Abstract
Germline mutations in BRCA1 predispose carriers to a high incidence of breast and ovarian cancers. BRCA1 functions to maintain genomic stability through critical roles in DNA repair, cell cycle arrest and transcriptional control. A major question has been why BRCA1 loss or mutation leads to tumors mainly in estrogen-regulated tissues, given that BRCA1 has essential functions in all cell types. Here we report that estrogen and estrogen metabolites can cause DNA double strand breaks (DSB) in estrogen receptor-α negative breast cells and that BRCA1 is required to repair these DSBs to prevent metabolite-induced genomic instability. We found that BRCA1 also regulates estrogen metabolism and metabolite-mediated DNA damage by repressing the transcription of estrogen-metabolising enzymes, such as CYP1A1, in breast cells. Lastly, we used a knock-in human cell model with a heterozygous BRCA1 pathogenic mutation to show how BRCA1 haploinsufficiency affects these processes. Our findings provide pivotal new insights into why BRCA1 mutation drives the formation of tumours in estrogen-regulated tissues, despite the general role of BRCA1 in DNA repair in all cell types.
Keywords: BRCA1, Estrogen, Estrogen Metabolism, DNA damage, Genomic Instability
INTRODUCTION
BRCA1 is a tumour suppressor protein which functions to preserve genomic stability by regulating key cellular processes including homologous recombination (HR) mediated DNA repair, cell cycle checkpoint control, transcriptional regulation, chromatin remodelling and post-replicative repair (1-3). Despite playing a role in processes essential to all cells, BRCA1 mutation predisposes to tumours predominantly in estrogen regulated tissues, such as the breasts and ovaries. Indeed, germ-line mutations in a single BRCA1 allele confer a lifetime risk of up to 90% of developing breast cancer and 30-40% of ovarian cancer (4, 5).
Several observations suggest estrogen has an important role in the development of BRCA1-dependent breast cancer. Pre- or post-menopausal oophorectomy in BRCA1 mutation carriers significantly reduces the risk of breast cancer onset and recurrence (6-8). Furthermore, pregnancy increases the risk of early-onset breast cancer in BRCA1 mutation carriers, in contrast to non-carriers for whom pregnancy is protective (9). It has also been reported that BRCA1 represses the expression of CYP19A1 (aromatase), which converts androgens to bioactive estrogens (10). Thus BRCA1 loss may increase CYP19 expression and subsequent estrogen production, further driving tumourigenesis (11).
Estrogen is postulated to promote tumourigenesis directly through stimulation of the estrogen receptor-α (ERα) and the downstream activation of pro-mitogenic transcriptional programs. However, this is confounded by observations that approximately 70-80% of BRCA1 mutated breast tumours are ERα negative (12, 13). Furthermore, BRCA1 drives ERα expression, suggesting the role of estrogen in BRCA1 dependent tumour development may be independent of ERα (14). Consistent with this, estradiol (E2; the predominant estrogen) induces tumour formation in ERα knockout mice (15). In these mice, reduction of endogenous E2, by either oophorectomy or treatment with aromatase inhibitors, delayed tumourigenesis, whereas the ERα antagonist fulvestrant had no effect (15).
The endogenous conversion of estrogen to genotoxic metabolites has been reported as an alternative, potentially ERα independent, mechanism for estrogen-dependent breast tumourigenesis. Estrogen is hydroxylated to form the catechol estrogens 2-hydroxyestradiol (2-OHE1(E2)) and 4-hydroxyestradiol (4-OHE1(E2)), a process which is catalysed by a number of cytochrome (CYP) P450 enzymes, including CYP1A1, CYP1A2, CYP1B1 and CYP3A4. The catechol estrogens are further oxidised (by the same enzymes) into semi-quinone and quinone forms, the latter of which can react with DNA to form adducts. Interestingly, urinary levels of 2-OHE2 and 4-OHE2 are elevated in breast cancer patients compared to healthy controls (16) and 4-OHE2 concentrations have been reported to be up to 3-times higher in breast cancer biopsies compared to normal breast tissue (17). Moreover, in-vivo studies have demonstrated that exogenous 2-OHE2 and 4-OHE2 can induce kidney and uterine cancers in mice (18, 19). The DNA adducts induced by these metabolites produce apurinic sites in the DNA which require repair, error-prone repair of which can lead to A-T to G-C mutations in DNA in the form of G.T heteroduplexes (20-22). Furthermore, high levels of depurinated estrogen adducts have been observed in serum and urine samples from breast cancer patients and women with a strong family history of breast cancer (23, 24). It has been suggested that these depurinating adducts are repaired through the nucleotide excision repair (NER) and base excision repair (BER) pathways, however, a study which examined chromosomal aberrations in DT40 cells after treatment with 4-OHE2, observed no difference between wild-type cells and cells depleted of XPA, a key protein in NER (25, 26). In contrast, there were enhanced chromosomal breaks following 4-OHE2 treatment of rad54 and ku70 mutant DT40 cells, both of which are required for repair of DSBs by HR and NHEJ, respectively. This suggests that estrogen metabolites may produce DNA DSBs.
The idea that estrogen metabolites may cause DNA DSBs, coupled with the role of BRCA1 in DSB repair, lead us to hypothesise that BRCA1-deficient cells, may be more susceptible to estrogen metabolite induced DNA damage and subsequent genomic instability. We therefore examined whether estrogen and its metabolites 2-OHE2 and 4-OHE2 can cause DSBs in human breast cells and examined the role of BRCA1 in both the induction and repair of estrogen metabolite induced DNA damage.
MATERIALS AND METHODS
Cell lines
MCF7 and MCF10A cells were obtained from ATCC and maintained according to the recommended instrucitons. MCF10A BRCA1 +/− 185delAG and matched control BRCA1 +/+ cells were generated as previously described (27). All cell-lines were verified by STR profiling.
siRNAs
siRNAs were obtained from Qiagen and reverse transfected into cells using RNAiMAX (Invitrogen) to a final concentration of 10nM. See Supplementary methods for sequences.
Immunofluorescence Microscopy
Cells were transfected with siRNAs as above and incubated for 48-hours. Cells were then plated onto coverslips and treated with E2, 2-OHE2 or 4-OHE2 (Sigma), or mock treated with vehicle and incubated for indicated time points. Cells were then fixed and stained with γ-H2AX (Millipore), 53BP1 (Millipore), Cyclin A (SCBT) or pATMSer1981 (Cell Signalling) primary antibodies and imaged using a Nikon Eclipse Ti microscope, using a 60x objective.
Comet assays
Neutral comet assays were carried out using the Cell Biolabs SCGE kit. Comets were scored using CometScore (TriTek Corp).
Western Blotting
Western blotting was carried out as previously described (28).
Metaphase spreads, FISH staining and chromosomal aberrations
Analysis was carried out as described previously (29).
qRT-PCR analysis
qRT-PCR was carried out using Roche LightCycler 480 RealTime ready catalogue assays for each gene (ACTB, CYP1A1, CYP1A2, CYP3A4, CYP1B1, COMT and NQO1) as per the manufactures instructions. A matched qRT-PCR reaction was carried out using the RT-ve control for each sample ensuring no genomic DNA contamination.
Chromatin immunoprecipitations (ChIPs)
ChIPs were performed as described previously (28). See supplementary methods for complete protocol and primer sequences.
Immunohistochemical staining of CYP1A1
Immunohistochemical staining was performed using a fully automated BondMax immunostainer with a polymer-based peroxidase detection system. (CYP1A1 (B4) SCBT primary antibody was used at a dilution of 1:50.
Ultra-performance-liquid-chromatography-tandem-mass spectrometry (UPLC-MS/MS)
Samples were extracted using liquid-liquid extraction (LLE) with diethyl ether, followed by dansyl chloride derivatisation as described by Xu et al. and analysed using UPLC-MS/MS. See supplementary methods for complete protocol (30).
