Abstract
Background
While risk factors for konzo are known, determinants of cognitive impairment in konzo-affected children remain unknown.
Method
We anchored cognitive performance (KABC-II scores) to serum levels of free-thyroxine (free-T4), thyroid-stimulating hormone (TSH), albumin, and motor proficiency (BOT-2 scores) in 40 children including 21 with konzo (median age: 9 years) and 19 without konzo (median age: 8 years). A multiple regression model was used to determine variables associated with changes in KABC-II scores.
Results
Age (β: − 0.818, 95%CI: − 1.48, − 0.152) (p=0.018), gender (β: − 5.72; 95% CI: − 9.87, −1.57 for females) (p=0.009), BOT-2 score (β: 0.390; 95% CI: 0.113, 0.667) (p=0.008), and free-T4 (β: 1.88; 95% CI: 0.009, 3.74) (p=0.049) explained 61.1% of variation in KABC-II scores. Subclinical hypothyroidism was not associated with poor cognition. A crude association was found between serum albumin and KABC-II scores (β: 1.26; 95% CI: 0.136, 2.39) (p=0.029). On spot urinary thiocyanate reached 688 μmol/l in children without konzo and 1032 μmol/L in those with konzo.
Conclusion
Female gender and low serum albumin are risk factors common to cognitive and proportionally associated motor deficits in children exposed to cassava cyanogens. The two types of deficits may share common mechanisms.
Keywords: serum albumin, cassava, cyanide, cognition, paralysis, thyroid
INTRODUCTION
Chronic dietary reliance on cyanogenic cassava (a.k.a manioc or tapioca) has been associated with malnutrition and neurodegeneration diseases in several countries of sub-Saharan Africa (Banea et al., 1992, Tylleskar et al., 1994, Cliff et al., 2011, Mlingi et al., 2011). Neurodegenerative syndromes include konzo, a distinct and non-progressive upper motor neuron disease characterized by visible signs of spasticity in legs (Figure 1) (Howlett et al., 1990, Tshala-Katumbay et al., 2001b, Tshala-Katumbay et al., 2002b, Chabwine et al., 2011), tropical ataxic neuropathy (TAN) and, reportedly, a motor neuron-cerebellar-parkinson-dementia syndrome (Osuntokun et al., 1968, Osuntokun, 1981, Oluwole et al., 2000).
Figure 1.

Spastic stance in a child affected by konzo. The child needs support to be able to move around. Hundreds of children with such disability are seen in konzo villages with prevalence as high as 5 % in the general population. Photograph by Tshala-Katumbay, Desire, with permission from subject and parents.
In a recent epidemiological survey in the Democratic Republic of Congo (DRC), we confirmed that outbreaks of konzo were associated with poor socioeconomic status, malnourishment, and reliance on insufficiently processed cyanogenic cassava as main source of food (Bonmarin et al., 2002, Boivin et al., 2013). For the first time, we showed that children with konzo had poor cognition relative to those without konzo and recruited from the same study population. We also showed that both children with konzo and those without konzo performed poorly when compared to a control group from a non-konzo area of the same province (Boivin et al., 2013). Current state of knowledge suggests that reliance on improperly processed cyanogenic cassava may be associated with a wide spectrum of abnormalities ranging from subclinical neurophysiological deficits to overt forms of deficits such as konzo and, possibly, impaired cognition (Katumbay et al., 2000, Tshala-Katumbay et al., 2001b, Ernesto et al., 2002, Tshala-Katumbay et al., 2002a, Tshala-Katumbay et al., 2002b, Mwanza et al., 2003a). Thus, cassava-associated neurological diseases may be seen either as discrete individual entities (discrete model), or overlapping entities i.e. a group of diseases with overlapping features (overlapping model), or members of a neurodegenerative continuum (continuum model); a classification scheme proposed for other types of neurodegenerative diseases (Armstrong, 2012). We favor the continuum model for several reasons. First, the above-mentioned disorders e.g. konzo and TAN consistently share common etiological factors including poor nutrition and cassava cyanogenic toxicity (Banea-Mayambu et al., 1997, Oluwole et al., 2000, Madhusudanan et al., 2008). Second, key biomarkers e.g. those of exposure to cyanogenic compounds such as the urinary levels of thiocyanate (SCN) do display poor fidelity to the individual diseases listed above (Lancet, 1984, Banea-Mayambu et al., 1997, Cliff et al., 1999). Third, there appear to be a wide spectrum of neuropathological features in the same individual diseases (Oluwole et al., 2000, Tshala-Katumbay et al., 2001b, Tshala-Katumbay et al., 2002a, Tshala-Katumbay et al., 2002b, Mwanza et al., 2003b, Madhusudanan et al., 2008). As such, the “continuum” model appears suitable for studies aimed at elucidating the biomarkers of cassava-associated neurodegeneration.
