Abstract
Background and Aim
Pre-transplant sarcopenia (reduced skeletal muscle mass) predicts poor outcome in cirrhosis. In contrast, whether muscle mass increases post orthotopic liver transplantation (OLT) is not known and was studied prospectively.
Methods
Consecutive patients who underwent a comprehensive nutritional evaluation in a liver transplant nutrition clinic were included. Core abdominal muscle area was measured on abdominal CT obtained pre- and post-OLT. Age and gender based controls were used to define sarcopenia. Measures of body composition pre-transplant were correlated with CT measurements. Predictors and clinical impact of post-OLT change in muscle area were examined. In 3 subjects post-OLT and 3 controls, expression of genes regulating skeletal muscle mass were quantified.
Results
During the study period, 53 patients (M:F 41:12; age 56.9±7.5 years) were followed up after OLT for 19.3±9 months. Five patients died and another 5 had acute graft rejection. Pre-OLT sarcopenia was present in 33 (66.2%). Pre-transplant clinical characteristics including Child’s score, MELD score and nutritional status or post transplantation immunosuppression regimen did not predict post transplant change in muscle mass. New onset post-OLT sarcopenia developed in 14 patients. Loss of muscle mass post-OLT increased risk of diabetes mellitus and a trend towards higher mortality. Skeletal muscle expression of myostatin was higher and that of ubiquitin proteasome proteolytic components lower post-OLT than in controls.
Conclusions
Post transplantation sarcopenia is common and could not be attributed to pre-transplant characteristics or the type or duration of post-OLT immunosuppression. Post-transplant sarcopenia contributes to adverse consequences and strategies targeting myostatin may be beneficial.
Keywords: Sarcopenia, cirrhosis, liver transplantation, outcome
Introduction
Reduction in skeletal muscle mass, or sarcopenia, is the most common complication in cirrhosis and adversely affects outcome before, during and after orthotopic liver transplantation (OLT)(1, 2). Liver transplantation reverses the biochemical abnormalities of cirrhosis as well as the complications of portal hypertension, including ascites and hepatorenal syndrome(3). Even though sarcopenia has not been specifically evaluated, studies of lean body mass using indirect measures of skeletal muscle mass failed to demonstrate an increase post-OLT (4-10). Muscle area quantified at standardized landmarks on abdominal CT is a more precise measure of whole body muscle mass(11, 12). Serial computed tomography (CT) scans form part of standard clinical care in cirrhotics pre-OLT(13). Although CT measurements of core abdominal muscle area have been studied in cirrhotics pre-OLT(13, 14), there are no systematic studies on serial changes in core muscle area before and after OLT(10). The present study was therefore performed to examine changes in skeletal muscle mass following liver transplantation, and its effect on clinical outcomes. The impact of OLT on visceral and subcutaneous fat areas on CT was also studied. Additionally, expression of genes regulating skeletal muscle mass was quantified in a subset of patients and controls in which this could be obtained.
Methods
We included consecutive adult patients with cirrhosis who had CT scans of the abdomen with pelvis before and after liver transplantation from July 2009 to July 2011. The pre-transplant diagnosis was confirmed by histology in the explanted liver. In 3 patients, the explanted liver had a small (<0.5 cm) hepatocellular carcinoma (HCC) that was not diagnosed pre-transplant.
All subjects had precise measurements of height and weight, anthropometric measurements for mid arm muscle area using a non-stretchable tape measure, triceps skinfold thickness using a Lange® skinfold calipers, and grip strength using the Jamar® grip strength meter prior to transplantation. Body composition was also quantified using a tetrapolar bioelectrical impedance analyzer (BIA) (RJL Quantum X, RJL Inc, Clinton Town, MI). The primary immunosuppressive regimen as well as the administration of any large-dose steroid pulses for acute rejection were documented. Given the slow turnover of muscle proteins, we documented only immunosuppressive medications administered for at least 4 consecutive weeks to have an impact on muscle area.
CT scan measures of skeletal muscle mass
All patients had a triphasic CT scan of the abdomen preoperatively on the date of measurement of body composition. Skeletal muscle mass was quantified by methods previously described by us (15). In brief, the mid fourth lumbar (L4) vertebral level was identified on each scan based on midline sagittal images that were reformatted from the unenhanced axial CT dataset. On the corresponding axial image, we determined total cross sectional area and mean attenuation (Hounsfield units) of the psoas, paraspinal (left and right quadratus lumborum) and abdominal wall muscles (rectus abdominis, oblique and transversus abdominis). Data were analyzed with and without normalization to height using the formula area/(ht2)(11). Psoas and paraspinal muscles were grouped together as core abdominal muscles(13, 14). Visceral and subcutaneous fat area, as well as total tumor volume in patients with HCC, were quantified by methods previously described by us(15).
