Abstract
Among γ-aminobutyric acid (GABA) interneurons, the Chandelier cell (ChC) has long captured the interest of neuroscientists as a subtype not described by Ramon y Cajal. ChCs feature an axonal arborization that selectively innervates the axon initial segments of pyramidal cells. Recent studies involving transgenic mice have identified intriguing features of ChCs, including a remarkably specific spatial and temporal origin, their capacity to have either excitatory or inhibitory influences on pyramidal neurons, and their synaptic alterations in schizophrenia. This review explores these and other developmental and functional aspects of this fascinating cortical neuronal subtype.
Introduction
In the cerebral cortex, γ-aminobutyric acid (GABA) interneurons modulate cortical excitability by feedback and feedforward inhibition, and are critical to the neuronal plasticity [1, 2] as well as to the synchronous activity of pyramidal neurons [3]. Among GABAergic cortical interneurons, one major subclass is defined by its property of discharging in trains of very rapid, non-accommodating action potentials (40–200 Hz), and thus are called “fast-spiking” (FS). Most FS interneurons are termed “basket” cells for their propensity to have curved axon terminals that mainly target the proximal dendrites and cell soma of pyramidal neurons. However, it is the other FS interneuron type that is arguably the most enigmatic of cortical interneurons, the “chandelier” cell (ChC). ChCs, also referred to as axo-axonic cells, have long captured the interest of neuroscientists as the interneuron subtype that was not described by Ramon y Cajal [4]. More recently, a number of intriguing features of ChC have come to light that further piques this interest, including the spatial and temporal specificity of their origin [5*, 6**], their capacity to have either excitatory or inhibitory influences on pyramidal neurons [7**], their remarkable elimination of axon terminals during adolescent-age range development in primates [8–10], and their synaptic alterations in schizophrenia [11]. This review delves into these and other developmental and functional aspects of this fascinating cortical interneuronal subtype.
Chandelier cells; initial identification and defining features
The chandelier cells (ChCs), so named because the axon terminals resembled a candelabrum, were first identified in the 1970s by Szentagothai, and independently, by Jones [12, 13]. ChCs feature a distinctive axonal arborization that includes a moderately dense weave of horizontal collaterals, from which vertically-oriented axon terminals hang (Figure 1). These terminals, also called cartridges, selectively innervate the axon initial segment (AIS) of pyramidal neurons [14]. ChCs are most abundant in layers 2 and 3, but are present in all cortical layers [15, 16]. Although originally described in neocortex [12], ChCs have also been found in the CA3 [17], CA1 [18] and dentate gyrus [19] regions of the hippocampus, and in the amygdala [20].
Figure 1. Variable expression of Parvalbumin in chandelier cells (ChCs).
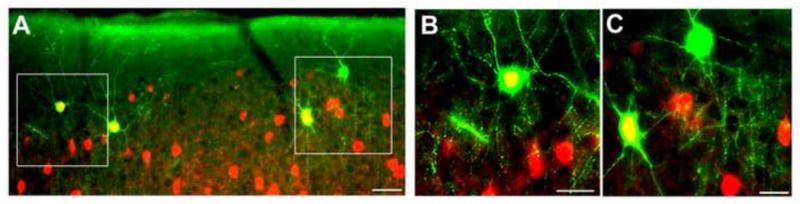
A. Immunofluorescence staining for GFP (green) and Parvalbumin (PV; red) in the superficial layers of neocortex in an Nkx2.1-Cre reporter line [31]. A. In this reporter line, cortical interneurons other than ChCs are also labeled, including the PV-expressing basket cells. PV expression is not detected in all ChCs. B. High magnification image of the ChC expressing PV (left box in A). C. High magnification image of the ChC with no detectable PV expression (right box in A). Scale bars: 50μm in A and 25μm in B and C.
