequences of songs that recur in the same sequence later in singing. In the repertoire curve, this translates into dots forming long ‘bands’ as in figure 2a, whereas bands are shorter and occur less often in birds singing less ordered as in figure 2c. The order randomized song sequence of bird ‘id 7’ does not show longer bands (figure 2b).
In the network graphs of these three song sequences (figure 2a–c, bottom), nodes are positioned in a way that edges are of about equal length and there are as few crossings edges as possible. In addition, the size of the nodes (song types) reflects their frequency of occurrence. As can be seen in figure 2a, the singing of an ‘orderly singing bird’ results in an ordered network with high average shortest path and transitivity values (table 1). The values for the non-ordered singer (figure 2c and table 1, bird id 5) are considerably lower, though they are still higher than the ones calculated for the 2000 respective randomized networks. Thus, even the ‘low-order’ of the song sequence in figure 2c is far from being a random sequence. This did hold true for all 19 birds under investigation: their network measures were always considerably higher than the measures for the corresponding randomized network. This is further corroborated when considering the degree distribution (i.e. probability that a song type has a certain number of connections or transitions). A bell-shaped distribution is derived from random networks; whereas a tail to the right would suggest the existence of hubs, i.e. highly connected song types. All sequences investigated had indeed a right-tailed degree distribution, confirming that some song types were highly connected. The two conventional repertoire measures repertoire size and distance to chance χ² were not correlated in the 19 birds analysed (Spearman's rank correlation: n = 19, r = 0.347, p = 0.145). In other words, the number of different song types a bird sang was not correlated to how orderly these song types followed each other. The two network measures transitivity and average shortest path length were also not correlated in our birds (Spearman's rank correlation: n = 19, r = 0.25, p = 0.299). Average shortest path length correlated highly with the distance to chance χ² measure (Spearman's rank correlation: n = 19, r = 0.842, p < 0.001).
The correlation of average shortest path length and repertoire size was not significant (r = 0.444, p = 0.059). By contrast, transitivity was negatively correlated with repertoire size (r = −0.61, p = 0.007) and was not correlated with distance to chance χ² (r = 0.31, p = 0.198).
The results on transitivity still hold true when we adjusted transitivity for repertoire size. Adjustment was performed by using the respective residuals of the original transitivity values to a regression line obtained by linear regression of transitivity on repertoire size in random graphs with systematically variation of graph sizes (92–208 nodes, 2000 random graphs per graph size, mean slope −2.019, mean intercept 7.044, mean p < 0.001). The correlation of adjusted transitivity with repertoire size was significant, r = −0.479, p = 0.039 in contrast to the correlation of adjusted transitivity and distance to chance χ²: r = 0.435, p = 0.064.
‘Small-world-ness’ was calculated as the relationship of average shortest path length and transitivity with respect to the values of these measures in the corresponding random networks. All 19 song sequence networks had small-world-ness values more than 1 (range 3.04–6.70, average 5.28 ± 1.03 s.d., table 1) indicating small-world network topology. Small-world-ness was correlated with repertoire size (Spearman's rank correlation: ρ = 0.533, p = 0.020) and even stronger correlated with distance to chance χ² (Spearman's rank correlation: ρ = 0.812, p < 0.001).
Small-world-ness was correlated with average shortest path lengths (Spearman's rank correlation: ρ = 0.819, p < 0.001) but not with transitivity (Spearman's rank correlation: ρ = 0.191, p = 0.431).
(a). Transition patterns of song types
The 19 investigated birds sang on average 54.7 ± 5.7% (mean ± s.d.) of their song types with identical in and out degree (one-way pattern and hourglasses). A total of 22.4 ± 2.7% of song types were identified as branches (higher out degree than in degree), and 22.9 ± 3.2% as bottlenecks (higher in degree than out degree, see figure 1c for illustration). More than half of the song types (51.8%) that were identified as opening branches were sung by more than one male—some of these were shared by up to six individuals. The same was found when considering the song types functioning as narrowing bottlenecks: 52.4% of these were shared by at least two (and up to six) individuals.