A cohort of 109 healthy control subjects (54.2±10.8 y; 63 M, 46 F) who had CT abdomen for unspecified abdominal pain was evaluated to establish normal values for muscle and fat area. All these subjects were carefully evaluated for presence of any chronic illnesses, medication use that could affect fat or muscle turnover, and were within the normal BMI range (18-25 kg/m2). Gender and age specific 5th percentile values of the normalized total muscle area were used to define sarcopenia (Table 1).
Table 1.
Gender- and age-specific cut-off values for sarcopenia
| Characteristics | Male |
Female |
||
|---|---|---|---|---|
| <50 years old | >50 years old |
<50 years old | >50 years old | |
|
| ||||
| Number | 20 | 43 | 16 | 20 |
|
| ||||
| Age yrs(mean±SD) | 40.5±6.7 | 59.9±6.6 | 43.5±4.5 | 60.9±6.3 |
|
| ||||
| BMI kg/m2(mean±SD) | 23.6±1.8 | 23.9±1.2 | 24.3±0.9 | 23.4±1.8 |
|
| ||||
| Psoas muscle | ||||
| Absolute cm2 | ||||
| 5th percentile | 39.48 | 31.80 | 35.10 | 29.25 |
| 20th percentile | 41.05 | 34.08 | 35.60 | 30.79 |
| Normalized (cm2/h2) | ||||
| 5th percentile | 12.27 | 10.12 | 10.47 | 10.33 |
| 20th percentile | 13.74 | 10.66 | 11.69 | 11.54 |
|
| ||||
| Paraspinal muscle | ||||
| Absolute cm2 | ||||
| 5th percentile | 61.03 | 53.51 | 48.49 | 45.51 |
| 20th percentile | 64.33 | 58.02 | 52.40 | 48.91 |
| Normalized (cm2/h2) | ||||
| 5th percentile | 20.43 | 16.21 | 17.79 | 15.26 |
| 20th percentile | 22.19 | 18.73 | 18.46 | 17.71 |
|
| ||||
|
Abdominal wall
muscle |
||||
| Absolute cm2 | 84.77 | 67.39 | 60.21 | 42.48 |
| 5th percentile | 89.58 | 73.05 | 63.65 | 50.32 |
| 20th percentile | ||||
| Normalized (cm2/h2) | 26.93 | 22.39 | 21.11 | 15.10 |
| 5th percentile | 30.96 | 23.61 | 23.22 | 18.99 |
| 20th percentile | ||||
|
| ||||
| Total muscle | ||||
| Absolute cm2 | ||||
| 5th percentile | 189.27 | 156.62 | 149.03 | 120.91 |
| 20th percentile | 199.56 | 164.60 | 152.94 | 132.84 |
| Normalized (cm2/h2) | ||||
| 5th percentile | 60.09 | 48.97 | 53.43 | 41.28 |
| 20th percentile | 67.00 | 53.81 | 54.69 | 49.59 |
|
| ||||
| Visceral fat | ||||
| Absolute cm2 | ||||
| 5th percentile | 78.91 | 101.33 | 103.04 | 127.40 |
| 20th percentile | 92.22 | 121.04 | 116.10 | 134.03 |
| Normalized (cm2/h2) | ||||
| 5th percentile | 24.71 | 32.67 | 34.48 | 47.47 |
| 20th percentile | 31.21 | 36.86 | 37.94 | 51.53 |
|
| ||||
| Subcutaneous fat | ||||
| Absolute cm2 | ||||
| 5th percentile | 157.66 | 182.64 | 198.19 | 209.73 |
| 20th percentile | 175.87 | 199.80 | 209.39 | 231.86 |
| Normalized (cm2/h2) | ||||
| 5th percentile | 51.16 | 53.57 | 59.45 | 78.91 |
| 20th percentile | 56.55 | 53.84 | 73.16 | 87.40 |
Muscle biopsy and quantification of genes
Rectus abdominis skeletal muscle biopsies were obtained in 3 subjects 24 months post-OLT, and 3 matched control subjects who underwent elective abdominal surgery. Total RNA was extracted, reverse transcribed to cDNA and expression of mRNA quantified using real time PCR on a Stratagene Mx3000P (Stratagene, LaJolla, CA) using a SYBR protocol on a fluorescence temperature cycler using methods described by us(16, 17). Relative differences were normalized to the expression of β-actin. The primer sequences for human C3,C5, C9, Atrogin and MuRF have been previously published by us(18, 19). Real time PCR products were then separated by gel electrophoresis to confirm specific product presence and size.