Risk factors of konzo have been reported (WHO, 1996). Determinants of cognitive impairment seen among children from konzo-affected areas need to be elucidated. Reports on the possible existence of pervasive cognitive deficits among children from konzo-affected areas (Katumbay et al., 2000, Boivin et al., 2013) justify the need for a thorough investigation to determine whether they share common biomarkers and/or mechanisms with the classical paralysis known as konzo. Possible common mechanisms may include the direct effect of cyanide or cyanate, neurotoxic metabolites of linamarin, the main cassava cyanogenic compound (Spencer, 1999, Kimani et al., 2013); the metabolic interference of thiocyanate (SCN), the downstream detoxification product of cyanide, with the uptake of iodine at the thyroid gland level (Erdogan et al., 2001) or glutamergic transmission (Spencer, 1999); a nutritional deficiency in select nutrients (Thilly et al., 1990, Thilly et al., 1993, Adamolekun, 2010); or a combination of the aforementioned factors (Elnour et al., 2000, Erdogan et al., 2001, Bonmarin et al., 2002, Di Filippo et al., 2008, Nyaradi et al., 2013). In this study, we sought to determine whether cognitive deficits observed among children from the most affected area of the DRC (Bonmarin et al., 2002, Boivin et al., 2013) were explained by changes in thyroid function while assessing the role of traditionally known risk factors for konzo e.g. low levels of serum albumin, often used as surrogate marker for protein malnutrition in konzo areas (Tshala-Katumbay and Spencer, 2007).
SUBJECTS AND METHOD
Subjects
Forty subjects were selected from cases of konzo and respective controls recruited during a recent DRC epidemiological survey which revealed cognition deficits among children relying on cyanogenic cassava as staple food (Boivin et al., 2013). All the 17 subjects with the severe form of the disease and the two pairs of twins with the mild form of konzo (median age: 9 years, interquartile range (IQR): 11 – 7 years) and their respective 19 presumably healthy controls (median age: 8 years, IQR: 12 – 7 years) (one control subject per pair of twins) were selected for the present study. Children with konzo had to fulfill the following WHO inclusion criteria: a visible symmetric spastic abnormality of gait while walking or running; a history of onset of less than 1 week followed by a non-progressive course in a formerly healthy person; and bilaterally exaggerated knee or ankle jerks without signs of disease of the spine. According to these criteria, the severity of the disease is graded as follows; mild case = able to walk; moderate case = need support (one or tow sticks) to walk, severe case = unable to walk (WHO, 1996). Children with history of illness that may affect the CNS (e.g., cerebral malaria) were excluded from the studies. Retroviral HIV I/II and HTLV-I/II infections were ruled out using antibody detection kits (Vironostika HIV Ag/Ab kit batch A61LY and Serodia kit batch SG 10804, respectively). Ethical approval was obtained from Oregon Health & Science University (OHSU) and the DRC Ministry of Health.
Kaufman Assessment Battery for Children, 2nd edition (KABC-II) measure of cognitive performance
Cognition was assessed using the KABC-II testing battery. The battery has been validated in Uganda and used in the DRC on several occasions including during the main epidemiological study (Boivin et al., 1993, Boivin et al., 1995, Bangirana et al., 2009, Boivin et al., 2013). Study subjects had a comprehensive evaluation of their core cognitive performance domains as previously described (Boivin et al., 2013). The KABC-II global mental processing index, herein referred to as KABC-II score, was used as outcome measure for this study.
Bruininks/Oseretsky Test, 2nd Edition (BOT-2) measure of motor proficiency
Motor proficiency was assessed using the BOT-2. This is regarded as one of the most comprehensive and sensitive instruments for motor assessment. Testing involves game-like tasks that hold the subject’s interest and are not verbally complex. Composite scores include fine manual control, manual coordination, body coordination, strength and agility and total composite score. The BOT-2 has proven adaptable and useful in characterizing the specific aspects of motor impairment associated with HIV in Ugandan children, a disease which also has pediatric neuromotor effects (Bagenda et al., 2006). The DRC main survey revealed BOT-2 deficits in all domains of motor function when assessed in relation to disease (konzo) status (Boivin et al., 2013). In this analysis, we used the BOT-2 total composite score as a measure of motor proficiency and independent predictor of cognitive performance.
Laboratory Analyses
Collection of samples was carried out in the rural and remote konzo area of Kahemba (DRC) by a team of trained laboratory technicians. Blood was collected through venipuncture in Vacutainer tubes with no anticoagulants and kept at room temperature for approximately 2 hours. Immediately thereafter, serum samples were collected, centrifuged at 15,000 rpm for 15 min, aliquoted in cryotubes, and flash-frozen in liquid nitrogen. Samples were then shipped to Kinshasa, the capital city of DRC, and stored at − 80°C until shipment to OHSU on dry ice for biochemical analyses. One-time on spot urine collections were carried out at the time of the general examination. Urine samples were also immediately flash-frozen in liquid nitrogen, shipped to Kinshasa, and stored at − 80°C until use for biochemical analyses. Cyanogenic exposure was ascertained by levels of urinary SCN, which were measured using a previously described semi-quantitative method (Haque and Bradbury, 1999). Thyroid function was assessed by measuring levels of serum thyroid stimulating hormone (TSH) using a chemiluminescence immunoassay quantitative 3rd generation sandwich assay and free-T4 (thyroxin) using a paramagnetic particle chemiluminescent immunoassay routinely performed in the OHSU clinical laboratories. Laboratory reference values ranged from 0.6–1.2 ng/dL for free-T4 and 0.34–5.60 μIU/mL for TSH. Four cases had no sufficient samples to carry out the dosage of free-T4. Normal values for free-T4 associated with elevated TSH were considered in favor of subclinical hypothyroidism. Serum albumin was determined using a piccolo automated system (Abaxis, Germany).