Chandelier cell origin and development
With respect to their origins at the subcortical forebrain, cortical interneurons can broadly be parsed into two: those that derive from Nkx2.1-expressing progenitors in the medial ganglionic eminence (MGE) or preoptic area, and those that derive from caudal ganglionic eminence (CGE). Fate-mapping studies using the Nkx2.1 Cre lines [6**, 21], in utero retroviral injections [22*], and transplantation studies [5*] have demonstrated that most or all ChCs originate in the MGE starting at embryonic day (E) 13.5 (Figure 2A). Studies involving MGE transplantations into mouse neonatal neocortex, as well as spatial and temporal fate mapping using Nkx2.1Cre-ERT2 mice, have indicated that ChCs are primarily generated by the most ventral region of the MGE and at the latest stages of cortical neurogenesis (Figure 2B) [5*, 6**]. In fact, tamoxifen injections in the neonatal period can label ChCs in the Nkx2.1Cre-ERT2 mice [6**]. However, this may reflect persistent expression of Nkx2.1, or the in-lineage ectopic expression of Cre that occurs in transgenic mice with Cre-poly A inserts to the Nkx2.1 gene [21, 23], rather than reflecting the neonatal neurogenesis of ChCs. While the late gestation generation of ChCs in mice is distinctive, it is not entirely unexpected since most ChCs in the mouse are found directly at the layer 1 - layer 2 boundary, and since MGE interneurons generally follow the same “inside-out” relationship of birthdate to laminar location as pyramidal neurons [24, 25].
Figure 2. Origin and development of chandelier cells in mice.
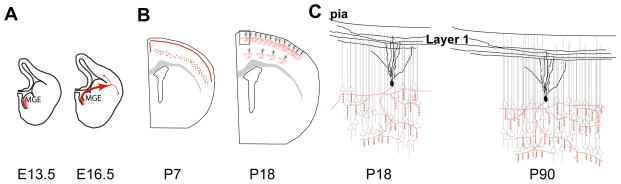
A, B. ChCs originate primarily from the ventral region of the Medial Ganglionic Eminence (vMGE, area in red) as early as E13.5 (A), although most are generated late in gestation. ChC precursors are thought to initially migrate along the periventricular region of the lateral ventricle and into the overlying cortex (B). C, D. Postnatal development and morphology of ChCs. At P7, immature ChCs form a dense plexus at the border of layers 2 and 1 (C). Some ChCs are also detected in layer 5. By P20, the majority of immature ChCs die and their distinct dendritic (black) and axonal morphology with its cartridges (red) are formed (D). E, F. Morphology of ChCs during postnatal development. A significant increase in the lateral span of the ChC axonal arbor is observed with age while number of cartridges increases slightly. However, the average density of cartridges does not differ significantly between different ages. The innervation pattern of ChC axon remains similar between P18 (E) and P90 (F) with pockets of dense innervation where every pyramidal cell is targeted by ChC cartridges. Additionally, the dendrites of ChCs at the layer 2 and 1 have an asymmetric morphology and they are strongly driven by the layer 1 axons.
The finding by Taniguchi and colleagues that late gestation and early postnatal administration of tamoxifen to Nkx2.1CreERT2 reporter mice labels cortical interneurons with a roughly 3:1 bias for ChCs over basket cells led to fascinating observations on ChC postnatal development. ChC precursors migrate along the lateral wall of the ventricle and follow a lateral or medial route (Figure 2B) once they reach the cortex around birth (at postnatal day (P) 0). Initially, they enter the ventricular zone and then invade the cortical plate, reaching layer 1 around P2-P3. Finally, these cells descend to their destination in the cortical plate between P3-P7. At P7, well prior to the formation of axonal cartridges, many labeled cells were present along the layer 1- layer 2 border where their processes seemed to form a dense plexus (Figure 2C) [6**]. By P20, some 90% of these cells were no longer detected, and the remaining cells were nearly all ChCs, raising the likelihood that ChCs form this plexus transiently, which is then pruned to adult levels (Figure 2D). Interestingly, cell death of mouse cortical interneurons was reported during a similar developmental period, although with loss of about 40% of the cells [26]. At this developmental stage, just after the close of pyramidal neuron radial migration, pyramidal neurons are extending and ramifying their apical dendrites and forming their terminal tufts. P7 is also a time when the gradient for chloride across the pyramidal neuron plasma membrane favors a depolarizing response to the activation of GABA-A receptors. This raises the possibility that ChCs may be providing a transient, excitatory drive to the cortical projection neurons when they are extensively involved in axonal and dendritic maturation and synaptogenesis. In line with this possibility, conditional loss of the Neuregulin receptor ErbB4 in MGE interneurons affect ChC but not basket cell synapses, and secondarily results in reduced spine density by cortical pyramidal neurons [27**].