Isolation and culture or primary breast progenitor cells
See supplementary methods for complete protocol. Ethical approval to obtain primary breast tissue was granted through the Northern Ireland Biobank. Tissue was dissociated and mammospheres cultured as previously described (31) in ultra-low attachment 75 cm^2 flasks for 7 days. Mammosphere cultured cells were then dissociated and plated into Lab-Tek II CC2 treated chamber slides (Nunc) in the media above.
RESULTS
Estrogen metabolites induce DNA DSBs in breast cells
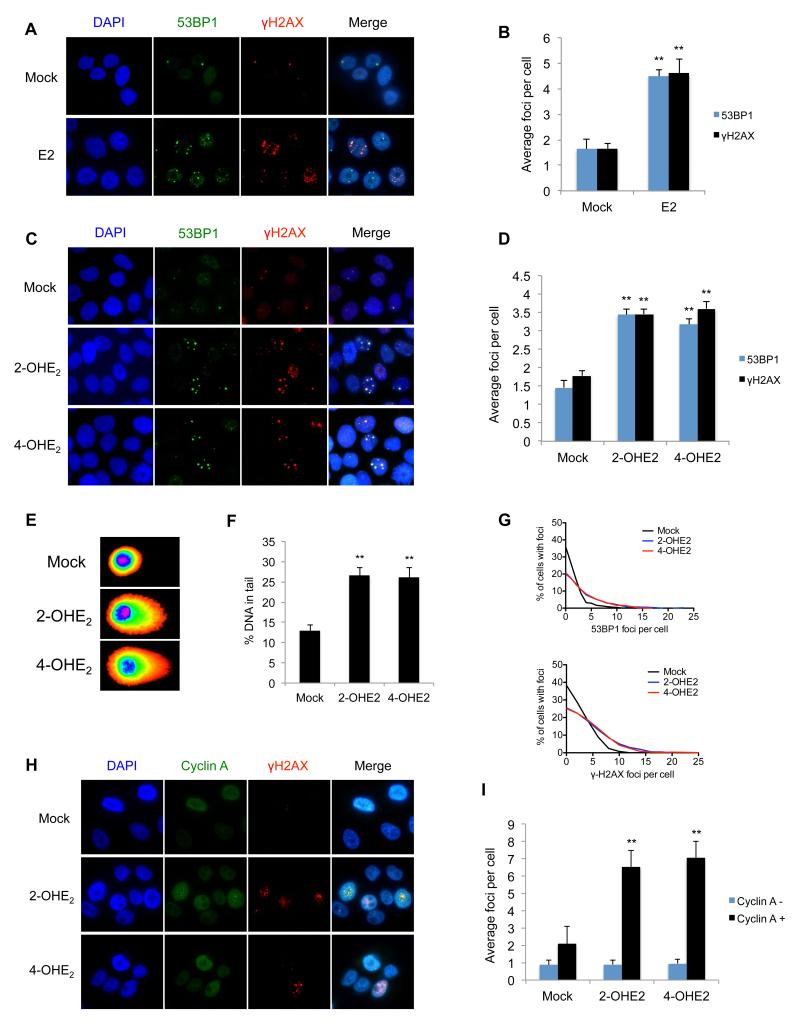
To determine if estrogen metabolites can generate DNA DSBs, we first assessed whether the parent hormone, Estradiol (E2), induces DSBs in breast cells. Normal like, ERα negative MCF10A breast cells, were treated with either E2 or mock treated for 3-hours and stained with the DSB markers 53BP1 and γH2AX. Treatment with E2 resulted in a significant increase in 53BP1 and γH2AX foci respectively, compared to controls (p<0.001) (Figure 1a-b). To investigate if estrogen metabolites cause DSBs, we treated MCF10A cells with the metabolic intermediates 2-OHE2 and 4-OHE2 for 3-hours and stained with 53BP1 and γH2AX. Similar to E2 treatment, we found that both metabolites induced a significant increase in DSB foci (p<0.01) (Figure 1c-d). These findings were also confirmed in the ERα positive breast cancer cell line, MCF7, indicating that E2 metabolite induced DSB induction is independent of ERα (Supplementary Figures 1a-b). To further confirm that 2-OHE2 and 4-OHE2 induce DSBs, we performed neutral comet assays. Indeed, MCF10A cells treated with 2-OHE2 and 4-OHE2 demonstrated a greater percentage of tail DNA, tail moment and tail length, indicative of increased DSBs (Figures 1e-f & Supplementary Figures 1c-d).
Figure 1. Estrogen and its metabolites cause DNA double strand breaks in S/G2 phase cells.
A) Representative images of 53BP1 and γ-H2AX marked DNA DSBs in MCF10A cells 3-hours after mock treatment or treatment with10nM Estradiol (E2). B) Quantification of 53BP1 and γ-H2AX foci in cells described above. Bars represent mean number of foci per cell +/− SEM from three independent experiments (>200 cells were counted per experiment). Significance of changes in foci numbers were assessed using students two-tailed t-test C) Representative images of 53BP1 and γ-H2AX marked DNA DSBs in MCF10A cells 3-hours after mock treatment or treatment with1μM 2-OHE2 or 4-OHE2. D) Quantification of 53BP1 and γ-H2AX foci in cells described above. E) Representative comet images of MCF10A cells treated as above and subjected to neutral single cell electrophoresis. Fluorescence intensity mapping of comet images has been applied using CometScore. F) Quantification of tail DNA in comets from above. Tail DNA was quantified using CometScore. Bars represent mean percentage of DNA in comet tails, +/− SEM, from three independent experiments (>100 comets were scored per experiment). G) Frequency distribution of 53BP1 and γ-H2AX foci in MCF10A cells treated with 2-OHE2 or 4-OHE2 H) Representative images of γ-H2AX marked DNA DSBs in MCF10A cells 3-hours after mock treatment or treatment with 1μM 2-OHE2 or 4-OHE2. Cells were also stained with Cyclin-A antibodies to identify cells in S/G2-phase. I) Quantification of γ-H2AX foci in Cyclin-A positive and negative cells treated as above.
DNA DSBs are known to result in activation of the ATM kinase, which is activated through autophosphorylation of ATM at serine-1981. In keeping with this, 2-OHE2 and 4-OHE2 treatment resulted in a 2.27-fold and 3.37-fold increase in ATMpSer1981 foci (co-localised with 53BP1 foci) compared to mock controls (Supplementary Figures 1e-f). Taken together, this data suggests that 2-OHE2 and 4-OHE2 induce DNA DSBs in breast cells.
Interestingly, when carrying out these experiments, we consistently observed uneven distribution of estrogen metabolite induced DSBs and rather than all cells containing slightly more foci than mock treated cells, we consistently observed that a fraction of cells (approx. 25-30%) incurred a greater number of DNA DSBs following 2-OHE2 and 4-OHE2 treatment. For example, in cells treated with 2-OHE2, 23% had no 53BP1 foci, whereas 26% of cells had 6 or more foci (Figure 1g). This suggested that estrogen metabolite mediated DNA damage may be affected by cell cycle distribution. To test this, we co-stained 2-OHE2 and 4-OHE2 treated MCF10A cells with γH2AX and cyclin-A, which is specifically expressed during S and G2 phases of the cell cycle. We found that the vast majority of estrogen metabolite mediated DNA damage occurred in cyclin-A positive cells, suggesting that 2-OHE2 and 4-OHE2 specifically induce DNA damage during S/G2 phase cells (Figures 1h-i). To confirm this, MCF10A cells treated with 2-OHE2 and 4-OHE2 were pulse labelled with 5-ethynyl-2′-deoxyuridine (EdU), which is incorporated into DNA during replication. Cells were then co-stained for γH2AX and EdU. Indeed, significantly more γH2AX foci were observed in EdU positive cells compared to EdU negative cells following estrogen metabolite treatment (Supplementary Figure 1g-h). This suggests that the DNA DSBs produced by 2-OHE2 and 4-OHE2 treatment occur specifically during S-phase.
