Abstract
Purpose
Detecting circulating plasma tumor DNA (ptDNA) in early stage cancer patients has the potential to change how oncologists recommend systemic therapies for solid tumors after surgery. Droplet digital polymerase chain reaction (ddPCR) is a novel sensitive and specific platform for mutation detection.
Experimental Design
In this prospective study, primary breast tumors and matched pre- and post-surgery blood samples were collected from early stage breast cancer patients (n=29). Tumors (n=30) were analyzed by Sanger sequencing for common PIK3CA mutations, and DNA from these tumors and matched plasma were then analyzed for PIK3CA mutations using ddPCR.
Results
Sequencing of tumors identified seven PIK3CA exon 20 mutations (H1047R) and three exon 9 mutations (E545K). Analysis of tumors by ddPCR confirmed these mutations and identified five additional mutations. Pre-surgery plasma samples (n=29) were then analyzed for PIK3CA mutations using ddPCR. Of the fifteen PIK3CA mutations detected in tumors by ddPCR, fourteen of the corresponding mutations were detected in pre-surgical ptDNA, while no mutations were found in plasma from patients with PIK3CA wild type tumors (sensitivity 93.3%, specificity 100%). Ten patients with mutation positive ptDNA pre-surgery had ddPCR analysis of post-surgery plasma, with five patients having detectable ptDNA post-surgery.
Conclusions
This prospective study demonstrates accurate mutation detection in tumor tissues using ddPCR, and that ptDNA can be detected in blood before and after surgery in early stage breast cancer patients. Future studies can now address whether ptDNA detected after surgery identifies patients at risk for recurrence, which could guide chemotherapy decisions for individual patients.
Keywords: PIK3CA, breast cancer, droplet digital PCR, plasma tumor DNA
Introduction
The goal of adjuvant systemic therapies in clinical oncology, such as chemotherapy administered after breast cancer surgery, is to eradicate microscopic residual disease that could lead to recurrence and incurable disease. Large randomized prospective trials support the use of adjuvant systemic therapies, however these trials also show the majority of patients (~ 60% to 70%) with early stage breast cancer are cured with local therapies alone and that the addition of adjuvant therapies improves disease free survival and cure rates by ~ 10% to 20% (1). Though this is a relatively small percentage of patients, this equates to tens of thousands of lives that are cured due to the administration of additional systemic therapies. However, it is also known that many systemic therapies have toxicities such as infection, neuropathy, heart failure and secondary leukemias from chemotherapies that can even lead to premature deaths. Since there is no reliable method for detecting microscopic residual disease after surgery, the current paradigm is to recommend adjuvant therapies to the majority of patients to benefit relatively few, resulting in overtreatment. The ability to develop accurate markers of microscopic residual disease after surgery could identify patients who are at higher versus lower risk for recurrence, and potentially spare patients toxic therapies that are not needed. This could lead to a paradigm shift of individualized adjuvant therapy recommendations based on the presence or absence of residual disease post-surgery.
During the past few years, studies have demonstrated the ability to reliably detect and quantify circulating cell-free cancer DNA and RNA, mostly in patients with metastatic disease (2–10). This is predicated on the principle that cancer DNA contains somatic mutations, that is, DNA alterations that are unique to cancer cells and not present in normal cells. Circulating cell-free DNA is thought to be shed or released from normal and cancer cells (11, 12). For clarity and precise nomenclature, we will refer to circulating cell-free plasma tumor DNA as ptDNA since circulating tumor DNA also refers to urine tumor DNA and tumor DNA in other bodily fluids. The ability to accurately detect the small proportion of ptDNA that exists within the larger population of non-cancerous derived plasma DNA holds great promise for guiding systemic therapy decisions in oncology (13). For example, using blood as a “liquid biopsy”, one can noninvasively identify specific mutations within a patient's cancer (14). In addition, due to the quantitative nature of ptDNA detection and the short half-life of plasma DNA (1 to 2 hours), detection of minimal tumor burden and monitoring response to therapies in metastatic disease has been shown to be feasible (4, 8).
Technologic advances have raised the possibility of incorporating ptDNA testing for use in clinical oncology. Specifically, digital polymerase chain reaction (PCR) and next generation sequencing allow for the detection of mutant DNA molecules that are present at 0.02% to 0.1% of the total amount of DNA assayed (2, 3). In essence, these technologies partition individual DNA molecules and then each molecule is amplified and queried for a given mutation (15). This dramatically increases the ability to detect rare mutant DNA molecules among a vast population of normal or wild type DNA. Prior studies have been conducted mostly in the metastatic setting where the amount of total plasma DNA and ptDNA tends to be greater, and sensitivity for detecting ptDNA is generally not an issue (2, 4). For example, using varying techniques studies have reported detection of ptDNA in metastatic breast cancer patients with high sensitivities and specificities (5, 7). Additionally, a recent study has demonstrated the ability to detect miRNAs in metastatic prostate cancer using digital PCR (10). Moreover, a recent prospective study in metastatic breast cancer patients compared ptDNA to circulating tumor cells (CTCs) and the breast cancer serum marker CA 15–3, and demonstrated ptDNA more accurately reflects tumor burden with a greater dynamic range for following response to therapies and disease progression (2). However, only a few studies have evaluated ptDNA in early stage cancer patients, and in these analyses the ability to identify ptDNA has varied, likely due to the technical limits of these methods to detect smaller tumor burden (9, 14, 16–19). Indeed, these studies suggest that more sensitive methods are needed to utilize ptDNA as a cancer biomarker for early stage disease.