All studies were approved by the Institutional Review Board at the Cleveland Clinic. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Statistical Analyses
Qualitative variables were compared using the Chi square test. Fat and muscle area on pre-OLT CT scans were correlated with measures of fat mass and fat-free mass obtained by BIA. The impact of these nutritional parameters on outcomes and length of hospital and intensive care (ICU) stay were determined. We also related grip strength to muscle CT attenuation, since lower CT attenuation has been related to higher muscle fat content, reduced muscle strength(20, 21), and consequently poorer outcomes(22, 23). Quantitative and rating variables were compared by the paired Student’s ‘t’ test. Predictors of skeletal muscle changes following liver transplantation were identified on univariate analysis and then their independent effects studied using multivariable analysis. Kaplan Meier survival analysis was performed to determine the impact of post-transplant change in muscle mass on survival. All values are expressed as mean± SD unless specified. A p value <0.05 was considered significant.
Results
During the study period, 225 patients had cadaveric OLT for nonacute liver failure related indications, of whom 53 also had serial CT scans with the interval between CT scans being 2.6-39.3 months. Their demographic and clinical characteristics are shown in table 2. Subjects excluded from the study were not significantly different in age (53.5±13.3 years), gender (66% male), or etiology of liver disease (viral (15%), combined alcohol and viral (4%), nonalcoholic steatohepatitis (14%), and HCC (28%)). MELD score was 18.7±8.8. Patients with HCC vs. no HCC had significantly shorter transplant wait times (4.1±2.9 vs. 6.9±6.0 months, p<0.05), lower Child’s score (6.9± 1.9 vs. 8.9±1.9, p<0.01) and lower chemical MELD score (11.0±4.1 vs. 15.3±6.0, p<0.01). Follow-up and clinical outcomes post-transplantation are shown in table 3.
Table 2.
Clinical, demographic and biochemical characteristics and body composition of cirrhotic patients pre-transplantation
| Characteristic | |
|---|---|
|
| |
| Number | 53 |
|
| |
| Gender (M:F) | 41:12 |
|
| |
| Age (yrs) | 56.9± 7.5 |
|
| |
| Child-Pugh score | 7.6±2.1 (5-12) |
|
| |
| MELD score | 12.6±5.3 (6-29) |
|
| |
| Etiology of cirrhosis (n, %) | |
| Viral | 22 (41.5) |
| Alcohol and viral | 12 (22.6) |
| NASH | 4 (7.5) |
| Other | 15 (28.3) |
|
| |
| Diabetic (n, %) | 6 (11.3) |
|
| |
| Serum sodium (mEq/L) | 136.5±4.8 |
|
| |
| S. creatinine (mg/dl) | 1.0±0.4 (0.6-3.3) |
|
| |
| S. bilirubin (mg/dl) | 3.4±4.4 (0.7-25.8) |
|
| |
| S. alanine amino transferase (IU/dL) | 77.2±73.2 (9-400) |
|
| |
| International normalized ratio | 1.3±0.3 (0.9-2.2) |
|
| |
| S. Albumin (g/dL) | 3.3±0.7 (1.6-4.6) |
|
| |
| Indication for OLT (n, %) | |
| HCC | 34 (64.2) |
| Cirrhosis without HCC | 15 (28.3) |
| Cholestasis | 4 (7.5) |
|
| |
| Body mass index (kg/m2) | 28.9±5.4 |
|
| |
| Mid arm circumference | |
| Left (cm) | 30.8±5.0 |
| Right (cm) | 30.9±5.1 |
|
| |
| Triceps skinfold thickness | |
| Left (mm) | 18.4±9.2 |
| Right (mm) | 18.6±9.4 |
|
| |
| Corrected mid arm muscle area | |
| Left (cm2) | 41.2±15.6 |
| Right (cm2) | 42.7±16.9 |
|
| |
| Grip strength | |
| Left (lb) | 57.9±22.3 |
| Right (lb) | 58.5±24.4 |
|
| |
| Whole body fat mass (kg) by BIA | 26.3±9.9 |
|
| |
| Whole body fat free mass (kg) by BIA | 63.2±16.8 |
NASH nonalcoholic steatohepatitis, OLT orthotopic liver transplantation, HCC hepatocellular carcinoma BIA bioelectrical impedance analysis
Table 3.