An additional interesting finding from the fate-mapping study based on Nkx2.1CreERT2 mice was that most ChCs that were not at the layer 1 - layer 2 boundary were present in layer 5 [6**]. A similar observation was reported for human neocortex [28] while analysis of chandelier axon terminals in monkey prefrontal cortex over postnatal development did not reveal this pattern [8]. In addition to the variations in the layer distribution of ChCs, the density of ChC terminals in different cortical regions were found to be distinct in mice, monkey and humans [9, 16, 28]. It is important to note that the differences observed in cartridge distribution may reflect the changes in the expression of synaptic markers used in each study. Still, one remaining issue is whether ChCs exist as subtypes of a general class of axo-axonic cells, as reported in the monkey [29], a possibility that is also raised by their variable expression of the calcium binding protein parvalbumin (Figure 1) [6**]. Whether layer 5 ChCs have distinct properties from those in layer 2 is not known, but within layer 2, ChCs appear to form a homogenous interneuron type as defined by dendritic or axonal morphology and various electrophysiological characteristics [7**]. In addition, these cells also show extensive electrical coupling, suggesting that their activity is coordinated [6**, 7**].
Chandelier cell targeting, physiological features, and function
ChC cartridges target the axon initial segment (AIS) of pyramidal neurons, where action potentials are generated [14, 30]. Cartridges of ChCs located at the border of layer 1 and 2 are visible by around P14, and were found to be surprisingly stable in their distribution and morphology from the P18 (late pre-weaning) to P90 (young adult) in mice [31]. The lateral span of the ChC axonal arbor and number of cartridges increases slightly with age although the average density of cartridges does not differ significantly between different ages (Figure 2E, F). Consistent with earlier findings [16, 28, 32, 33], ChC axon terminals contact an AIS with an average of 3–5 boutons per cartridge [31]. By comparing this number to the total number of putative synapses at the AIS, at least 4 ChCs are estimated to innervate each pyramidal neuron. On the other hand, the mean number of boutons innervating the AIS is reduced in ChCs of the monkey prefrontal cortex during development, and the number of boutons per cartridge is positively correlated with the AIS size [10].
In mouse somatosensory cortex, individual ChCs contact 35–50% of pyramidal neurons within the range of its axonal arbor, with clear pockets of higher or lower innervation densities (Figure 2E, F). It remains unclear how this arrangement forms or whether it reflects a previously unappreciated organizational construct in the cortical circuitry. Interestingly, AIS innervation by the cartridges was found to be highly variable also in the monkey prefrontal cortex ChCs during development [10]. Although regulation of AIS targeting is unknown in the cortex, an axo-axonic interneuron population in cerebellum has been found to initially target the axonal side of the cell soma and the AIS, then to refine this targeting to the AIS by signaling of the adhesion molecule L1CAM [34].
Both the Parvalbumin-expressing basket interneurons, and the far less common ChCs, fire high frequency, minimally adapting, “fast-spiking” trains of action potentials in response to supra-threshold current injections. Parvalbumin (PV) is an EF-hand domain containing, high affinity calcium (Ca2+)-binding protein [35]. In PV knockout mice, synapses with short-term depression are converted to synapses with short-term facilitation resulting in enhanced synaptic transmission [36]. Although PV is expressed in all FS basket cells, PV expression in ChCs located at different cortical areas of the Nkx2.1CreERT2 reporter line varied between 15% colabeling in the prefrontal cortex to 50% in the somatosensory cortex [6**]. It would be interesting to compare the short-term plasticity of ChCs in different cortical areas to elucidate whether PV-expressing and PV-negative ChCs have different short-term plasticity properties.
Prior to recent advances that employed transgenic mice to visualize ChC and basket cells in slice preparations and hence tremendously enhance electrophysiological studies, basket and ChCs were often seen as having similar effects on pyramidal neuron excitation. However, with the aid of transgenic reporter mice, a detailed comparison of cortical basket and ChC intrinsic firing properties, as well as more detailed analysis of ChC influences on pyramidal neuron excitability, has become possible. For example, in terms of intrinsic firing properties, basket cells have a greater latency of firing at threshold current injections [37], although this parameter varies by species [38]. Interestingly, this study also revealed that ChCs have a higher firing frequency and adaptation compared to basket cells in both rats and monkeys [38].
ChCs can have a great impact on the output of pyramidal neurons based on the localization of their synapses at the AIS, and they were proposed to be circuit switches [39]. On the other hand, the unusual location of ChC synapses at the AIS also renders them with complex physiological properties, such that whether they depolarize [37, 40] or hyperpolarize [41] their target pyramidal neurons had been controversial.