Here we describe a prospective study in which we examined the ability to detect mutant PIK3CA molecules in ptDNA from early stage (stage I–III) breast cancer patients using next generation digital PCR platforms. PIK3CA is an oncogene that encodes the p110α component of PI3 kinase, and there is currently intense interest to develop PI3 kinase inhibitors due to the high frequency of PIK3CA mutations in human cancers (20). There are three frequently recurring “hotspot” PIK3CA mutations within two exons (exon 9: E542K and E545K and exon 20: H1047R) which account for 80–90% of all PIK3CA mutations found in human malignancies (21). Multiple cancer sequencing studies have found PIK3CA mutations to be present in ~30% of all breast cancers, with a higher frequency (~45%) reported in estrogen receptor/progesterone receptor (ER/PR) positive and HER2 positive breast cancers (22–25). In prior work, we and others demonstrated that mutant PIK3CA DNA can be reliably detected in plasma from metastatic breast cancer patients using various technologies (2, 5, 16). We hypothesized that the newer technique of droplet digital PCR (ddPCR) could identify PIK3CA mutations in formalin fixed paraffin embedded (FFPE) primary tumor samples and corresponding plasma from early stage breast cancer patients before and after surgery with high sensitivity and specificity. If feasible, this would allow for future trials testing the clinical utility of ptDNA as a cancer biomarker to guide individual decisions regarding adjuvant systemic therapies.
Materials and Methods
Patients and Sample Collection
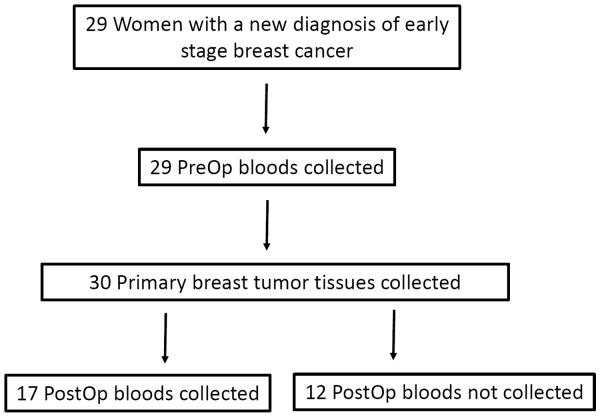
For each patient, a single pre-surgery blood sample was collected prior to definitive surgery, either lumpectomy or mastectomy. In addition, we collected a second blood sample after surgery. Ten milliliters (10ml) of blood was collected at each time point. Primary tumor samples were obtained as FFPE blocks and slides prepared for DNA analysis as previously described (5) (see also Supplementary Methods). Of the twenty-nine patients, all had primary cancer tissue and pre-surgery blood collected, while seventeen patients had post-surgery blood collected (Figure 1).
Figure 1. Enrollment of patients and collection of clinical samples.
A total of twenty-nine women were enrolled in this prospective study. PIK3CA mutational analysis was performed on thirty tumor specimens and pre- and post-surgery plasma samples collected. In twelve patients, post-surgery blood samples could not be collected. PreOp denotes pre-surgery specimens and PostOp denotes post-surgery specimens.
Tissue DNA sequencing and ddPCR of FFPE samples
Genomic DNA was extracted from tumor and adjacent normal tissues, and purified as previously described (5) (see also Supplementary Methods). PCR primers used for amplifying segments of PIK3CA exon 9 and exon 20, and the nested sequencing primers used for Sanger sequencing are shown in Table S1. Genomic DNA was also analyzed by ddPCR using TaqMan probes for wild type PIK3CA, as well as PIK3CA E545K and PIK3CA H1047R mutations to detect and quantitate these mutations (Table S2). ddPCR results were quantified using RainDrop Analyst software (RainDance Technologies) and are expressed as a percentage or fractional abundance of mutant to total (mutant plus wild type) PIK3CA molecules for each sample (see also Supplementary Methods).