Birds with a large repertoire sang more relative one-way patterns (Spearman's rank correlation r = 0.63, p = 0.004). Orderly singing birds with high values of distance to chance sang more relative one-way patterns: r = 0.54, p = 0.016.
Birds with a large proportion of hourglasses sang with shorter average path lengths (Spearman's rank correlation: r = −0.48, p = 0.049) and with smaller transitivity values (r = −0.64, p = 0.004), and birds with a large proportion of hourglasses sang with smaller distance to chance χ² values (r = −0.6, p = 0.006).
Birds with high amounts of relative one-way patterns sang with high average shortest path lengths (r = 0.66, p = 0.002) and with high small-world-ness values (r = 0.59, p = 0.007).
(b). Song measures and age of birds
Based on feather characteristics, six birds in our analysis were identified as 1-year old and 12 as older than 1 year. For one individual, age class was not identifiable. Confirming the results of prior studies [34], 1-year-old birds had smaller repertoires than older birds, (U-test, W = 4, p = 0.001). Older birds sang their songs in more orderly sequences (distance to chance χ², U-test, W = 13, p = 0.032). Comparing network measures for age classes, we found that older birds sang with longer average shortest path lengths (U-test, W = 12, p = 0.025) and older birds sang with larger small-world-ness values (U-test, W = 7, p = 0.005). Transitivity and transitivity adjusted for repertoire size did not differ between the age classes (U-tests, transitivity: W = 46, p = 0.385, adjusted transitivity: W = 41, p = 0.682).
(c). Playback experiment
In a playback experiment, we tested song responses to playback strings containing bottleneck or branch song types. Transition patterns refer to the ones determined by a sample of 19 birds of the population. The target birds used song types with different transition patterns when responding to a playback depending on playback treatment (n = 12, exact Wilcoxon signed-rank test, W = 0, p < 0.001, figure 3). When birds heard a playback consisting of song types with branch transition patterns, they responded with song types with bottleneck transition pattern (or low-value branch) in their population. On the contrary, when they heard song types with bottleneck transition patterns, they responded with song types that tended to be branching transitions in their population (figure 3).
Figure 3.
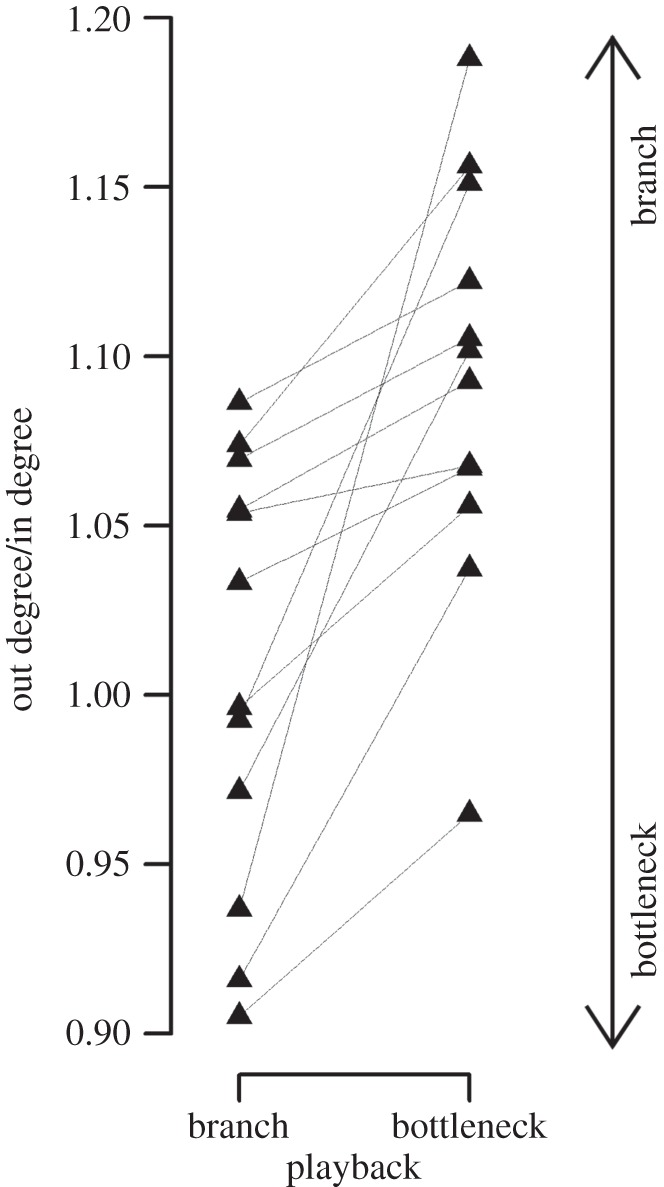
Response to playbacks containing song types with branch or bottleneck transition patterns (each triangle represents the mean per playback for one bird, the two values of each bird are connected by a thin line). Birds sang more ‘branch song types’ when hearing bottlenecks and vice versa (n = 12, exact Wilcoxon signed-rank test, W = 0, p < 0.001). High out degree together with low in degree values (proportion above 1) characterize branches and low out degree together with high in degree values (proportion below 1) define bottlenecks.
4. Discussion