Estrogen metabolite-mediated DNA damage is exacerbated by BRCA1 loss and not efficiently repaired in BRCA1-deficient cells
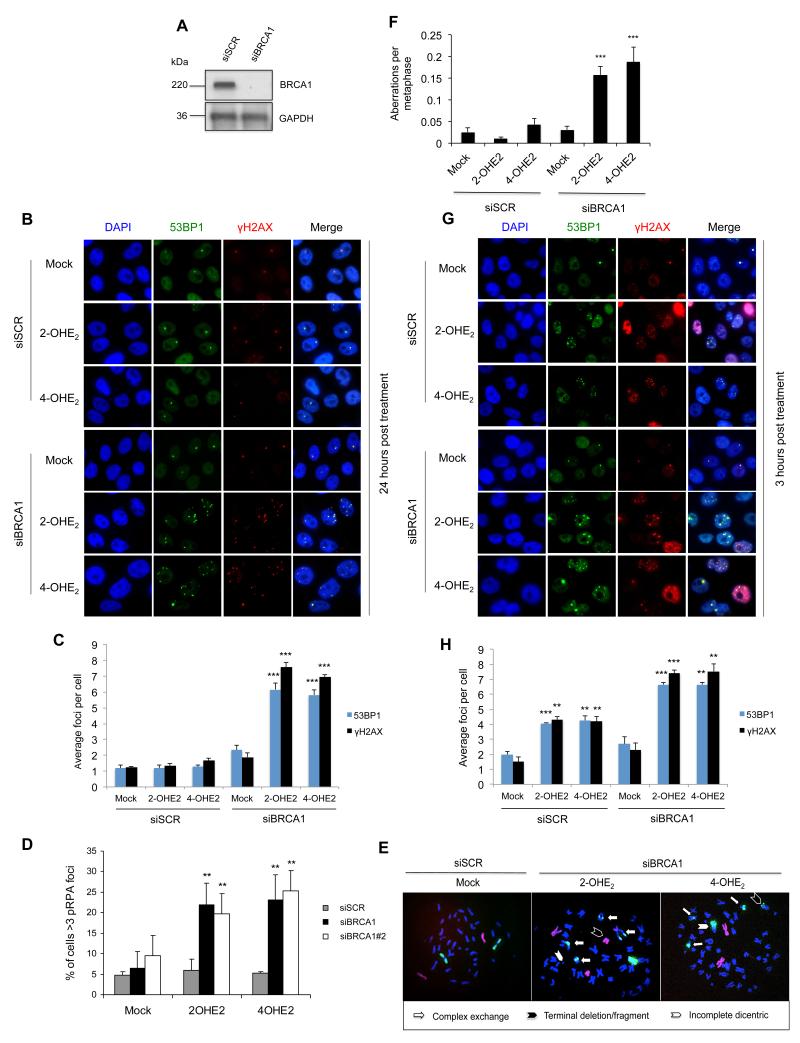
BRCA1 is known to play a pivotal role in DNA DSB repair. Additionally, a role for BRCA1 in the post-replicative repair of bulky DNA adducts induced by UV, which are structurally similar to E2 metabolite adducted bases, has been recently described (3). In light of this, we examined if BRCA1 was required for the repair of estrogen induced DSBs. Endogenous BRCA1 expression was depleted using two independent siRNAs (siBRCA1) compared to a non-targeting scrambled siRNA (siSCR) and the cells treated with 2-OHE2 and 4-OHE2 for 3-hours. Cells were then allowed to recover for twenty-four hours following treatment, before being fixed and stained for 53BP1 and γH2AX (Figure 2a-c & Supplementary Figure 2a-b). No significant difference in 53BP1 and γH2AX foci number was observed in siSCR cells treated with 2-OHE2 or 4-OHE2 relative to untreated control cells, suggesting that DSBs generated during estrogen metabolite treatment had been efficiently repaired. In contrast, significantly more 53BP1 and γH2AX foci remained in BRCA1-depleted cells treated with 2-OHE2 and 4-OHE2 compared to controls (p<0.001), indicating that BRCA1 is required for efficient repair of E2-metabolite induced DSBs. This was visualised at multiple time-points following 3-hours of 2-OHE2 or 4-OHE2 treatment (Supplementary Figure 2c d). Additionally, given that E2-metabolites induce DNA damage during S-phase, taken together with the known role for BRCA1 in the post-replicative repair of bulky DNA adducts, we assessed whether depletion of BRCA1, using two independent siRNAs, caused replication fork stalling, marked by residual pS4/8 RPA32 foci (3). Consistent with a role for BRCA1 in repairing E2-metabolite induced DNA damage in s-phase cells, we observed a dramatic increase in pS4/8 RPA32 positive cells upon BRCA1 depletion following 2OHE2 and 4OHE2 treatment (Figure 2d).
Figure 2. BRCA1 represses estrogen metabolite mediated DNA DSBs and is required for their repair.
A) Representative western blot demonstrating BRCA1 knockdown in MCF10A cells. B) Representative images of 53BP1 and γ-H2AX marked DNA DSBs in control (siSCR) and BRCA1-depleted (siBRCA1) MCF10A cells, 24-hours after treatment with 2-OHE2 or 4-OHE2 (1μM 3-hours). C) Quantification of 53BP1 and γ-H2AX foci in cells described above. Bars represent mean number of foci per cell +/− SEM from three independent experiments (>200 cells were counted per experiment). D) Quantification of cells containing >3 pS4/8-RPA foci (indicative of stalled replication forks) in control (siSCR) and BRCA1-depleted cells (siBRCA1 & siBRCA#2) treated with 2-OHE2 or 4-OHE2 (1μM 3-hours). E) Representative metaphase spreads of control (siSCR) and BRCA1-depleted (siBRCA1) MCF10A cells 24-hours after treatment with 2-OHE2 or 4-OHE2 (1μM 3-hours). Spreads were stained with FISH probes against chromosome 1 (Green) and Chromosome 2 (Red) in order to visualise gross chromosomal aberrations. F) Quantification of chromosomal aberrations in metaphase spreads from cells described above (>100 metaphases were scored per experiment). G) Representative images of 53BP1 and γ-H2AX marked DNA DSBs in MCF10A cells 3-hours after mock treatment or treatment with 1μM 2-OHE2 or 4-OHE2. H) Quantification of 53BP1 and γ-H2AX foci in cells described above.
We next examined if 2-OHE2 and 4OHE2 treatment also induced chromosomal instability in BRCA1-depleted cells. We assessed chromosomal aberrations in control and BRCA1-depleted MCF10A cells 24-hours following treatment with 2-OHE2 and 4-OHE2. Structural rearrangements were visualised and quantified in metaphase spreads using chromosome 1 & 2 FISH staining (Figure 2e-f). BRCA1 depletion resulted in a marked increase in chromosomal aberrations following both 2-OHE2 and 4-OHE2 treatment, demonstrating that E2 metabolite treatment induces genomic instability in BRCA1-deficient cells. Intriguingly, when we examined DSB production in these cells immediately following 2-OHE2 and 4-OHE2 treatment (3-hours) we found that 2-OHE2 and 4-OHE2 treatment resulted in a significant increase in 53BP1 and γH2AX foci in BRCA1-depleted cells compared to control cells (Figure 2g-h & Supplementary Figure 2e). Additionally, similar to that demonstrated earlier, E2 metabolite mediated DNA damage occurred specifically in S/G2 phase of the cell cycle in BRCA1-depleted cells (Supplementary Figure 2f). Due to the relatively short treatment time (3-hours), it is unlikely that this increase in DSBs observed in BRCA1-depleted cells is due solely to defective DNA repair. To confirm that this occurred at early time points and was not confounded by the dose and/or time points of E2-metabolite treatment used, we assessed γH2AX marked DNA damage in control and BRCA1-depleted cells following treatment with 1nm, 10nm, 100nm and 1μM E2, 2-OHE2 and 4-OHE2 at various time-points (Supplementary Figure 3a-c). This revealed increased DNA damage in BRCA1-depleted cells at all doses and treatment time-points assessed.