Isolation and Quantification of ptDNA by ddPCR
Blood samples and plasma DNA preparation were performed as previously described (5) (see also Supplementary Methods). Purified plasma DNA was subjected to high fidelity PCR amplification using the primers listed in Table S3. The PCR amplified products were then diluted and combined with mutant and wild type probes for PIK3CA E545K and PIK3CA H1047R mutation detection in separate reactions for each mutation specific probe (Table S3). ddPCR was then performed per the manufacturer's protocol with results reported as a percentage or fractional abundance of mutant DNA alleles to total (mutant plus wild type) DNA alleles.
Statistical Analysis
This was a feasibility study to determine the accuracy of detecting PIK3CA mutations using ddPCR on pre-surgery plasma from early stage breast cancer patients. We estimated that for 95% accuracy with a 95% lower limit confidence interval of 80%, thirty patient samples with approximately half containing PIK3CA mutations would be sufficient. Using the ddPCR results from the FFPE analysis as our reference, Java Stat 2-way contingency tables were used to calculate sensitivity, specificity, accuracy and 95% confidence intervals for the detection of ptDNA in the pre-surgery plasma samples taking into account both PIK3CA E545K and H1047R assays.
In order to quantify the percent ptDNA containing mutant PIK3CA in plasma samples, a fractional abundance calculation using the QuantaSoft program (Bio-Rad Technologies) was employed, using the total number of droplets (with and without DNA) to calculate the number of DNA molecules as copies/μl, and then dividing the number of mutant DNA molecules by the number of total DNA molecules (mutant plus wild type), multiplied by 100 to yield a percentage of mutant DNA molecules in a sample taking into account a Poisson distribution of occupied to unoccupied droplets (10). In addition, this program was employed for the summation of each category of droplets in multiple replicates creating a single meta-well for each sample with a 95% confidence interval calculated using Poisson statistics as previously described (10).
Results
Twenty-nine early stage (stage I–III) breast cancer patients were consented and enrolled between August 2010 and January 2011 in a prospective IRB-approved study at Johns Hopkins. Patient characteristics are described in Table 1. The median age of enrolled patients was 60. Though not pre-enriched, the majority of patients (93%) had ER/PR positive disease. One patient (patient 14) had bilateral breast cancers at diagnosis. At the time of data analyses, the median duration of follow up for all twenty-nine patients was 36 months and two patients had recurred.
Table 1.
Patient characteristics
| Patient | Age at Diagnosis (median = 60) | Stage | TNM | Histology | ER | PR | HER2 |
|---|---|---|---|---|---|---|---|
| 1 | 66 | IA | T1c N0(i+) Mx | lobular | positive | positive | negative |
| 2 | 63 | IIA | T1b N1 Mx | ductal | positive | positive | negative |
| 3 | 52 | IA | T1c N0 Mx | ductal/lobular | positive | positive | negative |
| 4 | 77 | IA | T1b N0 Mx | lobular | positive | positive | negative |
| 5 | 48 | IA | T1c N0 Mx | ductal | negative | negative | negative |
| 6 | 38 | IIB | T2 N1 Mx | ductal | positive | positive | negative |
| 7 | 49 | IIA | T2 N0 Mx | ductal | positive | positive | negative |
| 8 | 51 | IA | T1c N0 M0 | ductal | positive | positive | negative |
| 9 | 48 | IIA | T2 N0 Mx | ductal | positive | positive | negative |
| 10 | 55 | IA | T1c N0 M0 | ductal | positive | positive | negative |
| 11 | 71 | IA | T1c N0 Mx | ductal | positive | negative | negative |
| 12 | 69 | IIA | T2 N0(i−) Mx | lobular | positive | positive | negative |
| 13 | 52 | IA | T1c N0 Mx | ductal/lobular | positive | positive | negative |
| 14 | 64 | IIA IIIA |
R:T1a N1 Mx L: T1b N2 Mx |
ductal lobular |
positive positive |
positive positive |
negative negative |
| 15 | 75 | IA | T1b N0 Mx | lobular | positive | positive | negative |
| 16 | 51 | IIB | T2 N1 MX | metaplastic | negative | negative | negative |
| 17 | 67 | IA | T1c N0 Mx | ductal/lobular | positive | positive | negative |
| 18 | 63 | IA | T1c N0 Mx | ductal | positive | positive | negative |
| 19 | 60 | IIA | T1c N1 Mx | ductal | positive | positive | negative |
| 20 | 38 | IIB | T2 N1 Mx | ductal | positive | positive | negative |
| 21 | 72 | IIA | T2 N0 Mx | ductal | positive | positive | negative |
| 22 | 59 | IA | T1c N0 M0 | ductal | positive | positive | negative |
| 23 | 53 | IIIA | T2 N2 Mx | ductal | positive | positive | negative |
| 24 | 67 | IA | T1c N0 Mx | ductal/lobular | positive | positive | negative |
| 25 | 54 | IA | T1c N0 Mx | ductal | positive | positive | negative |
| 26 | 58 | IIB | T2 N1 MX | ductal | positive | positive | negative |
| 27 | 63 | IIA | T1 N1 Mx | ductal | positive | positive | negative |
| 28 | 55 | IIA | T1c N1 Mx | ductal | positive | positive | negative |
| 29 | 61 | IIB | T2 N1mi Mx | ductal | positive | positive | equivocal* |
n=29. ER: estrogen receptor, PR: progesterone receptor, HER2: Human Epidermal Growth Factor Receptor 2.
equivocal by immunohistochemical staining 2+, and FISH ratio of HER2:chromosome 17 = 2.1.