Statistical analyses
Linear regression was used to explore the association between KABC-II scores (the outcome variable) and demographic characteristics (age, sex), clinical (BOT-2 scores), and biochemical variables (serum albumin, free-T4, TSH). BOT-2 scores were significantly different between konzo and non-konzo children (Boivin et al., 2013); consequently it was retained as the primary gauge of motor proficiency (rather than konzo status) since it provided finer details of motor proficiency. Regression models initially examined each explanatory variable separately for association with mean KABC-II score, with estimated regression parameters providing crude (unadjusted) estimates of the association. Subsequent multivariable models were constructed using age and sex as balancing variables (for all models) with additional terms added based on initial univariate findings. Interactions were examined to see whether sex modified any of the variables retained in the multivariable model. Standard checks for model adequacy (Shaprio-Wilk test and Q-Q plot of residuals, plots of residuals against fitted values, screening for outliers/high leverage) were performed on this baseline model. This model was then supplemented with free-T4 and TSH (separately) to see whether either measure could further improve performance. The Bayesian Information Criterion (BIC) was used to compare these two non-nested models to determine which additional predictor (free-T4 or TSH) was a more potent supplement to the baseline model. Model diagnostics were again performed and the baseline model was run a second time on the final data set, which due to missing values for free-T4/TSH, had a slightly reduced sample size. This last step was done to ensure significance relative to the baseline model was not driven by cases that had to be excluded. Stata (version 11.2) was used for all analyses with significance level set at 0.05.
RESULTS
Impairments and Biochemical Profiles
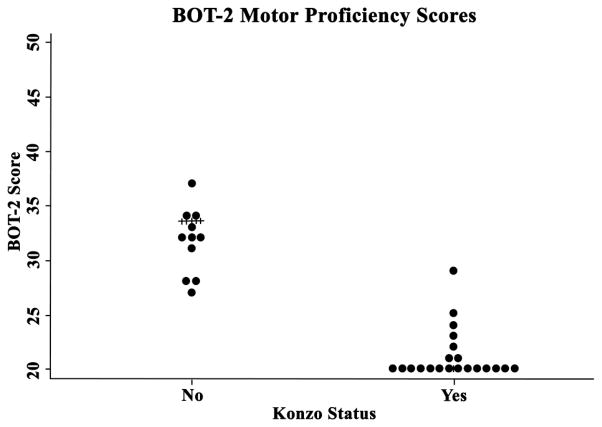
Motor proficiency and cognitive performance scores are summarized in Table 1. A significant difference was seen in BOT-2 and KABC-II scores, and levels of serum albumin, when comparing konzo vs. non-konzo groups using a crude (unadjusted) analysis (Table 2 and Figure 2) (p<0.05, Wilcoxon rank-sum test). The median (IQR) duration of paralysis in the konzo group was 17 (16 – 39) months.
Table 1.
Age, neurological proficiency scores, and biochemical characteristics by gender
| Characteristics | Male | Female | p-value** |
|---|---|---|---|
| Age, Years (21/19)* | |||
| Mean (SD) | 9.0 (3.3) | 9.5 (2.7) | |
| Median (IQR) | 9.0 (6.0 – 11.0) | 8.0 (8.0 – 12.0) | 0.50 |
| BOT-2 motor score (18/19)* | |||
| Mean (SD) | 28.3 (10.0) | 26.7 (7.60) | |
| Median (IQR) | 23.5 (20.0 – 37.0) | 27.0 (20.0 – 32.0) | 0.60 |
| KABC-II cognition score (18/19)* | |||
| Mean (SD) | 59.4 (9.00) | 54.8 (9.06) | |
| Median (IQR) | 57.5 (54.0 – 65.0) | 54.0 (50.0 – 57.0) | 0.07 |
| Albumin, μmol/L (20/15)* | |||
| Mean (SD) | 532.0 (134.4) | 523.9 (94.9) | |
| Median (IQR) | 535.5 (481.8 – 638.4) | 529.9 (449.5 – 619.4) | 0.52 |
| Free-T4, ng/dL (19/17)* | |||
| Mean (SD) | 1.08 (0.20) | 1.13 (0.27) | |
| Median (IQR) | 1.10 (0.90 – 1.30) | 1.00 (0.90 – 1.30) | 0.79 |
| TSH, μIU/mL (20/17)* | |||
| Mean (SD) | 3.75 (2.08) | 3.76 (1.59) | |
| Median (IQR) | 3.38 (2.10 – 5.20) | 3.50 (2.67 – 4.82) | 0.78 |
Indicates male/female N ratio;
Wilcoxon rank-sum test
Table 2.
