Abstract
Schizophrenia is a disorder of cognitive neurodevelopment. At least some of the core cognitive deficits of the illness appear to be the product of impaired gamma frequency oscillations which depend, in part, on the inhibitory actions of a subpopulation of cortical GABA neurons that express the calcium binding protein parvalbumin (PV). Recent studies have revealed new facets of the development of PV neurons in primate neocortex and of the nature of their molecular alterations in individuals with schizophrenia. Other recent studies in model systems provide insight into how these alterations may arise in the course of cortical circuitry development.
Keywords: basket neuron, chandelier neuron, gamma oscillations, parvalbumin
Dysfunction of inhibitory cortical circuits has emerged as a key substrate for the pathophysiology of cognitive dysfunction in schizophrenia, now recognized as the core clinical feature of the disorder. This perspective has been supported by the multiple reports, from different research groups using complementary methods in separate cohorts of subjects, that schizophrenia is associated with lower tissue levels of the mRNA for the 67 kD isoform of glutamic acid decarboxylase (GAD67; product of the GAD1 gene), the enzyme responsible for most GABA synthesis in the cortex [1]. Consistent with these findings, cortical GAD67 protein levels are also lower in schizophrenia [2;3]. Together, these data support the hypothesis that the capacity to synthesize cortical GABA is lower in the cerebral cortex of individuals with schizophrenia, and thus that levels of cortical GABA are reduced.
Attempts to test this hypothesis in vivo have included measures of total GABA levels by magnetic resonance spectroscopy. Unfortunately, the results of these studies have been variable, with cortical GABA levels reported to be lower, higher or not different in individuals with schizophrenia relative to comparison subjects [4]. The apparent inconsistencies in these findings may reflect a number of differences across studies including the cortical region examined, the medication status and age of the subjects, and the specific methods employed [4]. On the other hand, in vivo electrophysiological measures that index the functional activity of GABA neurons have been more consistent. For example, gamma frequency (30–80 Hz) oscillations require the synchronized inhibition of neighboring populations of pyramidal neurons by the subclass of cortical GABA neurons that express the calcium-binding protein parvalbumin (PV) [5]. In the human prefrontal cortex, gamma oscillations increase in proportion