Abstract
Circulating endothelial cells (CEC) are derived from multiple sources including bone marrow (circulating endothelial progenitors [CEP]) and established vasculature (mature CEC). Although CEC have shown promise as a biomarker for cancer patients, their utility has been limited in part by the lack of specificity for tumor vasculature and the different non-malignant causes that can impact CEC. Tumor endothelial markers (TEM) are antigens enriched in tumor vs non-malignant endothelia. We hypothesized that TEMs may be detectable on CEC and that these circulating TEM+ endothelial cells (CTEC) may be a more specific marker for cancer and tumor response than standard CEC. We found that tumor-bearing mice had a relative increase in numbers of circulating CTEC, specifically with increased levels of TEM7 and TEM8 expression. Following treatment with various vascular targeting agents, we observed a decrease in CTEC that correlated with the reductions in tumor growth. We extended these findings to human clinical samples and observed that CTEC were present in esophageal cancer and non-small cell lung cancer (NSCLC) patients (N=40) and their levels decreased after surgical resection. These results demonstrate that CTEC are detectable in preclinical cancer models and cancer patients. Further, they suggest that CTEC offer a novel cancer-associated marker that may be useful as a blood-based surrogate for assessing the presence of tumor vasculature and antiangiogenic drug activity.
Keywords: Circulating TEM+ endothelial cell (CTEC), Circulating endothelial cell (CEC), Tumor endothelial markers (TEMs)
Introduction
Angiogenesis is a key step in tumor progression, including the spread and growth of metastases. Therefore, considerable effort has been directed at targeting the vascular component of malignant disease. Anti-angiogenic agents targeting the vascular endothelial growth factor (VEGF) pathway, such as the monoclonal antibody bevacizumab and the tyrosine kinase inhibitor AZD2171, have demonstrated clinical activity in multiple malignancies, including non-small cell lung cancer (NSCLC), renal cell cancer, and colorectal cancer (1–5). Currently, clinical studies evaluating the efficacy of such agents are hindered by the lack of validated surrogate markers of drug activity. Availability of blood-based biomarkers would be paramount because they could predict which patients are most likely to benefit from treatment prior to clinical progression (6, 7).
In recent years, we and other investigators have studied a number of circulating biomarkers for VEGF pathway inhibition in peripheral blood that are derived from circulating endothelial cells (CECs) and myeloid lineage cells, circulating pro-angiogenic factors and receptors, and soluble markers of hypoxia and endothelial damage (7–11). Elevated levels of CECs (CD45−CD31+CD146+ cells) are detected in renal cell carcinoma cancer patients as compared with the levels found in normal control subjects and are associated with advanced disease (12). It has become evident that among CECs in blood, some express a progenitor-like phenotype defined as circulating endothelial progenitors (CEPs); whereas others show the characteristics of mature, terminally differentiated cells (mature CECs). Whereas mature CECs are thought to be shed from existing vessels, CEPs are derived from the bone marrow and can home to sites of angiogenesis and participate in the generation of new vessels in adults (13, 14).
Both CECs and CEPs have been investigated as biomarkers for anti-angiogenic therapy in preclinical models and clinical studies (8–10, 15–18). Levels of mature CECs typically change in patients treated with anti-angiogenic or vasculature-targeting agents (8, 15–17). We have also observed that among patients with gastrointestinal stromal tumors changes in CECs were a marker of clinical benefit for patients treated with the multitargeted kinase inhibitor sunitinib (8).
Not specific to cancer, CECs occur during pathologic conditions such as vasculitis (19), infection (20), and myocardial infarction (21). There is an unmet need to identify CEC populations that more precisely reflect the presence of, or changes in, tumor vasculature. Global analysis of altered gene expression patterns in endothelial cells from human colorectal cancer tissues revealed a series of genes termed tumor endothelial markers (TEMs) (22). Since being first described, TEMs have received considerable attention as potential markers or targets of tumor vasculature. Further studies have indicated that some TEM expression may not be limited to the tumor vasculature. In addition to TEMs, published reports have indicated that CD276 (B7-H3), a leukocyte co-stimulatory molecule, may be over expressed by endothelial cells during pathological but not physiological angiogenesis (23).
We hypothesized that a subset of CECs may originate from tumor endothelium sloughed into peripheral circulation, and that such CECs could be distinguished from CECs derived from other sources by the presence of TEMs. Here, we evaluated circulating endothelial cells in both murine models of human NSCLC as well as patients with esophageal carcinoma. Our data suggest that specific subpopulations of CECs bear TEMs, that CTECs may be useful as blood-based markers for detecting the presence of cancer or monitoring response to therapies that affect the tumor vasculature.
Materials and Methods
Cell lines
The adenocarcinoma cell line H1975 was provided by Drs. John Minna and Adi Gazdar (The University of Texas Southwestern Medical School, Dallas, TX). MS-1 and Lewis lung cancer cells were obtained from ATCC. All cell lines were maintained in RPMI 1640 medium with 10% FBS, penicillin-streptomycin, and L-glutamine.
Mice
Athymic nude mice (Ncr-nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). C57BL/6 mice were bought from Charles River Laboratories (Wilmington, MA). All animals were housed under pathogen-free conditions in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the United States Department of Agriculture and National Institutes of Health. At the time of the studies, the nude mice were 8–10 weeks old and the C57BL/6 mice were 6–8 weeks old.
Flow cytometry analysis of circulating endothelial cells in mice
For both humans and mice, CECs in peripheral blood were evaluated by flow cytometry as previously described (24, 25). Red cell lysis was performed using FACSLyse solution (BD Biosciences, San Jose, CA) as per the manufacturer's directions, and 100 µl of blood was analyzed for each mouse. The following directly conjugated antibodies were used for detection of CECs and CEPs in peripheral blood in mouse: anti-mouse CD45-PerCP, Flk-1-Alexa 700 (mouse VEGFR-2), CD31-APC (platelet/endothelial cell adhesion molecule-1), and CD117-PE-Cy7 (c-kit receptor; all from BD Biosciences). Syto 16 (Molecular Probes, Eugene, OR) was used as a nuclear stain to identify nucleated cells and exclude debris, platelets, and macroparticles. It was not used to distinguish between apoptotic or non-apoptotic cells (24). As previously described, in mice CECs were characterized as CD31+CD45−VEGFR-2+CD117−, and CEPs as CD31+CD45−VEGFR-2+CD117+ (7). For detection of CD276, cells were stained with PE-conjugated CD276 antibodies. For detection of TEM7, cells were stained with biotin-conjugated TEM7 antibodies and Texas red-conjugated streptavidin. Flow cytometry was done using a FACSCanto flow cytometer (BD Biosciences), and acquired data were analyzed with FLOWJO software (Treestar, Ashland, OR) with analysis gates designed to remove residual platelets and cellular debris. For each mouse, a minimum of 100,000 events were typically counted. MS-1 cells, a transformed murine endothelial cell line isolated from pancreatic islets of C57BL/6 mice, were used as a positive control for endothelial cells. The gating strategy from a representative mouse blood specimen is shown in Supplemental Figure 1.
