Abstract
We identified and sequenced from the squid Euprymna scolopes two isoforms of haemocyanin that share the common structural/physiological characteristics of haemocyanin from a closely related cephalopod, Sepia officinalis, including a pronounced Bohr effect. We examined the potential roles for haemocyanin in the animal's symbiosis with the luminous bacterium Vibrio fischeri. Our data demonstrate that, as in other cephalopods, the haemocyanin is primarily synthesized in the gills. It transits through the general circulation into other tissues and is exported into crypt spaces that support the bacterial partner, which requires oxygen for its bioluminescence. We showed that the gradient of pH between the circulating haemolymph and the matrix of the crypt spaces in adult squid favours offloading of oxygen from the haemocyanin to the symbionts. Haemocyanin is also localized to the apical surfaces and associated mucus of a juvenile-specific epithelium on which the symbionts gather, and where their specificity is determined during the recruitment into the association. The haemocyanin has an antimicrobial activity, which may be involved in this enrichment of V. fischeri during symbiont initiation. Taken together, these data provide evidence that the haemocyanin plays a role in shaping two stages of the squid–vibrio partnership.
Keywords: haemocyanin, host–symbiont interaction, oxygen provision, specificity
1. Introduction
The bobtail squid Euprymna scolopes establishes a light organ symbiosis with the luminous bacterium Vibrio fischeri. During embryogenesis, the host develops ciliated epithelial tissues on the surface of the nascent organ that, after the host hatches, promote both the harvesting of the symbiont cells from the seawater and their eventual colonization of the organ's deep tissues. After hatching, these superficial epithelia shed mucus, and ciliary-mucus currents entrain the bacterioplankton into regions above pores, through which the symbionts will enter the organ. Vibrio fischeri and other Gram-negative bacteria adhere to the cilia and aggregate in mucus near the pores; however, by approximately 3 h, V. fischeri has become the only resident of these aggregates, i.e. specificity of the symbiosis is determined on the surface, even before the symbiont enters the host [1].
The mechanisms underlying this specificity have not been well defined. However, several lines of data suggest that the biochemistry of the microenvironment plays a critical role. For example, antimicrobials such as nitric oxide (NO) [2] and a peptidoglycan-recognition protein (EsPGRP2), which breaks down bacterial cell walls, are present in the mucus [3]. Resistance to NO is critical for the ability of V. fischeri to colonize normally [4]. Further, recent analyses have shown that interaction with as few as three to five V. fischeri cells, which are bound to the cilia on the light organ surface during initiation of the association, induce changes in host gene expression [5], including the upregulation of those predicted to encode antimicrobial proteins that may be critical for determining specificity [5].
Vibrio fischeri cells that reach the crypts grow and, after attaining a critical density, begin to luminesce using host-derived nutrients and oxygen [6,7]. Key features of the symbiosis express profound diel rhythms; first, the symbiont population density dramatically varies over the day, i.e. approximately 95% of the bacteria are expelled from the light organ at dawn and the remaining bacteria subsequently repopulate the crypts. Second, symbiont light production also fluctuates during the day, increasing during the evening and peaking early at night [8]. Experiments manipulating the rhythm suggest that, even though the symbionts reach their maximum density during the day, the production of bioluminescence is limited by oxygen availability in the crypts until later during the night [8].
The relationships of the adult light organ tissues [9] suggest that the diffusion of oxygen from the surrounding seawater is unlikely to match the symbionts' needs. Further, the light organ is one of the most highly vascularized regions of the host's body, suggesting that oxygen is delivered to the symbionts through the circulatory system [10]. In the squid, and certain other invertebrates, the metalloprotein haemocyanin, which is dissolved in the haemolymph, transports oxygen throughout the body; oxygen binding in haemocyanins is cooperative, and its affinity can be affected by pH, temperature and various solutes [11]. A recent study of the squid–vibrio system demonstrated that the genes encoding the host's haemocyanin are regulated over the day–night cycle in the adult tissues that support the symbiont [12]. These data suggested a role for haemocyanin in the dynamics of the mature symbiosis.
Haemocyanins arose from ancestral copper-coordinating proteins, which had diverged into two protein families, the tyrosinases and the pro-phenol oxidases; the former gave rise to the haemocyanin of molluscs, and the latter to the haemocyanin of arthropods [13,14]. Thus, despite their common mechanism of oxygen binding, their separate origins resulted in structural and functional distinctions. Specifically, arthropod haemocyanin isoforms have one functional unit (FU), while the molluscan haemocyanin isoforms comprise seven to eight FUs, whose three-dimensional conformation slightly differs from that of the arthropods [15].
Apart from oxygen binding, the protein domains of the mollusc haemocyanins also have two distinct phenol oxidase (PO) activities: a cresolase, which hydrolyses monophenol into o-diphenols and is specific to tyrosinases, and a catechol oxidase, which oxidizes o-diphenols [15]. These enzymatic activities can be enhanced in vitro by proteolytic treatment or the use of detergents [15]. This proteolytic enhancement also occurs in vivo in crustaceans, with the activation of immune defence pathways in response to the recognition of bacterial products [15,16]. As such, the PO activity plays a role in controlling microbial pathogenesis through the production of highly reactive quinones.
In this study, we characterize biochemical features of the host haemocyanin and provide evidence for a role for this protein in the regulation of the mature light organ, as well as in the initial establishment of the symbiosis. We first describe the primary structure of haemocyanin. We next show that, in addition to being the major respiratory protein in the blood, haemocyanin co-occurs with the symbiont cells within the crypts and delivers oxygen to these regions of the organ. Finally, our in vitro data suggest that, through its PO activity, haemocyanin participates in the creation of an antimicrobial cocktail that selects for V. fischeri during initiation of the symbiosis.
2. Material and methods
(a). General procedures
Adult Hawaiian bobtail squid (E. scolopes) were caught in Oahu, Hawaii and transported to UW-Madison, where they were maintained and bred in a recirculating seawater system. Juvenile squid were incubated overnight either in the absence of V. fischeri (aposymbiotic) or in the presence of approximately 105 CFU ml−1 of either the wild-type V. fischeri strain ES114 (WT) or a deletion mutant derivative defective in light production, ΔluxCDABEG (Δlux) [17]. All animal protocols followed regulatory standards established by UW-Madison.
Microscope observations were either performed on a Zeiss 510 laser-scanning confocal or a Zeiss Axio Imager M2 epifluorescence microscope. Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (USA) and all molecular reagents and fluorochromes from Life Technologies (USA). Primers (electronic supplementary material, table T1), which were synthesized by Integrated DNA Technologies (USA), and buffer formulae are defined in the electronic supplementary material.