PIK3CA mutation detection in FFPE samples with ddPCR
Tumor specimens with corresponding clinical and pathological information were collected from all patients. Sanger sequencing of these thirty primary tumor specimens and adjacent normal tissues (controls) from twenty-nine patients identified seven PIK3CA exon 20 mutations (H1047R) and three exon 9 mutations (E545K) yielding a frequency of 33.3% (10 of 30 tumors). No other mutations within these coding regions were found. These DNA samples were then subjected to ddPCR which confirmed the ten mutations identified by Sanger sequencing. Mutant to wild type PIK3CA fractional abundance ranged from 13.8% to 55.6%. ddPCR also identified two additional PIK3CA H1047R mutations, one additional PIK3CA E545K mutation and one sample with both H1047R and E545K mutations. The prevalence of PIK3CA mutations detected per tumor by ddPCR was 46.7% (14 of 30). Results comparing the FFPE and ddPCR analyses with fractional abundance are shown in Table 2.
Table 2.
Analysis of PIK3CA mutations in tumors and blood.
| Patient | Sequencing FFPE tumor tissue | ddPCR FFPE tumor tissue | ddPCR pre-surgery plasma |
|---|---|---|---|
| 1 | Wild type | H1047R (9.4%) | H1047R (0.03%) |
| 2 | Wild type | Wild type | Wild type |
| 3 | Wild type | Wild type | Wild type |
| 4 | Wild type | E545K (28.9%) H1047R (6%) |
E545K (0.01%) H1047R (0.02%) |
| 5 | H1047R | H1047R (25.5%) | H1047R (0.02%) |
| 6 | Wild type | Wild type | Wild type |
| 7 | Wild type | Wild type | Wild type |
| 8 | H1047R | H1047R (26%) | H1047R (0.12%) |
| 9 | E545K | E545K (43.7%) | E545K (0.02%) |
| 10 | Wild type | Wild type | Wild type |
| 11 | H1047R | H1047R (13.8%) | H1047R (0.01%) |
| 12 | Wild type | Wild type | Wild type |
| 13 | Wild type | E545K (16.1%) | Wild type |
| 14 | R:Wild type L:Wild type |
Wild type Wild type |
Wild type Wild type |
| 15 | Wild type | Wild type | Wild type |
| 16 | H1047R | H1047R (32.2%) | H1047R (2.99%) |
| 17 | E545K | E545K (55.6%) | E545K (0.07%) |
| 18 | Wild type | Wild type | Wild type |
| 19 | E545K | E545K (20.3%) | E545K (0.01%) |
| 20 | Wild type | Wild type | Wild type |
| 21 | Wild type | Wild type | Wild type |
| 22 | H1047R | H1047R (28.9%) | H1047R (0.02%) |
| 23 | Wild type | Wild type | Wild type |
| 24 | Wild type | Wild type | Wild type |
| 25 | H1047R | H1047R (30.5%) | H1047R (0.01%) |
| 26 | Wild type | Wild type | Wild type |
| 27 | Wild type | Wild type | Wild type |
| 28 | Wild type | H1047R (9.5%) | H1047R (0.02%) |
| 29 | H1047R | H1047R (43.1%) | H1047R (0.01%) |
Tumor and blood samples from twenty-nine early stage breast cancer patients were analyzed for PIK3CA mutations using Sanger sequencing of formalin fixed paraffin embedded tumors (Sequencing FFPE tumor tissue), ddPCR of formalin fixed paraffin embedded tumors (ddPCR FFPE tumor tissue) and pre-surgery plasma DNA (ddPCR pre-surgery plasma). Percentage reflects fractional abundance of mutant PIK3CA (E545K or H1047R) to total PIK3CA DNA.