Follow-up and clinical outcomes post-transplantation
| Transplant waiting time after evaluation (months)a | 5.2±4.6 [3.8; 0.8-19.9] |
|
| |
| Mean interval between pre- and post-transplant CT scans (months)a |
19.3±11.9 [20.8; 2.6-39.3] |
|
| |
| Mean interval between transplant and post- transplant CT scan (months)a |
13.1±8.0 [12.6;0.5-25.2] |
|
| |
| Indication for follow-up CT scan (n, %) | |
| HCC surveillance | 31 (58.5) |
| Rise in transaminases | 6 (11.3) |
| Suspected infection | 7 (13.2) |
| Abdominal pain | 9 (16.9) |
|
| |
| Mean duration of hospital stay (days) | 13.0±9.3 |
|
| |
| Mean duration of ICU stay (days) | 3.2±1.7 |
|
| |
| New onset diabetes (n) | 11 |
|
| |
| Post-transplantation immunosuppression (n, %) | |
| Tacrolimus | 51 (96.2) |
| Mycophenolate mofetil | 41 (77.4) |
| Sirolimus | 8 (15.1) |
| Prednisone | 11 (20.8) |
| Cyclosporine | 3 (5.7) |
|
| |
| Cause of death (n) | |
| Coronary artery disease | 1 |
| Metastatic cancer | 2 |
| Recurrent HCC in graft | 1 |
| Recurrent HCV with renal failure | 1 |
Mean±SD [median; range]
CT computed tomography, HCC hepatocellular carcinoma, ICU intensive care unit
Pre-transplant Body composition
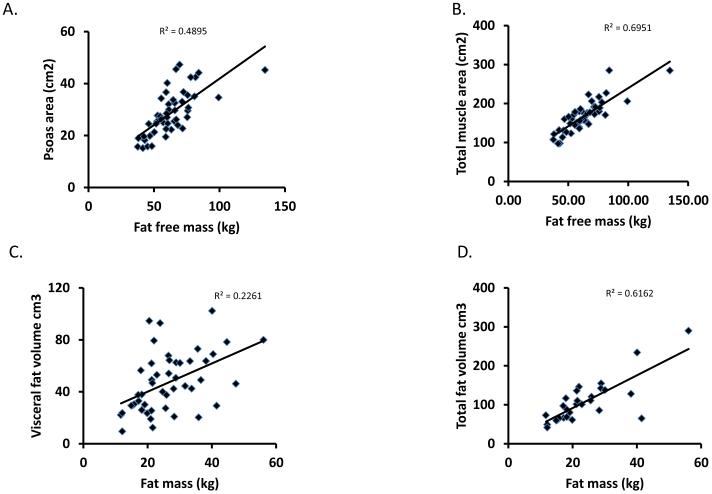
The pre-transplant body composition is shown in tables 2 and 3. Mean muscle area and attenuation, and visceral and subcutaneous fat areas, were significantly lower in cirrhotics compared to that previously published by us in a cohort of healthy controls(15). Sarcopenia was present in 33 (62.3%) cirrhotic patients before transplantation. When normalized psoas or total muscle area were used for analyses, the results were not different and for all the analyses, the normalized psoas area was used. Body composition measured using BIA correlated with the direct measures of fat free and fat mass (Figure 1). These correlations were weaker when BIA derived measures were normalized to body weight (supplementary figure 1). Grip strength correlated significantly with the psoas muscle area (r2=0.42; p<0.01) and CT attenuation of the psoas muscle (r2=0.41; p<0.01). Grip strength correlated inversely with total length of hospital stay (r2 −0.30, p=0.05) but not ICU stay. Psoas muscle area pre-transplant did not correlate with the duration of hospital stay, length of stay in the ICU, Child-Pugh score, or MELD score (p>0.1). The etiology of cirrhosis and the presence of HCC did not affect pre-transplant muscle or fat area. Tumor volume also did not affect muscle area. Eleven (20.8%) subjects had diabetes mellitus, but their muscle and fat area were not significantly different (p>0.05) from those without diabetes mellitus. The presence of ascites was associated with a significantly lower skeletal muscle mass both pre- and post-OLT (p<0.05).
Figure 1.
Panels A, B. Fat free mass measured by BIA correlated highly with psoas area and total muscle area measured by CT image analysis at L4 vertebral level. p<0.05.
Panels C, D. Whole body fat mass measured by BIA correlated with visceral fat mass but even more so with total fat area measured by CT image analysis at L4 vertebral level. p<0.05.
Post-transplant changes in body composition
The mean area of all 3 muscle groups decreased after liver transplantation, but mean visceral and subcutaneous fat areas were unchanged (Table 4). Muscle CT attenuation increased in all muscle groups. In 35 (66%) patients, psoas and paraspinal muscle area decreased post-OLT, while abdominal wall muscles decreased in 41 (77.4%). Fat area increased in 23 (43.4%) and remained unchanged or decreased in the remaining 30 (56.6%). Grip strength and bioelectrical impedance analysis were not done post-transplantation because the primary focus of the study was to determine the change in muscle area after liver transplantation.
Table 4.