Flow cytometry analysis of circulating endothelial cells in human specimens
For human specimens, CTEC and CEC/CEP populations were evaluated by eight-color flow cytometry. The following antibodies were used: CD276-PE (R&D Systems), CD146-PerCP-Cy5 (Beckman Coulter), CD31-PECy7 (BD Biosciences), CD133-APC (Miltenyi Biotec), CD45-APC-Cy7(eBiosciences). Syto16 (Molecular Probes, Eugene, OR) was used as a nuclear stain to identify nucleated cells and exclude debris, platelets, and macroparticles. It was not used to distinguish between apoptotic or non-apoptotic cells. Cells were gated based on side scatter (SSC) and forward scatter (FSC) to display major blood components of the Syto16+ PBMCs. Antibodies directed against CD45 were used to exclude hematopoietic cells. CD133 antibodies were used to identify progenitor cells. Endothelial cells were identified with antibodies against CD146 and CD31. CECs were identified as CD45−/CD133−/CD146+/CD31+. CEPs were identified as CD45−/CD133+/CD146+/CD31+. CTECs were identified as CD45−/CD133−/CD146+/CD31+/CD276+. To establish absolute CEC counts, the volume of blood analyzed was determined by using the lymphocyte or monocyte counts obtained from the patients' differential white cell counts. The gating strategy from a representative human blood specimen is shown in Supplemental Figure 2.
Xenograft model and anti-angiogenic treatment
A total of 1.0 × 106 murine Lewis lung carcinoma tumor cells and 1.0 × 107 H1975 human NSCLC cells were injected subcutaneously into the flanks of C57BL/6 mice and nude mice, respectively. When tumors reached a volume of 300 mm3, mice were stratified into treatment groups of 5 animals per group. Animals received vehicle (91% polysorbate 80) by oral gavage or the VEGF receptor 2 inhibitor AZD2171 (6 mg/kg; gavage) daily for 14 days. For VEGF treatment, mice were injected intraperitoneally with 10 µg of recombinant human VEGF (National Cancer Institute, Biological Resources Branch, Bethesda, MD) daily for 5 days. Bevacizumab was administered intraperitoneally at a dose of 10 mg/kg twice weekly.
Animals were weighed weekly and tumor volume was measured twice weekly. After treatment, mice were given isoflurane anesthesia, and blood was collected by retro-orbital puncture and anti-coagulated using 0.5 M EDTA. After mice were euthanized, tumors were harvested and frozen in OCT embedding medium (Sakura Finetek, Torrance, CA).
Immunohistochemistry
Frozen tumor sections were fixed in acetone for 10 minutes and washed three times with PBS. Sections were blocked for 1 hour with 5% normal horse serum and 1% normal goat serum in PBS and incubated with primary antibodies TEM7 (1:1000; Imgenex), TEM8 (1:500; Abcam) and CD276 (1:1000; eBioscience) overnight at 4°C. Slides were washed with PBS and incubated with secondary antibody Alexa 488 (1:600, Molecular Probes, Eugene, OR) for 1 hour at room temperature.
For double staining, each slide was washed with PBS and incubated with antibodies directed against CD31 (1:500, BD Biosciences) or smooth muscle actin (Dako, Carpinteria, CA) at 4°-C overnight. After being washed with PBS, slides were incubated with secondary antibody Alexa 594 (1:600, Molecular Probes) for 1 hour at room temperature. Cell nuclei were stained using 4,6-diamididino-2-phenylindole (Vector Laboratories).
Isolation of endothelial cells
Endothelial cells were isolated from tumor xenografts by using a MACS magnetic cell sorting system (Miltenyi Biotec, Auburn, CA). Tumors were excised when they reached approximately 1 cm in diameter. Normal endothelial cells were obtained from excised skin, lung, liver, and epididymal fat pads of the same mice. Excised tissue was minced and digested with collagenase II (Worthington, Freehold, NJ). Blood cells were removed by single sucrose step-gradient centrifugation with Histopaque 1077 (Sigma-Aldrich), and cell suspensions were filtered. Endothelial cells were isolated using FITC-conjugated anti-CD31 antibody and the MACS system according to the manufacturer’s instructions. CD31-positive cells were sorted, and the purity of endothelial cells was confirmed by flow cytometric analysis of cell surface protein FITC-conjugated BS1-B4.
Human subjects
Following institutional review board approval written informed consent were obtained from all the study participants. Forty-consecutive patients, treated surgically at MD Anderson Cancer Center for esophageal and non-small cell lung cancer were enrolled in the study. Exclusion criteria for this study included any condition that deemed a patient unsatisfactory for surgery after the pre anesthetic evaluation and patients from which the complete tumor resection was not possible. All patients were followed until death or for a period of 5 years post operatively. The clinical characteristics are presented in Table 1. All patients had blood drawn before and one month after surgery. Blood samples were also obtained from a group of 9 healthy volunteers for comparison analysis. The peripheral blood was collected in heparin tubes using a vacutainer system. All the samples were processed within two hours from the time of collection. Peripheral blood mononuclear cells (PBMCs) were isolated using density centrifugation over Histopaque-1077 (Sigma) according to the manufacturer’s instructions. Then, the cells were resuspended in cell freezing medium (20% DMSO, 80 % RPMI 1640) and stored in liquid nitrogen until the day of analysis. Baseline and follow-up samples for each patient were analyzed together to minimize inter assay variability.
Table 1.