to cognitive task demands, such as working memory load [6], and under such task demands the power of prefrontal gamma band oscillations is reduced in subjects with schizophrenia both in the chronic stage of the illness [7] and in the early stages before the initiation of treatment [8]. Thus, alterations in PV neurons could contribute to gamma oscillation disturbances and cognitive deficits in schizophrenia [9].
Alterations in cortical PV neurons in schizophrenia
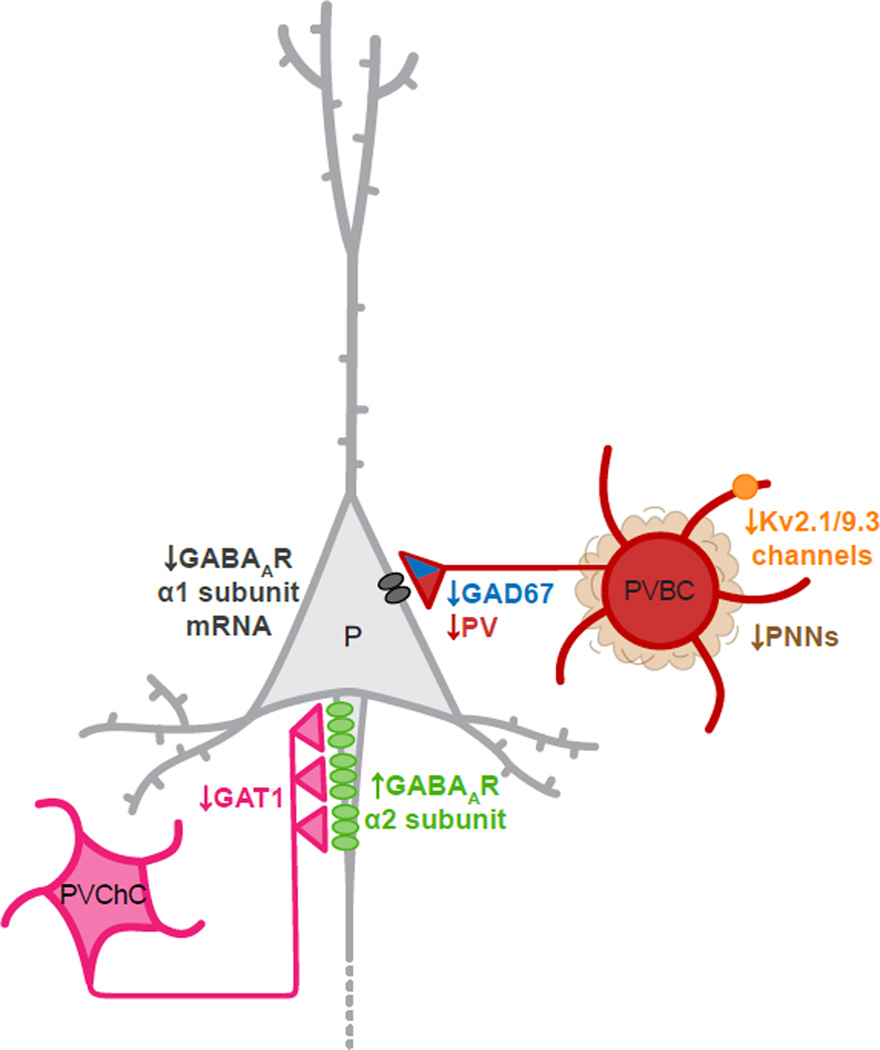
Postmortem studies have shown that the number of PV neurons does not differ between subjects with schizophrenia and comparison subjects [10–12], but these cells do exhibit abnormalities in critical molecular features that are likely to affect their function. For example, in subjects with schizophrenia mRNA levels of GAD67 are markedly lower in a substantial proportion of PV cells [10]. In addition, studies at the tissue, laminar and cellular levels have demonstrated lower levels of PV mRNA [10;13]. Cortical PV neurons include chandelier cells (PVChCs) and basket cells (PVBCs), which innervate the axon initial segment and soma/proximal dendrites of pyramidal cells, respectively. The alterations in PVChCs and their targets in schizophrenia have recently been reviewed [14]. The major findings are a reduction in the density of GABA membrane transporter 1 (GAT1)-immunoreactive axon terminals (cartridges) from PVChCs and a postsynaptic increase in the GABAA receptor α2 subunit in the axon initial segment of pyramidal neurons. These findings have been interpreted as compensatory responses that increase GABA neurotransmission at these synapses, but whether GAD67 levels are lower specifically in PVChC terminals in schizophrenia has not been directly assessed. In contrast, recent studies of PVBC axon terminals found that GAD67 protein levels are reduced by ~50% [3] and PV protein levels by ~25% [15]. In addition, mRNA levels of the GABAA receptor α1 subunit, which is commonly found in pyramidal neurons postsynaptic to PVBCs terminals, are selectively reduced in prefrontal layer 3 pyramidal cells in schizophrenia [16], the same laminar location where the changes in PV neurons are most prominent. Signaling through α1-containing GABAA receptors may also be impaired by abnormal N-glycosylation of this subunit in schizophrenia [17]. Thus, alterations in the capacity of PVBCs to synthesize and release (as influenced by terminal levels of PV [18]) GABA, and for the GABA released from PVBC terminals to inhibit pyramidal cells, could all contribute to impaired cortical gamma oscillations in schizophrenia.