(b). Analysis of the cDNA
Full-length cDNA sequences of HCY isoforms (GenBank accession numbers KF647897 and KF647898) were obtained by rapid-amplification of cDNA ends (RACE), using the GeneRacer kit, following the manufacturer's instructions (for details, see the electronic supplementary material, Material and methods).
We aligned full-length haemocyanin cDNA from a variety of mollusc species (electronic supplementary material, Material and methods) using MUSCLE v. 3.7 [18], and conserved sites were selected using GBlocks v. 0.91b [19]. Maximum-likelihood reconstruction was performed using PhyML v. 3.0 [20] with the WAG + Γ model, and 100 bootstrap replicates were conducted for support estimation (Dryad doi:10.5061/dryad.cq032). We used the interface from phylogeny.fr as a platform for the reconstruction [21].
Haemocyanin transcripts from both isoforms were quantified by quantitative reverse-transcription PCR (qRT-PCR) using specific transcripts for each isoform. The qRT-PCR protocol, previously described in [5], followed the MIQE guideline [22] (n = 4 replicates of one adult or 20 juveniles). For specifics, see the electronic supplementary material, Material and methods. In situ hybridization against haemocyanin2 transcripts was performed as described in [5].
(c). Protein localization
An affinity-purified polyclonal antibody (α-HCY2) was produced in chicken against the synthetic peptide CISFDNSETDRDPQP (GenScript, USA). Soluble proteins from juvenile light organs, extracted in phosphate-buffered saline (PBS) containing a protease inhibitor cocktail, were separated on a 7% SDS-polyacrylamide gel for western blot analysis as described in [5] (see the electronic supplementary material, Material and methods for details).
Immunocytochemistry (ICC) experiments on whole light organs were performed as previously described [3], with either the primary α-HCY2 antibody or α-IgY as a negative control, at a dilution of 1 : 1000 for 7 days (see the electronic supplementary material, Material and methods for details). ICC experiments in mucus were performed as before [3], except that squid were fixed for 3 h in Bouin's solution, permeabilized for 2 h in a marine PBS (mPBS) containing Triton X100 (mPBST), and the mucus was counterstained with Alexa633-wheatgerm agglutinin (WGA).
ICC experiments on paraffin sections were performed as follows: squid were fixed in 4% paraformaldehyde in mPBS overnight at 4°C and embedded in paraffin. Five-micrometre sections were deparaffinized in histoclear solution (National Diagnostics) and rehydrated in an ethanol series. Slides were brought to boiling in the antigen retrieval solution (RD Systems), then cooled to 65°C and incubated for 10 min. Slides were then rinsed in deionized water followed by mPBS and blocked in mPBS containing 0.5% BSA and 1% goat serum for 30 min at room temperature (RT). Slides were incubated in α-HCY2 or α-IgY antibodies 1 : 500 in the blocking solution overnight at 4°C and rinsed 3 × 5 min in mPBS. Slides were then incubated in blocking solution for 1 min, then for 1 h at RT in a goat-anti-chicken, FITC-conjugated secondary antibody diluted in blocking solution. Slides were rinsed 3 × 15 min in mPBS and counterstained for actin with 1 : 40 rhodamine phalloidin in mPBS overnight at 4°C, and for nuclei with a 1 : 1000 dilution of TOTO-3 for 10 min at RT. Slides were then mounted in Vectashield (Vector Laboratories), and samples were viewed by confocal microscopy.
(d). Determination of oxygen affinity
For oxygen affinity measurements, collected haemolymph samples were either: (i) used directly in a diffusion chamber apparatus, or (ii) the haemocyanin was separated from the small metabolites and smaller proteins present in the haemolymph by two passages through an Amicon Ultra-4 with Ultracel-100 membrane (Millipore) in 2 × 3.5 ml of stabilization buffer III and concentrated to the same volume as that of the initial haemolymph sample. The functional measurements were made in a diffusion chamber, using a step-by-step procedure, as previously described [23]. Five microlitres of the native sample, or of the ‘purified’ samples adjusted at different pH values (pH approx. 6.5, 7.0, 7.5, 7.8 and 8.0), were equilibrated in the chamber with pure N2, pure O2 and mixtures of the two gases to determine the oxygen equilibrium curves. The Bohr effect Φ, which characterizes the relationship between oxygen binding affinity and pH, was determined by the equation Φ = ΔlogP50/ΔpH. For details about the determination of oxygen affinity, see the electronic supplementary material, Material and methods.
(e). Protein purification, and the determination of phenol oxidase and antimicrobial activities
Haemolymph samples were centrifuged at 2000×g for 10 min at 4°C to pellet haemocytes. Next, to remove small molecules, haemolymph was filtered three times through an Amicon Ultra-4 with Ultracel-100 membrane (Millipore) in 3.5 ml of stabilization buffer I. The remaining haemocyanin protein was pelleted by centrifugation overnight at 25 000×g at 4°C. The pellet was resuspended in stabilization buffer II and passed through an anion-exchange chromatography column (MonoQ 4.6/100 PE, GE Healthcare) for high-performance liquid chromatography (HPLC). Buffer A and buffer B were run as a linear gradient from 0 to 100% B in A using over 80 column volumes at a flow rate of 1 ml min−1. Fractions absorbing at 560 nm were collected and exchanged two times with stabilization buffer I using the Amicon column as described above. Before storage at −80°C, these purified (more than 95%) haemocyanin extracts were pooled and concentrated to 66 mg protein ml−1, as calculated spectrophotometrically as: C = (A235 – A280)/2.51 × dilution factor [24].
PO activity of haemocyanin was determined based on the method described in [16] with some modifications. Briefly, 66 µg of purified haemocyanin was diluted in a PIPES-based buffer (pH 6.3) to obtain a final volume of 123 µl. Also, depending on the combination tested (figure 4a), 40 µg of protease (subtilisin Karlsberg, type XIV protease or cathepsin L) and/or 1 mM phenylthiourea (PTU) was added. Reactions were then incubated in microplate wells for 10 min at RT, and 2 µl of dopamine, catechol or tyramine was added to final concentrations of 6, 6 and 3.4 mM, respectively. In triplicate experiments, quinone production was recorded at 420 nm (Tecan spectrophotometer, GENios) after a 1-h (or 3.5-h, for tyramine) incubation at RT.
Figure 4.