Age, neurological proficiency scores, and biochemical characteristics by disease status according to the WHO discrete criteria
| Characteristics | Konzo | Non-Konzo | p-value** |
|---|---|---|---|
| Age, Years (21/19)* | |||
| Mean (SD) | 9.2 (3.2) | 9.3 (2.9.0) | |
| Median (IQR) | 9.0 (7.0 – 11.0) | 8 (7.0 – 12.0) | 0.71 |
| BOT-2 motor score (21/16)* | |||
| Mean (SD) | 21.2 (2.30) | 35.8 (7.00) | |
| Median (IQR) | 20.0 (20.0 – 21.0) | 33.5 (31.5 – 41.5) | 0.00 |
| KABC-II cognition score (21/16)* | |||
| Mean (SD) | 53.48 (6.78) | 61.8 (10.0) | |
| Median (IQR) | 52.0 (49.0 – 57.0) | 57.5 (54.5 – 68.0) | 0.01 |
| Albumin, μmol/L (20/15)* | |||
| Mean (SD) | 469.9 (116.1) | 606.8 (61.5) | |
| Median (IQR) | 462.9 (409.2 – 527.2) | 619.4 (540.0 – 675.3) | 0.00 |
| Free-T4, ng/dL (17/19)* | |||
| Mean (SD) | 1.09 (0.22) | 1.12 (0.25) | |
| Median (IQR) | 1.10 (0.90 – 1.20) | 1.10 (0.90 – 1.30) | 0.70 |
| TSH, μIU/mL (18/19)* | |||
| Mean (SD) | 3.88 (2.13) | 3.64 (1.57) | |
| Median (IQR) | 3.33 (2.42 – 5.02) | 3.48 (2.24 – 4.94) | 0.98 |
Indicates konzo/non-konzo N ratio;
Wilcoxon rank-sum test
Figure 2.
BOT-2 scores clearly differentiated between konzo and non-konzo children.
Twenty-three children had hormonal profiles suggestive of a normal thyroid function [median (IQR) free-T4: 1.0 (0.9 – 1.1) ng/dL and TSH: 2.67 (1.87 – 3.93) μIU/mL]. Five subjects, including one 7 year-old boy severely affected by konzo with a visible and palpable goiter, had subclinical hypothyroidism [median (IQR) free-T4: 1.1 (1.1 – 1.3) ng/dL and TSH: 5.85 (5.81 – 6.61) μIU/mL]. Three children severely affected by the disease and five without the disease were categorized as having possible hyperthyroidism [median (IQR) free-T4: 1.40 (1.35 – 1.55) ng/dL and TSH: 3.30 (2.94 – 5.05) μIU/mL]. No between-group differences were found in KABC-II scores across the thyroid functional groups (p>0.05, Wilcoxon rank-sum test). Twins had values that were not remarkably different from the remaining subjects. Levels of free-T4 and TSH showed no significant differences when comparing males to females (Table 1) or konzo vs. non-konzo (Table 2) using a crude analysis (p>0.05, Wilcoxon rank-sum test).
One-time on spot urine collections had a median (IQR) of urinary SCN of 344 (344–688) μmol/L in konzo subjects relative to 344 (172–344) μmol/L in subjects without konzo. Children with konzo had urinary SCN levels as high as 1032 μmol/L whereas subjects without konzo had a maximum of 688 μmol/l of urinary SCN. These measures were from one-time on-spot urine collections, not 24-hour collections, thus not suitable for statistical analyses of biological relevance. Twins had values that were not remarkably different from the remaining study subjects.
Association Models for the Prediction of Cognitive Performance
In simple (unadjusted) models, we found that age, disease status, motor proficiency (BOT-2 score), and albumin were significantly associated with cognitive performance. In a full multivariable model (adjusting for all listed terms), only age, gender, and motor proficiency were significantly associated with cognitive performance; albumin was still retained in the model (p=0.086) as it improved diagnostic measures (with respect to normality and reducing impact of outliers). Inclusion of T4 (p=0.049) made a marginal improvement to the model, while inclusion of TSH (p=0.198) instead produced no measureable improvement and BIC for each model showed a preference for the T4 rather than TSH (180.8 for T4 vs. 183.7 for TSH; lower is better). Among individuals of similar age, sex, albumin level and T4 level, each additional 1-unit improvement in motor proficiency was associated with a 0.39 (95% CI: 0.11–0.67) point increase in average KABC-II score (p=0.008). Likewise, after controlling for age, sex, albumin and motor proficiency, each 0.2 ng/dL increase in free-T4 level was associated with a 1.88 (95% CI: 0.01–3.74; p=0.049) point gain in average KABC-II score (Table 3).
Table 3.
Models showing association between various characteristics of interest and mean KABC-II score, with and without adjustment for key demographic variables
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| Variable | Crude estimates (95% CI) | Adj.* estimates (95% CI) | Adj.* estimates (95% CI) | Adj.* estimates (95% CI) |
| Age, Years, per 1 year increase | −1.55 (−2.44, −0.657) | −1.04 (−1.67, −0.407) | −1.01 (−1.69, −0.334) | −0.819 (−1.48, −0.152) |
| Gender | ||||
| Male | Reference | Reference | Reference | Reference |
| Female | −4.55 (−10.6, 1.47) | −3.94 (−7.71, −0.158) | −4.35 (−8.54, −0.164) | −5.72 (−9.87, −1.57) |
| Konzo status ($) | ||||
| Non-konzo | Reference | |||
| Konzo | −8.27 (−13.9, −2.66) | |||
| BOT-2 score (#), per 1 unit increase | 0.653 (0.372, 0.935) | 0.371 (0.109, 0.633) | 0.353 (0.060, 0.065) | 0.390 (0.113, 0.667) |
| Albumin (μmol/L)[^], per 50 μmol/L increase | 1.26 (0.136, 2.39) | 0.833 (−0.125, 1.79) | 0.664 (−0.532, 1.86) | 0.210 (−1.00, 1.42) |
| T4 (ng/dL)[&] | 0.967 (−1.94, 3.88) | 1.88 (0.01, 3.74) | ||
| TSH (μIU/L), per 1 μIU/L increase | 0.658 (−1.04, 2.36) | |||
| R-square (%) | 61% | 55.4% | 61.1% | |
| BIC (lower is better) | $$ | 182.5 (5df) | 180.8 (6df) |
All multivariable models include age and sex as control variables. Model 1 is based on n=32 records having complete data on those variables included. Model 3 is based on n=28 records; Model 2 is same as Model 1, but with sample size restricted to same set of n=28 on which Model 3 was constructed.