Other molecular alterations in PV cells could also contribute to impaired gamma oscillations in schizophrenia. For example, a recent study found evidence of schizophrenia-related alterations in expression of a PV cell type-specific subunit for a voltage-gated potassium channel. In the human prefrontal cortex, PV neurons selectively express KCNS3, the gene encoding the Kv9.3 potassium channel α-subunit [19]. This subunit assembles with delayed rectifier Kv2.1 α-subunits, which are expressed by the majority of cortical neurons including PV cells, to form heteromeric Kv2.1/Kv9.3 channels. In subjects with schizophrenia, levels of KCNS3 mRNA were 23% lower in prefrontal cortical gray matter and 40% lower in PV neurons captured by laser microdissection [20]. Interestingly, in the individually collected PV neurons, KCNB1 mRNA, which encodes the Kv2.1 subunit, showed a decrease in expression comparable to KCNS3 mRNA in the subjects with schizophrenia, and expression levels of both transcripts were highly correlated within subjects. These findings suggest a concomitant down-regulation of both Kv9.3 and Kv2.1 subunits, and thus a lower complement of Kv2.1/Kv9.3 heteromeric channels in prefrontal PV neurons in schizophrenia. Compared with homomeric Kv2.1 channels, Kv2.1/Kv9.3 channels are more effectively activated by small depolarization steps from the resting membrane potential, suggesting that Kv2.1/Kv9.3 channels are among those dendritic voltage-gated potassium channels that contribute to the fast EPSP decay in PV neurons [21]. Because such fast EPSPs summate in a narrow time window, PV neurons can efficiently detect, and fire in response to, temporally convergent excitatory inputs from neighboring pyramidal neurons [22]. Thus, the properties of Kv2.1/Kv9.3 channels impute a level of precision to the detection of coincident excitatory synaptic inputs in PV neurons that may be essential for the synchronization of cortical neural networks in gamma oscillations. Consequently, the deficient expression KCNS3 and KCNB1 mRNAs in PV neurons, if reflected in a lower complement of Kv2.1/Kv9.3 channels, would be predicted to slow the time course of EPSPs in PV neurons, impairing their ability to appropriately generate gamma oscillations, and thus contribute to cognitive dysfunction in schizophrenia [20].
Potential mechanisms for PV cell dysfunction in schizophrenia
A series of recent studies suggest a number of different mechanisms, not mutually exclusive, that could contribute to PV cell alterations in schizophrenia. The lower levels of GAD67 mRNA could reflect disturbances in upstream factors that regulate the GAD1 gene. For example, a variant in the GAD1 gene associated with increased risk for schizophrenia [23] and altered chromatin structures at the GAD1 promoter [24] have been associated with lower levels of GAD67 mRNA in schizophrenia. Recently, Akbarian and colleagues [25] reported that the GAD1 gene contains a promoter/enhancer loop that allows for distal regulatory elements to be positioned closer to the transcription start site, resulting in increased transcription. In a small sample of schizophrenia subjects with lower levels of GAD67 mRNA, this loop was decreased in the prefrontal cortex. Interestingly, the formation of the loop is sensitive to changes in neuronal activity, and the activity-driven expression of GAD67 critically controls the synthesis of GABA available for synaptic release [26]. In concert, these findings may provide insight into a molecular mechanism for how a reduction in cortical network activity, due to factors such as NMDA receptor hypofunction of PV neurons [27] or lower excitatory drive to the pyramidal neurons that innervate PV GABA neurons [9], could lead to lower GAD67 expression.
Other recent findings provide a potential link between immune- and/or inflammation-related abnormalities and cortical GABA neuron alterations in schizophrenia. Single-nucleotide polymorphisms in genes involved in immune and inflammatory signaling pathways have been associated with increased risk for schizophrenia [28–30], and maternal exposure to infection and elevated serum cytokine levels during pregnancy have been associated with an increased risk of schizophrenia in offspring [31]. In adult schizophrenia subjects, elevated levels of proinflammatory cytokines [32] and higher mRNA levels for interferon-induced transmembrane protein (IFITM) have been found in the prefrontal cortex [33;34]. A recent study in a very large sample of schizophrenia and control subjects replicated these findings and demonstrated that the elevated expression (over 2 fold increase) in schizophrenia was exclusively found in endothelial cells [35]. Interestingly, levels of IFITM mRNA were negatively correlated with both GAD67 and PV mRNAs in the same schizophrenia subjects [35], suggesting that pathological disturbances in immune activation may contribute to PV neuron alterations in at least some individuals with schizophrenia.