Clinical characteristics and number of CTEC counts in peripheral blood before and one month after major thoracic surgery.
| Characteristic | Before | One month after | P value | |
|---|---|---|---|---|
| N (%) | Median (Range) cells/µl | |||
| Overall | 40 (100) | 0.38 (0.00–2.40) | 0.11 (0.00–0.66) | <0.001 |
| Gender | ||||
| Female | 13 (32.5) | 0.49 (0.16–1.39) | 0.11 (0.01–0.66) | 0.003 |
| Male | 27 (67.5) | 0.33 (0.00–2.40) | 0.10 (0.00–0.37) | <0.001 |
| Ethnicity | ||||
| Caucasians | 34 (85) | 0.36 (0.00–2.40) | 0.10 (0.00–0.66) | <0.001 |
| Others | 6 (15) | 0.54 (0.05–0.97) | 0.13 (0.00–0.56) | 0.116 |
| Smoking History | ||||
| Never | 9 (22.5) | 0.24 (0.05–0.91) | 0.06 (0.00–0.30) | 0.028 |
| Ever | 31 (77.5) | 0.41 (0.00–2.40) | 0.11 (0.00–0.66) | <0.001 |
| Induction Therapy | ||||
| No | 13 (32.5) | 0.57 (0.16–1.49) | 0.14 (0.01–0.66) | 0.003 |
| Yes | 27 (67.5) | 0.35 (0.00–2.40) | 0.10 (0.00–0.56) | <0.001 |
| Complete Pathological Responsea | ||||
| No | 21 (52.5) | 0.40 (0.00–2.40) | 0.13 (0.00–0.66) | <0.001 |
| Yes | 6 (15) | 0.19 (0.04–1.31) | 0.00 (0.00–0.21) | 0.075 |
| Primary Tumor Localization | ||||
| Lung | 18 (45) | 0.47 (0.16–1.49) | 0.14 (0.00–0.66) | 0.002 |
| Esophagus | 22 (55) | 0.23 (0.00–2.40) | 0.06 (0.00–0.37) | <0.001 |
| Histology | ||||
| Adenocarcinoma | 33 (82.5) | 0.33 (0.00–2.40) | 0.11 (0.00–0.66) | <0.001 |
| Squamous cell carcinoma | 3 (7.5) | 0.39 (0.16–0.49) | 0.10 (0.00–0.14) | 0.109 |
| Other | 4 (10) | 0.68 (0.41–0.97) | 0.11 (0.01–0.23) | 0.680 |
| Clinical Stageb | ||||
| I | 10 (25) | 0.33 (0.16–1.39) | 0.11 (0.00–0.66) | 0.009 |
| II | 13 (32.5) | 0.37 (0.02–2.40) | 0.11 (0.00–0.36) | 0.005 |
| III | 17 (45.5) | 0.44 (0.00–1.43) | 0.10 (0.00–0.37) | 0.005 |
| Recurrence | ||||
| No | 22 (55) | 0.31 (0.00–1.43) | 0.12 (0.00–0.66) | 0.001 |
| Yes | 18 (45) | 0.47 (0.00–2.40) | 0.10 (0.10–0.56) | 0.001 |
Statistical Analysis
For animal studies, Mann-Whitney test was used to compare differences in CTECs, CECs, and CEPs between treatment groups. Mann-Whitney test was used to compare differences in CTECs/ECs between cancer patients and healthy volunteers. Wilcoxon signed-rank test was used to look at changes in those values in terms of absolute differences between the samples collected before and after surgery. Correlation between CTECs cell counts and tumor size was determined by Spearman's rho correlation test. Survival was analyzed using the Kaplan-Meier method.
Results
Expression of tumor endothelial markers
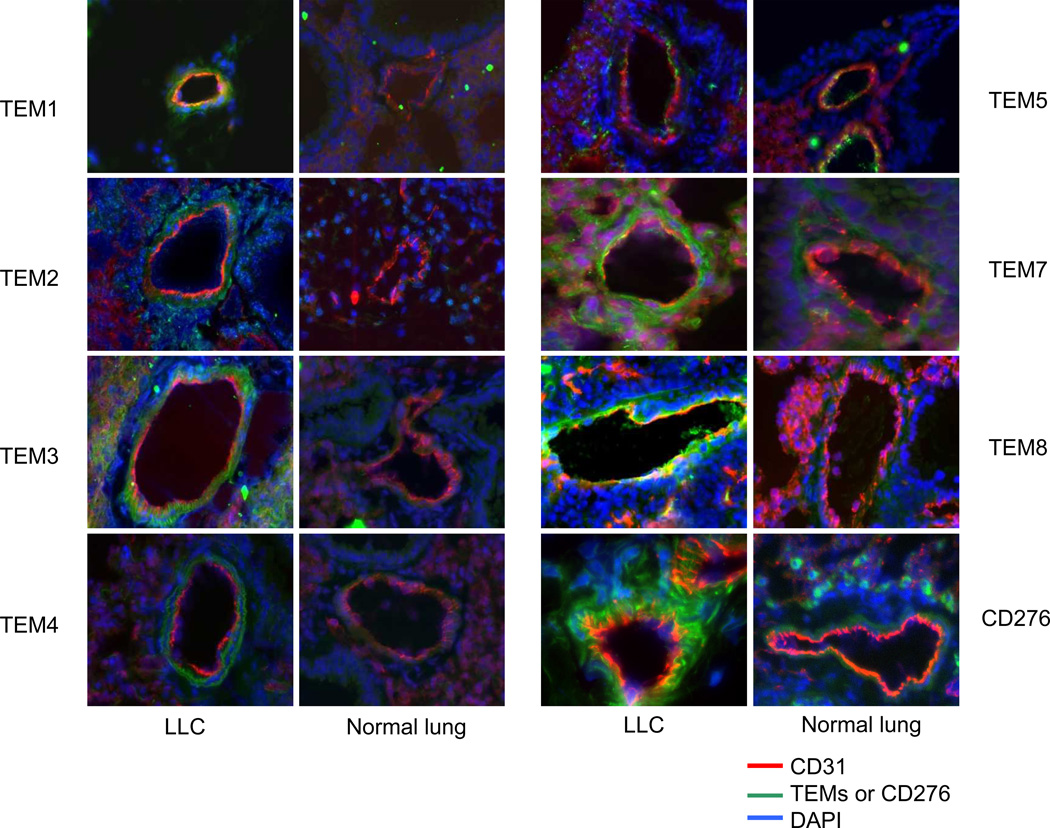
As a first step toward assessing whether TEMs could be detected on tumor-derived CECs, we evaluated the effect of expression of various TEMs and CD276 on the vasculature of normal tissue and Lewis lung carcinoma tumor tissue in the C57BL/6 mouse model by immunohistochemistry (Fig. 1). The immunoreactivity of TEMs was detectable in all tumor tissues and was absent or minimally detectable in normal lung tissue with the exception of TEM5, which was expressed on endothelium from both tumor and normal tissues in our analysis. TEM expression was also absent in normal skin from mice (data not shown). TEM7, TEM8, and CD276 co-localized with CD31, suggesting that their expression is primarily limited to the tumor endothelium (Fig. 1), although some staining of perivascular cells was noted for TEM7. TEM1 and TEM4 exhibited considerable expression in tumor pericytes, which was confirmed by double-staining with the smooth muscle marker actin (data not shown). Our observation that TEM1 and TEM5 are expressed on perivascular cells and normal endothelium, respectively, is consistent with previous reports(23, 26). These results indicated that the expression of most TEMs was relatively tumor specific and that for some TEMs expression was not limited to tumor endothelial cells. TEM7, TEM8, and CD276 appeared to be the most promising markers based on these immunohistochemical results.