Adjusted for individual predictors;
Konzo status based on WHO criteria,
Higher score indicates greater motor proficiency,
Based on n=32 subjects,
Based on n=33 subjects,
BIC not computed for Model 1 since sample sizes differ, not directly comparable to Models 2/3.
BOT-2 score was used as measure of motor proficiency in the multivariable analyses.
DISCUSSION
This study highlights cognitive deficits proportional to motor deficits in children who rely on improperly processed cyanogenic cassava as the main source of food. Girls appear to have higher risk for deficits when compared to boys. A crude analysis of study results indicates that age and low level of serum albumin were additional risk factors for poor cognition among the studied children. Low level of serum albumin may conceivably be considered as risk factor for cognitive deficits as a substantial body of knowledge suggests that chronic undernutrition (evidenced by low serum albumin) is a major risk for cyanide poisoning and, hence, neurodevelopmental deficits in konzo-affected areas (Vanderpas et al., 1984, Thilly et al., 1993, Katumbay et al., 2000, Tshala-Katumbay et al., 2001b, Odabas et al., 2005, Boivin et al., 2013, Nyaradi et al., 2013). Cognitive deficits increasing with age may just reflect long-term effects of malnutrition and/or cyanogenic exposure. Despite the small sample size, our association models revealed risk factors i.e. female gender and low serum albumin that are similar to those known for konzo, the paralytic disease associated with cassava cyanogenic poisoning (Tshala-Katumbay and Spencer, 2007).
Whether the motor and cognitive deficits in children relying on cyanogenic cassava as staple food share common mechanisms has to be further explored. Arguments in favor include motor deficits that are significantly and proportionally associated with changes in cognitive performances, common risk factors including female gender and low serum albumin, and lack of hypothyroidism to explain poor cognition (Vanderpas et al., 1984, Katumbay et al., 2000, Tshala-Katumbay et al., 2001b, Khandelwal and Tandon, 2012). Possible mechanisms include nutritional deficiencies and/or direct toxicity effects of cassava cyanogens. For example, low serum proteins may alter cyanide detoxification capabilities resulting in increased production of cyanate, a metabolite capable of inducing both motor and cognition deficits (Ohnishi et al., 1975, Tellez et al., 1979, Tor-Agbidye et al., 1999, Kimani et al., 2013). However, further studies are still needed to rule out a vast array of nutrient deficiencies to explain neurological deficits in konzo-affected areas (Nyaradi et al., 2013). The high susceptibility of females to the neurotoxic effects of cassava cyanogens has remained unclear. Hormonal influences have not been ruled out. A possible explanation is that in females are at disadvantage in a context of food scarcity where males tend to serve themselves bigger portions of meals, particularly those of animal origin needed for the detoxification of cyanide.
Consistent with previous findings on the biomarkers of konzo, it is not surprising that median levels of urinary SCN in children with konzo appear similar to those in children without konzo. Urinary SCN is function of cyanogenic exposure, cyanide detoxification capabilities, and metabolic clearance and excretion. Higher urinary SCN do not necessarily indicate cyanide poisoning. Instead, it may indicate efficient detoxification mechanisms. As such, urinary levels of SCN may not serve as a good maker for the neuropathological features observed at the individual level. Field measures of urinary SCN may, however, provide useful information with respect to the extent of cyanogenic exposure within a community (Lancet, 1984, Banea-Mayambu et al., 1997, Tshala-Katumbay et al., 2001a). In our study, levels of urinary SCN were well above the reportedly safe limits for exposure to cyanogenic compounds confirming that cyanogenic exposure is a key risk factor for konzo (Banea et al., 2013).
Current knowledge and evidence suggests cassava-associated neurological diseases may be viewed as a continuum of abnormalities including subclinical disorders, frank motor disability e.g. konzo, and, possibly, poor cognition (vide supra). A “continuum model” offers the advantage of anchoring biomarkers to a wide range of quantifiable clinical features. In our view, such a detailing approach may help determine the exact markers and/or mechanisms of cassava-associated neurological diseases. This study was the first attempt to explore and determine whether cognitive deficits observed in children recruited from konzo areas were modulated by risk factors traditionally described for konzo. Despite our relatively small sample size, we were able to confirm that children relying on cyanogenic cassava as staple food display cognitive that correlate with deficits in motor function. These deficits share common risk factors including female gender and low serum albumin, a putative marker of chronic malnutrition. Cognitive deficits may not be explained by changes in thyroid function. Instead, it is possible that both motor and cognitive deficits share common mechanisms possibly linked to the direct toxicity of cassava cyanogens and/or their metabolites.