Body composition changes before and after liver transplantation.
| Characteristic | Pre-OLT | Post-OLT |
|---|---|---|
|
| ||
| Number | 53 | 53 |
|
| ||
| Body mass index (kg/m2) | 28.9±5.4 | |
|
| ||
| Psoas area (cm2) | 28.6±8.3 | 26.8±8.1** |
|
| ||
| Normalized psoas area (cm2/ht2) | 9.2±2.3 | 8.6±2.4* |
|
| ||
| Paraspinal muscle area(cm2) | 58.1±10.5 | 55.8±9.3** |
|
| ||
| Normalized paraspinal area (cm2/ht2) |
18.9±2.9 | 18.1±2.8* |
|
| ||
| Abdominal wall muscle area (cm2) | 80.2±22.9 | 72.4±18.8*** |
|
| ||
| Normalized abdominal muscle area (cm2/ht2) |
25.9±6.4 | 23.4±5.2* |
|
| ||
| Psoas muscle CT attenuation (HU) | 33.0±9.9 | 28.8±10.4** |
|
| ||
| Paraspinal muscle CT attenuation (HU) |
12.8±17.5 | 5.9±19.5** |
|
| ||
| Abdominal wall muscle CT attenuation (HU) |
14.8±12.1 | 9.6±13.3** |
|
| ||
| Visceral fat area (cm2) | 155.01±75.3 | 134.3±89.3 |
|
| ||
| Normalized visceral fat area (cm2/ht2) |
15.0±7.1 | 13.1±8.5 |
|
| ||
| Subcutaneous fat (cm2) | 202.3±127.7 | 176.6±99.5 |
p<0.05
p<0.01
p<0.001
HU Hounsfield units, OLT orthotopic liver transplantation, CT computed tomography
Using the cutoff values from the normal controls, 46 (86.8 %) patients had sarcopenia post-OLT. Of the 33 patients who had pre-transplant sarcopenia, only 2 (6.1 %) patients had reversal of sarcopenia while the remaining patients (n=31; 93.9%) had persistent sarcopenia post-OLT. Of the 20 patients who did not have sarcopenia pre-OLT, 15 (75%) patients developed sarcopenia after OLT.
Consequences of post-transplantation loss of muscle mass
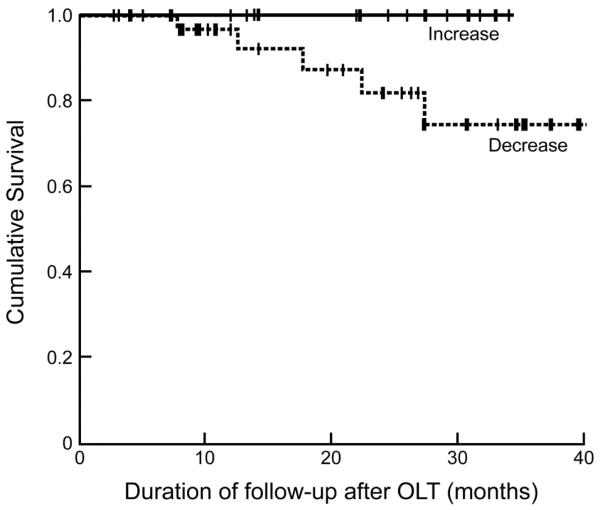
All 5 patients who died were sarcopenic pre-OLT and had a further reduction in muscle area after transplantation. Pre-OLT sarcopenia was associated with increased mortality (p=0.06). The number of patients who had reversal of sarcopenia was too small to perform more detailed characterization and hence the changes in muscle area pre- and post-OLT were used for the survival analyses. Kaplan Meier survival analysis showed a trend (p=0.08) towards higher mortality in patients with continued reduction in muscle area (Figure 2). Due to the small number of events, these trends did not reach statistical significance.
Figure 2.
Kaplan Meier survival curves in patients with an increase in muscle area post-OLT compared to those with a reduction in muscle area. Log rank test p=0.08.
A reduction in psoas muscle and paraspinal muscle area were significantly associated with the development (n=11) of post-OLT diabetes mellitus (DM) (p<0.05) with a 3.1 fold increased risk (95% CI 1.01-9.38). However, change in visceral or subcutaneous fat mass, whole body weight, body-mass index (BMI), or immunosuppressive regime was not associated with the development of post-transplant DM. Post-transplant hypertension was associated with an increase in whole body weight and BMI, rather than changes in muscle mass.
Predictors of post-transplant sarcopenia
A higher proportion of patients with HCC (15/33, 45.5%) had an increase in muscle area after transplantation compared to those without HCC (3/20, 15%). However, tumor volume did not predict post-transplant sarcopenia. All patients met the Milan criteria for transplantation for HCC (24). Pre-transplant Child-Pugh score, MELD score, bilirubin, diabetes, or psoas area did not predict the post-transplant change in psoas muscle area, paraspinal muscle, and anterior abdominal wall muscle area. Normalizing the muscle area or fat area to height did not alter these results.