We applied a network analysis to long sequences of nocturnal song of male nightingales. This analysis resulted in new measures to describe the song structure in the species. We selected two network measures that we expected to be particularly well suited for a reflection of the sequential order of nightingale singing: namely, the average shortest path length—a measure that should be particularly sensitive to long linear transitions (resulting in longer average path lengths), and the transitivity—a measure that emphasizes the connectivity of songs in sequential proximity. The combined relationship of both measures to their respective expression in corresponding random graphs revealed the small-world topology of nightingale song sequence networks. Similarly, a study on the song of a California thrasher also uncovered a small-world architecture in which subsets of phrases were strongly grouped and linked [26].
The network measures were correlated with either repertoire size or order measures or both (small-world-ness) and suggested considerable variation among males, apparently related to male characteristics, i.e. we found that older males sang with longer average path lengths and larger small-world-ness values than males in their first breeding season (1-year old).
A comparison of the ‘transition patterns’ allowed us to determine that certain song types had the same transition patterns across males. Learning experiments with nightingales have been shown that song types heard in the learning phases in sequential proximity are later sung together, forming ‘packages’ [10]. Considering this organizational principle, the song types that were identified as ‘branches’ or ‘bottlenecks’ by a network analysis might then be song types that are performed at the end of one and at the beginning of the next song package. The respective transition patterns of some song types were shared among several males suggesting that these transition properties may be a common and shared trait between males of a given population. As a consequence, knowledge about these transition properties might allow listening birds to anticipate the ‘upcoming’ sequence.
Based on the results of the network analysis, we conducted a playback experiment. We tested whether male birds that hear song types with certain transition patterns adjust their singing according to these patterns. The results were as follows: when the birds heard a playback consisting of branches, they respond with song types that have pre-dominantly a bottleneck character in their population and when they heard bottlenecks they respond with song types that tend to be branches.
A possible interpretation of this outcome is that birds tried to take over the ‘vocal leadership’ in the interaction simulated by means of the playback. The concept of vocal leadership was originally introduced in the context of vocal matching [48]. Afterwards, it was often used to describe temporal interaction patterns (i.e. the leader sings a song that is immediately followed by a song of the follower. The leader pauses slightly longer before he starts with the next song, again followed by the follower, etc.) [49]. This principle can also apply on the level of song sequences if interacting individuals share such sequences (i.e. the song types and the sequential order). Here, a stimulus song X is responded to by a song Y, which can be regarded as a sequential continuation of X [50–52]. The role of ‘vocal leader’ has been shown to bear some advantages in a contest [49].