Recently, extensive analysis of the synaptic transmission of ChCs at the layer 1 and 2 border of P16- P25 mouse somatosensory cortex revealed that the effect of ChC synaptic transmission relies on the resting membrane potential (Vrest) of the pyramidal neuron [7**]. If the pyramidal neuron Vrest is more hyperpolarized than the reversal potential of GABA (EGABA), ChC synaptic transmission was depolarizing and increased the spiking probability of the pyramidal neuron. However, ChC synapses were found to have a hyperpolarizing effect if the pyramidal neuron was depolarized to EGABA or above. In another set of experiments, an “in vivo-like” activity was induced in the pyramidal neurons. Activation of the ChCs shortly (5 ms) before the expected pyramidal neuron spike evoked reliable inhibition. On the other hand, ChC activation at earlier time points (15–40 ms) evoked both hyperpolarizing and depolarizing effects. These results are consistent with the findings that ChC synaptic response is dependent on the Vrest of the pyramidal neurons because shortly before the expected pyramidal neuron spike, the pyramidal neuron is already at a depolarized state whereas Vrest of the pyramidal neuron is expected to be variable at earlier time points.
Analyses of ChCs located at the border of layers 1 and 2 also revealed an asymmetric distribution of the dendrites that are mostly oriented towards layer 1 (Figure 2E). This finding led to the hypothesis that ChCs, similar to the apical dendrites of pyramidal neurons, may receive input from other cortical areas via axons in layer 1 and may act as circuit switches [7**]. Stimulation of layer 1 and dual recordings from both pyramidal neurons and ChCs at layer 2/3 showed that the stimulation strength necessary for activation by layer 1 inputs is significantly less for ChCs compared to pyramidal neurons. An analogous situation exists for fast-spiking interneurons and excitatory granule neurons in the hippocampus [42]. Hence, ChCs can affect pyramidal neuron responses to layer 1 input. In fact, at the stimulation strength that is sufficient to activate ChCs, pyramidal neurons were initially depolarized. However, the highest stimulation strength that activates the ChC but not the pyramidal neuron depolarized the pyramidal neuron to a value similar to EGABA, leading to a shunting effect. As the layer I stimulation was increased to induce pyramidal neuron spiking, the influence of the ChC input converted from shunting to hyperpolarizing. In sum, these experiments revealed a novel top-down regulation role for the ChCs providing feed-forward inhibition onto pyramidal neurons. That said, the possibility exists that in a “quiet” circuit, multiple depolarizing ChC inputs onto a pyramidal neuron may evoke an excitatory response. This possibility can now be tested on the Nkx2.1CreERT2 line by using optogenetics to activate multiple ChCs targeting the same pyramidal neuron [43].
Finally, PV+ interneuron activity can entrain gamma frequency oscillations and contribute to their synchrony [44, 45]. The rhythmic activity of PV+ interneurons synchronizes cortical circuits by generating a narrow window for effective excitation. However, since basket cells outweigh ChCs among PV+-expressing neurons, clarification is still needed for whether ChCs have a specific role in gamma oscillations or not. Once again, Nkx2.1CreERT2 line, combined with lines that allow Cre-mediated excitation or inhibition in vivo by optogenetics, should be a good model system to address this question.