Taken together, these data indicate that loss of BRCA1 expression results in delayed repair kinetics but also increased levels of DNA damage following treatment with E2 metabolites. To further confirm this, we examined induction and repair of DNA DSBs following 2-OHE2 and 4-OHE2 treatment in BRCA1 mutant MDA-MB-436 cells stably transfected with either empty vector (EV) or a BRCA1 expression plasmid (Supplementary figure 4a-c). Like, BRCA1-depleted cells, this revealed increased DNA damage induction at short time points (3-hours) in the BRCA1-deficient cells (Supplementary figure 4b). Similarly, defective repair of 2-OHE2 and 4-OHE2 induced DSBs was observed in BRCA1-deficient cells in comparison to BRCA1 reconstituted cells (Supplementary figure 4c).
Given that BRCA2 is also involved in HR mediated DSB repair and mutations in this gene also predispose to tumours in the breast and ovaries, we examined the effect of BRCA2 depletion on 2-OHE2 and 4-OHE2 induced DNA damage in both MCF10A and MCF7 cells. Intriguingly, BRCA2 depletion resulted in slightly increased DSBs following treatment with 2-OHE2 and 4-OHE2 for 3-hours, which appeared to remain unrepaired at 24-hours following recovery from 2-OHE2 and 4-OHE2 treatment (Supplementary Figure 5). Nevertheless, the increased level of DNA damage observed following treatment with 2-OHE2 and 4-OHE2 in BRCA2 depleted cells was minimal in comparison to that observed in BRCA1-depleted cells. Additionally, the defective repair of these DSBs in BRCA2 depleted cells is consistent with BRCA2’s role in HR mediated DSB repair.
BRCA1 regulates the expression of estrogen metabolising enzymes
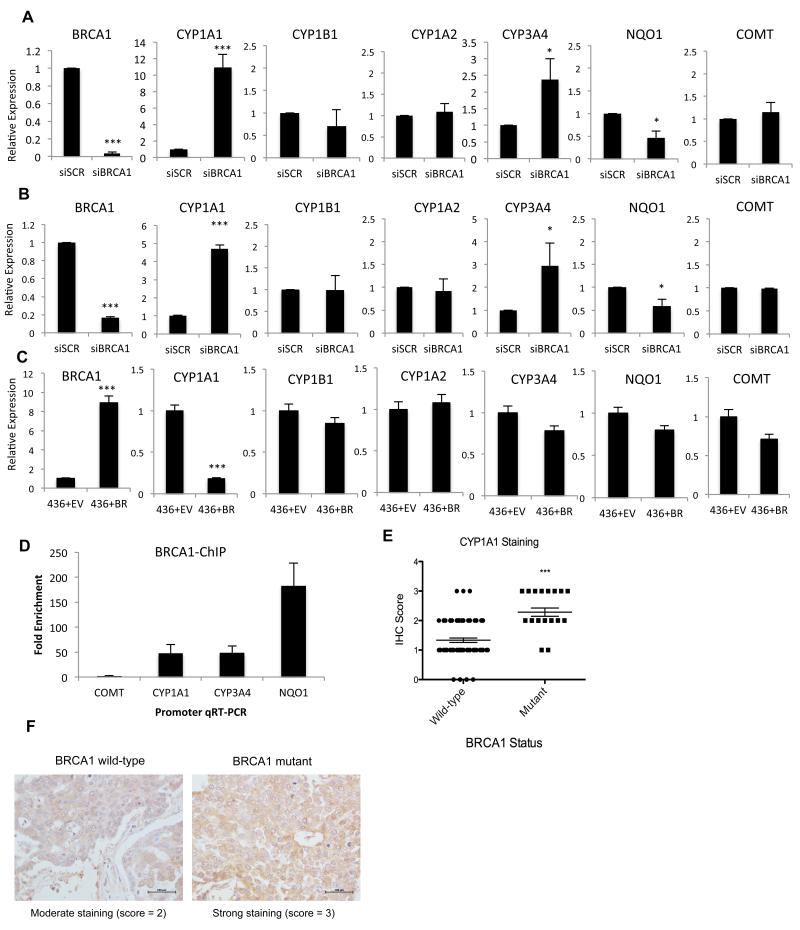
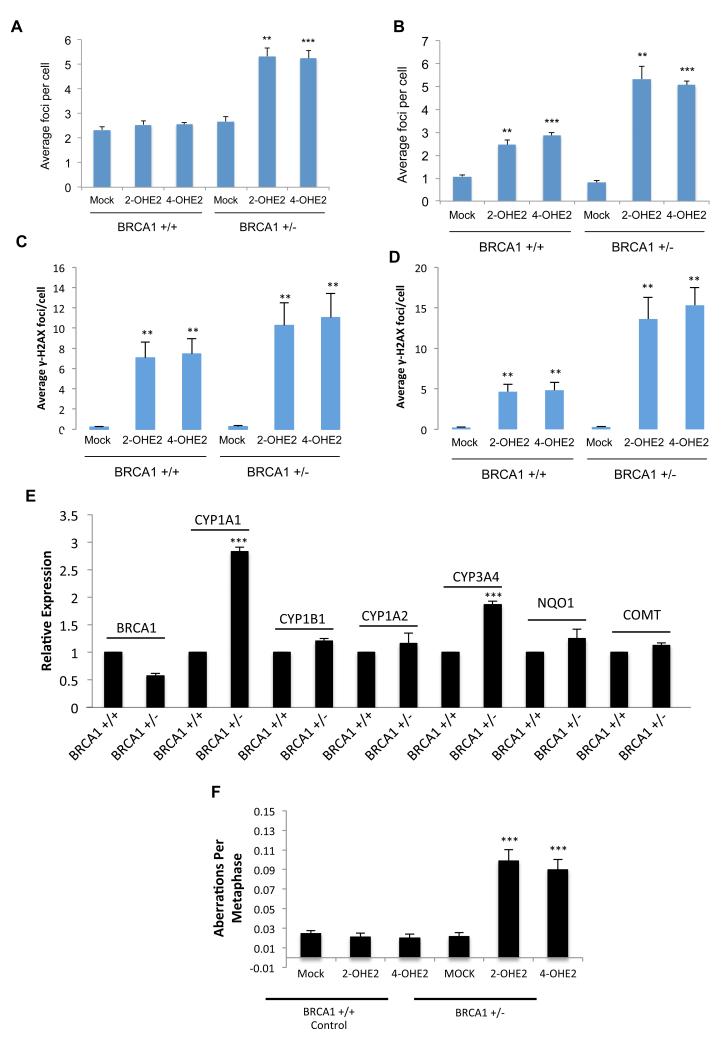
We have previously proposed that BRCA1 may transcriptionally repress the expression of estrogen metabolising genes. Specifically, we have demonstrated that CYP1A1, the enzyme responsible for conversion of E2 to 2-OHE2 and semi-quinone/quinone metabolites, is repressed by BRCA1 in a number of breast cancer cell lines (32). Additionally, BRCA1 has been found to transcriptionally activate a number of detoxification/antioxidant genes including NAD(P)H Quinone Oxoreductase 1 (NQO1), which reduces genotoxic quinones to non-reactive hydro-quinones (33). This led us to hypothesise that the exacerbated DNA damage observed in BRCA1-depleted cells at short time points following 2-OHE2 and 4-OHE2 treatment may be due, at least in part, to increased estrogen metabolism in these cells, mediated by upregulated expression of estrogen metabolising enzymes, and/or down-regulated expression of detoxification enzymes such as NQO1. To assess this, we examined the expression of a panel of estrogen metabolising and detoxification genes including CYP1A1, CYP1B1, CYP1A2, CYP3A4, NQO1 and COMT, using qRT-PCR, in MCF10A and MCF7 cells transfected with control and BRCA1 depleting siRNAs (Figure 3a-b). This revealed that CYP1A1 and CYP3A4 are consistently upregulated upon BRCA1 loss, suggesting BRCA1 represses the expression of these genes. Additionally, NQO1 was consistently down-regulated upon BRCA1 depletion in both cell lines. We also examined the expression of these genes in BRCA1-mutant MDA-MB-436 cells, stably transfected with EV or BRCA1 (Figure 3c). Ectopic expression of BRCA1 in these cells repressed expression of CYP1A1, but had a limited effect on CYP3A4 and NQO1 expression.