In the context of the vocal leadership concept, the results of our playback experiment can be interpreted as follows: if the loudspeaker plays a bottlenecking song, birds avoid continuing singing ‘in this direction’, because this would push them in the role of the sequential follower. Instead, they sing a branching song, hereby choosing alternative continuations of the song sequence with the possibility to obtain the sequential lead. If the playback plays a song with branching transition patterns, nightingales chose a song with bottleneck patterns in response. Thereby, they select a direction towards relatively determined passages of their song sequence. In the following, this would allow them to act as the sequential leader (at least as long as the ‘opponent’ agrees to sing in this direction).
This response strategy is almost ideally supported by a song organization following a small-world network topology as is the case for nightingale song sequences. We understand the meaning of small-world topology for singing as follows: some passages of singing show a highly determined sequential order, whereas in other passages, types have a high interconnectivity and several songs hold the potential to function as central linking hubs. The resulting overall song structure allows the generation of information encoding units, and at the same time, a fast and flexible switching between such units. Thus, the sequential patterning actually used in communication via song turns out to be a well-balanced ratio between rules of sequential organization on the one hand and flexibility in the application of these rules on the other hand resulting in an increased potential to transfer information compared to both: more randomized and more determined sequences. Older males singing with smaller world topology might then be interpreted as an experience-depending adjustment of the song sequence patterns.
It has repeatedly been shown that the connectivity and sequential activity of organizational and functional units of neurons in the brain follow a small-world topology (e.g. [53–55]). The application of online imaging techniques and online song analysis on a singing nightingale might thus result in a parallel representation of the small-world network of songs and the small-world network of neuronal activity patterns. Both networks should exhibit parallels. This scenario might serve as the basis for the investigation of further questions; for example, whether the information about song sequencing is stored together with the respective storage place for the song types, or whether sequential information is stored in separate places and circuits.
In the bird song systems studied in this regard so far, elements as ‘units of production’ of song are neuronally represented by a ‘synfire chain’. Element sequences arise by weighted connections between the last neuron of one synfire chain to the first neuron of a next synfire chain. The higher its synaptic load, the more probable is a given element sequence when compared with competing following elements with lower synaptic weight [56].
The discontinuous song of nightingales (and many other song birds) is characterized by pauses between songs that are considerably longer when compared with the pauses between elements within a song. This raises the question whether song sequences are encoded by the same mechanism as element sequences. Possible alternatives would be for example an indexing of song types and the storage of sequential information in totally different parts of the brain's song system. Though for the time being, this has to be considered a hypothetical scenario, it allows predictions to be to derived regarding future playback experiments. If the sequential order between song types is neuronally encoded by the connection of synfire chains between the last element of the preceding and the first element of the following song, then responses in playbacks should strongly depend on which final elements of songs are played. Exchanging final elements should result in differences in the sequential response pattern (which should not be the case when manipulating other elements within song).
To summarize, our results demonstrate that network approaches and measures provide biologically meaningful data to describe the song structure of individuals in a species with extremely large repertoires and complex rules of song retrieval. These measures certainly add new possibilities to inquire into principles of song structure and its neural foundations, learning mechanisms, functions and evolution and may provide promising starting points to inquire into comparative studies on the ontogenesis and evolution of the small worlds of bird song and human language.
Acknowledgements
Ringing was done on behalf of the Vogelwarte Radolfzell (Beringungszentrale Max Planck Institute for Ornithology), and the permission was granted by the Senatsverwaltung für Stadtentwicklung und Umweltschutz Berlin. We thank Sarah Kiefer, Kim Mortega and Tina Teutscher for their assistance in fieldwork and for valuable discussions throughout all phases of this study.