Potential molecular mechanisms of post-transplant sarcopenia
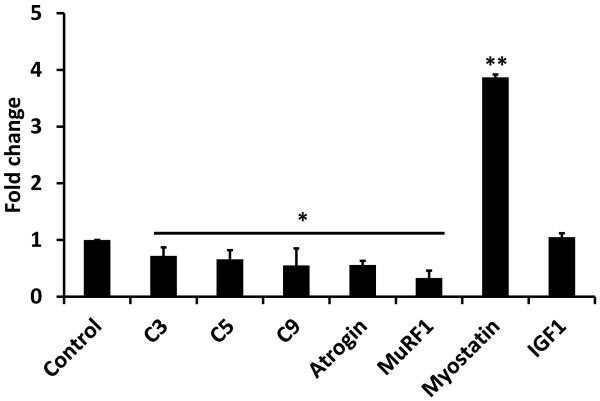
In 3 subjects who had reduction in muscle area post-OLT, expression of proteasome C3,C5,C9, atrogin and MuRF mRNA was lower than in controls, while that of myostatin was significantly elevated. Skeletal muscle IGF1 was not different between post-transplant and control subjects (Figure 3).
Figure 3.
Fold change in relative expression of genes regulating skeletal muscle mass in post-OLT compared to controls. * p<0.05 and ** p<0.01 compared to controls.
Discussion
This is the first systematic study to prospectively quantify skeletal muscle area and fat mass in cirrhotics pre- and post-transplantation using a precise and validated method. Pre-transplantation, patients had lower skeletal muscle mass as measured by multiple methods. Sarcopenia was defined using age and gender specific cutoff values from the control subjects in our population. The majority of patients had sarcopenia pre-OLT and a significant proportion developed new onset sarcopenia post-OLT. Post-OLT reduction in muscle mass increased the risk of diabetes mellitus, and a trend towards increased mortality. Unlike other malignancies, hepatocellular carcinoma in the background of cirrhosis did not worsen sarcopenia, and was associated with a lower risk of post-transplant sarcopenia. Our data on the expression of genes regulating skeletal muscle mass suggest that increased myostatin expression may be responsible for persistently low muscle mass, even after recovery of liver function.
Skeletal muscle area in this cohort of cirrhotic subjects pre-OLT was lower than that of controls, but higher than that reported by us earlier(15). This may be related to the significant proportion of patients with HCC undergoing transplantation in the present study with lower Child-Pugh and MELD scores. This suggests that muscle mass may be higher in patients undergoing OLT for HCC than for severe liver failure. Consistently, the mean chemical MELD score (and not the MELD finally granted) in patients with HCC was lower than the rest of our cohort. Increasing Child’s score and MELD score have both been reported to worsen nutritional status in cirrhosis (2, 25). Another potential explanation for the lesser degree of sarcopenia in these patients with HCC could be related to the shorter transplant waiting list time that has also been reported by others(26-28).
Post-transplant changes in body composition have previously been reported in small cohorts of subjects(5-8, 29). This is the first study to use predefined cut off values to define sarcopenia and showed that the majority of patients who were sarcopenic pre-OLT had persistent post-OLT sarcopenia. Our data on the normal controls was similar to that reported by others(11), but cutoff values to define sarcopenia, were determined for age and gender specific values. This was necessary because age related and gender specific differences in muscle mass are well recognized(30). The novel observation in our study was that 44% of cirrhotics who did not have sarcopenia pre-OLT developed new onset sarcopenia post-OLT. The data in the present study are similar to that in earlier reports, with patients failing to gain fat free or lean body mass on anthropometry(10). Clinical predictors, including the number of episodes of rejection or the type of immunosuppressive regimen, did not affect loss of muscle mass after liver transplantation. It is possible that alterations in dosage schedule as well as changes in immunosuppressive regimen affects muscle mass. However, this needs to be examined in large multicenter studies or as part of the ongoing studies like the A2ALL cohort(31). Surprisingly, muscle area increased in patients with HCC who underwent transplantation. Although the presence of HCC was not associated with lower muscle mass pre-transplantation, removal of the tumor seemed to result in an improvement in muscle area.