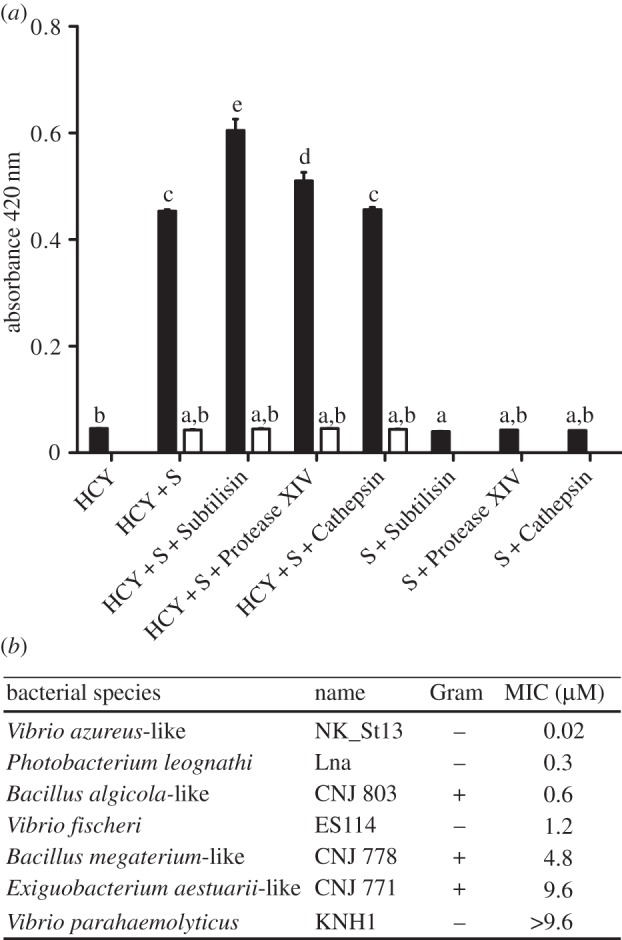
Antimicrobial properties of haemocyanin. (a) Determination of the PO activity of EsHCY (HCY). The reaction's absorbance was measured spectrophotometrically after a 1-h incubation in the presence of 6 mM dopamine substrate (S) and/or different proteases. White bars correspond to the PO-activity in the presence of the inhibitor (1 mM PTU). Mean ± s.e. (n = 3). Different letters indicate a statistical difference between samples (ANOVA with Tukey HSD adjustment for pairwise comparisons). (b) Minimal inhibitory concentration (MIC) of haemocyanin against various Gram-negative and Gram-positive bacterial strains in the presence of 625 µM dopamine in a PIPES-based buffer at pH 6.35.
To determine the lowest concentration of haemocyanin that inhibits the growth of different marine bacterial strains, a modified minimal inhibitory concentration (MIC) test was performed as described in [25]. Briefly, a dilution series of purified haemocyanin in PIPES buffer [16] containing 625 µM dopamine as substrate was placed into wells containing 100 bacterial colony-forming units (CFU). For each series, the presence or absence of bacterial growth (i.e. a circular pellet appearing at the bottom of the well) was recorded after 24 h of incubation at 28°C (see the electronic supplementary material, Material and methods, for details). No bactericidal activity was observed in the presence of dopamine alone.
(f). Characterization of flow between the haemolymph and the light organ crypts, and measurement of pH
After anaesthesia, adult squid were injected in the cephalic vessel with 50 µl of a 1 : 100 dilution of the fluorescent pH indicator carboxy-SNARF-4F [5] in seawater, or a seawater-only control. Following injection, the squid were revived and maintained for at least 12 h prior to assay. To monitor SNARF accumulation in the light organ crypts, the crypt contents (vented material) were collected as described previously [10]. A fluorescent SNARF signal was detected in the expelled contents of SNARF-injected, but not carrier-injected, animals using epifluorescence microscopy. The pH of haemolymph and expelled crypt contents was measured as described previously [5], except by epifluorescence. Briefly, the probe was excited at 488 nm and emission was measured at two different wavelengths (580 and 650 nm); the emission ratio depends on the pH of the solution. A pH calibration curve was obtained with 20 independently measured ratios from a series of 5 pH standards made with SNARF in mPBS.
3. Results and discussion
(a). Euprymna scolopes synthesizes two isoforms of haemocyanin
(i). Haemocyanin structure
The derived amino acid sequence of RACE products revealed two distinct isoforms of haemocyanin (EsHCY), consisting of 3342 and 3346 amino acids that share 80% identity. Each isoform comprised eight FUs, originating from ancestral duplication events, and sharing the ABCDD'EFG subunit organization first described in Sepia officinalis [26,27] (figure 1; electronic supplementary material, figure S1).
Figure 1.
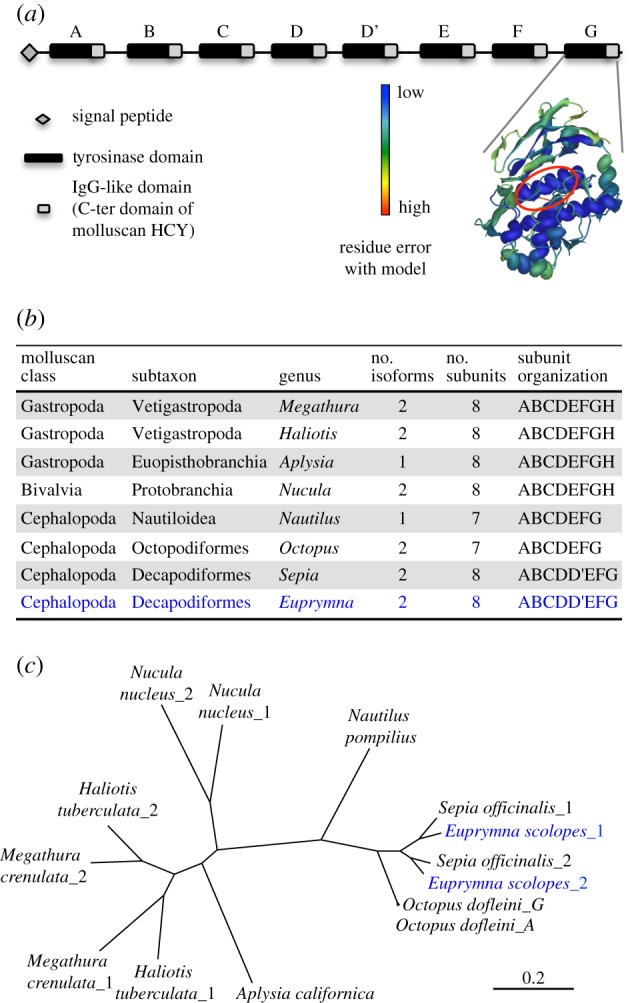
Characterization of haemocyanin from E. scolopes. (a) Schematic of EsHCY. Right: illustration of the predicted three-dimensional structure of FU-G of HCY2 against crystal structures from O. dofleini (Od-G; Swiss-Prot model (FU-G): 1js8B [28]); catalytic centre circled in red; colour scale indicates error level. Z-scores values, representing the deviation of the EsHCY's FU-G structures from that of the authentic crystal structure, are −1.4 and −0.64 for HCY1 and HCY2, respectively, with a percentage of BLAST sequence identity of 72.5 and 74.6%. Z = 0 for the crystal; valid models vary from −4 to +4 [29]. (b) Characteristics of haemocyanin proteins from different mollusc species. (c) Maximum-likelihood inference phylogeny based on haemocyanin sequences from different mollusc species. All nodes had a bootstrap value more than 99 (100 replicates).