Chandelier cells in disease
Recent advances in mouse genetics, imaging and electrophysiology techniques have greatly advanced our efforts to understand the cortical interneuron dysfunction in neuropsychiatric disorders. Alterations in the GABAergic signaling components have been reported in the pathogenesis of these disorders such as epilepsy, schizophrenia and autism [46–48]. In particular, PV+ interneuron abnormalities are prominent [47, 49], consistent with their role in gamma oscillations that are thought to mediate or to reflect cognitive functions disrupted in these disorders [50]. In the case of ChCs, evidence exists for an association between ChC abnormalities and schizophrenia [48, 49]. A genetic association between ChCs and schizophrenia was uncovered when the cortical interneuron-specific disruption of a candidate susceptibility gene for schizophrenia, ErbB4, decreased the number of chandelier synapses [22*]. ErbB4 is a receptor tyrosine kinase that signals via interaction with its ligand, Neuregulin, also a candidate susceptibility gene for schizophrenia [51]. Earlier studies suggested that ErbB4 signaling contributes to formation and/or stabilization of glutamatergic synapses onto PV+ cortical interneurons [22*, 52–54]. Recently, analysis of a conditional knockout mouse line (ErbB4 CKO) where ErbB4 was disrupted selectively in postmitotic, MGE-derived interneurons, also showed a reduction in the excitatory synapses onto both basket and ChC interneurons [27**]. Remarkably, the ChCs, but not the basket cells, had a marked reduction of synapses onto pyramidal neurons [27**]. The same mice also had several phenotypes associated with schizophrenia, some of which include decreased cortical pyramidal neuron spine density, disrupted synchrony in gamma-range oscillations between the frontal cortex and hippocampus, and reduced prepulse inhibition. This mouse model suggests a remarkable connection between human genetics, physiology, postmortem studies of schizophrenia, and ChC function as measured in mice. However, a definitive study on ChC function at the system level and in relation to ErbB4 or putative disease genes awaits a method to selectively affect most or all cortical chandeliers, but not other interneurons.
Although these alterations in ChCs certainly affect the cortical microcircuitry, whether they could be an effect, rather than a cause, of the core pathology is still unclear. Additionally, while some of this pathology probably results from disruptions that began close to the time of symptom onset, it is also likely that symptomatic dysfunction of cortical microcircuitry evolves from abnormalities that occurred earlier during development. However, linking microcircuitry dysfunction to the disruption of their developmental trajectories has been a major challenge. To this end, generation of the Nkx2.1CreERT2 line is a major improvement to overcome this challenge. Using this line, manipulations of genes associated with neuropsychiatric disorders during development have the potential to model the cause and effect relation of ChC deficits in these disorders.
Future challenges and opportunities
Despite the considerable progress made in understanding the development and function of ChCs, a number of major questions remain. First, what is the regulation of ChC fate determination? Like other MGE-derived interneurons, the transcription factors Nkx2.1 in mitotic progenitors, and Lhx6 [55–57] and Sox6 [58, 59] in postmitotic Nkx2.1-lineage interneurons, are likely to be important. But how is the fate of these cells being established relative to other MGE-derived interneuron subtypes? The recent discovery of the spatial and temporal bias for ChC generation during late gestation in the ventral-most MGE [5*] paved the way for identifying transcriptional regulators and signaling pathways based on their enrichment in this particular spatial and temporal coordinates. These studies have the potential to identify an embryonic marker for ChCs, which can circumvent the caveat that one third of the cells that express Cre in the Nkx2.1CreERT2 line are not ChCs. On a related topic, a retroviral lineage study suggests that Somatostain and PV expressing interneurons can be generated by the same radial progenitor [60*]. This raises the question of whether basket and chandelier interneurons related clonally and whether ChCs are generated from radial progenitors that are distinct from other MGE interneurons.
Second, how do ChCs function, in vivo, both at the local circuit and behavioral levels? The ability to enrich for ChC labeling via the Nkx2.1CreERT2 mouse not only allows the investigation of their postnatal development, but it also enables studying their function by expressing a) genetically encoded activity indicators, such as calcium (GCaMPs) or voltage indicators [61] b) optogenetic activators and silencers, or other proteins that alter membrane potential [62] c) selective cellular silencers or suicide mediators. Finally, genes linked to neuropsychiatric disorders, such as ErbB4, can be manipulated in ChCs by crossing the Nkx2.1CreERT2 line to the transgenic mice carrying floxed alleles of these genes. These studies will reveal which of these genes can induce disease-related endophenotypes in mice when mutated specifically in ChCs, and are, together with similar approaches targeting other cortical cell types, likely to revolutionize discovery of the etiological antecedents of neuropsychiatric disease.
Highlights.
Chandelier cells (ChCs) have specific spatial and temporal origins.
ChCs can depolarize or hyperpolarize the pyramidal cell based on its resting membrane potential.
Nkx2.1CreERT2 reporter line is the first mouse line for studying a specific interneuron subtype.
ChC-specific defects reported in interneuron-specific ErbB4 knockouts (ErbB4 CKO).
ErbB4 CKOs have schizophrenia-like endophenotypes, providing a direct link to ChCs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melis Inan, Weill Cornell Medical College, New York, NY.
Stewart Anderson, Children’s Hospital of Philadelphia/University of Pennssylvania School of Medicine Philadelphia, PA.