PIK3CA mutations detected in ptDNA by ddPCR before surgery
Matched pre-surgery plasma samples were collected for all patients (n=29). The amount of total plasma DNA varied among samples ranging from 3.07 to 22.60 nanograms, which is consistent with prior reports (26–29). These pre-surgery plasma samples were analyzed for PIK3CA E545K and H1047R mutations using ddPCR for detection. ddPCR detected four patients with PIK3CA exon 9 mutations in their pre-surgery ptDNA with a fractional mutation abundance ranging from 0.01% to 0.07%. Ten pre-surgery plasma samples were also identified as harboring a PIK3CA H1047R mutation with a fractional abundance ranging from 0.01% to 2.99%. Comparison of the ddPCR ptDNA results with corresponding ddPCR FFPE samples demonstrated that of the fifteen PIK3CA mutations detected in tissue samples, identical mutations were detected in fourteen of the matched ptDNA samples (Table 2). Notably, the single patient (patient 13) in whom the PIK3CA mutation was not detected in ptDNA had a low percentage of mutant PIK3CA in the tumor sample (not detected by Sanger sequencing) and a small amount of total plasma DNA (~5 nanograms). Importantly, none of the patients with PIK3CA wild type tumors had detectable PIK3CA mutations in pre-surgery plasma DNA. These data demonstrate a sensitivity of 93.3% (95% confidence interval 75.5% – 93.3%); specificity of 100% (95% confidence interval 82.2% – 100.0%) and accuracy of 96.7% (95% confidence interval 78.9% – 96.7%) of ddPCR for detecting mutant PIK3CA in pre-surgery ptDNA.
In total, from the fifteen PIK3CA mutations identified in primary tumors by ddPCR, fourteen PIK3CA mutations were detected in thirteen of the pre-surgery plasma samples, as one patient (patient 4) had concurrent E545K and H1047R mutations identified in both tumor and ptDNA. Of these PIK3CA positive ptDNA samples, eight patients, including patient 4, had stage IA disease with mutant PIK3CA ptDNA fractional abundance ranging from 0.01% to 0.12%. Three patients with stage IIA disease had plasma PIK3CA mutation levels with a fractional abundance of 0.02% and 0.01%, while two stage IIB patients' plasma samples demonstrated mutant PIK3CA at 0.01% and 2.99% fractional abundance. Fractional abundance for all ddPCR results are shown in Tables 2 and 3 with 95% confidence intervals in Table S4. Representative ddPCR pre-surgery plasma DNA analyses are shown in Figure S1.
Table 3.
ddPCR analysis of post-surgery plasma for patients with pre-surgery PIK3CA mutation positive ptDNA.
| Patient | ddPCR pre-surgery plasma (day 0) | Surgery Type, Day) | ddPCR post-surgery plasma (day) | Chemotherapy (Day) | Radiation Therapy (Day) | Endocrine therapy (Day) | Clinical information as of 5/2013 |
|---|---|---|---|---|---|---|---|
| 1 | H1047R (0.03%) | Mastectomy Day 24 | H1047R (0.06%) Day 72 | None | None | Day >72 | No recurrence |
| 4 | E545K (0.01%) H1047R (0.02%) |
Lumpectomy Day 9 | Wild Type H1047R (0.03%) Day 22 |
None | None | Day 45 | No recurrence |
| 5 | H1047R (0.02%) | Lumpectomy Day 23 | Wild Type Day 36 | Day >36 | Day >36 | None | No recurrence |
| 8 | H1047R (0.12%) | Mastectomy Day 31 | Wild Type Day 43 | None | None | Day >43 | No recurrence |
| 9 | E545K (0.02%) | Mastectomy Day 38 | Not collected | None | None | Day 77 | No recurrence |
| 11 | H1047R (0.01%) | Mastectomy Day 37 | Not collected | Day 101 | None | Day 186 | No recurrence |
| 16 | H1047R (2.99%) | Mastectomy Day 40 | H1047R (0.02%) Day 85 | Day 65 | Day 261 | None | Metastatic disease developed in brain, liver and lung at 26 months from initial diagnosis |
| 17 | E545K (0.07%) | Lumpectomy Day 28 | Wild Type Day 43 | Day > 78 | Day > 78 | Day > 78 | No recurrence |
| 19 | E545K (0.01%) | Lumpectomy Day 25 | Not collected | None | Day 92 | Day 128 | No recurrence |
| 22 | H1047R (0.02%) | Lumpectomy Day 13 | Wild Type Day 85 | None | Day 50 | Day 85 | No recurrence |
| 25 | H1047R (0.01%) | Lumpectomy Day 4 | H1047R (0.02%) Day 12 | None | Day 71 | Day 118 | No recurrence |
| 28 | H1047R (0.02%) | Lumpectomy Day 29 | H1047R (0.01%) Day 42 | Day >48 | Day >48 | Day >48 | No recurrence |
| 29 | H1047R (0.01%) | Lumpectomy Day 25 | Wild Type Day 45 | Day >45 | Day >45 | Day >45 | No recurrence |
ddPCR was performed on corresponding plasma DNA prepared from blood collected at various times post-surgery. Percentage reflects fractional abundance of mutant PIK3CA (E545K or H1047R) to total PIK3CA DNA. For patients who received adjuvant radiation and/or systemic therapies with their local oncologists, the exact day of initiating those therapies is unknown though clinical follow up at our institution documents the minimal time (e.g. Day > XX).