The two isoforms diverged before the split between the cephalopod orders Sepiida and Sepiolida. An analogous duplication event occurred independently in the gastropods, before the divergence of keyhole limpets and abalones. Each FU of both isoforms has retained its oxygen-binding residues, i.e. the essential histidine that coordinates copper in type-3 centres A and B [13]. The comparison of EsFU-G with the crystal structure of FU-G in Octopus dofleini [28–30] supports the structural conservation of this domain, particularly in the inner α-helixes, where the functional residues are located. Because the haemocyanin FU organization of E. scolopes is very similar to that of S. officinalis, we can predict its three-dimensional structure, based on crystallographic analyses of the cuttlefish's [27,31]: specifically, the subunits of the S. officinalis haemocyanin form a large cylindrical decamer that resembles a wall and collar, and that has a more compact appearance than that of other cephalopod haemocyanins.
(ii). Haemocyanin synthesis and expression
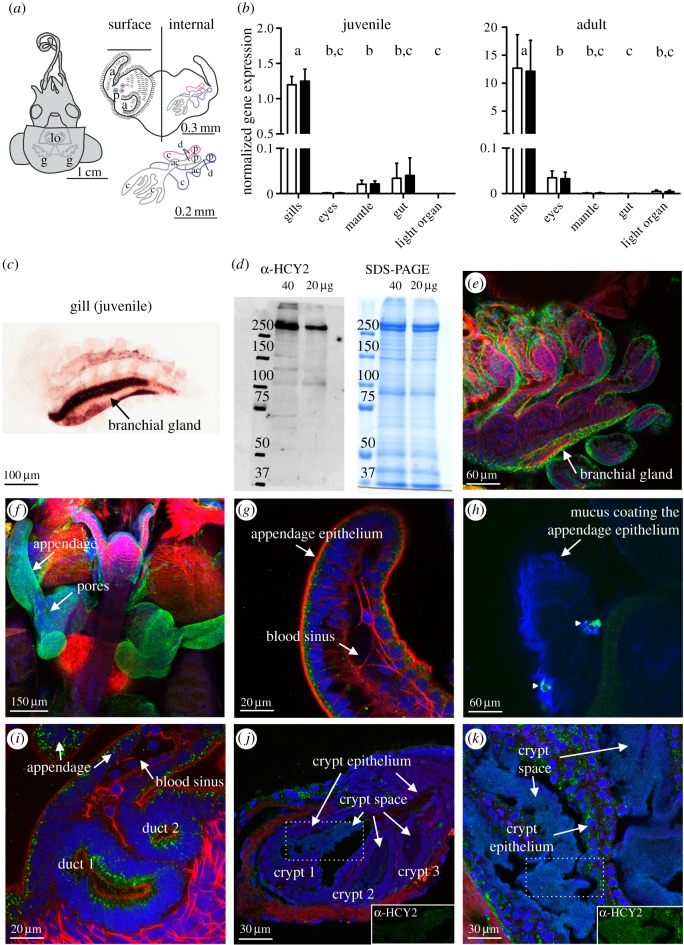
We quantified the expression of mRNA encoding each haemocyanin isoform by quantitative RT-PCR and localized the transcripts by in situ hybridization in both juvenile and adult squid tissues (figure 2b,c). These mRNAs were mainly synthesized in the gills, with particularly strong labelling in the branchial gland, as previously described in other cephalopods [32]. Secondary sites of synthesis were detected in the mantle and gut of juvenile squid, as well as in the eyes and symbiont-containing central core of the light organ of adult squid. Both isoforms were expressed at similar levels in all tissues. However, these data did not eliminate the possibility that fine-scale differences in localization of the two isoforms could occur.
Figure 2.
Localization of haemocyanin transcript and protein in squid tissues. (a) Left: ventral surface of the squid showing the gills (g) and the light organ (lo) deep in the mantle cavity. Right, top: enlargement of the light organ, showing the muco-ciliary epithelial surfaces (ce) in contact with seawater, as well as the internal features (enlarged below) through which V. fischeri cells pass during their migration to the crypts. a, appendage; ac, antechamber; c, crypt; d, duct; p, pore. (b) Normalized gene expression of Eshcy1 (white bars) and Eshcy2 (black bars) in different tissues from juvenile (n = 4 × 20 animals) and adult (n = 4 animals) squid, as mean ± s.e. In this set of comparisons, those pairs of bars that share letters are not significantly different, statistically (ANOVA with Tukey honestly significant difference (HSD) adjustment for pairwise comparisons). (c) In situ hybridization showing the strong staining of the Eshcy2 riboprobe in the gills, where cephalopods typically produce haemocyanin. (d) Coomassie staining of a 7% SDS-PAGE gel (right) of soluble protein (20 and 40 µg samples) extracted from juvenile light organs, and its associated western blot (left) against EsHCY antibody (α-HCY2); expected size of the monomer: 383 kDa. (e–i) ICC of EsHCY2 in juvenile tissues. The antigen labelling is particularly bright in the gills (e), and in the ciliated field (f), the cytosol of the appendage epithelium (g) and the pore/duct regions (i) of the light organ. Green, α-HCY2 (antibody); red, rhodamine phalloidin (f-actin); blue, TOTO-3 (nuclei). (h) ICC of EsHCY2 in the mucus coating the appendage epithelium. Triangles indicate foci of α-HCY2 cross-reactivity in the mucus. Green, α- HCY2; blue, Alexa633-WGA (mucus-binding lectin, WGA). (j–k) ICC of EsHCY2 in 5-µm sections of symbiotic juvenile (j) and adult (k) light organs. In both samples, antigen labelling is present in the lumen of the crypt space, in direct contact with bacteria. Green, α-HCY2; red, rhodamine phalloidin (f-actin); blue, TOTO-3 (nuclei). The inset shows α-HCY2 staining (green channel only) corresponding to the dotted area. Negative controls for the ISH (sense probe), western blot and ICC, are presented in the electronic supplementary material, figure S2. In all cases, these control tissues/extracts showed no detectable labelling.