Figure 3. BRCA1 transcriptionally regulates estrogen metabolizing genes.
A-B) qRT-PCR determined expression of genes involved in estrogen metabolism in control (siSCR) and BRCA1-depleted (siBRCA1) MCF10A (a) and MCF7 (b) cells. Gene expression was normalised to ACTB expression and is shown relative to expression in control (siSCR) cells. Bars represent mean relative expression +/− SEM from three independent experiments. C) qRT-PCR determined expression of genes as above in the BRCA1-deficient MDA-MB-436 cells stably transfected with either empty vector (+EV) or a BRCA1 expression plasmid (+BRCA1). D) BRCA1 Chromatin Immunoprecipitation (ChIP) qPCR using primers targeting the promoters of COMT (not regulated by BRCA1), CYP1A1, CYP3A4 and NQO1. Quantified amounts of immunoprecipitated DNA were normalised to inputs and reported relative to the amount quantified at a non-specific control region. Bars represent mean fold enrichment +/− SEM from three independent experiments. E) Immunohistochemistry (IHC) determined expression of CYP1A1 in BRCA1 mutant, and matched BRCA1 wild-type breast tumours. CYP1A1 expression in each tumour was scored as 0= absent, 1 = low, 2 = moderate, or 3 = high. Significance of changes in gene expression were assessed using students two-tailed t-test - ***p = 0.0007. F). Representative images of moderate and high CYP1A1 staining in BRCA1 wild-type and BRCA1 mutant breast tumours.
To assess if BRCA1 regulates the transcription of these genes directly, we performed BRCA1 Chromatin Immunoprecipitation (ChIP)-qPCR assays from MCF10A cells, using primers specific to the promoter regions of CYP1A1, CYP3A4, NQO1 and COMT as a negative control (Figure 3d). We observed enrichment of BRCA1 at all of these promoters with the exception of COMT, which is neither transcriptionally regulated by BRCA1 nor promoter bound by BRCA1.
To confirm that this occurs in BRCA1-deficient tumours, we assessed the levels of CYP1A1, the most highly de-regulated gene upon BRCA1 loss, using immunohistochemistry (IHC) in a panel of 21 BRCA1 mutant and 75 BRCA1 wild-type breast tumours. Intratumoural CYP1A1 expression was scored by a pathologist and an independent scorer as very weak/absent = 1, moderate = 2 or strong = 3 (Figure 3e-f). This revealed that the mean expression of CYP1A1 expression is significantly upregulated in this cohort of BRCA1 mutant tumours compared to BRCA1 wild-type sporadic tumours (p = 0.0007).
Given that we observed a slight increase in E2-metabolite induced DNA damage in BRCA2 depleted cells treated with 2-OHE2 and 4-OHE2 we also assessed the role of BRCA2 in regulating the expression of CYP1A1 in both MCF10A and MCF7 cells (Supplementary figure 6a-b). This revealed no significant difference in CYP1A1 expression between control and BRCA2-depleted cells.
BRCA1 supresses estrogen metabolite mediated DNA damage by suppressing estrogen metabolism
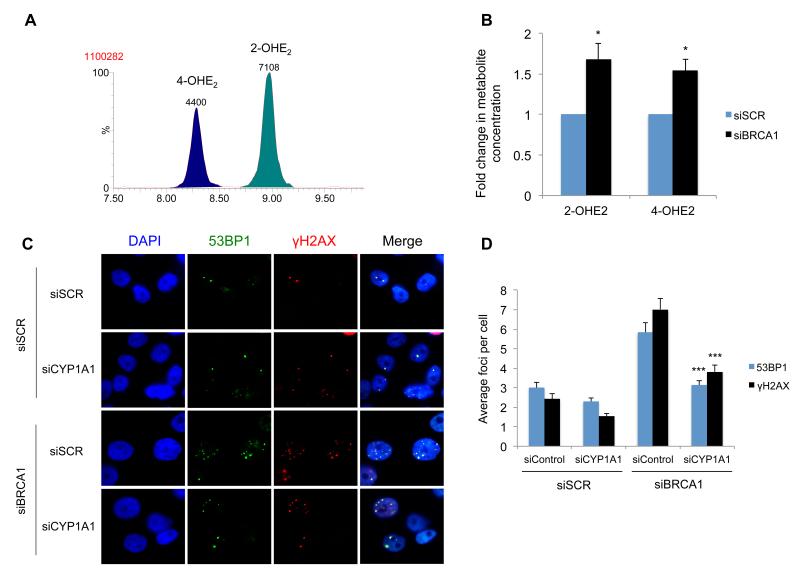
As BRCA1-depletion results in increased levels of CYP1A1 and CYP3A4 enzymes and decreased NQO1 expression, we hypothesised that the levels of 2-OHE2 and 4-OHE2 and 2,3/3,4 quinone products would be increased in BRCA1-depleted cells compared to control cells following E2 treatment. Quinone and semi-quinone metabolites are extremely unstable and have a very limited half-life making them difficult to quantify. In contrast, 2-OHE2 and 4-OHE2 (which are generated by CYP1A1 and CYP3A4 in breast cells) are relatively stable. We therefore developed an ultra-performance-liquid-chromatography-tandem-mass spectrometry (UPLC-MS/MS) method for the detection of 2-OHE2 and 4-OHE2. The method was optimised for baseline chromatographic separation and the accurate identification and quantification of both isomeric forms of the hydroxyl-estradiol metabolite (Figure 4a). Using this method, we found that the relative concentration of 2-OHE2 and 4-OHE2 was significantly higher in BRCA1-depleted cells compared to control cells (p<0.05) (Figure 4b).
Figure 4. BRCA1 Suppresses Estrogen Metabolite Mediated DNA Damage by Suppressing Estrogen Metabolism.