References
- 1.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 2.Kullmann DM, et al. Plasticity of inhibition. Neuron. 2012;75(6):951–62. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Buzsaki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012;14(4):345–67. doi: 10.31887/DCNS.2012.14.4/gbuzsaki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 2012;22(4):820–7. doi: 10.1093/cercor/bhr148. This is the first report on the origin of chandelier cells. By transplanting spatially and temporally distinct MGE progenitors, the authors identified that chandelier cells originate from the ventral MGE and are mainly generated at later stages of gestation in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339(6115):70–4. doi: 10.1126/science.1227622. This study described a method to label that allows the relatively selective labeling of chandelier interneurons. Using an inducible Nkx2.1CreERT2:dt tomato Loxp mouse line, the authors found that late gestation or early postnatal administration of tamoxifen labels cortical interneurons with a high enrichment (70%) of chandelier cells. This transgenic mouse model led to fascinating observations on chandelier cell postnatal development and has opened up novel avenues for investigating the role of chandelier cells in cortical microcircuitry development and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Woodruff AR, et al. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31(49):17872–86. doi: 10.1523/JNEUROSCI.3894-11.2011. In this thorough study of chandelier cell function, the authors identified the chandelier cells located at layer 1 - layer 2 border as a homogeneous group. Using dual patch clamp of chandelier and pyramidal neurons, they found that the depolarizing versus hyperpolarizing effect of chandelier cell synapses onto pyramidal neurons depended on the resting membrane potential of the pyramidal neuron. They also identified a novel top-down regulation role for the chandelier cells providing feed-forward inhibition onto pyramidal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson SA, et al. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 9.Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465(3):385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- 10.Fish KN, et al. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 2013;33(19):8352–8. doi: 10.1523/JNEUROSCI.0306-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28(6):310–6. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Szentagothai J. The ‘module-concept’ in cerebral cortex architecture. Brain Res. 1975;95(2–3):475–96. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- 13.Jones EG. Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J Comp Neurol. 1975;160(2):205–67. doi: 10.1002/cne.901600204. [DOI] [PubMed] [Google Scholar]
- 14.Somogyi P. A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res. 1977;136(2):345–50. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 15.DeFelipe J, et al. Variability in the terminations of GABAergic chandelier cell axons on initial segments of pyramidal cell axons in the monkey sensory-motor cortex. J Comp Neurol. 1985;231(3):364–84. doi: 10.1002/cne.902310307. [DOI] [PubMed] [Google Scholar]
- 16.Inda MC, DeFelipe J, Munoz A. Morphology and distribution of chandelier cell axon terminals in the mouse cerebral cortex and claustroamygdaloid complex. Cereb Cortex. 2009;19(1):41–54. doi: 10.1093/cercor/bhn057. [DOI] [PubMed] [Google Scholar]
- 17.Sik A, Tamamaki N, Freund TF. Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. Eur J Neurosci. 1993;5(12):1719–28. doi: 10.1111/j.1460-9568.1993.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 18.Somogyi P, et al. A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res. 1983;259(1):137–42. doi: 10.1016/0006-8993(83)91076-4. [DOI] [PubMed] [Google Scholar]
- 19.Soriano E, Cobas A, Fairén A. Neurogenesis of glutamic acid decarboxylase immunoreactive cells in the hippocampus of the mouse. II: Area dentata. Journal of Comparative Neurology. 1989;281(4):603–11. doi: 10.1002/cne.902810409. [DOI] [PubMed] [Google Scholar]
- 20.McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212(3):293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506(1):16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 22*.Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464(7293):1376–80. doi: 10.1038/nature08928. In this study, ErbB4, a risk gene for schizophrenia was found to be expressed in basket and chandelier interneuron synapses. The authors carried out gain and loss of function experiments in vitro and in vivo to investigate the role of ErbB4 in synapse formation and synaptic function. The results revealed that ErbB4 is required for Gabaergic synapse formation as well as proper function of glutamatergic synapses on to basket and chandelier interneurons. [DOI] [PubMed] [Google Scholar]
- 23.Fogarty M, et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27(41):10935–46. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobas A, Fairén A. GABAergic neurons of different morphological classes are cogenerated in the mouse barrel cortex. Journal of Neurocytology. 1988;17(4):511–9. doi: 10.1007/BF01189806. [DOI] [PubMed] [Google Scholar]