We used an antibody to EsHCY (α-HCY2) to define the fine-scale distribution of the protein in host tissues (figure 2e–k). Because haemocyanin is secreted and transported, protein localization is likely to differ from sites of gene transcription. The antibody reacted against a protein at the predicted size of EsHCY (expected monomeric molecular weight: 382 kDa) both in the haemolymph (data not shown) and in the light organ (figure 2d). Confocal analyses of the ICC with α-HCY2 revealed cross-reactivity in the gills and epithelia of the juvenile light organ (figure 2e–g). In addition, cross-reactive sites localized to the mucus adjacent to the ciliated epithelium (figure 2h). Thus, although gene expression was low in the ciliated epithelia, the protein was abundant, suggesting either (i) EsHCY is highly stable and, despite its low synthesis rate, can accumulate in the tissues; or (ii) the protein is transported from the blood sinus into the epithelial cells by transcytosis. Precedence for the latter hypothesis is supported by studies in S. officinalis, in which haemocyanin occurs in the intercellular space, and in vesicles and vacuoles of the renal and branchial epithelia, a phenomenon initially attributed to a possible role for haemocyanin in copper homoeostasis [33]. EsHCY was also detectable in the adult central core, mainly in the crypt epithelium but also in the crypt spaces, where symbionts are located (figure 2j,k). These results are consistent with the proteomic detection of haemocyanin in crypt contents [34]. By ICC, we could not detect an influence of symbiosis (WT versus aposymbiotic) or symbiont bioluminescence (WT versus Δlux) on the pattern of haemocyanin production in the light organ (electronic supplementary material, figure S3). However, these methods are insufficiently sensitive to discount the possibility that small differences occur between these conditions.
(b). Vascular haemocyanin provides oxygen to symbionts in the crypt space
The oxygen-binding properties of squid haemocyanin were determined using whole-blood samples collected from adult animals at different times during the day (figure 3) and were in the range reported for other cephalopod haemocyanins [35]. Specifically, blood samples collected during the night had a pH of 7.16 ± 0.08, and a P50 for oxygen, i.e. pressure at which haemocyanin is half-saturated with oxygen, of 1.62 ± 0.25 kPa, with a cooperativity coefficient of 2.43 ± 0.10 at 26°C. By contrast, samples collected during the day had a pH of 6.94 ± 0.04, a significantly decreased affinity (P50 of 5.40 ± 1.58 kPa), but an essentially unchanged cooperativity coefficient (2.67 ± 0.14). These values are equivalent to a half-saturation of 18 µM during the night and 61 µM during the day and indicate that at both times EsHCY has an oxygen affinity two to three orders of magnitude lower than the nanomolar values reported for V. fischeri luciferase and cytochromes [36]. These results indicate that haemocyanin helps deliver oxygen from the haemolymph to the symbionts to support the elevated oxygen demand of luminescence.
Figure 3.
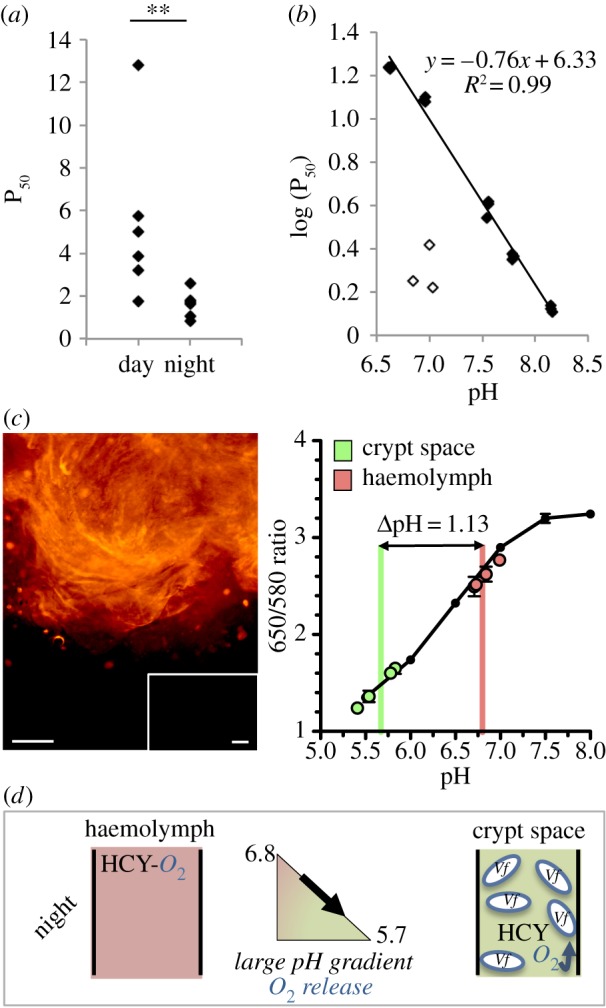
Characteristics of the haemocyanin protein and its putative role in the delivery of oxygen to the symbionts. (a) The affinity for oxygen in native haemolymph samples is lower during the day (14.00–16.00; n = 6) than during the night (2.00–4.00; n = 6). P50 in kPa. **p < 0.01 based on a Wilcoxon's test. (b) Determination of the Bohr effect of EsHCY using the regression between log(P50) and pH. Oxygen affinity of the EsHCY samples was measured after exchanging it in five stabilization buffers at pHs of approximately 6.5, 7.0, 7.5, 7.8 or 8.0 (closed diamonds). Affinity was also measured in native (unprocessed) haemolymph samples (open diamonds). (c) Circulation between the haemolymph and the crypt space, and the associated pH values. The pH-sensitive dye SNARF was injected into the cephalic artery and transported through the body. SNARF is excited at 488 nm, and selectively emits at two different wavelengths (580 and 650 nm). The 650/580 ratio is characteristic of a particular pH, which is estimated by a calibration curve, generated in pH-adjusted mPBS. SNARF was detected in the central core of the light organ (image, left). Injection with sterile seawater revealed no autofluorescence (inset). The pH was determined both in the circulating haemolymph and in the crypt space, i.e. expelled material, in adult squid during the night (figure, right; mean ± s.e., n = 5). Scale bars, 100 μm. (d) A model for the directional transport of oxygen into the crypt space during the night. During the night, circulating haemocyanin (left), which has a relatively high oxygen affinity, circulates to and enters the crypt spaces that have become acidic as a result of bacterial metabolism. The reduced pH in the crypts favours the offloading of oxygen from the haemocyanin (right) to the symbionts, where it is used by their high-affinity luciferase.