Future perspectives
Further studies are needed to determine the differential expression of markers of cyanogenic exposure in relation yet to be determined unique biomarkers of cassava-related neurological disease. Biomarkers of interests should be classified into (1) those of exposure to cassava cyanogens e.g. serum cyanide-adducts or urinary SCN, a more practical measure for field work, (2) markers of susceptibility to cyanogenic poisoning e.g. poor nutrition and/or genetic make up, (3) and markers of disease process e.g. oxidative and/or carbamoylation damage (Fasco et al., 2011, Tshala-Katumbay et al., 2013). Both motor and cognitive deficits should be anchored to these biomarkers in adequately selected research groups including children with konzo and those without konzo relying on cyanogenic cassava as the main source of food and those from similar background, culture, and dietary habits with the exception of dietary reliance on insufficiently processed food. Such studies should also help establish molecular signatures common to motor and cognition deficits and test for causal relationships through interventional trials. Strategies for intervention may include nutritional rehabilitation through adoption of balanced diet programs and proper processing of cassava prior to human consumption as previously indicated. Additional interventional arms may focus on mitigating the intermediate outcomes of cyanide poisoning e.g. oxidative stress and/or cyanate-associated carbamoylation of proteins.
Acknowledgments
This study was funded by a grant from the Fogarty International Center and the National Institute of Environmental Health and Science: R01ES019841 (National Institutes of Health, Bethesda, MD). We thank the community of Kahemba and Dr. Jean-Jacques Kaniki (Bandundu Province, DRC) and Grace Muange (OHSU) for their participation in the current research project.
Footnotes
CONFLICT OF INTEREST STATEMENT
All authors have no conflict interest to declare
References
- 1.Adamolekun B. Etiology of Konzo, epidemic spastic paraparesis associated with cyanogenic glycosides in cassava: role of thiamine deficiency? J Neurol Sci. 2010;296:30–33. doi: 10.1016/j.jns.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong RA. On the ‘classification’ of neurodegenerative disorders: discrete entities, overlap or continuum? Folia Neuropathol. 2012;50:201–208. doi: 10.5114/fn.2012.30521. [DOI] [PubMed] [Google Scholar]
- 3.Bagenda D, Nassali A, Kalyesubula I, Sherman B, Drotar D, Boivin MJ. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics. 2006;117:729–740. doi: 10.1542/peds.2004-2699. [DOI] [PubMed] [Google Scholar]
- 4.Banea JP, Bradbury JH, Mandombi C, Nahimana D, Denton IC, Kuwa N, Tshala Katumbay D. Control of konzo by detoxification of cassava flour in three villages in the Democratic Republic of Congo. Food Chem Toxicol. 2013;60:506–513. doi: 10.1016/j.fct.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Banea M, Bikangi N, Nahimana G, Nunga M, Tylleskar T, Rosling H. High prevalence of konzo associated with a food shortage crisis in the Bandundu region of zaire. Ann Soc Belg Med Trop. 1992;72:295–309. [PubMed] [Google Scholar]
- 6.Banea-Mayambu JP, Tylleskar T, Gitebo N, Matadi N, Gebre-Medhin M, Rosling H. Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neurone disease konzo in former Zaire. Trop Med Int Health. 1997;2:1143–1151. doi: 10.1046/j.1365-3156.1997.d01-215.x. [DOI] [PubMed] [Google Scholar]
- 7.Bangirana P, Seggane M, Allebeck P, Giordani B, John CC, Opoka OR, Byarugaba J, Ehnvall A, Boivin MJ. A preliminary examination of the construct validity of the KABC-II in Ugandan children with a history of cerebral malaria. Afr Health Sci. 2009;9:186–192. [PMC free article] [PubMed] [Google Scholar]
- 8.Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N, Muamba K. Effects of treatment for intestinal parasites and malaria on the cognitive abilities of schoolchildren in Zaire, Africa. Health Psychol. 1993;12:220–226. doi: 10.1037//0278-6133.12.3.220. [DOI] [PubMed] [Google Scholar]
- 9.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Boivin MJ, Okitundu D, Makila-Mabe Bumoko G, Sombo MT, Mumba D, Tylleskar T, Page CF, Tamfum Muyembe JJ, Tshala-Katumbay D. Neuropsychological effects of konzo: a neuromotor disease associated with poorly processed cassava. Pediatrics. 2013;131:e1231–1239. doi: 10.1542/peds.2012-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonmarin I, Nunga M, Perea WA. Konzo outbreak, in the south-west of the Democratic Republic of Congo, 1996. J Trop Pediatr. 2002;48:234–238. doi: 10.1093/tropej/48.4.234. [DOI] [PubMed] [Google Scholar]