A) UPLC-MS/MS MRM transition (m/z 755.1>521.1) chromatogram from control (siSCR) MCF10A cell culture medium. The transition (representing loss of one dansyl group at the O-S bond) detects both isobaric species 2- & 4-OHE2-bisdansyl with baseline chromatographic separation. Isomer identification was confirmed by ratio of a secondary ion transition (m/z 755.1>170.2) in comparison with analytical standards. Quantification accuracy was enhanced by use of isotopically labelled internal standards. B) Levels of 2- & 4-OHE2 in culture medium from control (siSCR) and BRCA1-depleted (siBRCA1) MCF10A cells quantified by an isotope dilution UPLC-MS/MS method. Cells were cultured in medium containing 10 nM E2 for 24h prior to media sampling. All values were normalised to cell counts. Bars represent mean concentration +/− SEM from three independent experiments (8 replicate cultures). Significance of changes in gene expression were assessed using Student’s two-tailed t-test with significant changes in concentration data indicated by; * = p < 0.05. C) Representative images of 53BP1 and γ-H2AX marked DNA DSBs in BRCA1 and CYP1A1 co-depleted MCF10A cells, 3-hours after mock treatment or treatment with 10nM Estradiol. Depletion of BRCA1 and CYP1A1 was confirmed by qRT-PCR (Supplementary figure 6c-d) (E2). D) Quantification of 53BP1 and γ-H2AX foci in cells described above (>200 cells were counted per experiment). Significance of changes indicated by; *** = p < 0.001.
As CYP1A1 was the most robustly up-regulated estrogen metabolising gene upon BRCA1 depletion, we sought to examine the role of this enzyme in estrogen dependent DNA damage. To investigate this, BRCA1 and CYP1A1 were co-depleted in MCF10A cells and DNA DSBs assessed 3-hours following treatment with E2 (Figure 4c-d). BRCA1 and CYP1A1 depletion was assessed by qRT-PCR (Supplementary Figure 6c-d). Strikingly, CYP1A1 depletion lead to a marked reduction in estrogen mediated DSBs in BRCA1-depleted cells (p<0.001), suggesting that estrogen mediated DNA damage in BRCA1-depleted cells is, at least in part, due to increased CYP1A1 levels in these cells.
This suggests that BRCA1, apart from mediating repair of E2-metabolite mediated DNA damage, transcriptionally regulates estrogen metabolising enzymes and subsequently represses estrogen metabolism, thereby protecting cells against estrogen induced DNA damage. This is particularly important in breast and ovarian cells, which are exposed to much higher levels of estrogen than other tissues within the body (34). Nevertheless, to confirm whether this mechanism may drive genomic instability in non-breast cells, we assessed E2-metabolite induced DNA damage in HEK293, kidney cells (Supplementary Figure 6e-f). This revealed that, although 2-OHE2 and 4-OHE2 are capable of inducing DNA DSBs in these cells, they induce much lower levels of DSBs in comparison to breast cells. Additionally, 2-OHE2 and 4-OHE2 treatment (3-hours) did not induce increased levels of DSBs in BRCA1-depleted cells (Supplementary Figure 6e). Moreover, we observed similar, low-levels of DNA damage in both BRCA1 and BRCA2 depleted cells. In contrast, consistent with the role of BRCA1 and BRCA2 in DSB repair, both BRCA1 and BRCA2 were required for repair of E2-metabolite induced DNA damage in these cells (Supplementary Figure 6f). Given that much lower levels of DNA damage were observed in these cells and that we did not observe any increased DNA damage in BRCA1-depleted cells in comparison to control, or BRCA2 depleted cells, we hypothesised that BRCA1 may not regulate the expression of CYP1A1 in these cells. Intriguingly, we were unable to detect any CYP1A1 transcript in these cells, suggesting that these cells may not metabolise estrogen at the same rate as breast cells (data not shown).
Estrogen metabolite mediated DNA DSBs are exacerbated in BRCA1 heterozygous breast cells
Taken together, our data suggests that in BRCA1-deficient breast cells, de-regulated estrogen metabolism results in increased levels of genotoxic metabolites resulting in increased DNA damage which, coupled with defective DNA repair, leads to genomic instability, a key hallmark of cancer initiation and progression. However, clinical evidence suggests a direct role for estrogen in breast cancer development in BRCA1 mutation carriers, suggesting that heterozygous loss of BRCA1 may result in haploinsufficiency in at least one of BRCA1’s functions. Indeed, a number of studies have reported increased sensitivity to ionising radiation in BRCA1 carrier/heterozygous cells suggesting that DNA DSB repair may be impaired in carriers (35). Additionally, a recent study by Konishi et al., using somatic cell gene targeting to introduce the common pathogenic BRCA1 mutation 185delAG into a single BRCA1 allele in MCF10A cells, demonstrated that this heterozygous BRCA1 mutation confers impaired HR mediated DSB repair, hypersensitivity to genotoxic stress and increased genomic instability (27). We therefore set out to determine if heterozygous mutation of BRCA1 impacts estrogen metabolite mediated DNA damage and estrogen metabolism.
Using the same cell line model developed by Konishi et al., heterozygous BRCA1 185delAG (BRCA1+/−) and control (BRCA1+/+) MCF10A cells were treated with 2-OHE2 and 4-OHE2 for 3-hours and the media replaced with normal media for 24-hours, before fixing and staining for 53BP1 and γH2AX. Consistent with a defect in BRCA1 function, a significant number of unresolved DNA damage foci were visible in 2-OHE2 and 4-OHE2 treated BRCA1+/− cells in comparison to control BRCA1+/+ cells (p<0.001) (Figure 5a). Surprisingly, the level of unrepaired DNA DSBs was similar to that observed in BRCA1-depleted MCF10A cells, suggesting that BRCA1 haploinsufficiency imparts a major defect in repair of E2-metabolite mediated DNA damage. We next examined if, estrogen metabolites generate more DSBs in BRCA1+/− cells. Indeed, although short term (3-hours) 2-OHE2 and 4-OHE2 treatment induced DNA damage in BRCA1+/+ cells, significantly more DNA DSB foci were observed in BRCA1+/− cells (p<0.01) (Figure 5b). In support of this, we treated normal primary breast progenitor cells, isolated from breast tissue obtained from a woman undergoing elective breast reduction, as well as BRCA1+/− primary breast progenitor cells from a BRCA1 mutation carrier undergoing a risk-reducing mastectomy, with both 2-OHE2 and 4-OHE2 and examined both DNA damage induction at 3-hours post treatment, as well as their ability to repair E2-metabolite induced DNA damage 24-hours following treatment with 2-OHE2 and 4-OHE2. In keeping with our previous findings, 2-OHE2 and 4-OHE2 treatment for 3-hours induced more DNA damage in the BRCA1+/− mammary progenitor cells compared to the BRCA1 wild-type cells and this DNA damage was not repaired as efficiently in the BRCA1+/− cells compared to the BRCA1 wild-type cells (Figure 5c-d). Taken together this data suggests that like BRCA1-depleted cells, BRCA1+/− cells may have increased rates of estrogen metabolism as well as defective repair of E2-metabolite induced DNA damage.
Figure 5. BRCA1 heterozygosity leads to increased estrogen metabolite mediated DNA damage, defective DNA repair, and genomic instability and loss of repression of estrogen metabolizing enzymes.
A-B) Quantification of γ-H2AX marked DNA DSBs in BRCA1 wild-type (BRCA1 +/+) and BRCA1 heterozygous 185delAG (BRCA1 −/+) MCF10A cells, 24-hours after treatment with 2-OHE2 or 4-OHE2 (a) or immediately following 3-hours of treatment with 2-OHE2 or 4-OHE2 (b). Bars represent mean number of foci per cell +/− SEM from three independent experiments (>200 cells were counted per experiment). C-D) Quantification of γ-H2AX marked DNA DSBs as above in BRCA1 wild-type (BRCA1 +/+) and BRCA1 heterozygous primary breast progenitor cells 24 hours after treatment with 2-OHE2 or 4-OHE2 (c) or immediately following 3-hours of treatment with 2-OHE2 or 4-OHE2 (d). E) qRT-PCR determined expression of genes involved in estrogen metabolism in BRCA1 wild-type (BRCA1 +/+) and BRCA1 heterozygous 185delAG (BRCA1 −/+) MCF10A cells. Bars represent mean relative expression +/− SEM from three independent experiments. F) Quantification of chromosomal aberrations in metaphase spreads from BRCA1 wild-type (BRCA1 +/+) and BRCA1 heterozygous 185delAG (BRCA1 −/+) MCF10A cells, 24-hours after treatment with 2-OHE2 or 4-OHE2. Bars represent mean aberrations per metaphase +/− SEM, from three independent experiments (>100 metaphases were scored per experiment).