The squid's alternating behaviours of hiding in the sand (day) and hunting in the water column (night) may differentially influence blood pH [37] and, thus, EsHCY's oxygen affinity through a Bohr effect. To determine the extent of this effect, we separated EsHCY from other blood components by repeated exchange with a Tris-based buffer adjusted to pH values between 6.0 and 8.2. Subsequent measurements indicated the protein's oxygen affinity (figure 3b), but not cooperativity (electronic supplementary material, figure S4a), was strongly reduced by a lower pH, with an observed Bohr effect of −0.76, typical of cephalopods [38] and other invertebrates [39]. Oxygen affinity decreased after the haemocyanin was separated from the other blood components (electronic supplementary material, figure S4b); however, addition of candidate blood components produced by the squid (taurine) or the symbionts (acetate and lactate) had no detectable effect on EsHCY oxygen affinity (electronic supplementary material, figure S4c).
These results led to an investigation of whether a pH gradient between the blood and the crypt spaces might affect oxygen delivery by haemocyanin to the symbionts. Haemocyanin is present in the crypt space of symbiotic light organs (figure 2; electronic supplementary material, figure S3); however, whether this haemocyanin comes from the circulating blood is unclear. Therefore, we tested whether haemocyanin travels from the general circulation to the crypt space and measured the associated pH values at those two sites. We injected into the cephalic artery a pH-sensitive fluorescent probe (SNARF), which does not permeate membranes by diffusion. We showed that (i) the light organ is highly vascularized, (ii) haemolymph passes from the vascular system into the crypt space and (iii) the pH is significantly lower in the crypt than the haemolymph (5.67 ± 0.18 versus 6.80 ± 0.12) (figure 3c). These data suggest that haemocyanin-bound oxygen taken up in the gills and transported in the haemolymph may be effectively offloaded into the acidic crypt spaces (figure 3d). The consumption of oxygen by V. fischeri (including that driving more than 10% of the cell's energy commitment going to bioluminescence [40]) will thus presumably lead to a gradient that requires a constant flow of oxygen into the crypts.
Bacterial transcriptomic profiles and metabolic modelling [12,36] collectively support the prediction that changes in symbiont, rather than host [37], metabolism over the day–night cycle may influence the pH of the crypt spaces and, thereby, the delivery of oxygen. Thus, we hypothesize that oxygen-saturated vascular haemocyanin migrates to the crypt spaces where a 1-unit pH differential (figure 3c) lowers its affinity for oxygen, driving the oxygen towards the high-affinity, high-demand activity of the symbiont's luciferase (figure 3d). Hence, a combination of EsHCY biochemistry and symbiont metabolism may work together to promote bioluminescence in the squid crypts.
The squid–vibrio system is not unique in recruiting an oxygen-carrier metalloprotein for the control of symbiont activities. In the nodules of legumes, oxygen is provided to symbionts via leghaemoglobin. This carrier poises the levels of oxygen in symbiotic tissues, providing optimal conditions for the activity of symbiont nitrogenase [41]; however, it can also sanction rhizobium cells whose nitrogen-fixation efficiency is low, by limiting their growth [42]. In hydrothermal vent and cold seep tubeworms [43], haemoglobins play a role in delivery of both oxygen and hydrogen sulfide to the symbiotic tissue [44]. In the squid–vibrio association, the haemocyanin provides both respiratory oxygen to host tissues and oxygen for bacterial light production, which is the basis of the symbiosis; in contrast, in tubeworms, it is the sulfide that allows its symbionts to fix carbon, which is ‘the currency’ of that symbiosis.
(c). Haemocyanin antimicrobial activity is linked to phenol oxidase activity
Haemocyanin is part of the tyrosinase family of enzymes [14] and exhibits a PO including tyrosinase activity in certain taxa [45]. The production of quinones by the PO activity is antimicrobial [16,46]. Thus, because haemocyanin is present in mucus coating the ciliated epithelium of the light organ, where symbionts are recruited and selected from the bacterioplankton (figure 2), we hypothesized that haemocyanin also has an antimicrobial function during these initial events. Therefore, we purified haemocyanin from squid haemolymph using size-exclusion and anion-exchange HPLC separation (see the electronic supplementary material, figure S5a). The PO activity of this purified protein was determined using dopamine, an o-diphenol common in cephalopods, especially in the adjacent ink sac. We characterized the PO activity in an acidic buffer mimicking the mucus pH, and in the presence/absence of several proteases that could enhance the PO activity [15,16]. Haemocyanin exhibited significant PO activity using dopamine as the substrate and, similar to arthropod and molluscan haemocyanins whose PO activity is enhanced by proteases [16,47], EsHCY exhibited a higher PO activity in the presence of certain proteases, particularly a subtilisin-type bacterial serine-protease with broad specificity towards proteins (figure 4a). This activation might be linked to the cleavage of either the N- or C-terminus of the protein or to a conformation change that opens a substrate-binding pocket [45]. Similar results were obtained using other substrates for PO, such as catechol, another o-diphenol and tyramine, a mono-phenol, for which slower kinetics were observed. These results showed that EsHCY has a catecholase activity and, to a lesser extent, a cresolase activity, typical of tyrosinases (see the electronic supplementary material, figure S5b). Contrary to the PO activity of crustacean haemocyanins [16], the PO activity of EsHCY was not affected by the presence of LPS (the ratio of dopamine-based activity with/without Vf-LPS was 1.07; t-test, p = 0.08).
To test whether the PO activity of EsHCY is antimicrobial, we determined the minimal concentration of haemocyanin required to inhibit growth of various strains of marine bacteria in the presence of dopamine. Vibrio fischeri exhibited an intermediate resistance compared with other marine Gram-negative and Gram-positive strains tested (figure 4b). In addition, when we assayed the antimicrobial activity of unpurified blood, which potentially contains proteases, but without the addition of dopamine, no antimicrobial activity was detected (data not shown). These results suggest that the main antimicrobial activity of haemocyanin is linked to its PO activity. Taken together, the data suggest that the EsHCY protein, which is secreted into the mucus, could play a role in the selection of the symbiont, perhaps in combination with other antimicrobial factors, e.g. NO, PGRP2, lysozymes [2,3,5], that occur in the mucus matrix.