- 12.Chabwine JN, Masheka C, Balol’ebwami Z, Maheshe B, Balegamire S, Rutega B, Wa Lola M, Mutendela K, Bonnet MJ, Shangalume O, Balegamire JM, Nemery B. Appearance of konzo in South-Kivu, a wartorn area in the Democratic Republic of Congo. Food Chem Toxicol. 2011;49:644–649. doi: 10.1016/j.fct.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Cliff J, Muquingue H, Nhassico D, Nzwalo H, Bradbury JH. Konzo and continuing cyanide intoxication from cassava in Mozambique. Food Chem Toxicol. 2011;49:631–635. doi: 10.1016/j.fct.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Cliff J, Nicala D, Saute F, Givragy R, Azambuja G, Taela A, Chavane L, Gani A. Ankle clonus and thiocyanate, linamarin, and inorganic sulphate excretion in school children in communities with Konzo, Mozambique. J Trop Pediatr. 1999;45:139–142. doi: 10.1093/tropej/45.3.139. [DOI] [PubMed] [Google Scholar]
- 15.Di Filippo M, Tambasco N, Muzi G, Balucani C, Saggese E, Parnetti L, Calabresi P, Rossi A. Parkinsonism and cognitive impairment following chronic exposure to potassium cyanide. Mov Disord. 2008;23:468–470. doi: 10.1002/mds.21871. [DOI] [PubMed] [Google Scholar]
- 16.Elnour A, Hambraeus L, Eltom M, Dramaix M, Bourdoux P. Endemic goiter with iodine sufficiency: a possible role for the consumption of pearl millet in the etiology of endemic goiter. Am J Clin Nutr. 2000;71:59–66. doi: 10.1093/ajcn/71.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Erdogan MF, Erdogan G, Sav H, Gullu S, Kamel N. Endemic goiter, thiocyanate overload, and selenium status in school-age children. Biol Trace Elem Res. 2001;79:121–130. doi: 10.1385/BTER:79:2:121. [DOI] [PubMed] [Google Scholar]
- 18.Ernesto M, Cardoso AP, Nicala D, Mirione E, Massaza F, Cliff J, Haque MR, Bradbury JH. Persistent konzo and cyanogen toxicity from cassava in northern Mozambique. Acta Trop. 2002;82:357–362. doi: 10.1016/s0001-706x(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 19.Fasco MJ, Stack RF, Lu S, Hauer CR, 3rd, Schneider E, Dailey M, Aldous KM. Unique cyanide adduct in human serum albumin: potential as a surrogate exposure marker. Chem Res Toxicol. 2011;24:505–514. doi: 10.1021/tx100344e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque MR, Bradbury JH. Simple method for determination of thiocyanate in urine. Clin Chem. 1999;45:1459–1464. [PubMed] [Google Scholar]
- 21.Howlett WP, Brubaker GR, Mlingi N, Rosling H. Konzo, an epidemic upper motor neuron disease studied in Tanzania. Brain. 1990;113 (Pt 1):223–235. doi: 10.1093/brain/113.1.223. [DOI] [PubMed] [Google Scholar]
- 22.Katumbay DT, Lukusa VM, Eeg-Olofsson KE. EEG findings in Konzo: a spastic para/tetraparesis of acute onset. Clin Electroencephalogr. 2000;31:196–200. doi: 10.1177/155005940003100408. [DOI] [PubMed] [Google Scholar]
- 23.Khandelwal D, Tandon N. Overt and Subclinical Hypothyroidism. Who to Treat and How. Drugs. 2012;72:17–33. doi: 10.2165/11598070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Kimani S, Sinei K, Bukachi F, Tshala-Katumbay D, Maitai C. Memory deficits associated with sublethal cyanide poisoning relative to cyanate toxicity in rodents. Metab Brain Dis. 2013 doi: 10.1007/s11011-013-9459-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancet . Epidemic spastic paraparesis. Lancet. 1984;2:904–905. [PubMed] [Google Scholar]
- 26.Madhusudanan M, Menon MK, Ummer K, Radhakrishnanan K. Clinical and etiological profile of tropical ataxic neuropathy in Kerala, South India. Eur Neurol. 2008;60:21–26. doi: 10.1159/000127975. [DOI] [PubMed] [Google Scholar]
- 27.Mlingi NL, Nkya S, Tatala SR, Rashid S, Bradbury JH. Recurrence of konzo in southern Tanzania: rehabilitation and prevention using the wetting method. Food Chem Toxicol. 2011;49:673–677. doi: 10.1016/j.fct.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Mwanza JC, Lysebo DE, Kayembe DL, Tshala-Katumbay D, Nyamabo LK, Tylleskar T, Plant GT. Visual evoked potentials in konzo, a spastic paraparesis of acute onset in Africa. Ophthalmologica. 2003a;217:381–386. doi: 10.1159/000073066. [DOI] [PubMed] [Google Scholar]
- 29.Mwanza JC, Tshala-Katumbay D, Kayembe DL, Eeg-Olofsson KE, Tylleskar T. Neuro-ophthalmologic findings in konzo, an upper motor neuron disorder in Africa. Eur J Ophthalmol. 2003b;13:383–389. doi: 10.1177/112067210301300409. [DOI] [PubMed] [Google Scholar]