Figure 1. Expression of Tumor Endothelial Markers (TEMs).
Representative images showing CD31 (red) and TEM (green) markers of histological sections of Lewis lung carcinoma (LLC) (56) and normal lung tissues. TEM8, TEM7 and CD276 colocalized with CD31, suggesting that its expression is localized to the tumor endothelium. TEM1 and TEM exhibited perivascular expression patterns.
Expression of TEMs on endothelial cells isolated from normal and tumor tissues assessed using flow cytometry
Using flow cytometry, we next investigated whether TEMs could be detected on endothelial cells isolated from tumor tissue or normal tissue. In preliminary studies, we identified antibodies against TEM7 and CD276, but not TEM8, which were suitable for use in flow cytometry. Murine endothelial cells were purified from both the normal and tumor-derived tissues of H1975 xenografts using CD31-immunomagnetic beads. The purity of endothelial cells was confirmed using the FITC-conjugated cell surface protein BSI-B4.
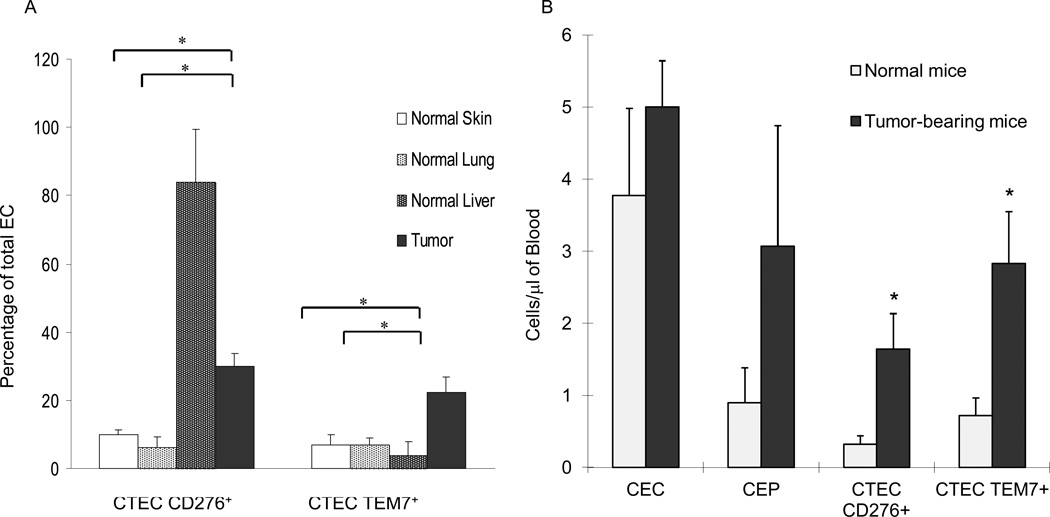
CTECs were defined based on the presence of CEC markers (CD31+CD45−VEGFR-2+CD117−) (7) as well as CD276 or TEM7 immunoreactivity. CD276 or TEM7 immunoreactivity was detected in 6% to 10% of total endothelial cells from normal skin or lung tissue, but in 30% and 22%, respectively, of tumor ECs (Fig. 2). Because CD276 expression on liver endothelium has previously been described (27), liver ECs were included in our analysis. Consistent with earlier reports, CD276 was detectable in 84% of liver ECs but TEM7 was detectable in only 4% (Fig. 2A). Therefore, TEM7 and CD276 ECs could be detected on tumor ECs by flow cytometry, and the expression of CD276 could also be detected on ECs from liver tissue in non-tumor-bearing mice.
Figure 2. Expression of TEM7 and CD276 in endothelial cells.
(A) Endothelial cells from both normal and tumor-derived tissues in H1975 xenografts were immunopurified using CD31-immunomagnetic beads. TEM7 and CD276 expression was evaluated using flow cytometry for established markers for mouse endothelium (CD31+CD45−VEGFR-2+CD117−). Values are means ± SEM. *p < 0.05 (B) CECs and CEPs in peripheral blood were evaluated using four-color flow cytometry. CTECs were first identified based on the staining pattern of CECs and further characterized using CD276 and TEM7 antibodies. CD276 CTECs and TEM7 CTECs were expressed at significantly higher levels in tumor-bearing mice than in normal controls (p = 0.041 and 0.032, respectively). Values are means ± SEM.
Circulating TEM+ endothelial cells (CTECs) are elevated in tumor-bearing animals
We used blood from mice bearing H1975 human NSCLC xenografts to test our hypothesis that the number of CTECs would be higher in tumor-bearing than non-tumor-bearing mice. As previously described, CECs and CEPs were characterized as CD31+CD45−VEGFR-2+CD117− and CD31+CD45−VEGFR-2+CD117+, respectively (7, 9, 17, 28, 29). We refer to the CECs co-expressing TEM7 and CD276 as TEM7 CTECs and CD276 CTECs, respectively. We found that levels of CECs and CEPs were higher in tumor-bearing than non-tumor-bearing mice, although the differences were not significant (p = 0.41 and 0.26, respectively). In contrast, the numbers of CD276 CTECs and TEM7 CTECs were significantly increased in the blood of tumor-bearing mice compared with the controls (p = 0.041 and 0.032 respectively) (Fig. 2B), demonstrating relative specificity of CTECs for the presence of tumor. In this experiment, 77% of CD276+ CTECs were also TEM7+, and 57% of TEM7+ cells also expressed CD276. Because CTECs are a subset of CECs, we evaluated the population of CTECs as a fraction of CECs (Supplemental Fig. 3). In tumor-bearing mice, the mean percentage of CD276+ or TEM7+ CECs was 31% and 55%, respectively. Whereas, in normal mice the mean percentage of CD276+ or TEM7+ CECs was 16% and 15.8%, respectively.