4. Conclusion
The data presented here suggest a role for haemocyanin in the dynamics of the squid–vibrio symbiosis, both in its initiation and its maintenance. We showed that haemocyanin exhibits antimicrobial properties that, in combination with other antimicrobials present in the mucus, may be involved in selecting V. fischeri during harvesting. The synergistic mechanisms between these various antimicrobials remain to be determined, but promise to provide valuable insight into how specificity is achieved even before symbionts enter host tissues. This activity might also be critical for controlling symbionts throughout the life of the animal, e.g. by limiting growth of the symbionts, preventing colonization by non-specific bacteria or even sanctioning cheaters. Determining whether EsHCY has these functions will require further study.
We are beginning to develop a clearer concept of the elements of the squid–vibrio system that are controlled on a diel rhythm, and how they are regulated. Earlier studies showed a day–night cycle on features ranging from bacterial bioluminescence to gene expression [8,12], and recently this symbiosis was the first in which the bacteria were found to drive host circadian rhythms [48]. Our data reveal the possibility that haemocyanin is an important component of these rhythms, at least in the adult animal. Our current model for how the symbiosis modulates oxygen delivery for luminescence by the microbial partner relies in part on the pH gradient between haemolymph and the crypt environment. The capacity of the symbiont to lower the crypt pH favours the release of oxygen from haemocyanin, and host provision of nutrients is likely to have a profound influence on the symbiont's ability to modulate pH in these tissues. Not unlike the host–symbiont conversation that goes on during the establishment of the association [5], the permanent adjustment of the partners' biochemistry and physiology may play a critical role in the maintenance of the symbiosis as well.
Acknowledgement
N.K., M.J.M-N. and E.G.R. conceived and designed the study. N.K. performed all the experiments and data analyses, except the blood circulation experiment (J.S.) and a preliminary experiment for dopamine sensitivity (L.Z.). R.A. helped with the experimental design of protein purification and antimicrobial activity. S.H. was involved in the design and supervision of the oxygen affinity experiments. N.K., M.J.M-N. and E.G.R. wrote the manuscript. All the authors have read and approved the final version of the manuscript.
Funding statement
This work was supported by the Marie Curie Actions FP7-PEOPLE-2010-IOF/272684/SymbiOx to N.K., NSF Graduate Research Fellowship and NIH NIGMS T32 GM008505 to J.S., NIH grant nos. AI 50661 to M.J.M-N. and OD 011024 to E.G.R. and M.J.M-N., and the Region Bretagne HYPOXEVO research programme to S.H.
References
- 1.Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid–vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642. ( 10.1038/nrmicro957) [DOI] [PubMed] [Google Scholar]
- 2.Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ. 2004. NO means ‘yes’ in the squid–vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cell. Microbiol. 6, 1139–1151. ( 10.1111/j.1462-5822.2004.00429.x) [DOI] [PubMed] [Google Scholar]
- 3.Troll JV, Bent EH, Pacquette N, Wier AM, Goldman WE, Silverman N, McFall-Ngai MJ. 2010. Taming the symbiont for coexistence: a host PGRP neutralizes a bacterial symbiont toxin. Environ. Microbiol. 12, 2190–2203. ( 10.1111/j.1462-2920.2009.02121.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Dunn AK, Wilneff J, McFall-Ngai MJ, Spiro S, Ruby EG. 2010. Vibrio fischeri flavohaemoglobin protects against nitric oxide during initiation of the squid–vibrio symbiosis. Mol. Microbiol. 78, 903–915. ( 10.1111/j.1365-2958.2010.07376.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer N, et al. 2013. Initial symbiont contact orchestrates host-organ-wide transcriptional changes that prime tissue colonization. Cell Host Microbe 14, 183–194. ( 10.1016/j.chom.2013.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruby EG, McFall-Ngai MJ. 1999. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri. Trends Microbiol. 7, 414–420. ( 10.1016/S0966-842X(99)01588-7) [DOI] [PubMed] [Google Scholar]
- 7.Stabb EV. 2005. Shedding light on the bioluminescence ‘paradox’. ASM News 71, 223–229. [Google Scholar]
- 8.Boettcher KJ, Ruby EG, McFall-Ngai MJ. 1996. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J. Comp. Physiol. A 179, 65–73. ( 10.1007/BF00193435) [DOI] [Google Scholar]
- 9.McFall-Ngai MJ, Montgomery MK. 1990. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae). Biol. Bull. 179, 332–339. ( 10.2307/1542325) [DOI] [PubMed] [Google Scholar]
- 10.Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. 2009. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ. Microbiol. 11, 483–493. ( 10.1111/j.1462-2920.2008.01788.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decker H, Hellmann N, Jaenicke E, Lieb B, Meissner U, Markl J. 2007. Minireview: recent progress in hemocyanin research. Integr. Comp. Biol. 47, 631–644. ( 10.1093/icb/icm063) [DOI] [PubMed] [Google Scholar]
- 12.Wier AM, et al. 2010. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc. Natl Acad. Sci. USA 107, 2259–2264. ( 10.1073/pnas.0909712107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Holde KE, Miller KI, Decker H. 2001. Hemocyanins and invertebrate evolution. J. Biol. Chem. 276, 15 563–15 566. ( 10.1074/jbc.R100010200) [DOI] [PubMed] [Google Scholar]
- 14.Martín-Durán JM, de Mendoza A, Sebé-Pedrós A, Ruiz-Trillo I, Hejnol A. 2013. A broad genomic survey reveals multiple origins and frequent losses in the evolution of respiratory hemerythrins and hemocyanins. Genome Biol. Evol. 5, 1435–1442. ( 10.1093/gbe/evt102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker H, Jaenicke E. 2004. Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev. Comp. Immunol. 28, 673–687. ( 10.1016/j.dci.2003.11.007) [DOI] [PubMed] [Google Scholar]
- 16.Jiang N, Tan NS, Ho B, Ding JL. 2007. Respiratory protein-generated reactive oxygen species as an antimicrobial strategy. Nat. Immunol. 8, 1114–1122. ( 10.1038/ni1501) [DOI] [PubMed] [Google Scholar]