References
- 1.Catchpole CK, Slater PJB. 2008. Bird song: biological themes and variations, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Byers B, Kroodsma D. 2009. Female mate choice and songbird song repertoires. Anim. Behav. 77, 13–22. ( 10.1016/j.anbehav.2008.10.003) [DOI] [Google Scholar]
- 3.Gil D, Gahr M. 2002. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141. ( 10.1016/S0169-5347(02)02410-2) [DOI] [Google Scholar]
- 4.Kipper S, Mundry R, Hultsch H, Todt D. 2004. Long-term persistence of song performance rules in nightingales (Luscinia megarhynchos): a longitudinal field study on repertoire size and composition. Behaviour 141, 371–390. ( 10.1163/156853904322981914) [DOI] [Google Scholar]
- 5.Mundinger P. 1982. Microgeographic and macrogeographic variation in the acquired vocalizations of birds. In Acoustic communication in birds II (eds Kroodsma D, Miller E.), pp. 147–208. New York, NY: Academic Press. [Google Scholar]
- 6.Petruskova T, Osiejuk TS, Petrusek A. 2010. Geographic variation in songs of the tree pipit (Anthus trivialis) at two spatial scales. Auk 127, 274–282. ( 10.1525/auk.2009.09077) [DOI] [Google Scholar]
- 7.Hailman J, Ficken M. 1986. Combinatorial animal communication with computable syntax: chick-a-dee calling qualifies as ‘language’ by structural linguistics. Anim. Behav. 34, 1899–1901. ( 10.1016/S0003-3472(86)80279-2) [DOI] [Google Scholar]
- 8.Clucas B, Freeberg T, Lucas J. 2004. Chick-a-dee call syntax, social context, and season affect vocal responses of Carolina chickadees (Poecile carolinensis). Behav. Ecol. Sociobiol. 57, 187–196. ( 10.1007/s00265-004-0847-9) [DOI] [Google Scholar]
- 9.Templeton C, Greene E, Davis K. 2005. Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937. ( 10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- 10.Hultsch H, Todt D. 1989. Memorization and reproduction of songs in nightingales (Luscinia megarhynchos): evidence for package formation. J. Comp. Physiol. A 165, 197–203. ( 10.1007/BF00619194) [DOI] [Google Scholar]
- 11.Hultsch H, Todt D. 1989. Song acquisition and acquisition constraints in nightingales, Luscinia megarhynchos. Naturwissenschaften 76, 83–85. ( 10.1007/BF00396717) [DOI] [Google Scholar]
- 12.Todt D, Hultsch H. 1998. How songbirds deal with large amounts of serial information: retrieval rules suggest a hierarchical song memory. Biol. Cybern. 79, 487–500. ( 10.1007/s004220050498) [DOI] [Google Scholar]
- 13.Doupe A, Kuhl P. 1999. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631. ( 10.1146/annurev.neuro.22.1.567) [DOI] [PubMed] [Google Scholar]
- 14.Margoliash D, Nusbaum H. 2009. Language: the perspective from organismal biology. Trends Cogn. Sci. 13, 505–510. ( 10.1016/j.tics.2009.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolhuis J, Okanoya K, Scharff C. 2010. Twitter evolution: converging mechanisms in birdsong and human speech. Nat. Rev. Neurosci. 11, 747–759. ( 10.1038/nrn2931) [DOI] [PubMed] [Google Scholar]
- 16.Berwick RC, Okanoya K, Beckers GJL, Bolhuis J. 2011. Songs to syntax: the linguistics of birdsong. Trends Cogn. Sci. 15, 113–121. ( 10.1016/j.tics.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 17.Hauser M, Chomsky N, Fitch W. 2002. The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579. ( 10.1126/science.298.5598.1569) [DOI] [PubMed] [Google Scholar]
- 18.Slater P. 1983. Sequences of song in chaffinches. Anim. Behav. 31, 272–281. ( 10.1016/S0003-3472(83)80197-3) [DOI] [Google Scholar]
- 19.Okanoya K. 2004. The Bengalese finch: a window on the behavioral neurobiology of birdsong syntax. Ann. NY Acad. Sci. 1016, 724–735. ( 10.1196/annals.1298.026) [DOI] [PubMed] [Google Scholar]