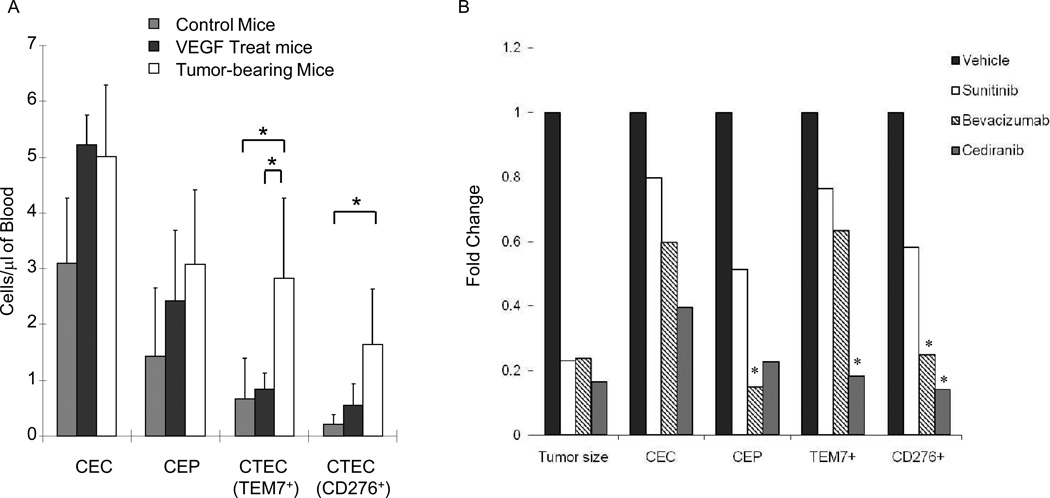
One potential shortcoming of CECs and CEPs as cancer-specific biomarkers is that their levels increase in response to stimuli other than cancer, including ischemia, infection, and organ regeneration, and in response to pro-angiogenic factors such as VEGF and SDF-1α (13, 24, 28, 30–39). Using H1975 xenografts, we examined the impact of VEGF injection or the presence of tumors on different EC populations. The number of TEM7 CTECs in tumor-bearing mice was significantly higher than that in non-tumor-bearing mice that had (VEGF treated) or had not (control) received a VEGF injection (p = 0.018 and 0.020, respectively (Fig. 3A). The level of CD276 CTECs was also significantly higher in tumor-bearing than non-tumor-bearing control mice (p = 0.015). By contrast, levels of CECs and CEPs were not significantly higher in tumor-bearing mice than in control or VEGF-treated mice suggesting that CTECs had greater specificity for the presence of tumor than CECs or CEPs did.
Figure 3. Effect of VEGF and vascular targeting agents on CEP, CEC, and CTEC levels.
(A) CECs, CEPs, and CTECs from H1975 xenograft tissue were evaluated by flow cytometry.. TEM7 CTEC and CD276 CTEC levels were significantly higher in tumor-bearing mice than in non-tumor-bearing controls (p = 0.03 and 0.01, respectively). The addition of 10 µg of VEGF (i.p.) had a non-significant effect on TEM7 CTECs and CD276 CTECs. TEM7 CTECs were significantly higher in tumor-bearing mice than in mice treated with VEGF (p = 0.03). Values are means ± SEM. (B) H1975 tumor-bearing mice were treated with vehicle only or with VEGFR tyrosine kinase inhibitor AZD2171 (cediranib) or sunitinib or with the human VEGF monoclonal antibody bevcizumab for 2 weeks. Tumor volume was significantly decreased after treatment (p < 0.05 for each treatment arm). TEM7+ CTECs were significantly reduced following treatment with cediranib (p<0.05). CD276+ CTECs were significantly reduced with bevacizumab and cediranib treatment (p<0.05).
CTECs as surrogate markers for anti-angiogenic activity
We next examined whether CTECs may serve as markers of angiogenesis inhibitor activity, as has been previously reported for CECs and CEPs (7, 8, 16, 17, 24, 29, 40). We injected mice with H1975 tumor cells subcutaneously, and once tumors reached a volume of 300 mm3, the animals were treated with vehicle only or with the VEGFR tyrosine kinase inhibitor AZD2171 (cediranib) or sunitinib, or the human VEGF monoclonal antibody bevcizumab. After two weeks of treatment, tumor growth was inhibited by 77% to 83% in each treatment arm compared with vehicle-only controls (Fig. 3B). The levels of CECs, CEPs, TEM7 CTECs, and CD276 CTECs in the collected blood were measured by flow cytometry. We found that each of the three inhibitors decreased the number of these cell types, although the degree of reduction varied. Reduced levels of CD276 CTECs (relative to control) was significantly correlated with the reductions in tumor growth (R2=0.96, p<0.05).
Detection of CTECs in clinical specimens
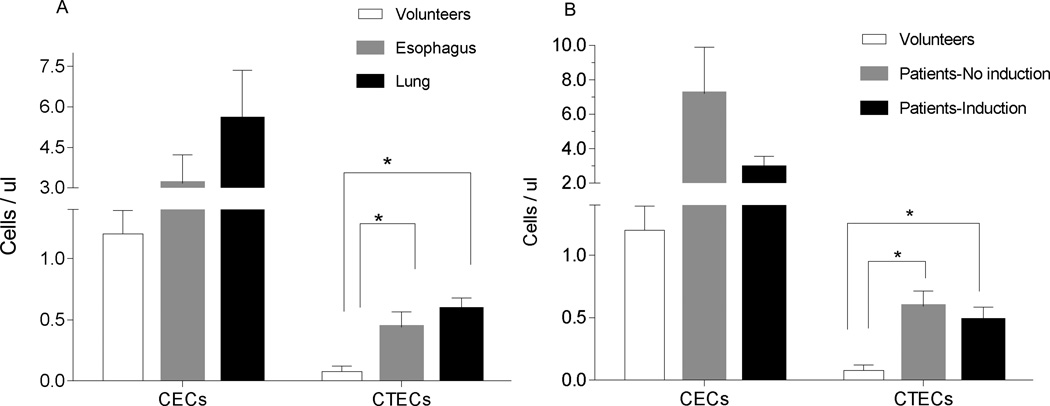
To investigate whether CECs and CTECs could be detected in cancer patients and were associated with the presence of cancer, we investigated CTECs in patients prior to and after resection of their tumor. The cancer patient group was comprised of 22 (55 %) patients with esophageal cancer and 18 (44 %) with lung cancer with a mean age of 60 ± 10.45 and 64 ± 9.28 respectively. Twenty-seven patients received induction chemotherapy, 19 patients with esophageal carcinoma and 8 with lung carcinoma. The tumor was successfully resected in all patients. There was no post-operative mortality. Eighteen patients had recurrence in the course of their follow up (Table 1). The control group of normal healthy volunteers consists of 9 patients, 6 men and 3 women, aged from 22 to 58 years (median 52 years). Although the primary goal of this analysis was to assess changes in CEC and CTECs after resection, we first explored levels of CECs and CTEC levels in esophageal and lung cancer patients compared to normal volunteers. CTECs (CD45−CD133−CD276+) were present at significantly higher levels in cancer patients (p<0.001) (Fig. 4A). This was true for both patients with lung and those with esophageal cancer (Fig. 4A). Consistent with earlier studies, mature CECs (CD45−CD133−CD31+) were present at a higher level in patients than in healthy volunteers, although this difference was not significant (p =0.13). CTECs were elevated in cancer patients compared to healthy volunteers regardless of whether they received induction chemotherapy (Fig. 4B). Given the limited size of the normal control group, however, this analysis was merely exploratory.