Most PV cortical neurons are surrounded by complex extracellular structures called perineuronal nets (PNNs). PNNs are composed of chondroitin sulfate proteoglycans and related components of the extracellular matrix, form in an activity-dependent manner, and serve as a cation buffer which may facilitate the fast-spiking nature of PV cells. The densities of PNNs, as assessed by labeling with Wisteria floribunda agglutinin which binds to the carbohydrate components of PNNs, were reported to be decreased in the prefrontal but not the primary visual cortex of subjects with schizophrenia [36]. Recent work suggests that PNNs are protective against oxidative stress [37], providing a potential link between reports of reduced levels of cortical glutathione, an antioxidant [38], a lower complement of PNNs and PV cell dysfunction in schizophrenia. Although intriguing from the perspective of an explanatory cascade of events, the robustness of each finding in schizophrenia, and the strength of their mechanistic links, awaits further study. For example, prefrontal glutathione levels were reported to be lower in both schizophrenia and bipolar subjects [38], but PNN densities were reported to be unaffected in bipolar subjects [36].
Genetic variants in both neuregulin and its ErbB4 receptor, which is heavily but not uniquely expressed by PV neurons in primate cortex [39], have been associated with liability to schizophrenia. A recent study [40] provides a potential mechanistic link by demonstrating that selective deletion of ErbB4 in PV neurons reproduces many of the anatomical and molecular findings in schizophrenia as well as alterations in cortical oscillations. Interestingly, total ErbB4 mRNA levels are not altered in schizophrenia, but the expression levels of two minor splice variants are markedly increased [41;42]. If the products of these splice variants serve as dominant negatives in relation to ErbB4 signaling, then increased expression of ErbB4 splice variants in schizophrenia could be mimicked by the deletion of ErbB4 in the mouse model.
Recent studies of the maturation of PVBCs and PVChCs in monkey prefrontal cortex might also provide insight into how cell type-specific differences in schizophrenia could arise during developmental periods that appear to be critical for the emergence of the clinical features of the illness. For example, earlier studies reported that the density of PVChC axon terminals (cartridges) decreased [43;44] and the density of PVBC terminals increased [45] during adolescence in monkey prefrontal cortex. However, it could not be determined whether these inverse changes reflected developmental differences in terminal number and/or in PV protein levels per terminal such that their detection differed with age. Using a new method that permits the quantification of both terminal number and protein levels per terminal, Fish and colleagues [46] found that the number of PVChC terminals was significantly lower in adult than in infant monkeys, whereas the density of PVBC terminals did not change with age. In contrast, PV protein levels in PVBC terminals were much higher in adult than infant monkeys but did not differ with age in PVChC terminals. Thus, PVChC and PVBC appear to utilize fundamentally different mechanisms to achieve adult levels of innervation density and biochemical properties. These findings suggest a cell type-specific mechanism of maturation for PVBC and PVChC axon terminals that might help explain the patterns of disturbances of these cell types in schizophrenia. For example, disease processes operating during adolescence might be expected to disrupt the PV protein content, but not the number, of PVBC terminals; this prediction matches the pattern of findings observed in schizophrenia [15].
The molecular and functional properties of PV neurons may also be influenced by disease-related alterations in the inputs they receive from other affected cell populations. A recent study in mouse visual cortex demonstrated that PV cells strongly inhibit each other, but provide little inhibition to other populations of GABA neurons, whereas somatostatin-containing GABA cells avoid inhibiting each other and strongly inhibit other populations, including PV cells [47]. Convergent findings indicate that mRNA levels of somatostatin [13;48;49] and somatostatin receptors [50] are altered in the prefrontal cortex of subjects with schizophrenia. Thus, altered inputs from somatostatin cells could also contribute to PV cell dysfunction in schizophrenia.
In concert, the findings reviewed above provide additional evidence that alterations in PV cell function are likely to be key contributors to cortical dysfunction and cognitive impairments in schizophrenia. The relationships among these various types and potential causes of PV cell dysfunction, and which subjects with schizophrenia they are operative in [13;48;49], awaits further study.
Figure.
Schematic drawing of pyramidal cell (P), parvalbumin basket call (PVBC) and PV chandelier cell (PVChC) circuitry in layer 3 of human DLPFC, illustrating the reported changes in schizophrenia. GAT1, GABA membrane transporter; PNNs, perineuronal nets.
Highlights.
Parvalbumin (PV) cell alterations contribute to cognitive deficits in schizophrenia
PV cells have multiple gene expression disturbances that impair their function
These disturbances may arise from either cell-autonomous factors or altered inputs
Acknowledgements
The author appreciates the assistance of Laura English in the preparation of the manuscript.