To examine this, we assessed the expression levels of the same panel of estrogen metabolising and detoxification enzymes in BRCA1 +/+ and BRCA1 +/− cells. Indeed, as in BRCA1-depleted cells, CYP1A1 and CYP3A4 were upregulated in BRCA1+/− compared to BRCA1+/+cells (Figure 5e). Intriguingly, NQO1 expression was maintained at similar levels in both cells lines suggesting that BRCA1 haploinsufficiency does not negatively impact all BRCA1 regulated transcriptional targets. Finally, consistent with the defective DSB repair observed in these cells following 2-OHE2 and 4-OHE2 treatment, we found that treatment with either of these metabolites induced genomic instability in BRCA1 +/− cells but not BRCA1 +/+ cells (Figure 5f).
DISCUSSION
One of the most perplexing features of BRCA1 biology, is that despite playing a central role in the DNA damage response and DSB repair pathways in all cells, mutation carriers predominantly develop tumours in the breast and ovaries; both estrogen driven tissues exposed to high levels of estrogen. Here, we show that treatment with both E2 and the E2-metabolites 2-OHE2 and 4-OHE2, induces DNA DSBs in human breast cancer cells in an ERα independent manner. We also found that E2-metabolite mediated DSBs occur specifically in S-phase cells, suggesting that induction of these lesions is coupled to DNA replication. We hypothesise that E2-metabolite adducted DNA bases represent replication barriers, which lead to replication fork stalling during DNA synthesis. Indeed, a number of studies have shown that 4-hydroxyequilenin (4-OHEN) a metabolite of the equine estrogen equilenin (which is almost identical to 4OHE-2 in humans) causes identical DNA-adducts to those caused by 4-OHE2 and that these adducts cause replication fork stalling (36, 37).
In general, stalled replication forks do not collapse and form DSBs, but are instead stabilised by the ATR kinase through the signalling and recruitment of a plethora of checkpoint signalling and repair proteins, resulting in resolution of the stalled fork through a HR-mediated repair process involving BRCA1. However, recent studies have shown that E2 inhibits ATR signalling, suggesting that E2 and its metabolites may lead to replication fork stalling and subsequent fork collapse and DSB formation through the combined effect of replication fork stalling and ATR inhibition (38). Further to this, BRCA1 has been shown to be required for both resolution of stalled replication forks as well as HR mediated repair of DSBs caused following stalled fork collapse (39). BRCA1 is also required for the removal and repair of bulky base adducts, a mechanism through which BRCA1 may supress adduct induced mutagenesis (3). Consistent with this, we observed a dramatic increase in pS4/8 RPA32 positive cells, upon BRCA1 depletion in 2OHE2 and 4OHE2 treated cells.
Interestingly, we found that BRCA1 depletion also resulted in increased levels of E2-metabolite induced DNA damage, even at very early time points. This suggested that BRCA1 may also play a more direct role in regulating the physical levels of DNA damage induced by estrogen metabolites. Previous data from our laboratory had indicated that BRCA1 loss leads to upregulation of CYP1A1, a major regulator of E2 metabolism in breast tissues (32). We therefore tested whether BRCA1 may also regulate the expression of other estrogen metabolising enzymes, thereby regulating the levels of estrogen derived metabolites. This analysis revealed that BRCA1 directly represses the transcription of CYP1A1 and CYP3A4 and promotes the expression of the NAD(P)H:quinone oxidoreductase, NQO1. We confirmed, using IHC in a cohort of BRCA1 mutant and matched BRCA1 wild-type tumours, that CYP1A1, the major enzyme involved in conversion of E2 to 2-OHE2 in breast tissues, is significantly upregulated in BRCA1 mutant tumours. This is consistent with the increased levels of both 2-OHE2 and 4-OHE2 observed in BRCA1-depleted cells. We also demonstrated that depletion of CYP1A1 significantly reduces the amount of DNA damage induced in BRCA1-depleted cells exposed to short term E2 treatment, confirming that E2-mediated DNA damage in BRCA1-depleted cells is, at least in part, due to increased estrogen metabolism. Intriguingly, when examining the impact of BRCA2 on estrogen metabolite-induced DNA damage, we found that although BRCA2 is required for the repair of these breaks, loss of BRCA2 does not lead to deregulated estrogen metabolism and the associated increased DNA damage. Perhaps this explains why BRCA2 mutations are less penetrant than BRCA1 mutations in predisposing carriers to breast and ovarian cancers.
We have also demonstrated that 2-OHE2 and 4-OHE2 treatment leads to increased DSB production in MCF10A cells and primary breast cells harbouring a pathogenic heterozygous BRCA1 mutation, and that like BRCA1-depleted cells, these cells are unable to repair E2-metabolite mediated DNA damage leading to increased genomic instability. Importantly, we found that BRCA1 heterozygous mutant cells also have upregulated levels of CYP1A1 and CYP3A4, suggesting that increased estrogen metabolism may contribute to E2-mediated DNA damage in these cells. Consistent with this, higher levels of urinary excreted 2-OHE2 and 4-OHE2 have been observed in BRCA1 carriers compared to healthy control women with no BRCA1 mutation (40).
Taken together, these findings suggest that exposure to estrogen and its subsequent metabolism in BRCA1-deficient breast cells, is capable of driving genomic instability, a well-defined early event in breast cancer development. Given that estrogen levels in normal/benign breast tissue are known to be 6-7 times that of circulating estrogen levels, our findings suggest a mechanism through which BRCA1 carriers, through enhanced production of DNA damaging estrogen metabolites, may acquire the genetic alterations that initiate neoplastic transformation in breast tissue (34). Similarly, levels of estrogen in ovarian tissues greatly exceed that of circulating estrogen, suggesting that this model may also explain the substantially increased risk of ovarian cancer in BRCA1 carriers (41).
A phase III trial termed, Prevention of Breast Cancer by Letrozole in Postmenopausal Women carrying a BRCA1/2 Mutation (LIBER), (ClinicalTrials.gov number, NCT00673335) is currently enrolling postmenopausal women for treatment with letrozole, an Aromatase Inhibitor (AI), to evaluate its ability to prevent the development of breast cancer in patients with a BRCA1/2 mutation. Our results coupled with the finding that aromatase levels are substantially higher in prophylactic mastectomy and oophorectomy tissue from BRCA1 carriers (10), provides further mechanistic data to support this approach.
However, AIs may have little preventative effect in premenopausal women, in whom the majority of BRCA1-linked tumours develop, and in whom estrogen production occurs predominantly in the ovaries through an aromatase independent biosynthesis pathway. In these women, oophorectomy has been shown to reduce the risk of breast cancer by up to 60% (42). Taking our findings into account, it may also be worth considering the use of AIs as an additional chemopreventative strategy in premenopausal women, whom have undergone risk-reducing oophorectomy without mastectomy.
Finally, in premenopausal women who have opted not to undergo risk-reducing oophorectomy or mastectomy, Luteinising hormone releasing hormone agonists (LHRHa’s), may prove useful as chemopreventative agents. These drugs cause reversible ovarian suppression/ablation and are currently used in combination with tamoxifen or AIs for the treatment of premenopausal women with ERα positive breast cancer (43, 44).