Figure 4. Detection of CECs and CTECs in human clinical blood specimens.
(A) CEC and CTEC levels were evaluated in cancer patients (22 patients with esophageal cancer and 18 patients with lung cancer) and healthy volunteers (n=9). When compared to CEC, the level of CTECs (CD45−CD133−CD276+CD31+) was significantly higher among patients with resectable lung cancer than among normal control volunteers. (p=0.001). (B) CTECs were elevated in cancer patients compared to healthy volunteers regardless of whether they received induction chemotherapy. Twenty-seven patients received induction chemotherapy, 19 patients with esophageal carcinoma and 8 with lung carcinoma.
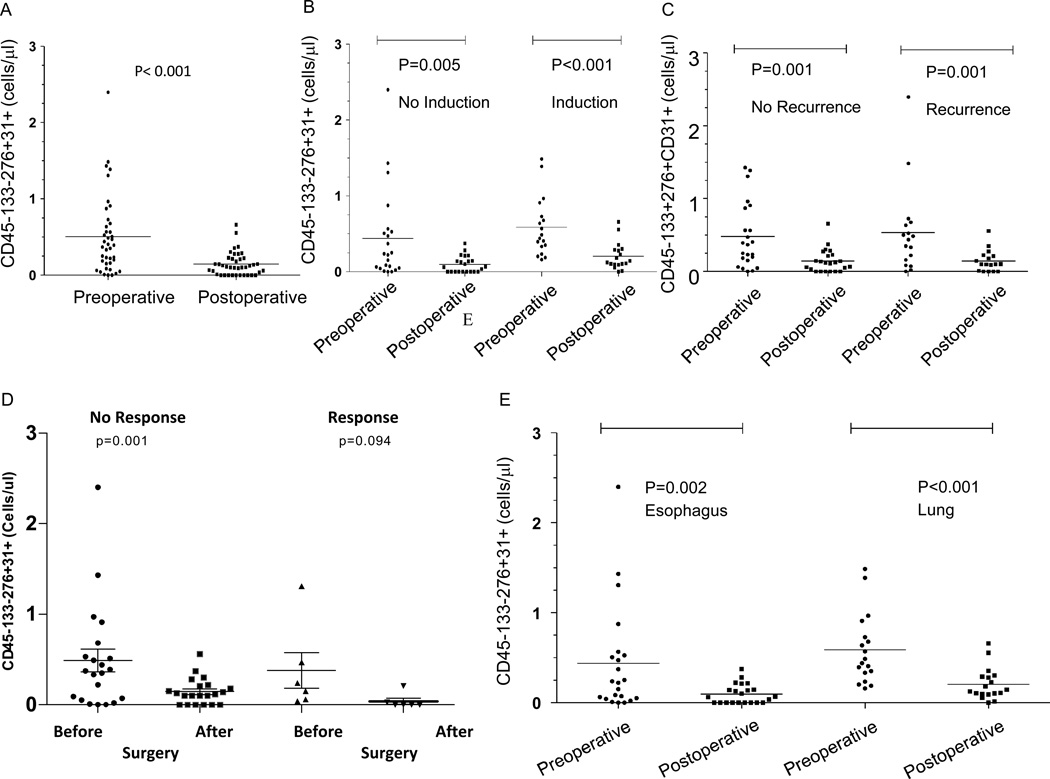
To investigate whether CTEC counts decreased following surgery, we analyzed blood samples collected before and one month following surgery in the same group of patients. We observed a significant decrease in patient CTEC levels following surgical removal of the tumor (p<0.001; Figure 5A). The effect was similar whether the patients had lung or esophageal cancer but more pronounced with lung cancer (Figure 5E). This post-surgical decrease in CTECs was observed regardless of whether patients received induction chemotherapy (Figure 5B). Patients who had induction therapy responded in a similar fashion after surgery indicating that despite the clinical response, surgery decreased the counts even further (Figure 5C).
Figure 5. Evaluation CTECs in patients with esophageal and lung cancer.
(A) Levels of CTECs decreased in patients following surgical removal of the tumor (p < 0.001). (B) This post-surgical decrease in CTECs was observed with or without induction chemotherapy. (C) This pattern was preserved whether the patients had a recurrence (D) or if they had a clinical response. (E) Levels of CTEC in patients who had esophagectomy (N=22) and those who had resection for lung cancer (N=18).
Discussion
CECs (41) are known to consist of at least two distinct populations: bone marrow-derived CEPs (42), which may contribute to pathologic neovascularization, and mature CECs derived from existing mature vasculature. CECs have emerged as a potentially useful biomarker for highly vascular and angiogenic tumors (7, 9, 16, 17). Increased levels of CECs have been observed in cancer patients relative to normal subjects and have been associated with disease progression (43, 44). In murine models, VEGF pathway inhibitors can have differential effects on mature CECs and CEPs in that inhibition of tumor angiogenesis is associated with an initial increase in mature CECs (7), followed by a subsequent reduction. Preclinical studies have demonstrated that CECs can be used as a surrogate marker for therapeutic activity of antiangiogenic agents (9, 29, 45). In a previous study of breast cancer patients receiving metronomic chemotherapy, an increase in apoptotic CECs, presumably being shed from the tumor vasculature, was shown to correlate with clinical benefit (16). Despite these discoveries, the clinical application of CECs as a cancer-specific biomarker is limited due at least in part because CECs and CEPs have been shown to increase in response to stimuli other than cancer, including pathological conditions (e.g., infection, ischemia, and sickle cell anemia) and physiological conditions (e.g., pregnancy and menstrual cycles), or in response to pro-angiogenic factors (e.g., VEGF and SDF-1α) (13, 24, 28, 30–39).