In contrast to previous reports on the development of obesity and increase in fat mass after transplantation(32), mean visceral and subcutaneous fat mass were unaltered in our cohort after transplantation. This may be due to the use of different methods to quantify body composition by previous investigators (4, 6, 9). Even though fat free mass and fat mass obtained from BIA correlated well with the muscle and fat mass quantified by CT image analysis, regional distribution of fat cannot be determined by BIA. Furthermore, BIA derived fat mass and fat free mass normalized to whole body weight had weaker correlation with CT derived measures of muscle and fat area. Therefore whole body weight may not be the best way to normalize body composition in cirrhotics. Use of clinical indication rather than protocol CT scans may also have affected our results. However, since the majority of patients had CT scans for HCC surveillance, with no evidence of tumor recurrence, the indication for CT did not seem to explain the observed differences in fat mass.
The consequences of changes in body composition post-transplantation have not been reported to date. In obese subjects, the risk of diabetes mellitus has been associated with higher adipose tissue mass(33). However, there is limited data on the risk of diabetes with lower fat free and skeletal muscle mass, even though skeletal muscle insulin resistance occurs in diabetes mellitus and prediabetes(34-36). In this cohort of post-transplant patients, risk of new onset diabetes during follow-up was increased in patients who had a reduction in muscle area. Based on these observations, it is not certain whether there is a causal relationship or an association between diabetes and reduction in muscle mass. On the other hand, hypertension, another component of the post-transplant metabolic syndrome, was associated with an increase in whole body weight but not loss of muscle mass. Although a reduction in muscle area was accompanied by a trend towards increased mortality, the number of deaths was too small to determine if post-OLT sarcopenia was associated with increased mortality.
Molecular evaluation of skeletal muscle responses was examined after OLT albeit in a small subset of patients. Compared to control subjects, expression of myostatin was increased, but the ubiquitin proteasome proteolytic components were unaltered. Even though serial biopsies were not performed, our highly novel data demonstrate for the first time a potential molecular mechanism for post-transplant sarcopenia secondary to impaired muscle protein synthesis by upregulation of myostatin via calcineurin inhibitors(37).
The major strengths of the present study include the prospective nature and precise measures of muscle area using serial CT measurements. The unusually large number of patients with HCC is a reflection of the current trends in OLT in the United States, and our data are the first of their kind to demonstrate the changes in body composition in this population of patients. Finally, despite previous data demonstrating post-transplant obesity, in the current cohort fat mass or BMI did not change post-OLT. This may be related to the selection of patients who had serial CTs, or the unusually large number of patients with HCC. Although demographic factors were similar in patients included and excluded from the study, a larger proportion of patients with HCC (with lower calculated MELD score) may have contributed to a lower occurrence of post-transplant sarcopenia. These observations suggest the need to identify the cohort of post-OLT patients who are likely to develop obesity and sarcopenia so that the metabolic consequences can be prevented. Given that adipose tissue and skeletal muscle with the liver form a “metabolic axis”, detailed molecular studies on both adipose tissue and skeletal muscle are necessary to target these organs to improve outcomes post-OLT.
In conclusion, post-OLT sarcopenia is a clinically significant disorder that has adverse clinical consequences including post transplant diabetes mellitus and possibly increased mortality. Extensive work has been done on the impact of malnutrition and muscle loss in cirrhotics before transplantation, but the impact and mechanisms of post-transplant sarcopenia need to be examined in order to improve the long-term outcome of transplant survivors. Even though sarcopenia, obesity and visceral adiposity with adiposopathy have adverse effects on cardiovascular and metabolic outcomes in the non-transplant population(38), our studies suggest that these may contribute to increased post-transplant morbidity, mortality and health care related costs. Quantifying body composition with an emphasis on muscle mass, muscle strength and regional fat mass should be part of management of the post-OLT patient.
Supplementary Material
Acknowledgements
This work was partly supported by NIH RO1 DK 83341 (SD).
Footnotes
No conflicts of interest for any of the authors.