- 20.Briefer E, Osiejuk T, Rybak F, Aubin T. 2010. Are bird song complexity and song sharing shaped by habitat structure? An information theory and statistical approach. J. Theor. Biol. 262, 151–164. ( 10.1016/j.jtbi.2009.09.020) [DOI] [PubMed] [Google Scholar]
- 21.Markowitz JE, Ivie E, Kligler L, Gardner TJ. 2013. Long-range order in canary song. PLoS Comput. Biol. 9, e1003052 ( 10.1371/journal.pcbi.1003052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todt D, Cirillo J, Geberzahn N, Schleuss F. 2001. The role of hierarchy levels in the vocal imitations of songbirds. Cybern. Syst. 32, 257–283. ( 10.1080/019697201300001876) [DOI] [Google Scholar]
- 23.Lachlan R, Verhagen L, Peters S, Cate C. 2010. Are there species-universal categories in bird song phonology and syntax? A comparative study of chaffinches (Fringilla coelebs), zebra finches (Taenopygia guttata) and swamp sparrows (Melospiza georgiana). J. Comp. Physiol. A 124, 92–108. [DOI] [PubMed] [Google Scholar]
- 24.Ivanitskii V, Marova I. 2012. Huge memory in a tiny brain: unique organization in the advertising song of Pallas's warbler (Phylloscopus proregulus). Bioacoustics 21, 87–105. ( 10.1080/09524622.2012.655939) [DOI] [Google Scholar]
- 25.Newman M. 2010. Networks: an introduction. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Sasahara K, Cody ML, Cohen D, Taylor CE. 2012. Structural design principles of complex bird songs: a network-based approach. PLoS ONE 7, e44436 ( 10.1371/journal.pone.0044436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrer I, Cancho R. 2010. Network theory. In The Cambridge encyclopedia of the language sciences (ed. Colm Hogan P.), pp. 555–557. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 28.Hultsch H, Todt D. 1981. Repertoire sharing and song-post distance in nightingales (Luscinia megarhynchos B.). Behav. Ecol. Sociobiol. 8, 183–188. ( 10.1007/BF00299828) [DOI] [Google Scholar]
- 29.Kipper S, Mundry R, Sommer C, Hultsch H, Todt D. 2006. Song repertoire size is correlated with body measures and arrival date in common nightingales (Luscinia megarhynchos). Anim. Behav. 71, 211–217. ( 10.1016/j.anbehav.2005.04.011) [DOI] [Google Scholar]
- 30.Kiefer S, Scharff C, Kipper S. 2011. Does age matter in song bird vocal interactions? Results from interactive playback experiments. Front. Zool. 29, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartsch C, Weiss M, Kipper S. 2012. The return of the intruder: immediate and later effects of different approach distances in a territorial songbird. Ethology 118, 876–884. ( 10.1111/j.1439-0310.2012.02081.x) [DOI] [Google Scholar]
- 32.Svensson L. 1992. Identification guide to European passerines. Thetford, UK: British Trust for Ornithology. [Google Scholar]
- 33.Mundry R, Sommer C. 2007. A new pattern of feather colouration for age determination in common nightingales. Limicola 21, 131–139. [Google Scholar]
- 34.Amrhein V, Korner P, Naguib M. 2002. Nocturnal and diurnal singing activity in the nightingale: correlations with mating status and breeding cycle. Anim. Behav. 64, 939–944. ( 10.1006/anbe.2002.1974) [DOI] [Google Scholar]
- 35.Vokurková J, et al. 2013. The causes and evolutionary consequences of mixed singing in two hybridizing songbird species (Luscinia spp.). PLoS ONE 8, e60172 ( 10.1371/journal.pone.0060172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiefer S, Spiess A, Kipper S, Mundry R, Sommer C, Hultsch H, Todt D. 2006. First year common nightingales (Luscinia megarhynchos) have smaller songtype repertoire sizes than older males. Ethology 112, 1217–1224. ( 10.1111/j.1439-0310.2006.01283.x) [DOI] [Google Scholar]