In an effort to identify more specific markers reflecting tumor vasculature, we investigated subpopulations of CECs bearing tumor endothelial cell-specific markers, which we term CTECs. In an earlier study by St. Croix et al. (22), 46 TEMs were identified by isolating the ECs in human colorectal tumors by serial analysis of gene expression, and further studies on a subset of TEMs (TEM1–TEM9) revealed their highly elevated levels specifically in the angiogenic state (22). Subsequent studies demonstrated counterparts of many of these TEMs in mice (23, 46, 47). Consistent with these earlier studies, we observed tumor endothelial staining for TEM8 and CD276 in our murine cancer models (23, 46). Prior studies of TEM7 have shown more varied results. Expression in human tumor endothelium has been observed in a number of studies (22, 23, 48, 49). In mouse studies, however, TEM7 expression was demonstrated by RT-PCR in one study of human melanoma and liposarcoma xenografts (47) but not observed in syngeneic melanoma tumors or human colon cancer xenografts by in situ hybridization or by SAGE analysis (23, 46). These differences may be due in part to the different methodologies used (e.g. SAGE which identifies differentially expressed gene transcripts versus immunohistochemistry and flow cytometry which evaluate protein levels of TEM7). Furthermore, given that vascular beds from different sites are morphologically and functionally distinct (50), our preclinical findings may be influenced by the fact that the tumors were grown subcutaneously. In addition, because tumor cells can influence tumor-associated endothelial cells though paracrine signaling, differences between the preclinical studies may also be due differences in the tumor models used (23).
TEM8, a cell-surface glycoprotein identified as an anthrax toxin receptor, is also upregulated on tumor vessels in mouse and human cancers(46, 51). In TEM8 knockout mice, developmental angiogenesis and wound healing occur normally, whereas tumor growth is impaired, indicating that TEM8 may be required for tumor angiogenesis but not physiological vascular processes(52, 53). In preclinical cancer models, targeting of TEM8 inhibited tumor angiogenesis and tumor growth(53).
Expression of CD276, a leukocyte co-stimulatory molecule, on endothelial cells has been evaluated previously. Using mouse models and comprehensive SAGE analysis, CD276 was identified as being expressed during pathological but not physiological angiogenesis(23). CD276 levels are elevated in colon and lung tumors compared to normal tissue, and although the tumor cells themselves expressed low levels of CD276, tumor-associated endothelial cells expressed high levels of CD276(23).
Consistent with prior reports, in our initial immunohistochemical analysis of Lewis lung cancer xenograft tissue we found that with the exception of TEM5, TEMs were present at higher levels in these tumors than in normal tissues (skin and lung). In some cases localization was not exclusively limited to tumor ECs; for example, immunoreactivity for TEM1, TEM4, TEM7 and CD276 was also detected in perivascular cells in tumors. Consistent with earlier reports, CD276 was also expressed in normal liver endothelium (27).
To further investigate whether TEMs could be detected on ECs isolated from tumor or normal tissue, we used four-color flow cytometry with a panel of established antibodies (24, 54). As expected, ECs isolated from tumors expressed significantly higher levels than ECs detected from normal skin and lung tissues but not normal liver tissue, the ECs of which expressed high levels of CD276.
We next examined whether we could detect CTECs in the blood of mice. We found that CTECs, but not CECs or CEPs, were present at significantly higher levels in tumor-bearing mice than in non-tumor-bearing mice or, for TEM7 CTECs, mice treated with VEGF. This finding suggested that CTECs are relatively more specific for tumors than CECs or CEPs are and relatively less responsive to a pro-angiogenic stimulus (i.e., VEGF) in the absence of tumor. As a subpopulation of CECs, CTECs may provide information distinct from that of CEPs or CECs. This difference may be due to CTECs being derived in part from the shedding of fragile, mature endothelium from tumor vasculature into the circulation whereas CEPs are mobilized from the bone marrow in response to a variety of pro-angiogneic stimuli, such as VEGF, and subsequently differentiate into mature CECs. It is worth noting, however, that a low level of CTECs was detected in non-tumor bearing mice. The source of these cells is not clear but, given that TEMs are relatively but not perfectly specific for tumor ECs, this may reflect a true population of rare TEM+ ECs in non-tumor bearing mice or alternatively a small degree of antibody cross-reactivity.
To address whether the increase in CTECs occurred after treatment with agents thought to selectively reduce tumor angiogenesis, we tested the effect of the VEGF pathway inhibitors AZD7121 (cediranib), sunitnib, and bevacizumab in our H1971 xenograft mouse model. Each of these agents decreased the number of CTECs as well as CECs and CEPs. The reduction of all three types correlated with decreased tumor volume. An anti-angiogenic agent may affect these cell populations differently; for instance, an angiogenic inhibitor could reduce VEGF-induced CEP mobilization but increase mature CEC and CTEC shedding. Additional studies are needed to determine whether these changes could serve as markers of biological activity.
Finally, we observed that in both NSCLC and esophageal cancer patients, CTEC levels decreased significantly after resection. A post-resection decrease was observed both in patients who had previously received induction chemotherapy and those who did not, suggesting that CTEC changes were not merely changes induced by chemotherapy, as previously observed for CECs(55). We also observed that CTECs are present in patients with both NSCLC and esophageal cancer at levels higher than normal, healthy control subjects but given the small size of this control group (N=9), this finding is hypothesis-generating and larger studies would be needed to more thoroughly evaluate the potential use of CTECs for the detection of cancer.
Based on the findings presented here, CTECs may be an appealing marker for several reasons. CTECs are more specific for cancer than CECs and CEPs and less influenced by non-malignant stimuli. Circulating tumor cells (CTCs) are also specific for cancer but, unlike CTCs, it appears that a limited number of TEMs are present on the majority of tumor endothelium although the number of different tumor types whose TEMs have been characterized is limited. In theory, therefore, CTECs may be more cancer-specific than CECs and a limited number of TEM markers on CECs could cover the vast majority of tumor types. More studies are needed to determine the clinical utility of CTECs.
The current study indicated that TEMs and CD276 are potentially useful diagnostic and prognostic markers for lung and esophageal cancer. CTECs are detectable and are found in increased numbers in tumor-bearing mice than in non-tumor-bearing mice. We propose that CTECs derive from ECs shed from the tumor and that they are a novel cancer-specific subset of CECs and, given the number of non-malignant conditions that can influence CEC levels, may be more useful than CECs for detecting tumors. However, many questions pertaining to TEMs remain and will need to be addressed in future mechanistic and prospective clinical studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elizabeth Hess and Emily Roarty, Ph.D. for editorial assistance. This work was supported by The University of Texas Southwestern Medical Center and The University of Texas MD Anderson Cancer Center Lung SPORE grant 5 P50 CA070907; LUNGevity Foundation Grant; M.D. Anderson Cancer Center Physician Scientist Award; CCSG grant 5 P30 CA016672; Damon Runyon Cancer Research Foundation (CI 24-04).