Reference List
- 1.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012 Dec;3(4):225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012 Feb;16(1):95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberg F, Isoniemi H, Hockerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100(1):14–21. doi: 10.1177/145749691110000104. [DOI] [PubMed] [Google Scholar]
- 4.Merli M, Giusto M, Giannelli L, Lucidi C, Riggio O. Nutritional Status and Liver Transplantation. (1 ed) 2011:190–198. doi: 10.1016/S0973-6883(11)60237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merli M, Giusto M, Riggio O, Gentili F, Molinaro A, Attili A, et al. Improvement of nutritional status in malnourished cirrhotic patients one year after liver transplantation. (6 ed) 2011:e142–e147. [Google Scholar]
- 6.Plank LD, Metzger DJ, McCall JL, Barclay KL, Gane EJ, Streat SJ, et al. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann Surg. 2001 Aug;234(2):245–255. doi: 10.1097/00000658-200108000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schutz T, Hudjetz H, Roske AE, Katzorke C, Kreymann G, Budde K, et al. Weight gain in long-term survivors of kidney or liver transplantation--another paradigm of sarcopenic obesity? Nutrition. 2012 Apr;28(4):378–383. doi: 10.1016/j.nut.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Hussaini SH, Oldroyd B, Stewart SP, Soo S, Roman F, Smith MA, et al. Effects of orthotopic liver transplantation on body composition. Liver. 1998 Jun;18(3):173–179. doi: 10.1111/j.1600-0676.1998.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 9.Hussaini SH, Soo S, Stewart SP, Oldroyd B, Roman F, Smith MA, et al. Risk factors for loss of lean body mass after liver transplantation. Appl Radiat Isot. 1998 May;49(5-6):663–664. doi: 10.1016/s0969-8043(97)00088-2. [DOI] [PubMed] [Google Scholar]
- 10.Dasarathy S. Post transplant sarcopenia- an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013 Nov;58(11):3103–3111. doi: 10.1007/s10620-013-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008 Oct;33(5):997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 12.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004 Dec;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 13.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012 Feb;10(2):166–73. 173. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010 Aug;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013 Jan;25(1):85–93. doi: 10.1097/MEG.0b013e328359a759. [DOI] [PubMed] [Google Scholar]
- 16.Dasarathy S, Dodig M, Muc SM, Kalhan SC, McCullough AJ. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol. 2004 Dec;287(6):G1124–G1130. doi: 10.1152/ajpgi.00202.2004. [DOI] [PubMed] [Google Scholar]
- 17.Dasarathy S, Muc S, Hisamuddin K, Edmison JM, Dodig M, McCullough AJ, et al. Altered expression of genes regulating skeletal muscle mass in the portacaval anastomosis rat. Am J Physiol Gastrointest Liver Physiol. 2007 Apr;292(4):G1105–G1113. doi: 10.1152/ajpgi.00529.2006. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012 Oct;303(8):E983–E993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013 Nov 5;110(45):18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001 Jun;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005 Mar;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 22.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003 May;51(5):636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 23.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000 Mar;55(3):M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996 Mar 14;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 25.Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006 Aug 15;24(4):563–572. doi: 10.1111/j.1365-2036.2006.03003.x. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012 Apr;18(4):434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachdev M, Hernandez JL, Sharma P, Douglas DD, Byrne T, Harrison ME, et al. Liver transplantation in the MELD era: a single-center experience. Dig Dis Sci. 2006 Jun;51(6):1070–1078. doi: 10.1007/s10620-006-8011-1. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004 Jan;10(1):36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 29.Wagner D, Adunka C, Kniepeiss D, Jakoby E, Schaffellner S, Kandlbauer M, et al. Serum albumin, subjective global assessment, body mass index and the bioimpedance analysis in the assessment of malnutrition in patients up to 15 years after liver transplantation. Clin Transplant. 2011 Jul;25(4):E396–E400. doi: 10.1111/j.1399-0012.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997 Jul;83(1):229–239. doi: 10.1152/jappl.1997.83.1.229. (1985 ) [DOI] [PubMed] [Google Scholar]
- 31.Gillespie BW, Merion RM, Ortiz-Rios E, Tong L, Shaked A, Brown RS, et al. Database comparison of the adult-to-adult living donor liver transplantation cohort study (A2ALL) and the SRTR U.S. Transplant Registry. Am J Transplant. 2010 Jul;10(7):1621–1633. doi: 10.1111/j.1600-6143.2010.03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009 Dec;15(12):1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 33.Bays H, Dujovne CA. Adiposopathy is a more rational treatment target for metabolic disease than obesity alone. Curr Atheroscler Rep. 2006 Mar;8(2):144–156. doi: 10.1007/s11883-006-0052-6. [DOI] [PubMed] [Google Scholar]
- 34.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 2010 Dec;34(Suppl 2):S4–17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 35.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de RN, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009 Nov;32(11):1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castaneda C, Bermudez OI, Tucker KL. Protein nutritional status and function are associated with type 2 diabetes in Hispanic elders. Am J Clin Nutr. 2000 Jul;72(1):89–95. doi: 10.1093/ajcn/72.1.89. [DOI] [PubMed] [Google Scholar]
- 37.Michel RN, Dunn SE, Chin ER. Calcineurin and skeletal muscle growth. Proc Nutr Soc. 2004 May;63(2):341–349. doi: 10.1079/PNS2004362. [DOI] [PubMed] [Google Scholar]
- 38.Bays HE, Laferrere B, Dixon J, Aronne L, Gonzalez-Campoy JM, Apovian C, et al. Adiposopathy and bariatric surgery: is ‘sick fat’ a surgical disease? Int J Clin Pract. 2009 Sep;63(9):1285–1300. doi: 10.1111/j.1742-1241.2009.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.