- 30.Nyaradi A, Li J, Hickling S, Foster J, Oddy WH. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front Hum Neurosci. 2013;7:97. doi: 10.3389/fnhum.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odabas D, Caksen H, Sar S, Unal O, Tuncer O, Atas B, Yilmaz C. Cranial MRI findings in children with protein energy malnutrition. Int J Neurosci. 2005;115:829–837. doi: 10.1080/00207450590882082. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi A, Peterson CM, Dyck PJ. Axonal degeneration in sodium cyanate-induced neuropathy. Arch Neurol. 1975;32:530–534. doi: 10.1001/archneur.1975.00490500050005. [DOI] [PubMed] [Google Scholar]
- 33.Oluwole OS, Onabolu AO, Link H, Rosling H. Persistence of tropical ataxic neuropathy in a Nigerian community. J Neurol Neurosurg Psychiatry. 2000;69:96–101. doi: 10.1136/jnnp.69.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osuntokun BO. Cassava diet, chronic cyanide intoxication and neuropathy in the Nigerian Africans. World Rev Nutr Diet. 1981;36:141–173. doi: 10.1159/000393156. [DOI] [PubMed] [Google Scholar]
- 35.Osuntokun BO, Durowoju JE, McFarlane H, Wilson J. Plasma amino-acids in the Nigerian nutritional ataxic neuropathy. Br Med J. 1968;3:647–649. doi: 10.1136/bmj.3.5619.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer PS. Food toxins, ampa receptors, and motor neuron diseases. Drug Metab Rev. 1999;31:561–587. doi: 10.1081/dmr-100101936. [DOI] [PubMed] [Google Scholar]
- 37.Tellez I, Johnson D, Nagel RL, Cerami A. Neurotoxicity of sodium cyanate. New pathological and ultrastructural observations in Maccaca nemestrina. Acta Neuropathol. 1979;47:75–79. doi: 10.1007/BF00698277. [DOI] [PubMed] [Google Scholar]
- 38.Thilly CH, Contempre B, Vanderpas JB. Excess of thiocyanate and selenium deficiency: cofactors in the etiology of endemic goiter and cretinism in North Zaire. Bull Mem Acad R Med Belg. 1990;145:440–448. discussion 448–450. [PubMed] [Google Scholar]
- 39.Thilly CH, Swennen B, Bourdoux P, Ntambue K, Moreno-Reyes R, Gillies J, Vanderpas JB. The epidemiology of iodine-deficiency disorders in relation to goitrogenic factors and thyroid-stimulating-hormone regulation. Am J Clin Nutr. 1993;57:267S–270S. doi: 10.1093/ajcn/57.2.267S. [DOI] [PubMed] [Google Scholar]
- 40.Tor-Agbidye J, Palmer VS, Lasarev MR, Craig AM, Blythe LL, Sabri MI, Spencer PS. Bioactivation of cyanide to cyanate in sulfur amino acid deficiency: relevance to neurological disease in humans subsisting on cassava. Toxicol Sci. 1999;50:228–235. doi: 10.1093/toxsci/50.2.228. [DOI] [PubMed] [Google Scholar]
- 41.Tshala-Katumbay D, Banea-Mayambu JP, Kazadi-Kayembe T, Nunga M, Bikangi N, Eeg-Olofsson KE, Tylleskär T. Neuroepidemiology of Konzo a Spastic Para-Tetraparesis of Acute Onset in a New Area of the Democratic Republic of Congo. Af J Neuro Sci. 2001a;20:8–12. [Google Scholar]
- 42.Tshala-Katumbay D, Edebol Eeg-Olofsson K, Kazadi-Kayembe T, Fallmar P, Tylleskar T, Kayembe-Kalula T. Abnormalities of somatosensory evoked potentials in konzo--an upper motor neuron disorder. Clin Neurophysiol. 2002a;113:10–15. doi: 10.1016/s1388-2457(01)00705-2. [DOI] [PubMed] [Google Scholar]
- 43.Tshala-Katumbay D, Eeg-Olofsson KE, Kazadi-Kayembe T, Tylleskar T, Fallmar P. Analysis of motor pathway involvement in konzo using transcranial electrical and magnetic stimulation. Muscle Nerve. 2002b;25:230–235. doi: 10.1002/mus.10029. [DOI] [PubMed] [Google Scholar]
- 44.Tshala-Katumbay D, Eeg-Olofsson KE, Tylleskar T, Kazadi-Kayembe T. Impairments, disabilities and handicap pattern in konzo--a non-progressive spastic para/tetraparesis of acute onset. Disabil Rehabil. 2001b;23:731–736. doi: 10.1080/09638280110055075. [DOI] [PubMed] [Google Scholar]
- 45.Tshala-Katumbay D, Mumba N, Okitundu L, Kazadi K, Banea M, Tylleskar T, Boivin M, Muyembe-Tamfum JJ. Cassava food toxins, konzo disease, and neurodegeneration in sub-Sahara Africans. Neurology. 2013;80:949–951. doi: 10.1212/WNL.0b013e3182840b81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tshala-Katumbay DD, Spencer PS. Toxic disorders of the upper motor neuron system. In: Eisen A, Shaw P, editors. Handb Clin Neurol. Vol. 82. Elsevier; New York: 2007. pp. 353–372. [DOI] [PubMed] [Google Scholar]
- 47.Tylleskar T, Legue FD, Peterson S, Kpizingui E, Stecker P. Konzo in the Central African Republic. Neurology. 1994;44:959–961. doi: 10.1212/wnl.44.5.959. [DOI] [PubMed] [Google Scholar]
- 48.Vanderpas J, Bourdoux P, Lagasse R, Rivera M, Dramaix M, Lody D, Nelson G, Delange F, Ermans AM, Thilly CH. Endemic infantile hypothyroidism in a severe endemic goitre area of central Africa. Clin Endocrinol (Oxf) 1984;20:327–340. doi: 10.1111/j.1365-2265.1984.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 49.WHO. Konzo– a distinct type of upper motor neuron disease. Wkly Epidemiol Rec. 1996;71:225–232. [Google Scholar]