PIK3CA mutations detected in ptDNA by ddPCR after surgery
We then sought to determine whether any patients continued to have detectable mutant PIK3CA after breast surgery. Post-surgical plasma specimens were collected from seventeen of the twenty-nine patients including nine patients with a positive mutant PIK3CA pre-surgical ptDNA sample, and a tenth patient (patient 4) whose tumor and pre-surgical ptDNA harbored both E545K and H1047R PIK3CA mutations for a total of ten potentially informative samples with eleven mutations identified in pre-surgery ptDNA. We collected post-surgery blood samples within 14 days after surgery in five of these patients as initially planned, while logistical issues allowed us to collect the remaining five patients' post-surgery blood at times ranging from 15 to 72 days after surgery. Two patients, patients 16 and 22, had their blood collected while receiving adjuvant chemotherapy and radiation therapy, respectively (Table 3).
Of the ten patients with PIK3CA mutations detected in ptDNA prior to surgery, five patients had detectable ptDNA post-surgery. For patient 1, there was a lengthy period between surgery and the post-surgery blood specimen (48 days), though adjuvant endocrine therapy was initiated after this sample was taken. Interestingly, patient 4 had both PIK3CA E545K and H1047R mutations present in tumor and pre-surgery plasma, but only the H1047R mutation was detected in the post-surgery sample taken 13 days after surgery and prior to adjuvant endocrine therapy. Thus far, patient 16 is the only patient with a PIK3CA mutation who has had a cancer recurrence (26 months after initial diagnosis). She presented with a triple negative (ER/PR/HER2 negative) metaplastic breast cancer, received standard adjuvant chemotherapy and radiation, and had the highest fractional abundance of pre-surgery mutant PIK3CA ptDNA (2.99%). Her post-surgery blood obtained after cycle 2 of adjuvant chemotherapy had persistent albeit lower fractional abundance of mutant PIK3CA ptDNA. Patients 25 and 28 had a relatively smaller fractional abundance of mutant PIK3CA ptDNA that was similar before and after surgery (lumpectomy for both) with a relatively short period of time between surgery and post-surgery blood draw at days 8 and 13, respectively. Both patients' post-surgery blood samples were collected prior to adjuvant systemic and radiation therapies.
Discussion
We employed the new technique of ddPCR in order to identify PIK3CA mutations in formalin fixed paraffin embedded (FFPE) primary tumor samples and corresponding plasma from early stage breast cancer patients. We detected PIK3CA mutations in pre-surgery blood samples with 93.3% sensitivity (14 of 15 mutations) and 100% specificity. In addition, post-surgery blood samples were available for ten mutation positive patients and ptDNA was detected in five of these patients. Importantly, these patients had no clinical evidence of disease after surgery. This high level of sensitivity and specificity for cancer mutation detection in peripheral blood offers a number of potential clinical applications for oncology practice. First, ddPCR could augment or replace mutation detection in primary cancer tissues. The fact that ddPCR can detect additional mutations in primary tumor tissues not found by traditional Sanger sequencing could increase the number of patients who are candidates for targeted therapies. Interestingly, patient 4's sample was detected by ddPCR with a high fractional abundance (E545K, 28.9%) but was not identified by sequencing, though in theory 28.9% is within the reported sensitivity of Sanger sequencing. Repeat sequencing of this sample was negative for mutation. Although we do not have a definitive explanation for this, in our hands the use of FFPE DNA for PCR and Sanger sequencing often leads to “allelic drop out” due to degradation of the sample, which could explain these results. In contrast, ddPCR requires much smaller amounts of input DNA and a smaller amplicon size, and therefore is less prone to this phenomenon. Second, the improved sensitivity of ddPCR allows for detection of ptDNA with even low levels of tumor burden. Eight of our patients in whom a PIK3CA mutation was detected in pre-surgery ptDNA had T1 (≤ 2.0cm) tumors and node negative disease, with one of these patients harboring a T1b (< 1.0cm) tumor. This presents the opportunity of using blood as a liquid biopsy for mutation detection in early stage breast cancer patients without the need for accessing tumor tissues. Third, the short half-life of ptDNA and wide dynamic range may allow for a rapid assessment of therapeutic response for both early and late stage disease. Finally, the use of ptDNA detection for minimal residual disease may help guide individualized decisions regarding adjuvant systemic therapies, and be used for surveillance of patients with a high risk for cancer recurrence.