Funding Source
Studies by the author cited in this paper were supported by NIH grants MH043784 and MH051234.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 3.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: Clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. doi: 10.1093/schbul/sbs092. This study represents an important addition to the literature on in vivo measures of cortical GABA levels in subjects with schizophrenia and helps explain why current findings are discrepant.
- 5.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 7.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharm. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillozet-Bongaarts AL, Hyde TM, Dalley RA, Hawrylycz MJ, Henry A, Hof PR, Hohmann J, Jones AR, Kuan CL, Royall J, et al. Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stan AD, Lewis DA. Altered cortical GABA neurotransmission in schizophrenia: Insights into novel therapeutic strategies. Curr Pharm Biotechnol. 2012;13:1557–1562. doi: 10.2174/138920112800784925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.152. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharm. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller TM, Haroutunian V, Meador-Woodruff JH. N-glycosylation of GABA receptor subunits is altered in schizophrenia. Neuropsychopharm. 2013 doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggermann E, Jonas P. How the 'slow' Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci. 2012;15:20–22. doi: 10.1038/nn.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiev D, Gonzalez-Burgos G, Kikuchi M, Minabe Y, Lewis DA, Hashimoto T. Selective expression of KCNS3 potassium channel alpha-subunit in parvalbumin-ontaining GABA neurons in the human prefrontal cortex. PLoS One. 2012;7:e43904. doi: 10.1371/journal.pone.0043904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Georgiev D, Arion D, Enwright J, Kikuchi M, Minabe Y, Corradi JP, Lewis DA, Hashimoto T. Lower gene expression for KCNS3 potassium channel subunit in parvalbumin-containing neurons in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.13040468. In Press. The findings of this study reveal a novel molecular disturbance, specific to PV neurons, that could alter their capacity to integrate and fire in the temporal fashion required for gamma oscillations.
- 21.Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327:52–58. doi: 10.1126/science.1177876. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 24.Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bharadwaj R, Jiang Y, Mao W, Jakovcevski M, Dincer A, Krueger W, Garbett K, Whittle C, Tushir JS, Liu J, et al. Conserved chromosome 2q31 conformations are associated with transcriptional regulation of GAD1 GABA synthesis enzyme and altered in prefrontal cortex of subjects with schizophrenia. J Neurosci. 2013;33:11839–11851. doi: 10.1523/JNEUROSCI.1252-13.2013. This interesting study revealed a novel molecular mechanism that could underlie lower expression of the major synthesizing enzyme for GABA in subjects with schizophrenia.
- 26.Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. J Neurosci. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: A pathway analysis of GWAS data. Schizophr Res. 2010;122:38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. The findings of this study, in concert with the report of Siegel et al., provide convergent support for inflammation as a contributory factor in cortical GABA neuron dysfunction in schizophrenia.
- 33.Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siegel BI, Sengupta EJ, Edelson JR, Lewis DA, Volk DW. Elevated viral restriction factor levels in cortical blood vessels in schizophrenia. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.019. In Press. The findings of this study, in concert with the report of FIllman et al., provide convergent support for inflammation as a contributory factor in cortical GABA neuron dysfunction in schizophrenia.
- 36.Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo TU. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 39.Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, Lewis DA, McBain CJ, Buonanno A. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: Implications for schizophrenia. Biol Psychiatry. 2011;70:636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, Ciceri G, Gabaldon MV, Moratal D, Dierssen M, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. This comprehensive analysis of a mouse genetic model provides compelling evidence for alterations in ErbB4 signaling as a plausible cause of PV cell dysfunction in schizophrenia.
- 41.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 42.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: Association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 43.Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- 44.Cruz DA, Lovallo EM, Stockton S, Rasband M, Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. J Comp Neurol. 2009;514:353–367. doi: 10.1002/cne.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- 46.Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 2013;33:8352–8358. doi: 10.1523/JNEUROSCI.0306-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beneyto M, Morris HM, Rovensky KC, Lewis DA. Lamina- and cell-specific alterations in cortical somatostatin receptor 2 mRNA expression in schizophrenia. Neuropharmacology. 2012;62:1598–1605. doi: 10.1016/j.neuropharm.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]