- 17.Bose JL, Rosenberg CS, Stabb EV. 2008. Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch. Microbiol. 190, 169–183. ( 10.1007/s00203-008-0387-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar RC, Drive RM, Valley M. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. BMC Bioinform. 32, 1792–1797. ( 10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552. ( 10.1093/oxfordjournals.molbev.a026334) [DOI] [PubMed] [Google Scholar]
- 20.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. ( 10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 21.Dereeper A, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469. ( 10.1093/nar/gkn180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustin S, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. ( 10.1373/clinchem.2008.112797) [DOI] [PubMed] [Google Scholar]
- 23.Weber RE. 1981. Cationic control of O2 affinity in lugworm erythrocruorin. Nature 292, 386–387. ( 10.1038/292386a0) [DOI] [Google Scholar]
- 24.Whitaker JR, Granum PE. 1980. An absolute method for protein determination based on difference in absorbance at 235 and 280nm. Anal. Biochem. 109, 156–159. ( 10.1016/0003-2697(80)90024-X) [DOI] [PubMed] [Google Scholar]
- 25.Fedders H, Leippe M. 2008. A reverse search for antimicrobial peptides in Ciona intestinalis: identification of a gene family expressed in hemocytes and evaluation of activity. Dev. Comp. Immunol. 32, 286–298. ( 10.1016/j.dci.2007.06.003) [DOI] [PubMed] [Google Scholar]
- 26.Lamy J, You V, Taveau JC, Boisset N, Lamy JN. 1998. Intramolecular localization of the functional units of Sepia officinalis hemocyanin by immunoelectron microscopy. J. Mol. Biol. 284, 1051–1074. ( 10.1006/jmbi.1998.2235) [DOI] [PubMed] [Google Scholar]
- 27.Markl J. 2013. Evolution of molluscan hemocyanin structures. Biochim. Biophys. Acta 1834, 1840–1852. ( 10.1016/j.bbapap.2013.02.020) [DOI] [PubMed] [Google Scholar]
- 28.Cuff ME, Miller KI, van Holde KE, Hendrickson WA. 1998. Crystal structure of a functional unit from Octopus hemocyanin. J. Mol. Biol. 278, 855–870. ( 10.1006/jmbi.1998.1647) [DOI] [PubMed] [Google Scholar]
- 29.Benkert P, Biasini M, Schwede T. 2011. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27, 343–350. ( 10.1093/bioinformatics/btq662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201. ( 10.1093/bioinformatics/bti770) [DOI] [PubMed] [Google Scholar]
- 31.Boisset N, Mouche F. 2000. Sepia officinalis hemocyanin: a refined 3D structure from field emission gun cryoelectron microscopy. J. Mol. Biol. 296, 459–472. ( 10.1006/jmbi.1999.3460) [DOI] [PubMed] [Google Scholar]
- 32.Schipp R, Höhn P, Ginkel G. 1973. Elektronenmikroskopische und histochemische Untersuchungen zur Funktion der Branchialdriise (Parabranchialdrtise) der Cephalopoda. Z. Zellforsch. 139, 253–269. ( 10.1007/BF00306525) [DOI] [PubMed] [Google Scholar]
- 33.Beuerlein K, Westermann B, Ruth P, Schimmelpfennig R, Schipp R. 2000. Hemocyanin re-uptake in the renal and branchial heart appendages of the coleoid cephalopod Sepia officinalis. Cell Tissue Res. 301, 413–421. ( 10.1007/s004410000254) [DOI] [PubMed] [Google Scholar]
- 34.Schleicher TR, Nyholm SV. 2011. Characterizing the host and symbiont proteomes in the association between the bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fischeri . PLoS ONE 6, e25649 ( 10.1371/journal.pone.0025649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melzner F, Mark FC, Pörtner H-O. 2007. Role of blood-oxygen transport in thermal tolerance of the cuttlefish, Sepia officinalis. Integr. Comp. Biol. 47, 645–655. ( 10.1093/icb/icm074) [DOI] [PubMed] [Google Scholar]
- 36.Dunn AK. 2012. Vibrio fischeri metabolism: symbiosis and beyond, 1st edn Elsevier, The Netherlands: Elsevier Ltd. [Google Scholar]
- 37.Pörtner H-O. 1994. Coordination of metabolism, acid–base regulation and haemocyanin function in cephalopods. Mar. Freshw. Behav. Physiol. 25, 131–148. ( 10.1080/10236249409378913) [DOI] [Google Scholar]
- 38.Pörtner H-O. 1990. An analysis of the effects of pH on oxygen binding by squid (Illex illecebrosus, Loligo pealei) hemocyanin. J. Exp. Biol. 424, 407–424. [Google Scholar]
- 39.Van Holde KE, Miller KI. 1995. Hemocyanins. Adv. Prot. Chem. 47, 1–81. ( 10.1016/S0065-3233(08)60545-8) [DOI] [PubMed] [Google Scholar]
- 40.Karl DM, Nealson KH. 1980. Regulation of cellular metabolism during synthesis and expression of the luminous system in Beneckea and Photobacterium. J. Gen. Microbiol. 117, 357–368. ( 10.1099/00221287-117-2-357) [DOI] [Google Scholar]
- 41.Bergersen FJ. 1997. Regulation of nitrogen fixation in infected cells of leguminous root nodules in relation to O2 supply. Plant Soil 191, 189–203. ( 10.1023/A:1004236922993) [DOI] [Google Scholar]
- 42.Kiers ET, Rousseau RA, West SA, Denison RF. 2003. Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81. ( 10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- 43.Childress JJ, Girguis PR. 2011. The metabolic demands of endosymbiotic chemoautotrophic metabolism on host physiological capacities. J. Exp. Biol. 214, 312–325. ( 10.1242/jeb.049023) [DOI] [PubMed] [Google Scholar]
- 44.Flores JF, Hourdez SM. 2006. The zinc-mediated sulfide-binding mechanism of hydrothermal vent tubeworm 400-kDa hemoglobin. Cah. Biol. Mar. 47, 371–377. [Google Scholar]
- 45.Decker H, Schweikardt T, Nillius D, Salzbrunn U, Jaenicke E, Tuczek F. 2007. Similar enzyme activation and catalysis in hemocyanins and tyrosinases. Gene 398, 183–191. ( 10.1016/j.gene.2007.02.051) [DOI] [PubMed] [Google Scholar]
- 46.Cerenius L, Lee BL, Söderhäll K. 2008. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271. ( 10.1016/j.it.2008.02.009) [DOI] [PubMed] [Google Scholar]
- 47.Decker H, Tuczek F. 2000. Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem. Sci. 25, 392–397. ( 10.1016/S0968-0004(00)01602-9) [DOI] [PubMed] [Google Scholar]
- 48.Heath-Heckman EAC, Peyer SM, Whistler CA, Apicella MA, Goldman WE, McFall-Ngai MJ. 2013. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid–vibrio symbiosis. mBio 4, e00167–13. ( 10.1128/mBio.00167-13) [DOI] [PMC free article] [PubMed] [Google Scholar]