It should be reemphasized that this is a feasibility study with a relatively small sample size. Thus, the clinical relevance of detectable versus undetectable ptDNA post-surgery is currently unclear. However, it is tempting to speculate that ptDNA may be a useful marker of residual micrometastatic disease and response to adjuvant therapies. The fact that half of our patients with detectable ptDNA before surgery also had ptDNA present after surgery demonstrates that it is feasible to design future trials with longer term follow up to test this hypothesis. Notably, patient 16 with triple negative metaplastic disease had a short disease-free interval despite adjuvant chemotherapy, consistent with the known natural history of this phenotype (30). In contrast, ER/PR positive breast cancers can recur decades after the diagnosis of early stage disease, and the use of adjuvant endocrine therapy may play a role in inducing tumor dormancy and increased risk of late recurrences beyond five years. While this would require many years of follow up to address conclusively, such studies could potentially identify patients who might benefit from prolonged or lifelong endocrine therapy, an emerging issue given recent data supporting extended use of these agents in some patients (31, 32).
One of our patients had two detectable mutations in her primary tumor as detected by ddPCR. Since there is no biologically known reason why a cancer would have two oncogenic mutations in the same gene within a given cell, we would presume that this breast cancer was multi-clonal arising from independent progenitors. Given the high risk nature of some patients for developing breast cancer, this explanation is plausible and in fact has also been reported by us and others in prior studies (5, 33).
Our study provides a proof of concept and attests to the feasibility of ptDNA analysis for early stage breast cancer patients; however there are limitations of our study including the small sample size and low number of recurrences partly due to the natural history of ER/PR positive breast cancers. In addition, we assayed only for hotspot mutations in the PIK3CA gene and thus were unable to follow approximately half of the patients enrolled in this study. We are currently expanding this study to include multiple blood draws before, during and after adjuvant therapies and performing broader mutational analyses to obtain additional “markers” to measure patients' ptDNA.
While the sensitivity and specificity of ddPCR for detecting ptDNA is a technologic achievement, applying these assays for routine use in clinical oncology requires further prospective studies. However, the ability to detect ptDNA before and after surgery lends credence to the idea that assessment of ptDNA may allow for more informed individualized decisions regarding adjuvant systemic therapies. This could potentially change the current paradigm of overtreatment for early stage breast cancer patients.
Supplementary Material
Statement of translational relevance.
In this study the feasibility and accuracy of plasma tumor DNA (ptDNA) detection was prospectively assessed by screening for the presence of tumor PIK3CA mutations in patients' plasma prior to and after breast surgery using ddPCR, a second generation digital PCR platform. We observed in early stage breast cancer patients that ddPCR could detect PIK3CA mutations preoperatively with 93.3% sensitivity (14 of 15 mutations detected) and 100% specificity (no false positives in 30 tumors). Moreover, 5 of 10 patients with PIK3CA mutation positive tumors continued to have mutant PIK3CA detected in their postoperative blood sample despite having no clinical evidence of disease. This work is the first to demonstrate in a prospective fashion that droplet digital PCR (ddPCR) can be used to accurately detect cancer DNA mutations in the blood of early stage breast cancer patients including those with minimal, clinically undetectable disease. Our results suggest that ddPCR provides an accurate and relatively non-invasive method for measuring residual disease and could be developed as a biomarker to identify early stage breast cancer patients at higher risk for recurrence to help make informed decisions regarding adjuvant therapies.
Acknowledgments
We thank Francis Long for technical assistance.
Financial Support:
This work was supported in part by The Stetler Fund, DOD Breast Cancer Research Program, the Flight Attendant Medical Research Institute (FAMRI); the V Foundation, the Maryland Cigarette Restitution Fund (J.L.); The Avon Foundation; The Sandra Garcia Foundation; The Santa Fe Foundation; NIH CA088843, CA109274, GM007309 CA009071, CA167939, CA168180; the Susan G. Komen for the Cure and the Breast Cancer Research Foundation. None of the funding sources influenced the design, interpretation or submission of this manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest B.H.P. is a paid consultant for GlaxoSmithKline and Novartis. B.H.P. is a paid member of the scientific advisory boards of Horizon Discovery, LTD and Loxo Oncology. Under separate licensing agreements between Horizon Discovery, LTD and The Johns Hopkins University, B.H.P. is entitled to a share of royalties received by the University on sales of products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. M.L.S. is an employee of RainDance Technologies. D.M. is an employee of Bio-Rad. All other authors declare no potential conflicts.
Author contributions Conception and design: JAB, DJ, VS, ACW and BHP
Development of methodology: JAB, DJ, SB, RC, SC, DJZ, HYW, PJH, PVT, MLS, DM, BHP
Acquisition of data: JAB, DJ, SB, RC, SC, DJZ, HYW, PVT, JC, BGB, DC, TB, MJH, VS, LJ, MH, JLange, JLauring, DV, JK, SJ, MLS, DM, LC, ACM, PA, BHP
Analysis and interpretation of data: All authors
Writing, review, and/or revision of the manuscript: All authors
We apologize to our colleagues whose important work could not be cited due to space constraints.