- 25.Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501(3):369–80. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- 26.Southwell DG, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491(7422):109–13. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Del Pino I, et al. Erbb4 Deletion from Fast-Spiking Interneurons Causes Schizophrenia-like Phenotypes. Neuron. 2013;79(6):1152–68. doi: 10.1016/j.neuron.2013.07.010. The authors studied the function of ErbB4 in MGE-derived cortical interneurons. Loss of ErbB4 reduced excitatory inputs to fast-spiking interneurons (basket and chandelier cells), and selectively reduced chandelier but not basket cell inputs to pyramidal neurons. Remarkably, loss of ErbB4 in MGE-derived interneurons also reduced spine density on the cortical pyramidal neurons, and resulted in multiple schizophrenia-related behavioral phenotypes. [DOI] [PubMed] [Google Scholar]
- 28.Inda MC, Defelipe J, Munoz A. The distribution of chandelier cell axon terminals that express the GABA plasma membrane transporter GAT-1 in the human neocortex. Cereb Cortex. 2007;17(9):2060–71. doi: 10.1093/cercor/bhl114. [DOI] [PubMed] [Google Scholar]
- 29.Lewis DA, Lund JS. Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990;293(4):599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- 30.Fairen A, Valverde F. A specialized type of neuron in the visual cortex of cat: a Golgi and electron microscope study of chandelier cells. J Comp Neurol. 1980;194(4):761–79. doi: 10.1002/cne.901940405. [DOI] [PubMed] [Google Scholar]
- 31.Inan M, et al. Dense and overlapping innervation of pyramidal neurons by chandelier cells. J Neurosci. 2013;33(5):1907–14. doi: 10.1523/JNEUROSCI.4049-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somogyi P, et al. Identified axo-axonic cells are immunoreactive for GABA in the hippocampus and visual cortex of the cat. Brain Res. 1985;332(1):143–9. doi: 10.1016/0006-8993(85)90397-x. [DOI] [PubMed] [Google Scholar]
- 33.Li XG, et al. Axonal and dendritic arborization of an intracellularly labeled chandelier cell in the CA1 region of rat hippocampus. Exp Brain Res. 1992;90(3):519–25. doi: 10.1007/BF00230934. [DOI] [PubMed] [Google Scholar]
- 34.Ango F, et al. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119(2):257–72. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, et al. Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. J Physiol. 2000;525(Pt 2):405–18. doi: 10.1111/j.1469-7793.2000.t01-3-00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caillard O, et al. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A. 2000;97(24):13372–7. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodruff A, et al. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Povysheva NV, et al. Electrophysiological Heterogeneity of Fast-Spiking Interneurons: Chandelier versus Basket Cells. PLoS One. 2013;8(8):e70553. doi: 10.1371/journal.pone.0070553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodruff A, Yuste R. Of mice and men, and chandeliers. PLoS Biol. 2008;6(9):e243. doi: 10.1371/journal.pbio.0060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabadics J, et al. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311(5758):233–5. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 41.Glickfeld LL, et al. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12(1):21–3. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30(38):12597–607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson S, Coulter D. Neuroscience. Neuronal birth to cortical circuitry. Science. 2013;340(6136):1058–9. doi: 10.1126/science.1235778. [DOI] [PubMed] [Google Scholar]
- 44.Tamas G, et al. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3(4):366–71. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 45.Bartos M, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99(20):13222–7. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122(Pt 10):1807–22. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 47.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–20. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 48.Inan M, Petros TJ, Anderson SA. Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol Dis. 2013;53:36–48. doi: 10.1016/j.nbd.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis DA, et al. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in neurosciences. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 51.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. American journal of human genetics. 2002;71(4):877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(7):3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26(2):443–55. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 54.Ting AK, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(1):15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du T, et al. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135(8):1559–67. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, et al. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J Comp Neurol. 2008;510(1):79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fragkouli A, et al. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009;136(22):3841–51. doi: 10.1242/dev.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaglin XH, et al. The origin of neocortical nitric oxide synthase-expressing inhibitory neurons. Front Neural Circuits. 2012;6:44. doi: 10.3389/fncir.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma T, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013 doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 60*.Brown KN, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334(6055):480–6. doi: 10.1126/science.1208884. In this interesting study, the authors found that different subgroups of cortical interneurons, such as Somatostatin- and Parvalbumin-expressing, can originate from the same progenitor in the MGE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–40. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]