- 37.R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) (accessed 23 June 2012). [Google Scholar]
- 38.Butts C, Handcock M, Hunter D. 2012. Network: classes for relational data. Irvine, CA: R package version 1.7–1. [Google Scholar]
- 39.Fruchterman T, Reingold E. 1991. Graph drawing by force-directed replacement. Softw. Pract. Exp. 21, 1129–1164. ( 10.1002/spe.4380211102) [DOI] [Google Scholar]
- 40.Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJ. Complex Syst. p. 1695 See http://igraph.org. [Google Scholar]
- 41.West D. 1996. Introduction to graph theory. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 42.Watts DJ, Strogatz SH. 1998. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442. ( 10.1038/30918) [DOI] [PubMed] [Google Scholar]
- 43.Humphries MD, Gurney K. 2008. Network ‘small-world-ness’: a quantitative method for determining canonical network equivalence. PLoS ONE 3, e0002051 ( 10.1371/journal.pone.0002051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cancho RFi, Sole RV. 2001. The small world of human language. Proc. R. Soc. Lond. B 268, 2261–2265. ( 10.1098/rspb.2001.1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amaral LAN, Scala A, Barthelemy M, Stanley HE. 2000. Classes of small-world networks. Proc. Natl Acad. Sci. USA 97, 11 149–11 152. ( 10.1073/pnas.200327197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bassett DS, Bullmore ET. 2006. Small world brain networks. Neuroscientist 12, 512–523. ( 10.1177/1073858406293182) [DOI] [PubMed] [Google Scholar]
- 47.Brumm H, Todt D. 2004. Male–male vocal interactions and the adjustment of song amplitude in a territorial bird. Anim. Behav. 67, 281–286. ( 10.1016/j.anbehav.2003.06.006) [DOI] [Google Scholar]
- 48.Kroodsma DE. 1979. Vocal dueling among male marsh wrens: evidence for ritualized expressions of dominance/subordinance. Auk 96, 506–515. [Google Scholar]
- 49.Naguib M, Fitchel C, Todt D. 1999. Nightingales respond more strongly to vocal leaders of simulated dyadic interactions. Proc. R. Soc. Lond. B 266, 537–542. ( 10.1098/rspb.1999.0669) [DOI] [Google Scholar]
- 50.Todt D. 1975. Short term inhibition of vocal outputs occurring in the singing behaviour of blackbirds (Turdus merula). J. Comp. Physiol. A 98, 289–306. ( 10.1007/BF00709802) [DOI] [Google Scholar]
- 51.Todt D, Hultsch H. 1996. Acquisition and performance of repertoires: ways of coping with diversity and versatility. In Ecology and evolution of acoustic communication in birds (eds Kroodsma DE, Miller EH.), pp. 79–96. Ithaca, NY: Cornell University Press. [Google Scholar]
- 52.Geberzahn N, Hultsch H. 2004. Rules of song development and their use in vocal interactions by birds with large repertoires. Anais Da Academia Brasileira De Ciencias 76, 209–218. ( 10.1590/S0001-37652004000200004) [DOI] [PubMed] [Google Scholar]
- 53.Sporns O, Chialvo D, Kaiser M, Hilgetag CC. 2004. Organization, development and function of complex brain networks. Trends Cogn. Sci. 8, 418–425. ( 10.1016/j.tics.2004.07.008) [DOI] [PubMed] [Google Scholar]
- 54.Reijneveld JC, Ponten SC, Berendse HW, Stam CJ. 2007. The application of graph theoretical analysis to complex networks in the brain. Clin. Neurophysiol. 118, 2317–2331. ( 10.1016/j.clinph.2007.08.010) [DOI] [PubMed] [Google Scholar]
- 55.Bullmore E, Sporns O. 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. ( 10.1038/nrn2575) [DOI] [PubMed] [Google Scholar]
- 56.Jin D. 2009. Generating variable birdsong syllable sequences with branching chain networks in avian premotor nucleus HVC. Phys. Rev. E 80, 51902 ( 10.1103/PhysRevE.80.051902) [DOI] [PubMed] [Google Scholar]