Portions of this study were presented at the American Society of Clinical Oncology Annual Meetings, Chicago, IL, June 2013
References
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 3.Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3:551–9. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 4.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins MJ, Jelovac D, Barnathan E, Blair B, Slater S, Powers P, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18:3462–9. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leary RJ, Sausen M, Kinde I, Papadopoulos N, Carpten JD, Craig D, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4:162ra54. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013 doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 9.Chen WW, Balaj L, Liau LM, Samuels ML, Kotsopoulos SK, Maguire CA, et al. BEAMing and Droplet Digital PCR Analysis of Mutant IDH1 mRNA in Glioma Patient Serum and Cerebrospinal Fluid Extracellular Vesicles. Molecular therapy Nucleic acids. 2013;2:e109. doi: 10.1038/mtna.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10:1003–5. doi: 10.1038/nmeth.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroun M, Maurice P, Vasioukhin V, Lyautey J, Lederrey C, Lefort F, et al. The origin and mechanism of circulating DNA. Ann N Y Acad Sci. 2000;906:161–8. doi: 10.1111/j.1749-6632.2000.tb06608.x. [DOI] [PubMed] [Google Scholar]
- 12.Choi JJ, Reich CF, 3rd, Pisetsky DS. The role of macrophages in the in vitro generation of extracellular DNA from apoptotic and necrotic cells. Immunology. 2005;115:55–62. doi: 10.1111/j.1365-2567.2005.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippman M, Osborne CK. Circulating tumor DNA--ready for prime time? N Engl J Med. 2013;368:1249–50. doi: 10.1056/NEJMe1301249. [DOI] [PubMed] [Google Scholar]
- 14.Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102:16368–73. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker M. Digital PCR hits its stride. Nat Methods. 2012;9:541–4. [Google Scholar]
- 16.Board RE, Wardley AM, Dixon JM, Armstrong AC, Howell S, Renshaw L, et al. Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat. 2010;120:461–7. doi: 10.1007/s10549-010-0747-9. [DOI] [PubMed] [Google Scholar]
- 17.Castells A, Puig P, Mora J, Boadas J, Boix L, Urgell E, et al. K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol. 1999;17:578–84. doi: 10.1200/JCO.1999.17.2.578. [DOI] [PubMed] [Google Scholar]
- 18.Kopreski MS, Benko FA, Borys DJ, Khan A, McGarrity TJ, Gocke CD. Somatic mutation screening: identification of individuals harboring K-ras mutations with the use of plasma DNA. J Natl Cancer Inst. 2000;92:918–23. doi: 10.1093/jnci/92.11.918. [DOI] [PubMed] [Google Scholar]
- 19.Dianxu F, Shengdao Z, Tianquan H, Yu J, Ruoqing L, Zurong Y, et al. A prospective study of detection of pancreatic carcinoma by combined plasma K-ras mutations and serum CA19-9 analysis. Pancreas. 2002;25:336–41. doi: 10.1097/00006676-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Cidado J, Park BH. Targeting the PI3K/Akt/mTOR pathway for breast cancer therapy. J Mammary Gland Biol Neoplasia. 2013;17:205–16. doi: 10.1007/s10911-012-9264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–9. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Distribution of Somatic Mutations in PIK3CA. Catalogue of Somatic Mutations in Cancer (COSMIC) 2011 cited; Available from: http://www.sanger.ac.uk/genetics/CGP/cosmic/
- 23.Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 24.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 26.Allen D, Butt A, Cahill D, Wheeler M, Popert R, Swaminathan R. Role of cell-free plasma DNA as a diagnostic marker for prostate cancer. Ann N Y Acad Sci. 2004;1022:76–80. doi: 10.1196/annals.1318.013. [DOI] [PubMed] [Google Scholar]
- 27.Chun FK, Muller I, Lange I, Friedrich MG, Erbersdobler A, Karakiewicz PI, et al. Circulating tumour-associated plasma DNA represents an independent and informative predictor of prostate cancer. BJU Int. 2006;98:544–8. doi: 10.1111/j.1464-410X.2006.06352.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzenbach H, Stoehlmacher J, Pantel K, Goekkurt E. Detection and monitoring of cell-free DNA in blood of patients with colorectal cancer. Ann N Y Acad Sci. 2008;1137:190–6. doi: 10.1196/annals.1448.025. [DOI] [PubMed] [Google Scholar]
- 29.Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer--a survey. Biochim Biophys Acta. 2007;1775:181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, et al. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65:441–6. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 31.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013 doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goss PE, Muss HB, Ingle JN, Whelan TJ, Wu M. Extended adjuvant endocrine therapy in breast cancer: current status and future directions. Clin Breast Cancer. 2008;8:411–7. doi: 10.3816/CBC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 33.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.