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the Northern Ireland Biobank for providing breast tumour sections and fresh normal breast tissues. This work was supported by grants from Cancer Research UK (C538/A8132 (D.P.H, K.B.M., M.B.), the Research and Development Office Northern Ireland (G.W.I.) and Cancer Focus Northern Ireland (K.I.S).
Footnotes
CONFLICT OF INTERESTS: The authors declare that they have no conflict of interest
REFERENCES
- 1.Huen MS, Sy SM, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11:138–48. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Q, Pao GM, Huynh AM, Suh H, Tonnu N, Nederlof PM, et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477:179–84. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathania S, Nguyen J, Hill SJ, Scully R, Adelmant GO, Marto JA, et al. BRCA1 is required for postreplication repair after UV-induced DNA damage. Mol Cell. 2011;44:235–51. doi: 10.1016/j.molcel.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 6.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–15. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 7.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–22. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 8.Narod SA, Kotsopoulos J, Lubinski J, Lynch H, Kim-Sing C, Neuhausen SL, et al. Oophorectomy after Menopause and the Risk of Breast Cancer in BRCA1 and BRCA2 Mutation Carriers? Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jernstrom H, Lerman C, Ghadirian P, Lynch HT, Weber B, Garber J, et al. Pregnancy and risk of early breast cancer in carriers of BRCA1 and BRCA2. Lancet. 1999;354:1846–50. doi: 10.1016/s0140-6736(99)04336-6. [DOI] [PubMed] [Google Scholar]
- 10.Chand AL, Simpson ER, Clyne CD. Aromatase expression is increased in BRCA1 mutation carriers. BMC Cancer. 2009;9:148. doi: 10.1186/1471-2407-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Ghosh S, Amleh A, Yue W, Lu Y, Katz A, et al. Modulation of aromatase expression by BRCA1: a possible link to tissue-specific tumor suppression. Oncogene. 2005;24:8343–8. doi: 10.1038/sj.onc.1208985. [DOI] [PubMed] [Google Scholar]
- 12.Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of Breast and Ovarian Cancers among BRCA1 and BRCA2 Mutation Carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomarkers Prev. 2012;21:134–47. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–8. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosey AM, Gorski JJ, Murray MM, Quinn JE, Chung WY, Stewart GE, et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99:1683–94. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127:1748–57. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Sun J, Chen Y, Song Y, Dong L, Zhan Q, et al. Analysis of multiplex endogenous estrogen metabolites in human urine using ultra-fast liquid chromatography-tandem mass spectrometry: A case study for breast cancer. Anal Chim Acta. 2012;711:60–8. doi: 10.1016/j.aca.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 17.Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 18.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–7. [PubMed] [Google Scholar]
- 19.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24:353–6. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarti D, Mailander PC, Li KM, Higginbotham S, Zhang HL, Gross ML, et al. Evidence that a burst of DNA depurination in SENCAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–53. doi: 10.1038/sj.onc.1204969. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Kosinska W, Khmelnitsky M, Cavalieri EL, Rogan EG, Chakravarti D, et al. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem Res Toxicol. 2006;19:475–9. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 22.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G.C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J Steroid Biochem Mol Biol. 2006;101:204–15. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Gaikwad NW, Yang L, Muti P, Meza JL, Pruthi S, Ingle JN, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 2008;122:1949–57. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaikwad NW, Yang L, Pruthi S, Ingle JN, Sandhu N, Rogan EG, et al. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer. 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani A, Okada T, Shibutani S, Sonoda E, Hochegger H, Nishigori C, et al. Extensive chromosomal breaks are induced by tamoxifen and estrogen in DNA repair-deficient cells. Cancer Res. 2004;64:3144–7. doi: 10.1158/0008-5472.can-03-3489. [DOI] [PubMed] [Google Scholar]
- 27.Konishi H, Mohseni M, Tamaki A, Garay JP, Croessmann S, Karnan S, et al. Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc Natl Acad Sci U S A. 2011;108:17773–8. doi: 10.1073/pnas.1110969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorski JJ, Savage KI, Mulligan JM, McDade SS, Blayney JK, Ge Z, et al. Profiling of the BRCA1 transcriptome through microarray and ChIP-chip analysis. Nucleic Acids Res. 2011;39:9536–48. doi: 10.1093/nar/gkr679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manti L, Durante M, Grossi G, Ortenzia O, Pugliese M, Scampoli P, et al. Measurements of metaphase and interphase chromosome aberrations transmitted through early cell replication rounds in human lymphocytes exposed to low-LET protons and high-LET 12C ions. Mutat Res. 2006;596:151–65. doi: 10.1016/j.mrfmmm.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–54. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 31.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harte MT, O’Brien GJ, Ryan NM, Gorski JJ, Savage KI, Crawford NT, et al. BRD7, a subunit of SWI/SNF complexes, binds directly to BRCA1 and regulates BRCA1-dependent transcription. Cancer Res. 2010;70:2538–47. doi: 10.1158/0008-5472.CAN-09-2089. [DOI] [PubMed] [Google Scholar]
- 33.Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang HJ, et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64:7893–909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 34.Lonning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol. 2009;117:31–41. doi: 10.1016/j.jsbmb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Ernestos B, Nikolaos P, Koulis G, Eleni R, Konstantinos B, Alexandra G, et al. Increased chromosomal radiosensitivity in women carrying BRCA1/BRCA2 mutations assessed with the G2 assay. Int J Radiat Oncol Biol Phys. 2010;76:1199–205. doi: 10.1016/j.ijrobp.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki N, Yasui M, Santosh Laxmi YR, Ohmori H, Hanaoka F, Shibutani S. Translesion synthesis past equine estrogen-derived 2′-deoxycytidine DNA adducts by human DNA polymerases eta and kappa. Biochemistry. 2004;43:11312–20. doi: 10.1021/bi049273n. [DOI] [PubMed] [Google Scholar]
- 37.Yasui M, Suzuki N, Liu X, Okamoto Y, Kim SY, Laxmi YR, et al. Mechanism of translesion synthesis past an equine estrogen-DNA adduct by Y-family DNA polymerases. J Mol Biol. 2007;371:1151–62. doi: 10.1016/j.jmb.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedram A, Razandi M, Evinger AJ, Lee E, Levin ER. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell. 2009;20:3374–89. doi: 10.1091/mbc.E09-01-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Z, Zhang J. A dual role of BRCA1 in two distinct homologous recombination mediated repair in response to replication arrest. Nucleic Acids Res. 2012;40:726–38. doi: 10.1093/nar/gkr748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berstein LM, Koskela A, Boyarkina MP, Adlercreutz H. Excretion of estrogens, catecholestrogens and phytoestrogens in carriers of BRCA1 gene mutations: effects of metformin. Neoplasma. 2010;57:333–8. doi: 10.4149/neo_2010_04_333. [DOI] [PubMed] [Google Scholar]
- 41.Lindgren PR, Backstrom T, Cajander S, Damber MG, Mahlck CG, Zhu D, et al. The pattern of estradiol and progesterone differs in serum and tissue of benign and malignant ovarian tumors. Int J Oncol. 2002;21:583–9. [PubMed] [Google Scholar]
- 42.Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23:7491–6. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 43.Del Mastro L, Levaggi A, Giraudi S, Pronzato P. Luteinising hormone releasing hormone agonists (LH-RHa) in premenopausal early breast cancer patients: current role and future perspectives. Cancer Treat Rev. 2011;37:208–11. doi: 10.1016/j.ctrv.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Rossi E, Morabito A, De Maio E, Di Rella F, Esposito G, Gravina A, et al. Endocrine effects of adjuvant letrozole + triptorelin compared with tamoxifen + triptorelin in premenopausal patients with early breast cancer. J Clin Oncol. 2008;26:264–70. doi: 10.1200/JCO.2007.13.5319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.