Conflicts of Interest: J.V.H. serves on advisory boards and receives research funding from Pfizer and AstraZeneca.
References
- 1.Hanrahan E, Lin H, Du D, Yan S, Kim E, Lee J, et al. Correlative analyses of plasma cytokine / angiogenic factor (C/AF) profile, gender and outcome in a randomized, three-arm, phase II trial of 1st-line vandetanib (VAN) and / or carboplatin plus paclitaxel (CP) for advanced non small cell lung cancer (NSCLC) J Clin Oncol, 2007 ASCO Annual Meeting Proocedings. 2007;25:7593. [Google Scholar]
- 2.Hanrahan EO, Heymach JV. Vascular endothelial growth factor receptor tyrosine kinase inhibitors vandetanib (ZD6474) and AZD2171 in lung cancer. Clin Cancer Res. 2007;13:s4617–s4622. doi: 10.1158/1078-0432.CCR-07-0539. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis DW, McConkey DJ, Abbruzzese JL, Herbst RS. Surrogate markers in antiangiogenesis clinical trials. Br J Cancer. 2003;89:8–14. doi: 10.1038/sj.bjc.6601035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaudry P, Force J, Naumov GN, Wang A, Baker CH, Ryan A, et al. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11:3514–3522. doi: 10.1158/1078-0432.CCR-04-2271. [DOI] [PubMed] [Google Scholar]
- 8.Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 9.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Bertolini F, Mancuso P, Braidotti P, Shaked Y, Kerbel RS. The multiple personality disorder phenotype(s) of circulating endothelial cells in cancer. Biochimica et biophysica acta. 2009;1796:27–32. doi: 10.1016/j.bbcan.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Shaked Y, Voest EE. Bone marrow derived cells in tumor angiogenesis and growth: are they the good, the bad or the evil? Biochimica et biophysica acta. 2009;1796:1–4. doi: 10.1016/j.bbcan.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Gruenwald V, Beutel G, Schuch-Jantsch S, Reuter C, Ivanyi P, Ganser A, et al. Circulating endothelial cells are an early predictor in renal cell carcinoma for tumor response to sunitinib. BMC Cancer. 2010;10:695. doi: 10.1186/1471-2407-10-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 15.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monestiroli S, Mancuso P, Burlini A, Pruneri G, Dell'Agnola C, Gobbi A, et al. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res. 2001;61:4341–4344. [PubMed] [Google Scholar]
- 18.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 19.Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet. 2003;361:206–210. doi: 10.1016/S0140-6736(03)12269-6. [DOI] [PubMed] [Google Scholar]
- 20.George F, Brouqui P, Boffa MC, Mutin M, Drancourt M, Brisson C, et al. Demonstration of Rickettsia conorii-induced endothelial injury in vivo by measuring circulating endothelial cells, thrombomodulin, and von Willebrand factor in patients with Mediterranean spotted fever. Blood. 1993;82:2109–2116. [PubMed] [Google Scholar]
- 21.Mutin M, Canavy I, Blann A, Bory M, Sampol J, Dignat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93:2951–2958. [PubMed] [Google Scholar]
- 22.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 23.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuch G, Heymach JV, Nomi M, Machluf M, Force J, Atala A, et al. Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res. 2003;63:8345–8350. [PubMed] [Google Scholar]
- 25.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 26.MacFadyen J, Savage K, Wienke D, Isacke CM. Endosialin is expressed on stromal fibroblasts and CNS pericytes in mouse embryos and is downregulated during development. Gene expression patterns : GEP. 2007;7:363–369. doi: 10.1016/j.modgep.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 28.Beaudry P, Hida Y, Udagawa T, Alwayn IP, Greene AK, Arsenault D, et al. Endothelial progenitor cells contribute to accelerated liver regeneration. J Pediatr Surg. 2007;42:1190–1198. doi: 10.1016/j.jpedsurg.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factoR165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 32.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, et al. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70:829–834. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 34.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 37.Rafii S, Heissig B, Hattori K. Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Ther. 2002;9:631–641. doi: 10.1038/sj.gt.3301723. [DOI] [PubMed] [Google Scholar]
- 38.Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, et al. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med. 2001;163:195–200. doi: 10.1164/ajrccm.163.1.9912036. [DOI] [PubMed] [Google Scholar]
- 39.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 40.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 41.Cech I, Burau KD, Al-Hashimi R. Factors contributing to elevated indoor radon in the Paso Del Norte region of the Texas-Mexico border: information for physicians. South Med J. 2009;102:701–706. doi: 10.1097/SMJ.0b013e3181a940d0. [DOI] [PubMed] [Google Scholar]
- 42.Khachatryan V, Sirunyan AM, Tumasyan A, Adam W, Bergauer T, Dragicevic M, et al. Search for quark compositeness with the dijet centrality ratio in pp collisions at radicals=7 TeV. Phys Rev Lett. 2010;105:262001. doi: 10.1103/PhysRevLett.105.262001. [DOI] [PubMed] [Google Scholar]
- 43.Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 44.Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 45.Schneider M, Tjwa M, Carmeliet P. A surrogate marker to monitor angiogenesis at last. Cancer Cell. 2005;7:3–4. doi: 10.1016/j.ccr.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 47.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 48.Bagley RG, Rouleau C, Weber W, Mehraein K, Smale R, Curiel M, et al. Tumor endothelial marker 7 (TEM-7): a novel target for antiangiogenic therapy. Microvasc Res. 2011;82:253–262. doi: 10.1016/j.mvr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Davies G, Cunnick GH, Mansel RE, Mason MD, Jiang WG. Levels of expression of endothelial markers specific to tumour-associated endothelial cells and their correlation with prognosis in patients with breast cancer. Clin Exp Metastasis. 2004;21:31–37. doi: 10.1023/b:clin.0000017168.83616.d0. [DOI] [PubMed] [Google Scholar]
- 50.Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102:430–437. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 52.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, et al. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhary A, Hilton MB, Seaman S, Haines DC, Stevenson S, Lemotte PK, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21:212–226. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mancuso P, Antoniotti P, Quarna J, Calleri A, Rabascio C, Tacchetti C, et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- 55.Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.